Transcranial direct current stimulation (tDCS) has been identified as a potential tool in the rehabilitation of cerebellar disease. We investigated whether tDCS of the cerebellum and primary motor cortex could alleviate motor impairments of subjects with cerebellar degeneration. The present study did not find stimulation effects of tDCS in young controls, aging controls, and individuals with cerebellar degeneration during reach adaptation. Our results require a re-evaluation of the clinical potential of tDCS in cerebellar patients.
Keywords: tDCS, cerebellar degeneration, motor adaptation, rehabilitation
Abstract
Several studies have identified transcranial direct current stimulation (tDCS) as a potential tool in the rehabilitation of cerebellar disease. Here, we tested whether tDCS could alleviate motor impairments of subjects with cerebellar degeneration. Three groups took part in this study: 20 individuals with cerebellar degeneration, 20 age-matched controls, and 30 young controls. A standard reaching task with force-field perturbations was used to compare motor adaptation among groups and to measure the effect of stimulation of the cerebellum or primary motor cortex (M1). Cerebellar subjects and age-matched controls were tested during each stimulation type (cerebellum, M1, and sham) with a break of 1 wk among each of the three sessions. Young controls were tested during one session under one of three stimulation types (anodal cerebellum, cathodal cerebellum, or sham). As expected, individuals with cerebellar degeneration had a reduced ability to adapt to motor perturbations. Importantly, cerebellar patients did not benefit from anodal stimulation of the cerebellum or M1. Furthermore, no stimulation effects could be detected in aging and young controls. The present null results cannot exclude more subtle tDCS effects in larger subject populations and between-subject designs. Moreover, it is still possible that tDCS affects motor adaptation in cerebellar subjects and control subjects under a different task or with alternative stimulation parameters. However, for tDCS to become a valuable tool in the neurorehabilitation of cerebellar disease, stimulation effects should be present in group sizes commonly used in this rare patient population and be more consistent and predictable across subjects and tasks.
NEW & NOTEWORTHY Transcranial direct current stimulation (tDCS) has been identified as a potential tool in the rehabilitation of cerebellar disease. We investigated whether tDCS of the cerebellum and primary motor cortex could alleviate motor impairments of subjects with cerebellar degeneration. The present study did not find stimulation effects of tDCS in young controls, aging controls, and individuals with cerebellar degeneration during reach adaptation. Our results require a re-evaluation of the clinical potential of tDCS in cerebellar patients.
the cerebellum is widely regarded as an essential structure for motor control and motor adaptation. Damage to the cerebellum leads to a number of specific motor impairments, commonly referred to as ataxia (Flourens 1824; Holmes 1908). One specific symptom of ataxia, easily reproduced in the laboratory, is the difficulty in adapting to perturbations of the motor system (Sanes et al. 1990). Specifically, with regard to reaching movements, patients with cerebellar degeneration demonstrate impaired motor adaptation during reaching tasks with force-field (Maschke et al. 2004) and visuomotor perturbations (Tseng et al. 2007). Whereas the motor performance deficits of patients with cerebellar degeneration are well described, the therapeutic options for the treatment of cerebellar disease are limited (Ilg et al. 2014; Marsden and Harris 2011).
In recent years, transcranial direct current stimulation (tDCS) has accrued considerable interest of the neuroscientific community for its scientific applications and therapeutic potential (Fregni and Pascual-Leone 2007; Nitsche et al. 2008; Stagg and Nitsche 2011). The technique has been identified as a possible tool in the rehabilitation after stroke (Hummel and Cohen 2006), and it has also been suggested recently that it may provide benefits to patients with cerebellar disease (Grimaldi et al. 2016). Several studies have explored the physiological basis of tDCS effects and provide us with likely mechanisms of how tDCS can aid in neurorehabilitation. The studies demonstrated that polarity-specific excitability changes and long-term potentiation-like plasticity are induced by stimulation of the primary motor cortex (M1) (Nitsche et al. 2000; Stagg and Nitsche 2011). Furthermore, it was demonstrated that cerebellar tDCS can influence the excitability of the cerebellum, which in turn, has a polarity-specific effect on cerebellar–M1 connectivity (Galea et al. 2009). These underlying mechanisms likely form the basis of tDCS effects that are observed in motor adaptation experiments. For instance, in healthy subjects, adaptation to motor perturbations is quicker when anodal tDCS is applied over the cerebellum (Avila et al. 2015; Block and Celnik 2013; Herzfeld et al. 2014; Jayaram et al. 2012). Moreover, short-term retention (Galea et al. 2011; Hunter et al. 2009; Panouillères and Jenkinson 2015) and long-term retention (Reis et al. 2009) are improved when anodal tDCS is applied over M1. Motor adaptation experiments using noninvasive brain stimulation in cerebellar patients are much rarer, but two recent pilot experiments have revealed behavioral improvements in cerebellar patients during tDCS stimulation (Grimaldi et al. 2014; Pozzi et al. 2014). This has further established the potential role for tDCS in the treatment of degenerative cerebellar ataxia. However, it remains unclear if all aforementioned effects of tDCS apply to cerebellar ataxia patients, since cerebellar degeneration may hamper tDCS excitability effects (Ugawa et al. 1994).
On the other hand, a recent study identified several areas of the cerebellum that degenerate similarly in both cerebellar ataxia patients and healthy, aging subjects (Hulst et al. 2015). Although older adults are generally slower to adapt to perturbations of the motor system (Seidler et al. 2010), motor adaptation in healthy, elderly subjects is enhanced when anodal tDCS is applied to the cerebellum (Hardwick and Celnik 2014). Therefore, motor adaptation may be enhanced in patients after cerebellar tDCS as well. Likewise, M1 stimulation improves motor learning and retention in aging subjects (Goodwill et al. 2013; Panouillères and Jenkinson 2015; Zimerman et al. 2013), which in turn, indicates potential beneficial effects of M1 stimulation for cerebellar patients.
The aim of the present study was to compare motor adaptation in cerebellar patients with age-matched controls and to test whether the positive effects of tDCS, as described in healthy aging, could also be established in cerebellar patients. Furthermore, a second between-subject experiment was carried out in a group of young controls to control for possible carryover effects. A standard reaching task with force-field perturbations was used to measure motor adaptation. Neuroimaging was used to identify the degree and pattern of cerebellar degeneration in patients. We expected to find impaired motor adaptation in cerebellar patients, characterized by slower and incomplete adaptation to the force-field perturbations. If tDCS could significantly alleviate the difficulties that patients have in adapting to perturbations, then this would further suggest a potential role for tDCS as a supportive treatment in cerebellar disease.
METHODS
Experiment 1
Participants.
Twenty individuals with cerebellar degeneration (8 women; mean age ± SD of 53.7 ± 10.8 yr; range 30–74 yr) and 20 age-matched controls without any known neurological diseases (9 women; mean age ± SD of 54.6 ± 11.2 yr; range 28–74 yr) participated in this study. All subjects were right handed, as assessed by the Edinburgh Handedness Inventory (Oldfield 1971). An overview of the subjects’ characteristics can be found in Table 1. The severity of cerebellar symptoms in cerebellar participants was assessed by one of two experienced neurologists (D. Timmann and M. Küper), based on the International Cooperative Ataxia Rating Scale (ICARS) (Trouillas et al. 1997) and the Scale for the Assessment and Rating of Ataxia (Schmitz-Hübsch et al. 2006). Eight cerebellar participants had a genetically defined spinocerebellar ataxia (SCA). Four participants presented with autosomal dominant cerebellar ataxia (ADCA) type III. Seven cerebellar participants had sporadic adult onset ataxia (SAOA) of unknown etiology. One cerebellar participant presented with cerebellar degeneration caused by cerebellitis. These disorders are known primarily to affect the cerebellum (Gomez et al. 1997; Timmann et al. 2009). All subjects gave informed oral and written consent. The experiment was approved by the Ethics Committee of the medical faculty of the University of Duisburg-Essen and conducted in accordance with the Declaration of Helsinki.
Table 1.
Overview of cerebellar subjects and control subjects
| Cerebellar Subjects |
Controls |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Sex | Diagnosis | Disease Duration, yr | ICARS (total/100) | ICARS UL (total/20) | LI | ID | Age | Sex | LI |
| P01 | 30 | M | SAOA | 9 | 38.5 | 7.5 | 0.16 | C01 | 28 | M | 0.79 |
| P02 | 34 | M | SAOA | 22 | 17 | 3 | 0.46 | C02 | 33 | M | 0.69 |
| P03 | 46 | M | SAOA | 7 | 8 | 0 | 0.50 | C03 | 47 | M | 0.55 |
| P04 | 47 | M | ADCA III | 17 | 32.5 | 4.5 | 0.18 | C04 | 47 | M | 0.77 |
| P05 | 48 | M | SCA 14 | 25 | 20 | 3 | 0.22 | C05 | 50 | M | 0.81 |
| P06 | 48 | F | ADCA III | 28 | 19 | 1 | 0.33 | C06 | 47 | F | 0.75 |
| P07 | 50 | F | SCA 14 | 17 | 17 | 1 | 0.30 | C07 | 52 | F | 0.61 |
| P08* | 51 | M | SAOA | 20 | 61 | 9 | −0.08 | C08* | 55 | F | 0.50 |
| P09 | 51 | M | ADCA III | 11 | 47 | 5 | 0.30 | C09 | 51 | M | 0.59 |
| P10 | 52 | M | ADCA III | 6 | 19.5 | 3 | 0.43 | C10 | 54 | M | 0.67 |
| P11 | 53 | M | Cerebellitis | 10 | 46 | 5 | 0.25 | C11 | 63 | M | 0.72 |
| P12 | 54 | F | SCA 14 | 25 | 27 | 3.5 | 0.40 | C12 | 55 | F | 0.68 |
| P13 | 54 | F | SAOA | 18 | 31 | 4.5 | 0.30 | C13 | 55 | F | 0.57 |
| P14 | 58 | F | SCA 6 | 8 | 43.5 | 10 | 0.34 | C14 | 57 | F | 0.67 |
| P15 | 61 | M | SCA 6 | 4 | 9 | 0 | 0.20 | C15 | 63 | M | 0.74 |
| P16 | 63 | M | SAOA | 9 | 20.5 | 5 | 0.56 | C16 | 64 | M | 0.70 |
| P17 | 66 | F | SCA 6 | 12 | 43.5 | 5 | 0.19 | C17 | 64 | F | 0.71 |
| P18 | 66 | F | SCA 6 | 15 | 47 | 5 | 0.28 | C18 | 65 | F | 0.78 |
| P19 | 67 | F | SCA 6 | 3 | 33 | 5 | 0.22 | C19 | 68 | F | 0.64 |
| P20 | 74 | M | SAOA | 16 | 12 | 2 | 0.22 | C20 | 74 | M | 0.58 |
Cerebellar subjects were age matched with the control subject on the right. ICARS, International Cooperative Ataxia Rating Scale (Trouillas et al. 1997); ICARS UL, score of right upper limb in finger-to-nose test, finger-to-finger test, pronation/supination, and Archimedes spiral drawing; LI, average final learning index over 3 experimental sessions; SAOA, sporadic adult onset ataxia; ADCA III, autosomal dominant ataxia type III; SCA 6/14, spinocerebellar ataxia types 6/14. Disease duration is years since presentation of the first symptoms.
Cerebellar subject and age-matched control, which were removed due to a high number of disregarded movements in the cerebellar subject.
Task.
All subjects participated in a standard force-field task (Shadmehr and Mussa-Ivaldi 1994) on a setup largely similar to the one used by Rabe and colleagues (2009). Subjects held the handle of a two-joint robotic manipulandum in their right hand while seated in a comfortable chair. The handle of the robotic manipulandum was able to move freely in the horizontal plane underneath a horizontal projection screen located above the subject’s hand (Fig. 1A). Hand position was recorded using encoders on each of the manipulandum’s motors with a resolution of 106 counts per revolution and a sample rate of 200 Hz using the analog inputs of a motor controller card (DMC-1826; Galil Motion Control, Rocklin, CA). The distance to the manipulandum and the chair height were adjusted individually to ensure a comfortable position and good vision of the projection screen. Vision of a subject’s arm was blocked by a cloth stretched from the projection screen to the subject’s neck. The position of the handle was represented by a green circular cursor with a diameter of 6 mm and directly corresponded with the position of the right hand of the subject. The origin was indicated by a black circle with a diameter of 14 mm in the middle of the projection screen. Participants were instructed to move the cursor into the origin at the start of each trial. After a delay of 2,500 ms, a black circular target with a diameter of 14 mm appeared in one of six target locations positioned 10 cm from the origin. The target locations were located at an angle of 30, 90, 150, 210, 270, and 330° from the origin and were presented in a pseudorandom order so that each target position appeared once every six trials. Subjects were instructed to move the cursor from the origin to the target by making a rapid hand movement as soon as the target appeared (Fig. 1B). Subjects received instructions to move through the target and not stop at the target location, as the handle was gently brought to a stop by a simulated cushion implemented by the manipulandum motors. The cursor was extinguished after it passed out of a 10-cm radius from the position of the origin. The disappearance of the cursor indicated the end of a movement, at which point, a cushioning force was applied by the robot to slow the hand to a stop safely. After the movement ended, the robot motors pushed the handle of the manipulandum back to the starting position. The cursor reappeared when the handle came within 2 cm of the origin location. During the cushion phase and push-back phase, subjects received feedback on movement speed and whether they hit the circular target. When subjects hit the target, and the movement was neither too fast nor too slow, the target became green, and a sound was played to indicate success. When subjects hit the target, but the movement was too fast, the target turned yellow. The target turned blue when the cursor hit the target, but the movement was too slow. If participants failed to hit the target, then the target was turned off and provided no further feedback. An adaptive mechanism based on movement duration was used to determine whether a movement was too fast or too slow or had the correct speed. Initially, a movement was considered to have the correct speed when it was completed within a time window centered at 500 ms with an upper and lower bound of 250 ms. In other words, movement durations between 250 and 750 ms were considered as having the correct speed, movement durations below 250 ms were considered too fast, and movement durations above 750 ms were considered as too slow. From the first movement onward, the upper and lower bound of the time window was reduced by 10% each time a movement was the correct speed and increased by 10% when a movement was either too fast or too slow. The adaptive time window and feedback from the experimenter encouraged subjects to move at similar speeds, and it also made sure participants received similar amounts of positive feedback, thereby preventing motivational differences that might be driven by differences in performance.
Fig. 1.
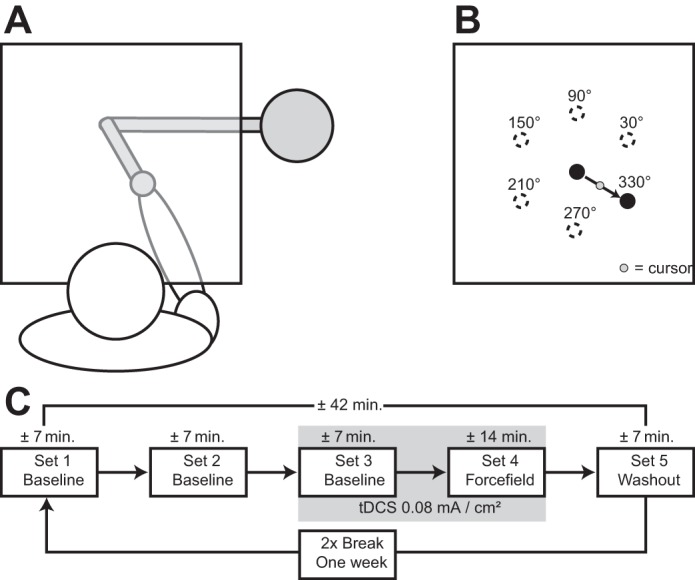
A: an overview of the setup used in the behavioral task. Drawings are not to scale. The horizontal projection screen is illustrated as transparent for the purpose of this figure. Subjects were unable to see the position of their own hand and robot manipulandum in the experiment, because the horizontal screen was covered with a blank paper. A piece of cloth stretched from the screen to the subject’s neck blocked vision of the subject’s arm. B: targets were located 10 cm from the origin at 30, 90, 150, 210, 270, and 330° around the origin location. Subjects were instructed to move the cursor from the origin to the target as soon as the target appeared. C: subjects performed 5 sets of movements in quick succession, after which, a break of exactly 1 wk followed. Upon resuming the experiment 1 wk later, subjects started with the first baseline set.
Each participant performed five sets of movements. The first three sets consisted of 84 trials without perturbations to allow participants to familiarize with the task (baseline phase). The fourth set consisted of 168 trials, of which 144 trials were force-field (FF) trials, and 24 trials were catch trials. In FF trials, a velocity-dependent force of 13 N⋅m−1⋅s−1 was applied by the robot motors perpendicular to the movement direction, pushing each participant’s hand in a clockwise direction. In catch trials, no external forces were applied, which in adapted participants, produced movements in the opposite direction of the perturbation. Catch trials to each of the six target locations were pseudorandomly interspersed over the entire set. The final set consisted of 84 trials without the application of external forces and without feedback on hand position by the cursor, as well as no visual or auditory feedback on movement time and success (washout phase; Fig. 1C).
tDCS stimulation parameters.
Participants were invited for three experimental sessions, separated by exactly 1 wk. During each session, participants performed the same motor adaptation task but under different stimulation regimes. In two of the three sessions, each participant received anodal tDCS stimulation: once over M1 and once over the cerebellum. In the third session, the recipient received sham tDCS stimulation over either M1 or cerebellum. The order of the three sessions was counterbalanced among participants. Stimulation parameters were largely similar to Galea et al. (2011). In short, anodal tDCS was delivered through two rubber electrodes (surface area: 25 cm2) covered with conductive paste (Ten20 Conductive Paste; Weaver and Company, Aurora, CO) via a NeuroConn device (DC-Stimulator Plus; NeuroConn, Ilmenau, Germany). For cerebellar stimulation, the anodal electrode was placed on the position of the right cerebellar cortex, with the center of the anodal electrode 3 cm lateral to the inion, and the cathodal electrode was placed on the right buccinator muscle. The anodal electrode for M1 stimulation was placed by finding the area of the left cortex that elicited a response of the first dorsal interosseous muscle after single transcranial magnetic stimulation (TMS) pulses. TMS was delivered by a Dantec MagPro magnetic stimulator (Tonica Elektronik, Farum, Denmark). The cathodal electrode was placed on the skin overlying the contralateral supraorbital region. During each experimental session, electrodes were placed over all of the stimulation locations, so participants were blinded for stimulation location.
In both cerebellar and M1 anodal stimulation, the target stimulation intensity was set at 2 mA, resulting in a current density of 0.08 mA/cm2. At the start of the third baseline set, current was ramped up from 0 to 2 mA in a period of 30 s, after which, anodal tDCS was applied for the entirety of the third and fourth sets (last baseline set and force-field set). Due to variances in movement times among subjects, this resulted in slightly different stimulation times for each subject. On average, subjects were stimulated for ∼22 min (mean duration 1,289 ± SD 150 s). At the end of tDCS stimulation, current was ramped down from 2 to 0 mA in 30 s. In sham stimulation, current was ramped up in 30 s and remained at 2 mA for a duration of 60 s, after which, current was ramped down again for the remainder of the experiment. This method of applying anodal and sham stimulation has shown achievement of a good level of blinding for participants (Gandiga et al. 2006). One experimenter (L. John) ran all of the behavioral experiments and used a prepared set of stimulation codes to remain blind for stimulation polarity (sham or anodal). An experimenter (B. Brol), who was not involved in the collection of behavioral data, deblinded the stimulation codes after data collection had ended.
Analysis of behavioral data.
Behavioral data were analyzed using MATLAB with the Statistics Toolbox (MATLAB 8.5; MathWorks, Natick, MA). Baseline aiming errors (AEs) in the second baseline set (before tDCS onset) were averaged per target direction and subtracted from AEs in the force-field and washout phase to correct for movement biases. Short movements with <2 cm of travel distance, movements in which hand velocity did not exceed 0.12 m/s, and movements with AEs more than four absolute deviations from the median were discarded. Effectively, this removed trials in which a subject did not move or made a movement with no effective movement toward the target direction (i.e., oscillating movements around the starting location). In the case of one cerebellar participant (P08 in Table 1), this led to >50% of all trials being filtered, so we decided to exclude this participant and his age-matched control from group analyses. On average, ∼2.7% of all movements were filtered, with the amount of filtered trials not being significantly different between cerebellar subjects and healthy controls (paired t-test, t18 = 0.06, P = 0.94; range 0.8–9.1%).
Movement onset was defined to be the first time in a trial at which hand speed exceeded 0.04 m/s. For each trial, a straight movement would be along a line from the position at movement onset to the target. For each time point in each movement, the perpendicular distance of the cursor from the line determining a straight movement was called the error. The moment of maximum error was determined by finding the maximum perpendicular displacement (PD) after movement onset. The AE—our primary measure of the error in each movement—was then the angle between a straight-line movement and a line from the starting position to the position of maximum error. A learning index (LI) was also calculated based on the amount of AE in FF trials vs. catch trials. This measure of learning has been described previously (Criscimagna-Hemminger et al. 2003; Maschke et al. 2004) and corrects for subjects stiffening the arm in FF trials to compensate for movement errors. When stiffening the arm, performance in FF trials will improve, but catch trials will not show a significant AE in the opposite direction (Smith and Shadmehr 2005). The LI was calculated as follows:
In this definition, LIs can range between −1 and 1, with values between −1 and 0 indicating no learning and a LI of 1 indicating maximum learning. The LI was calculated per bin of seven consecutive trials (6 FF trials and 1 catch trial). To assess final performance values, the LI was calculated over a bin of 36 trials (30 FF trials and 6 catch trials in each target direction). Mixed-design ANOVAs in SPSS (SPSS Statistics 23.0; IBM, Armonk, NY) were used to test for differences in average movement times and speeds, with the between-factor group (cerebellar subjects or healthy controls) and within-factors stimulation type (cerebellum, M1, or sham) and set number. Greenhouse-Geisser adjustments were performed where appropriate. Differences in AEs during adaptation and washout were tested with a linear mixed model, assuming compound symmetry for the fixed effects of group, simulation type, and bin number. Differences in final LIs were tested with the fixed effects of group and stimulation type. The unknown parameters in all mixed models were estimated via restricted maximum likelihood estimation, and we report adjusted type III errors for the fixed effects. The degrees of freedom were estimated via Satterthwaite approximation. The linear mixed models described are essentially similar to a repeated-measures (RM) ANOVA but also allow us to control for a continuous covariate (movement time). Furthermore, order effects were analyzed by testing with measurement day as a factor, instead of stimulation type, for both AEs and final LIs. The P values of pairwise comparisons were adjusted for multiple comparisons using the Bonferroni correction. Null results were tested for equivalence using the two, one-sided test procedure (Schuirmann 1987; Seaman and Serlin 1998).
Magnetic resonance imaging.
MR images in cerebellar subjects and their age-matched controls consisted of high-resolution, three-dimensional, T1-weighted Magneticization Prepared Rapid Acquisition Gradient Echo scans using a Magnetom Skyra 3T MRI scanner (Siemens Medical Solutions, Malvern, PA) with a 20-channel head/neck coil (repetition time = 2,500 ms, echo time = 4.37 ms, inversion time = 1,100 ms, flip angle = 7°, matrix = 256 × 100, voxel size = 1.0 × 1.0 × 1.0 mm3). All MR scans were evaluated by an experienced neuroradiologist (S. L. Göricke). None of the participants had radiological pathologies outside of the cerebellum.
Voxel-based morphometry.
A voxel-based morphometry analysis was applied to the cerebellum of each subject, as described previously (Hulst et al. 2015; Taig et al. 2012). The procedure will be explained briefly. The analysis was automated with an in-house program written for MATLAB 8.5, using the spatially unbiased infratentorial template (SUIT Toolbox, version 3.1) (Diedrichsen et al. 2009), implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). First, each subject’s brain was segmented into gray matter, white matter, and cerebrospinal fluid. Next, the gray matter of each subject’s cerebellum was normalized onto the template SUIT cerebellum. Each normalized cerebellum was then smoothed using a 6 × 6 × 6-mm3 median filter. To test for differences in gray matter volume between cerebellar subjects and age-matched controls, a paired sample t-test was performed on the gray-matter volume of individual voxels. The resulting map of t-scores was smoothed using a minimum filter, substituting each voxel with the minimum t-score in a 3 × 3 × 3-mm3 neighborhood. To correct for multiple testing, 500 permutations maps of the original data set were generated, where for each permutation, the match between MRI data and subject category (cerebellar subject or control) was randomized. The maximum t-score of each minimum-filtered permutation map was determined, and a significance threshold was calculated by taking the 95th percentile of all maximum t-scores. Voxels with t-scores above the significance threshold in the original gray-matter map were defined as significant. The result of this analysis was an assessment of the gray-matter volume difference between cerebellar subjects and healthy controls on a voxel-by-voxel basis. To assess the correlation of the final LI with cerebellar volume, Spearman’s correlations between gray-matter volume and final LI were calculated for both subject groups separately. Similarly to our analysis of t-scores, 500 permutations of the original data set were generated, and the significance threshold was calculated by taking the 95th percentile of all maximum correlations. The result of this analysis was an assessment of the correlation between gray-matter volume and learning within the group of cerebellar subjects and within the group of healthy controls.
Experiment 2
The first experiment was carried out using a crossover design, testing the same subjects over multiple sessions under different stimulation conditions. A within-subject design has increased power over a between-subject design, which is an advantage when it is difficult to recruit enough subjects for a sufficiently powered between-subject experiment (such as in the case of cerebellar patients). However, crossover designs can introduce carryover effects between measurement sessions. Although literature has described limited carryover effects during force-field adaptation when proper washout is applied (Caithness 2004), to eliminate the possibility of carryover effects influencing consecutive measurement sessions, an additional between-subject experiment was carried out. The experimental procedures of this second experiment were largely similar to the first experiment, with several important differences described below.
Thirty young, healthy controls were recruited (17 women; mean age ± SD of 23.9 ± 2.7 yr; range 18–29 yr) and gave informed oral and written consent. All subjects were measured during one session of the task described in experiment 1. Ten subjects received sham stimulation of the cerebellum, 10 subjects received anodal stimulation of the cerebellum, and 10 subjects received cathodal stimulation of the cerebellum. No subjects received stimulation of M1. Other stimulation parameters were equal to experiment 1. On average, subjects were stimulated for ∼22 min (mean duration 1,343 ± SD 114 s). The processing and analysis of behavioral data matched the methods described in experiment 1. Approximately 2.7% of all movements were filtered, with the amount of filtered trials not significantly different among groups (F2, 29 = 1.98, P = 0.16; range 0.2–9.9%). No MRI data were collected from these subjects.
RESULTS
Experiment 1
Movement times and speeds.
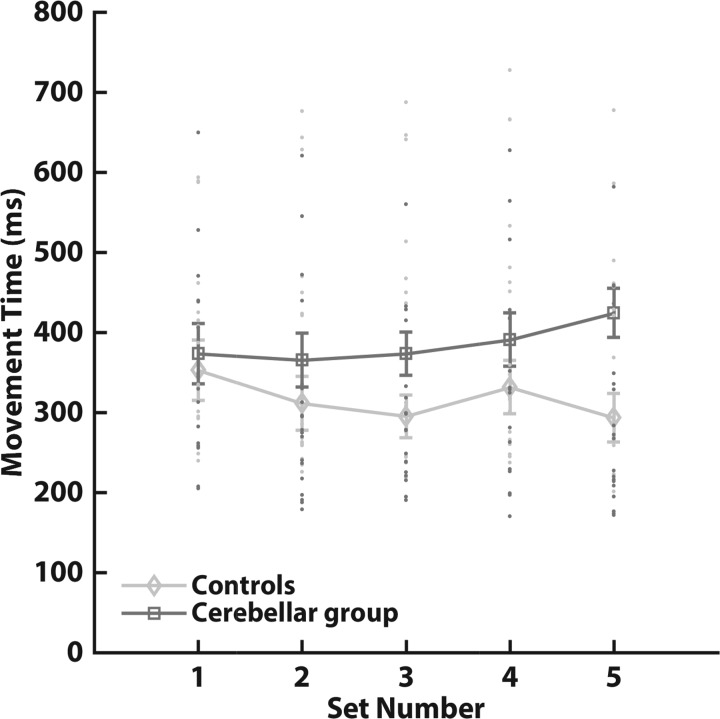
An analysis of average movement times indicated no differences between cerebellar subjects and controls or a main effect of set and stimulation but did uncover an interaction of set and group (Table 2). Analysis of the average movement speeds revealed a significant main effect of group and an interaction between set and group (Table 2). Whereas control subjects, on average, tended to speed up during the experiment, cerebellar subjects slowed down (Fig. 2). Importantly, movement times and speeds in the set with velocity-dependent FF trials (set 4) were not significantly different (movement time, pairwise comparisons, P = 0.22; speed, pairwise comparisons, P = 0.10); thus control subjects and cerebellar subjects were exposed to similar perturbation magnitudes.
Table 2.
ANOVAs of average movement times and speeds
| ANOVA | Factors | F Statistics | P |
|---|---|---|---|
| Average movement time | |||
| Between | Group | F(1, 36) = 2.48 | 0.12 |
| Within | Set | F(1.76, 63.4) = 2.81 | 0.07 |
| Set × group | F(1.76, 63.4) = 6.183 | 0.005* | |
| Stimulation | F(2, 72) = 0.08 | 0.92 | |
| Stimulation × group | F(2, 72) = 0.70 | 0.5 | |
| Set × stimulation | F(3.81, 137) = 1.12 | 0.35 | |
| Set × stimulation × group | F(3.81, 137) = 0.61 | 0.65 | |
| Average movement speed | |||
| Between | Group | F(1, 36) = 4.50 | 0.04* |
| Within | Set | F(2.18, 78.5) = 2.42 | 0.09 |
| Set × group | F(2.18, 78.5) = 8.5 | <0.001* | |
| Stimulation | F(2, 72) = 0.03 | 0.97 | |
| Stimulation × group | F(2, 72) = 1.06 | 0.35 | |
| Set × stimulation | F(3.19, 115) = 1.35 | 0.26 | |
| Set × stimulation × group | F(3.19, 115) = 0.84 | 0.48 |
Boldface highlights important (in-text) comparisons.
Significant at the α = 0.05 level.
Fig. 2.
Average movement times of age-matched controls (n = 19; diamonds) and cerebellar subjects (n = 19; squares). Movement times in this figure are averaged over all stimulation types. Movement times were calculated from movement onset to the moment the cursor extinguished after moving out of a 10-cm radius from the starting position. Set 4 refers to the set with force-field perturbation trials. Error bars indicate SE. There was no significant difference in movement times of the fourth set between age-matched controls and cerebellar subjects (pairwise comparisons, P = 0.22).
Data per subject.
An overview of the raw data of one healthy control subject and one cerebellar subject from an adaptation set can be found in Fig. 3. Movement trajectories initially deviate strongly from a straight trajectory in both the control and cerebellar subject. The control subject is able to adapt to the force-field perturbation and produces straighter movement trajectories later in the set (Fig. 3A). As a consequence, the average AE of the control subject during the force-field set decreases, whereas the AE in the negative direction in catch trials increases. This, in turn, leads to a higher LI for the control subject, indicating adaptation to the force-field perturbation. In contrast, the cerebellar subject maintains curved movement trajectories throughout the adaptation set (Fig. 3B). The average AE does not decrease significantly during the set, and catch trials do not show an increase in AEs in the negative direction. Evidently, the LI in the cerebellar subject does not consistently increase during the adaptation set. These observations generalized to other control and cerebellar subjects in the experiment and are consistent with observations of force-field adaptation in controls and patients in previous studies (Donchin et al. 2012; Smith and Shadmehr 2005).
Fig. 3.
Movement trajectories, aiming errors, and learning indices from 2 typical subjects during the adaptation set of a single experimental session. A: healthy control subject (C05; Table 1); B: cerebellar subject (P19; Table 1). Left: movement trajectories early and late in adaptation. Dashed lines represent movement trajectories of the first 12 movements in the set, and solid lines represent movement trajectories of the last 12 movements in the set. The target locations are depicted by white circles. Middle: aiming errors (AE; degrees) of perturbed trials and catch trials corresponding to the same set of the movement trajectories. Solid lines represent the average aiming error during force-field trials. Aiming errors were averaged over bins of 6 trials. Stars represent the aiming error of catch trials. When a subject adapts to the force field, the average aiming error will decrease, and catch trials will show increasing errors in the opposite direction of the force. Right: learning index (LI) during the force-field set. The learning index was calculated over bins of 6 perturbed trials and 1 catch trial. Solid lines represent the average learning index during adaptation. Shaded areas of the plot represent the bins over which the final learning index was calculated (last 6 bins).
Movement trajectories and perpendicular velocities.
Next, average movement trajectories and perpendicular velocities were compared among stimulation types in control subjects and cerebellar subjects. Figure 4 depicts the movement trajectories and perpendicular velocities during the last baseline trials and various stages of the force-field set (early, middle, and late adaptation). During the last trials of the baseline set (bins 11 and 12), the movement trajectories were relatively straight in both control and cerebellar subjects, and there was no apparent difference among the stimulation types (Fig. 4, A and C). During early adaptation (bins 1 and 2 of the force-field set), movement trajectories of control and cerebellar subjects deviated strongly from a straight trajectory to the target. Control subjects adapted more quickly and adequately to the force-field perturbation than cerebellar subjects, illustrated by straighter movement trajectories in the middle of the set (bins 12 and 13) and late in the set (bins 23 and 24). Importantly, in both groups, the development of the average movement trajectories was indistinguishable among stimulation types. This observation will be quantified when AEs are analyzed below (see Average AEs).
Fig. 4.
Average movement trajectories and perpendicular velocity traces of age-matched controls (n = 19) and cerebellar subjects (n = 19). Movements were rotated so that each movement was toward the same target direction and then averaged over 2 bins (12 trials). Movement trajectories and velocity traces show means ± SE A: movement trajectories of control subjects. Movements start at the bottom of the panel and end at the top. Baseline refers to the last 12 baseline trials (bins 11 and 12), early refers to bins 1 and 2 of the force-field set, middle refers to bins 12 and 13, and late refers to bins 23 and 24. CB, Cerebellar stimulation. B: velocity perpendicular to target direction in control subjects. C: movement trajectories of cerebellar subjects. D: velocity perpendicular to target direction in cerebellar subjects. There was no significant difference in perpendicular velocity at 55 ms among stimulation types (F2, 71.1 = 1.03, P = 0.36).
The perpendicular velocity traces painted a similar picture. Control subjects demonstrated smaller perpendicular velocities than cerebellar subjects earlier in the set (Fig. 4, B and D). Furthermore, only control subjects developed a slight overcompensation for the perturbation early in the movement, illustrated by the negative perpendicular velocity, ∼50–60 ms in the second half of the set. This is regarded as a characteristic of force-field learning in healthy individuals (Izawa et al. 2008) and is reduced in individuals with cerebellar damage (Criscimagna-Hemminger et al. 2010). Previously, tDCS effects have been described on the development of overcompensation in force-field learning (Herzfeld et al. 2014). However, tDCS effects on perpendicular velocity during overcompensation were not apparent in this experiment. This observation was quantified by performing a statistical analysis on perpendicular velocity at the moment of maximum overcompensation, defined as 55 ms after movement onset (between factor: group; within factor: stimulation type, bin number). As with the movement trajectories, the perpendicular velocity traces were indistinguishable among stimulation types in control subjects and cerebellar subjects [F2, 71.1 = 1.03, P = 0.36, 95% confidence intervals (CIs) of difference (−0.19 cm/s, 0.06 cm/s) and (−0.19 cm/s, 0.06 cm/s) for sham vs. M1 stimulation and sham vs. cerebellum stimulation, respectively], whereas cerebellar subjects did not show overcompensation compared with controls (F1, 35.1 = 13.4, P = 0.001).
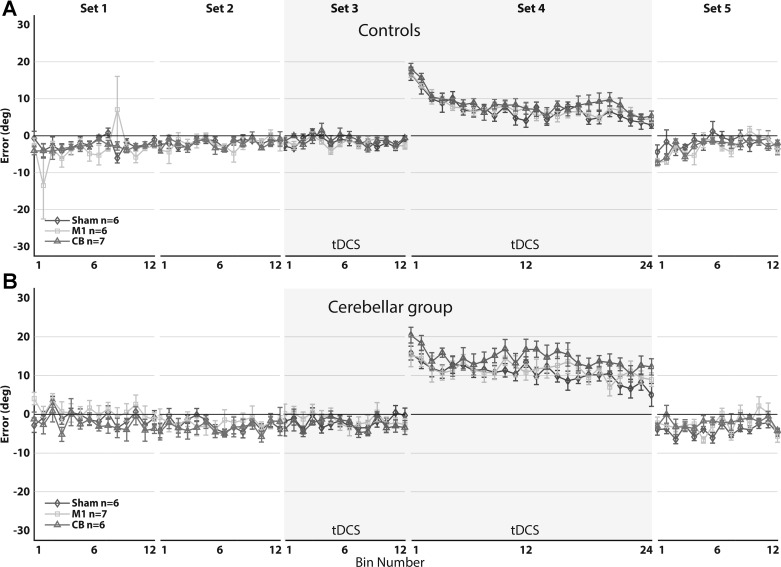
Average AEs.
Figure 5 provides an overview of the development of average AEs during all sets in control subjects and cerebellar subjects. AEs in control and cerebellar subjects reach near-zero values during baseline and do not differ among stimulation conditions [F2, 71.1 = 0.67, P = 0.51, 95% CIs of difference (−0.43°, 1.12°) and (−0.50°, 1.06°) for sham vs. M1 stimulation and sham vs. cerebellum stimulation, respectively]. Equivalency among stimulation types during baseline was established with the smallest effect size of interest (SESOI), set at −1° and +1° (P = 0.02 and P = 0.01 for sham vs. M1 stimulation and sham vs. cerebellum stimulation, respectively).
Fig. 5.
A: mean aiming errors of healthy, aging controls (n = 19). B: mean aiming errors of cerebellar subjects (n = 19). Mean aiming errors (degrees) are shown during baseline (sets 1–3), force-field adaptation (set 4), and washout (set 5) for sham (diamonds), M1 (squares), and cerebellum (triangles) stimulation. Aiming errors were averaged over bins of 6 movements. Sets 4 and 5 were corrected for baseline movement biases. Shaded areas represent the sets in which tDCS was applied. Error bars indicate SE. There was no significant difference in mean aiming errors of the adaptation set among stimulation types (F2, 71.2 = 2.31, P = 0.11).
Initially, AEs are high in the positive direction during the force-field set but decrease as subjects adapt to the perturbation. Control subjects make movements with negative AEs (toward the direction of the force field) in the beginning of the washout set, exhibiting an aftereffect of the perturbation. To assess the effects of tDCS during adaptation and washout, we analyzed the means of AEs of healthy controls and cerebellar subjects in the fourth and fifth set. This analysis did not reveal a main effect of stimulation type in both the adaptation set and the washout set, and there was no significant interaction of stimulation and group in the adaptation set and washout set (Table 3). When comparing mean AEs of controls and cerebellar subjects, we found a significant difference during adaptation but not of aftereffects in the washout set (Table 3). The analysis further revealed a main effect of bin number in the adaptation set and interaction effects of bin number and group, indicating that subjects significantly decrease AEs as the set progresses but healthy controls more than cerebellar subjects (Table 3). In the washout set, we saw similar main effects of bin number and interaction effects of bin number and group, indicating different washout effects between healthy controls and cerebellar subjects (Table 3).
Table 3.
Linear mixed model aiming errors with movement time as continuous covariate
| Linear Mixed Model | Fixed Effects | F Statistics | P |
|---|---|---|---|
| Aiming errors adaptation | |||
| Between | Group | F(1, 36.2) = 23.6 | <0.001* |
| Within | Bin | F(23, 828) = 39.9 | <0.001* |
| Bin × group | F(23, 828) = 6.16 | <0.001* | |
| Stimulation | F(2, 71.2) = 2.31 | 0.11 | |
| Stimulation × group | F(2, 71.6) = 0.86 | 0.43 | |
| Bin × stimulation | F(46, 1656) = 0.84 | 0.77 | |
| Bin × stimulation × group | F(46, 1656) = 0.74 | 0.90 | |
| Aiming errors washout | |||
| Between | Group | F(1, 38) = 0.30 | 0.59 |
| Within | Bin | F(13, 470) = 10.9 | <0.001* |
| Bin × group | F(13, 468) = 6.94 | <0.001* | |
| Stimulation | F(2, 71.9) = 1.01 | 0.37 | |
| Stimulation × group | F(2, 71.7) = 1.07 | 0.35 | |
| Bin × stimulation | F(26, 934) = 1.36 | 0.11 | |
| Bin × stimulation × group | F(26, 933) = 0.95 | 0.54 |
Boldface highlights important (in-text) comparisons.
Significant at the α = 0.05 level.
To investigate the null result of stimulation on average AEs during the adaptation set, group means were tested for equivalency, and 95% CIs of differences were calculated. Although equivalency among group means could not be established [SESOI = (−1°, +1°), P = 0.35 for sham vs. M1 stimulation and P = 0.29 for sham vs. cerebellum stimulation], the CIs show that the differences between sham and M1 stimulation [95% CI of difference (−1.91°, 0.22°)] and sham and cerebellum stimulation [95% CI of difference (−1.83°, 0.30°)] are small. Furthermore, the mean AE of sham stimulation (8.98 ± SE 0.58°) was, on average, slightly lower than M1 stimulation (9.83 ± SE 0.58°) and cerebellar stimulation (9.74 ± SE 0.58°), which is against the prediction of improved learning with anodal tDCS.
Similar analyses were performed to investigate the null result of stimulation in the washout set. Equivalency could not be established between sham and M1 stimulation [SESOI = (−1°, +1°), P = 0.06] but could be established between sham and cerebellar stimulation (P = 0.01). During washout, the differences between sham and M1 stimulation [95% CI of difference (−1.30°, 0.39°)] and sham and cerebellar stimulation [95% CI of difference (−0.91°, 0.77°)] were also small. Two additional analyses were performed to compare AEs in the second baseline set with AEs in the washout set. This revealed that control subjects initially made movements with negative AEs in the washout set, which differentiated significantly from AEs in the second baseline set (F1, 35.9 = 11.1, P = 0.002), indicating an aftereffect of the force-field perturbation. Cerebellar subjects made movements in the washout set, which could not be distinguished from baseline set movements (F1, 35.3 = 0.80, P = 0.38), indicating no aftereffects in cerebellar subjects.
Next, AEs were analyzed over measurement day to test for carryover effects. The analysis of AEs over measurement day was carried out separately for control subjects and cerebellar subjects, because we noticed a marked decrease in the means of controls with measurement day but not in cerebellar subjects. For control subjects, there was a significant effect of measurement day (F2, 35.0 = 4.82, P = 0.01). The mean AE of the first day (7.71° ± SE 0.52°) was significantly higher than the third day (6.44° ± SE 0.52°, P = 0.02), and there was a trend of a difference between the first day and second day (6.67 ± SE 0.52°, P = 0.06), both indicating a slight carryover effect. The 95% CIs for the difference in means were (−0.04°, 2.14°) for day 1 vs. day 2 and (0.17°, 2.38°) for day 1 vs. day 3.
No significant effect of measurement day could be detected in the cerebellar group (F2, 36.0 = 0.11, P = 0.89). Although equivalency between measurement days in cerebellar subjects was not established [SESOI = (−1°, +1°), P = 0.19 and P = 0.15 for day 1 vs. day 2 and day 1 vs. day 3, respectively], no indication of a carryover effect was observed in the means of AEs (day 1: 12.2° ± SE 1.01°, day 2: 11.9° ± SE 1.01°, day 3: 12.2° ± SE 1.01°) or 95% CIs of differences in means [(−1.54°, 2.20°) for day 1 vs. day 2 and (−1.82°, 1.93°) for day 1 vs. day 3]. We also separately analyzed AEs of subjects during the first session only. This can be regarded as a between-subject comparison of the stimulation effect during the first session (i.e., without possible carryover effects). Here, as well, we could not detect stimulation effects on average AEs (F2, 31.8 = 1.66, P = 0.21). The power of this between-subject analysis is low, due to the limited amount of subjects (n = 6 or 7 for each stimulation type), but the results are in accordance with the findings of the main results of the within-subject experiment. Figure 6 provides an overview of the development of AEs on the first day of measurement. From the figure, one could conclude that there is a trend of a difference among the stimulation types in the cerebellar patient group; i.e., the cerebellar stimulation group seems to be learning slower than other stimulation types. However, this is due to one particularly slow learner in the cerebellar stimulation group on the first day.
Fig. 6.
A: mean aiming errors of healthy, aging controls on the first measurement day (n = 6/7). B: mean aiming errors of cerebellar subjects on the first measurement day (n = 6/7). Mean aiming errors (degrees) are shown during baseline (sets 1–3), force-field adaptation (set 4), and washout (set 5) for sham (diamonds), M1 (squares), and cerebellum (triangles) stimulation. Aiming errors were averaged over bins of 6 movements. Sets 4 and 5 were corrected for baseline movement biases. Shaded areas represent the sets in which tDCS was applied. Error bars indicate SE. There was no significant difference in mean aiming errors of the adaptation set among stimulation types (F2, 31.8 = 1.66, P = 0.21).
To test whether nonsignificant stimulation effects were due to a lack in statistical power, a post hoc power analysis was conducted in G*Power (Faul et al. 2007), as well as a simulation-based power analysis. The simulation-based power analysis was carried out to control for differences in RM ANOVAs and our statistical model, since G*Power can calculate power of RM ANOVAs but not of linear mixed models. In the stimulation-based approach, we approximated power by generating many (n = 1,000 per effect size) permutations of the original data set, where the alternative hypothesis was true. That is, for each of the permutations, the true effect of tDCS was not zero, with effect sizes ranging between 0.05 and 1.00. Then, we calculated the fixed effect of tDCS for each permutation and determined the proportion of significant simulations (P < 0.05) for each effect size. We wanted to find out what the minimum effect size necessary was to reject reliably the null hypothesis and how sensitive our experiment was in picking up clinically relevant stimulation effects. Whereas calculated power was generally similar between G*Power and simulations, in cases where there was a small difference, we report the results of the analysis with the lowest power.
For two groups of 19 subjects (total n = 38) with α = 0.05 and 1 − β = 0.80, we calculated that we could reliably reject the null hypothesis if the true effect size were Cohen’s f ≥ 0.21 or higher (Cohen’s d ≥ 0.42). By taking into account carryover effects, we also calculated the minimum true effect size required if we only conducted the experiment in one subject group (total n = 19; e.g., only the cerebellar group). With α = 0.05, 1 − β = 0.80, and n = 19, we could reliably reject the null hypothesis if the true effect size were f = 0.30 or higher (d ≥ 0.6). This would mean that given the SD of the cerebellar group, we could reliably detect AEs of 2.6° and larger. To put this into perspective, the difference in AE between control subjects and cerebellar subjects that we found is ∼5°. Galea et al. (2011) report a difference of ∼5° between sham and cerebellar stimulation during reaching adaptation in a group of young controls. A recent study by Jalali et al. (2017) reports d = 0.7 of pooled experimental data (difference of ± 2.6°) in similar tasks and young control subjects. Others (Hashemirad et al. 2016; Minarik et al. 2016) have suggested a true effect size of tDCS ∼d = 0.5, depending on the task. We thus fully expected to pick up on clinically relevant stimulation effects, even in groups of 19 subjects.
Learning index.
The final measure of performance for each stimulation type was calculated by taking the LI of the last six bins (30 FF trials and 6 catch trials in each target direction). An overview of the development of the LI through the adaptation set averaged over stimulation types is shown in Fig. 7. As expected, healthy controls initially improve their performance strongly, after which, they plateau around a mean LI of 0.68 ± SD 0.10. The increase of the LI in cerebellar subjects is much less consistent, with a mean LI of 0.31 ± SD 0.11 at the end of the adaptation set.
Fig. 7.
Learning index during the adaptation set averaged across stimulation types for age-matched controls (n = 19, diamonds) and cerebellar subjects (n = 19, squares). Learning index was calculated for each bin of 7 trials (6 force-field trials and 1 catch trial). A value of 1 indicates full adaptation to the force-field perturbation, whereas values between 0 and −1 indicate no learning. Shaded area depicts SE. There was no significant difference in the final learning index among stimulation types (F2, 72.1 = 0.41, P = 0.67).
The final LI was significantly different between cerebellar participants and healthy subjects (Table 4). Importantly, the final LI was not affected by stimulation type, and there was no interaction of stimulation type and group (Table 4). Equivalency among stimulation types could not be established [SESOI = (−0.05, 0.05), P = 0.09 for sham vs. M1 stimulation and P = 0.27 for sham vs. cerebellum stimulation]. There was a trend for an effect of session number when comparing final learning over experimental sessions instead of stimulation type, indicating possible interference of previous measurement days (Table 4). As was investigated when AEs were analyzed, the source of this difference was a carryover effect of measurement day in the healthy control group. With the analysis of the final LI in controls over measurement day, pairwise comparisons revealed significantly higher final learning indices of the third measurement day compared with the first day (mean difference = 0.07, P = 0.02).
Table 4.
Linear mixed model final learning index with movement time as continuous covariate
| Linear Mixed Model | Fixed Effects | F Statistic | P |
|---|---|---|---|
| Final learning index (over stimulation type) | |||
| Between | Group | F(1, 35.7) = 185 | <0.001* |
| Within | Stimulation | F(2, 72.1) = 0.41 | 0.67 |
| Stimulation × group | F(2, 73.0) = 1.42 | 0.25 | |
| Final learning index (over measurement order) | |||
| Between | Group | F(1, 35.6) = 187 | <0.001* |
| Within | Day | F(2, 73.0) = 2.78 | 0.07 |
| Day × group | F(2, 72.5) = 1.87 | 0.16 |
Boldface highlights important (in-text) comparisons.
Significant at the α = 0.05 level.
The LI required the use of catch trials to calculate. Catch trials can cause a nontrivial amount of trial-by-trial unlearning (Thoroughman and Shadmehr 2000), which in combination with improved (un)learning, due to tDCS, could lead to complex mixed results. However, nonuse of catch trials during the adaptation set is not optimal, as catch trials give us a measure of the internal state of the motor system and allow us to assess whether subjects are stiffening the arm in response to the force-field perturbation. As a way to assure unlearning by catch trials was not affected by stimulation type, the difference between PD right before a catch trial and directly after a catch trial in the healthy control group was assessed as follows
The analysis revealed no effect of stimulation on unlearning but did reveal a significant effect of catch trial number (Table 5). The mean value of unlearning per catch trial was ∼0.80 ± SE 0.06 cm for each of the stimulation types. Unlearning was stronger in the beginning of the adaptation set than later in the adaptation set, which can be explained by a motor memory that is more resistant to unlearning later in the set (i.e., more “slow learning”) (Smith 2006). In all, the analysis shows that unlearning by catch trials during the adaptation set is not affected by stimulation type, and complex mixed results are thus unlikely.
Table 5.
Linear mixed model unlearning with movement time as continuous covariate
| Linear Mixed Model | Fixed Effects | F Statistic | P |
|---|---|---|---|
| Unlearning | |||
| Within | Stimulation | F(2, 35.0) = 1.00 | 0.38 |
| Catch trial | F(23, 412) = 17.8 | <0.001* | |
| Stimulation × catch trial | F(46, 821) = 0.83 | 0.79 |
Boldface highlights important (in-text) comparisons.
Significant at the α = 0.05 level.
Voxel-based morphometry.
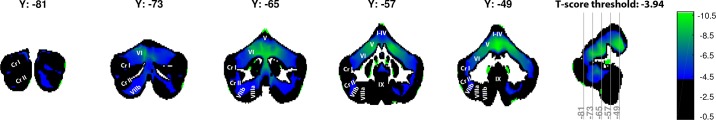
Figure 8 shows a cerebellar map of the difference in gray-matter volume between healthy controls and cerebellar subjects on a voxel-by-voxel basis, reported in t-scores. The significance threshold, as estimated by the permutation analysis, was set at t = −3.94 and t = 3.93, meaning that voxels with t-scores below −3.94 and t-scores above 3.93 were significant. Voxel t-scores ranged between −10.62 and 1.66, yielding 44.02% of voxels as significant, with significant voxels only being negative t-scores. The highest percentage of significant voxels was found in the anterior lobe (lobules I–V) and superior parts of the posterior cerebellar lobe (lobule VI). This confirms earlier observations, where the strongest degeneration in cerebellar patients was also found in the anterior and superior cerebellum (Hulst et al. 2015), although that study also reports strong degeneration of the more inferior parts of the posterior cerebellum (in particular, lobule VII). Previous studies have found that integrity of the anterior lobe of the cerebellum, in particular, lobules IV and V, is important for adaptation to force-field perturbations (Donchin et al. 2012; Rabe et al. 2009). Thus the loss of cerebellar volume in the anterior lobe of the cerebellum is the likely cause of the observed motor impairments in cerebellar patients.
Fig. 8.
Slices of the cerebellum showing t-scores of the gray-matter volume difference between cerebellar subjects (n = 19) and controls [n = 19; Montreal Neurological Institute coordinates (Y)]. A threshold was set at the calculated significance threshold, meaning that each voxel with a t-score that is less strong than −3.94 is color coded as black. Low significant t-scores are color coded as blue, whereas high significant t-scores are color coded as green. Definition of lobule anatomy and nomenclature is as described in Diedrichsen et al. (2009). Cr I/II, Crus I/II. I–IX, lobules.
Analysis of the correlation between the final LI and gray-matter volume yielded no results in both healthy control subjects (correlation thresholds between r = −0.72 and r = 0.75, voxel correlations between r = −0.47 and r = 0.45, no significant voxels) and cerebellar subjects (correlation thresholds between r = −0.74 and r = 0.75, voxel correlations between r = −0.39 and r = 0.67, no significant voxels). This is likely due to conservative corrections for multiple testing and the low amount of variance in the final LI of cerebellar subjects.
Experiment 2
The second experiment was analyzed using the same methodology described in experiment 1. We will summarize the most important results and the effects of cerebellar stimulation on our main performance measures.
Average movement times and movement speed indicated no differences among stimulation types but did uncover a main effect of set number (Table 6). Like elderly controls, young controls tended to speed up during the course of the experiment. Movement times in the first set were ∼100 ms longer (353 ms ± SE 19.9) than in the last set (255 ms ± SE 18.2), and were comparable with movement times of the elderly control group (unpaired t-test, t84.9 = −1.66, P = 0.10). A univariate test of mean movement times (F2, 27 = 0.37, P = 0.69) and movement speeds (F2, 27 = 0.30, P = 0.74) in the fourth set revealed no differences among the three stimulation types, indicating that all groups were exposed to similar perturbation magnitudes.
Table 6.
ANOVAs of average movement times and speeds
| ANOVA | Factors | F Statistics | P |
|---|---|---|---|
| Average Movement Time | |||
| Between | Stimulation | F(2, 27) = 0.69 | 0.51 |
| Within | Set | F(2.04, 64.1) = 13.5 | <0.001* |
| Set × stimulation | F(4.08, 64.1) = 0.28 | 0.71 | |
| Average Movement Speed | |||
| Between | Stimulation | F(2, 27) = 0.41 | 0.67 |
| Within | Set | F(2.47, 66.6) = 10.9 | <0.001* |
| Set × stimulation | F(4.93, 66.6) = 0.28 | 0.92 |
Boldface highlights important (in-text) comparisons.
Significant at the α = 0.05 level.
Second, perpendicular velocities were analyzed at the moment of maximal overcompensation. Like elderly controls, young control subjects developed an overcompensation to the force-field perturbation around the 55-ms mark of a movement. Negative perpendicular velocities during the beginning of a movement were observed in all stimulation types as the adaptation set progressed. No difference among stimulation types could be detected at 55 ms (F2, 26.8 = 0.27, P = 0.78). Here, as well, the magnitude of the overcompensation in young controls was similar to that of healthy, elderly controls (−0.75 cm/s ± SE 0.14 vs. −0.65 cm/s ± SE 0.10 in the final bin of the set, unpaired t-test, t85 = −0.51, P = 0.61).
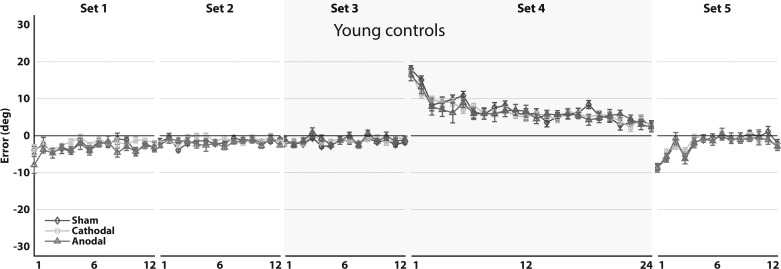
Next, the development of average AEs during the experiment was plotted and investigated (Fig. 9). Young controls, like healthy, elderly controls, learn to adapt to the perturbation quickly and exhibit AEs at the end of learning with near-zero values. Furthermore, like in elderly controls, an aftereffect was observed during the washout phase of the experiment (Fig. 9). No difference was found between the average of AEs of the adaptation set between young and elderly controls (unpaired t-test, t85 = −0.32, P = 0.75).
Fig. 9.
Mean aiming errors of young controls (n = 30, n = 10 for each stimulation type). Mean aiming errors (degrees) are shown during baseline (sets 1–3), force-field adaptation (set 4), and washout (set 5) for sham (diamonds), cathodal (squares), and anodal (triangles) stimulation of the cerebellum. Aiming errors were averaged over bins of 6 movements. Sets 4 and 5 were corrected for baseline movement biases. The shaded area represents the sets in which tDCS was applied. Error bars indicate SE. There was no significant difference in mean aiming errors of the adaptation set among stimulation types (F2, 25.7 = 0.10, P = 0.91).
Importantly, no stimulation effects could be detected in young controls in the baseline set [F2, 27.4 = 0.44, P = 0.65, 95% CIs of difference (−1.95°, 1.31°) for sham vs. cathodal and (−1.34°, 1.90°) for sham vs. anodal], as well as the adaptation set [F2, 25.7 = 0.10, P = 0.91, 95% CIs of difference (−3.19°, 4.10°) for sham vs. cathodal and (−3.05°, 4.24°) for sham vs. anodal] and washout set [F2, 27.1 = 0.03, P = 0.97, 95% CIs of difference (−2.87°, 3.11°) for sham vs. cathodal and (−2.72°, 3.25°) for sham vs. anodal]. Here, as well, we were interested in how sensitive our experiment was in detecting stimulation effects. Given α = 0.05 and 1 – β = 0.80, we calculated that we could reliably reject the null hypothesis if the true effect size were f = 0.43, d ≥ 0.86, and AEs of 2.72°. This meant that we could detect a true effect in AEs of 3.79° or more. Although less sensitive than experiment 1, we would argue that it is still reasonable to expect stimulation effects that are as large or larger than this.
Lastly, we investigated the effect of stimulation of the cerebellum on the final LI. The final LI reached values that were, on average, a little higher than the final LI of elderly controls (0.74 ± SD 0.12, unpaired t-test, t85 = 2.22, P = 0.03), indicating that young controls achieve slightly higher final learning than elderly controls. Importantly, here as well, no effects of stimulation were detected (F2, 26 = 0.52, P = 0.60). Equivalency among group means could not be established for all of the aforementioned measures under the equivalency criteria from experiment 1.
DISCUSSION
As expected, individuals with cerebellar degeneration were slower to adapt to force-field perturbations and displayed no aftereffects in the washout phase. These findings are in line with earlier work on motor adaptation in cerebellar patients (Maschke et al. 2004; Tseng et al. 2007). Against our expectations, cerebellar subjects did not benefit from tDCS. Cerebellar subjects did not adapt more quickly to force-field perturbations during stimulation of the cerebellum or M1 when compared with sham stimulation. Similarly, retention in the washout phase did not improve after stimulation of the cerebellum or M1 when compared with sham stimulation. No effects of tDCS were observed in the elderly age-matched controls, as well as young controls.
In short, we were unable to detect faster learning rates and higher retention after anodal stimulation, which is in contrast with previous reports [for review, see Buch et al. (2017)]. We cannot exclude, however, that tDCS elicits behavioral improvements under different task and stimulation parameters in larger subject populations or that carryover effects have masked potential benefits of tDCS in elderly controls. Nonetheless, our results suggest that tDCS of the cerebellum or M1, using currently available stimulation techniques, is unlikely to lead to improvements that are clinically relevant for cerebellar patients. When stimulation effects cannot be detected in the controlled environment of a laboratory, any potential benefits of stimulation are likely going to be small, and stimulation techniques should first be further developed before they can be applied in the clinic. Possible reasons for the lack of detectable stimulation effects and several limitations of this study are discussed below.
tDCS Effects May Be Highly Task Dependent
Studies investigating tDCS effects in reach adaptation in young, healthy subjects have reported different effects of stimulation, depending on the task and the performance measures. A recent study demonstrated that anodal stimulation of the cerebellum led to quicker overcompensation of a force-field perturbation, and cathodal stimulation of the cerebellum led to slower overcompensation, as evidenced by a significant difference in perpendicular velocities among stimulation types (Herzfeld et al. 2014). We were unable to replicate this finding in both of our experiments. In agreement with our results, the study by Herzfeld et al. (2014) did not find anodal stimulation effects on maximum PD—a measure that closely relates to our main performance measure—as well as no effects of anodal stimulation of M1. This contrasts with a study by Galea et al. (2011), who report anodal stimulation effects of M1 in a visuomotor task. Moreover, the authors found that anodal stimulation of the cerebellum sped up adaptation to visuomotor perturbations when measuring angular end-point error, a measure that closely relates to our performance measure. Although the experiments by Herzfeld et al. (2014) and Galea et al. (2011) appear similar to our experiment, there are significant differences in experimental design and task parameters. Differences in task parameters include the types of perturbation, the amount of movements, the number and direction of target locations, the projection of the hand position (horizontal plane or vertical monitor), the inclusion of clamp or catch trials, and the type and amount of feedback. Since nuances in task parameters have an effect on how the nervous system learns and retains a motor adaptation (Joiner and Smith 2008; Kitago et al. 2013), it is possible that these differences in task parameters explain the different stimulation effects. A recent study by Jalali et al. (2017) has further established the task specificity of stimulation effects in reach adaptation, suggesting that the parameters of a task influence how tDCS affects performance, and stimulation effects in one task and performance measure might not generalize to others.
tDCS Effects on Critical Areas May Have Been Insufficient
Other factors that could possibly have influenced tDCS efficacy in both our control subjects and cerebellar subjects were the stimulation parameters. A recent modeling study of the cerebellar electrode placement demonstrated that the majority of current is distributed over the cerebellar hemisphere under the anode (Rampersad et al. 2014). The highest electrical-field strengths are found on the inferior surface of the cerebellum, below the primary fissure. This could indicate that when applying tDCS with an electrode, placed 3 cm right from the inion, inferior areas of the cerebellum are mainly stimulated and to a lesser extent, the anterior cerebellum. Because adaptation to force-field perturbations depends on lobules IV and V of the anterior cerebellum (Donchin et al. 2012; Rabe et al. 2009), this could possibly explain the lack of cerebellar stimulation effects in our study. Due to the anatomical structure of the neck region and cerebellum, it is, however, unlikely that alternative cerebellar montages will alter the distribution of current, as variations in the cerebellar montage produce only small changes in current distribution (Parazzini et al. 2014). The same modeling study also revealed that the placement of electrodes during M1 stimulation in our study might be suboptimal for stimulation of the target area, which could have affected tDCS efficacy during M1 stimulation (Rampersad et al. 2014). However, as Rampersad et al. (2014) point out, several simplifications and assumptions are made when modeling tDCS (e.g., in tissue conductivity), and the models still need to be validated in animal studies.
The tDCS montages used in our experiment are the most commonly agreed upon montages for stimulation of the cerebellum and M1 (Nitsche et al. 2008; Woods et al. 2016), and the physiological basis for using these stimulation locations is well established [cerebellum: Galea et al. (2009); M1: Stagg and Nitsche (2011)]. Furthermore, several studies have found stimulation effects during motor learning using the exact cerebellar montage and exact M1 montage used in this experiment [for review, see Buch et al. (2017)]. It is therefore unlikely that the montage of electrodes was the driving force behind the lack of stimulation effects in this experiment, but alternative electrode montages could be considered depending on the task parameters and stimulation target.
tDCS Effects May Depend on Cerebellar Integrity
The specific task and stimulation parameters are the most likely candidates for the lack of stimulation effects in this experiment, but even when these methodological difficulties have been worked out (i.e., tDCS effects can robustly and predictably be elicited across healthy subjects and tasks), it could be difficult to elicit behavioral improvements in subjects with cerebellar atrophy. When stimulation is applied over an area of the cerebellum that is atrophied, the amount of cerebellar neurons left could be too low to institute a behavioral change. This can be demonstrated by the absence of cerebellar excitability effects on cerebellar–M1 connectivity in hereditary ataxia, for instance (Ugawa et al. 1994). Furthermore, since internal model formation is impaired in cerebellar patients, due to degeneration of the cerebellum (Smith and Shadmehr 2005), it is likely that consolidation of an impaired internal model does not lead to functional improvements. The M1 plays a crucial role in the retention of a newly formed motor memory (Hadipour-Niktarash et al. 2007), and any potential benefits of anodal stimulation of M1 in cerebellar patients could thus be masked by impaired internal model formation by the cerebellum. Since both cerebellar patients and aging subjects are affected by cerebellar atrophy of the anterior cerebellum (Hulst et al. 2015), the effect of stimulation on behavior might be limited. Of course, loss of cerebellar volume cannot have impeded tDCS efficacy in our group of young control subjects, and the null results in healthy, elderly and cerebellar patients are likely independent from cerebellar atrophy, but this caveat should be considered when further exploring the clinical potential of tDCS.
Limitations
Several limitations have to be taken into account for the interpretation of the results in this study. Since the focus of this experiment was to determine whether tDCS could be effective in reducing motor-learning deficits of cerebellar patients, we chose to stimulate anodally in cerebellar patients exclusively. Cathodal stimulation most commonly impairs the ability of healthy subjects to learn and retain a motor adaptation (Herzfeld et al. 2014; Jayaram et al. 2012), likely due to a decrease of cortical and cerebellar excitability (Galea et al. 2009; Nitsche et al. 2000), but positive cathodal stimulation effects have also been reported (Orban de Xivry et al. 2011; Pope and Miall 2012). It is therefore still possible that cathodal stimulation would have improved the ability of cerebellar patients to adapt to force-field perturbations, although we consider this unlikely because of the lack of effects of cathodal stimulation in the second experiment.
Furthermore, we did not assess physiological effects of tDCS. Even when we could not establish behavioral effects of tDCS, stimulation of the cerebellum and M1 has likely had an effect on neuronal excitability. If physiological effects of cerebellar or M1 stimulation could have been established, but not behavioral effects, then it would have further cemented the task dependency of tDCS effects. Moreover, we did not control for brain-derived neurotrophic factor polymorphisms, which are known to affect cortical plasticity among individuals (Antal et al. 2010; Fritsch et al. 2010), and did not quantify the sensitivity to TMS in subjects, which is a promising proxy measure of an individual’s sensitivity to brain stimulation (Labruna et al. 2015). After review of individual behavioral data, however, we could not distinguish between responders and nonresponders—something one would have expected if some of the subjects carried a brain-derived neurotrophic factor polymorphism or in the case of interindividual differences in TMS sensitivity.
Another difference between our application of tDCS and several aforementioned studies is the use of rubber electrodes, which are covered in conductive paste instead of covered by saline-soaked sponges. Rubber electrodes covered in conductive paste are expected to apply current over the scalp more consistently than electrodes covered in saline-soaked sponges, for which improper use can lead to oversaturation and alter the distribution of current between subjects and experimental sessions (Woods et al. 2016). Whereas we cannot rule out the possibility of different stimulation effects, due to the use of different electrode configurations, we do not think this has an impact on the interpretation of our findings.
Two additional limitations of our experimental design have to be taken into account. First, our LI required catch trials during adaptation to calculate, which can cause significant trial-to-trial unlearning. Although unlearning due to catch trials was not affected by stimulation type, it would have been desirable to use error-clamp trials instead. Error-clamp trials cause unlearning as well but less than catch trials (Kitago et al. 2013).
Second, experiment 1 was carried out using a within-subject design, which has the advantage of increased power but comes with the disadvantage of introducing possible carryover effects between sessions. Indeed, the healthy, elderly control group demonstrated a carryover effect between measurement days, which might have masked potential stimulation effects. However, the carryover effect was only present in the group of healthy, elderly controls and relatively minor. If conducting more learning sessions in the healthy elderly is more effective than stimulation, it is unlikely that tDCS can affect learning in cerebellar patients to a degree that is therapeutically relevant. Furthermore, a second experiment was carried out to control for stimulation effects, possibly being masked by carryover effects. The second experiment also did not detect an effect of tDCS in a between-subject design, which makes it more likely that improper task and stimulation parameters were at the root of our null result.
Conclusions
The present study did not find stimulation effects of tDCS in young control subjects; healthy, aging subjects; and individuals with cerebellar degeneration during reach adaptation. Not fully developed task and stimulation parameters may explain the lack of stimulation effects. Carryover effects were present in healthy, elderly controls and could have masked stimulation effects, but carryover effects were not present in the group of cerebellar patients. Furthermore, the second experiment, which controlled for carryover effects, was also unable to establish a significant relationship between tDCS and behavior. The second experiment was performed in a relatively small group of young control subjects and still needs to be replicated in a larger group of elderly controls. Despite these limitations, the results of our study require a re-evaluation of the clinical potential of tDCS in cerebellar patients. Currently, this study does not provide evidence that tDCS changes learning or retention rates in cerebellar patients. For tDCS to become a valuable tool in the neurorehabilitation of cerebellar disease, stimulation effects should be consistent and predictable between subjects and tasks and lead to behavioral improvements, which are large enough to be clinically relevant, in cerebellar patients.
GRANTS
Funding for the study was provided by a grant of the German Research Foundation (DFG TI 239/16-1), awarded to O. Donchin and D. Timmann, and a scholarship by the Else Kröner Fresenius Foundation, awarded to L. John.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.T., T.H., L.J., M.K., and O.D. conceived and designed research; L.J. and M.K. performed experiments; T.H. and S.L.G. analyzed data; T.H., L.J., and D.T. interpreted results of experiments; T.H. prepared figures; T.H. drafted manuscript; T.H., L.J., J.N.v.d.G., O.D., and D.T. edited and revised manuscript; T.H. and D.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Beate Brol for her support in the analysis of this experiment.
REFERENCES
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, Nitsche MA, Shoukier M, Ludwig H, Paulus W. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimulat 3: 230–237, 2010. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Avila E, van der Geest JN, Kengne Kamga S, Verhage MC, Donchin O, Frens MA. Cerebellar transcranial direct current stimulation effects on saccade adaptation. Neural Plast 2015: 968970, 2015. doi: 10.1155/2015/968970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block H, Celnik P. Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum 12: 781–793, 2013. doi: 10.1007/s12311-013-0486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, Gerloff C, Hallett M, Hummel FC, Nitsche MA, Pascual-Leone A, Paulus WJ, Reis J, Robertson EM, Rothwell JC, Sandrini M, Schambra HM, Wassermann EM, Ziemann U, Cohen LG. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 128: 589–603, 2017. doi: 10.1016/j.clinph.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004. doi: 10.1523/JNEUROSCI.2214-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. doi: 10.1152/jn.00622.2002. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46, 2009. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 107: 134–147, 2012. doi: 10.1152/jn.00007.2011. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191, 2007. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Flourens P. Recherches expérimentales sur les propriétés et les fonctions du système nerveux, dans les animaux vertébrés. Paris: J.-B. Ballière, 1824. [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3: 383–393, 2007. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66: 198–204, 2010. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29: 9115–9122, 2009. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117: 845–850, 2006. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, Zee DS, Clark HB, Anderson JH. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol 42: 933–950, 1997. doi: 10.1002/ana.410420616. [DOI] [PubMed] [Google Scholar]
- Goodwill AM, Reynolds J, Daly RM, Kidgell DJ. Formation of cortical plasticity in older adults following tDCS and motor training. Front Aging Neurosci 5: 87, 2013. doi: 10.3389/fnagi.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, Ferrucci R, Fregni F, Galea JM, Hamada M, Manto M, Miall RC, Morales-Quezada L, Pope PA, Priori A, Rothwell J, Tomlinson SP, Celnik P. Cerebellar transcranial direct current stimulation (ctDCS): a novel approach to understanding cerebellar function in health and disease. Neuroscientist 22: 83–97, 2016. doi: 10.1177/1073858414559409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Oulad Ben Taib N, Manto M, Bodranghien F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci 8: 9, 2014. doi: 10.3389/fnsys.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27: 13413–13419, 2007. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Celnik PA. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging 35: 2217–2221, 2014. doi: 10.1016/j.neurobiolaging.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemirad F, Zoghi M, Fitzgerald PB, Jaberzadeh S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: A systematic review and meta-analysis. Brain Cogn 102: 1–12, 2016. doi: 10.1016/j.bandc.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O’Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage 98: 147–158, 2014. doi: 10.1016/j.neuroimage.2014.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. A form of familial degeneration of the cerebellum. Brain 30: 466–489, 1908. doi: 10.1093/brain/30.4.466. [DOI] [Google Scholar]
- Hulst T, van der Geest JN, Thürling M, Goericke S, Frens MA, Timmann D, Donchin O. Ageing shows a pattern of cerebellar degeneration analogous, but not equal, to that in patients suffering from cerebellar degenerative disease. Neuroimage 116: 196–206, 2015. doi: 10.1016/j.neuroimage.2015.03.084. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712, 2006. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol 587: 2949–2961, 2009. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg W, Bastian AJ, Boesch S, Burciu RG, Celnik P, Claaßen J, Feil K, Kalla R, Miyai I, Nachbauer W, Schöls L, Strupp M, Synofzik M, Teufel J, Timmann D. Consensus paper: management of degenerative cerebellar disorders. Cerebellum 13: 248–268, 2014. doi: 10.1007/s12311-013-0531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Rane T, Donchin O, Shadmehr R. Motor adaptation as a process of reoptimization. J Neurosci 28: 2883–2891, 2008. doi: 10.1523/JNEUROSCI.5359-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali R, Miall RC, Galea JM. No consistent effect of cerebellar transcranial direct current stimulation (tDCS) on visuomotor adaptation. J Neurophysiol. First published March 15, 2017. doi: 10.1152/jn.00896.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol 107: 2950–2957, 2012. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol 100: 2948–2955, 2008. doi: 10.1152/jn.90706.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitago T, Ryan SL, Mazzoni P, Krakauer JW, Haith AM. Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci 7: 307, 2013. doi: 10.3389/fnhum.2013.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna L, Dabit M, Vanderschelden B, Nitsche M, Ivry RB. Individual differences in TMS sensitivity influence the effect of tDCS in a motor adaptation task. Neuroscience 2015. Chicago, IL: 21 October 2015. Poster 710.15/V14. [Google Scholar]
- Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil 25: 195–216, 2011. doi: 10.1177/0269215510382495. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol 91: 230–238, 2004. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- Minarik T, Berger B, Althaus L, Bader V, Biebl B, Brotzeller F, Fusban T, Hegemann J, Jesteadt L, Kalweit L, Leitner M, Linke F, Nabielska N, Reiter T, Schmitt D, Spraetz A, Sauseng P. The importance of sample size for reproducibility of tDCS effects. Front Hum Neurosci 10: 453, 2016. doi: 10.3389/fnhum.2016.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1: 206–223, 2008. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Marko MK, Pekny SE, Pastor D, Izawa J, Celnik P, Shadmehr R. Stimulation of the human motor cortex alters generalization patterns of motor learning. J Neurosci 31: 7102–7110, 2011. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panouillères M, Joundi RA, Brittain JS, Jenkinson N. Reversing motor adaptation deficits in the ageing brain using non-invasive stimulation. J Physiol 593: 3645–3655, 2015. doi: 10.1113/JP270484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol 125: 577–584, 2014. doi: 10.1016/j.clinph.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimulat 5: 84–94, 2012. doi: 10.1016/j.brs.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi NG, Minafra B, Zangaglia R, De Marzi R, Sandrini G, Priori A, Pacchetti C. Transcranial direct current stimulation (tDCS) of the cortical motor areas in three cases of cerebellar ataxia. Cerebellum 13: 109–112, 2014. doi: 10.1007/s12311-013-0524-5. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- Rampersad SM, Janssen AM, Lucka F, Aydin Ü, Lanfer B, Lew S, Wolters CH, Stegeman DF, Oostendorp TF. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans Neural Syst Rehabil Eng 22: 441–452, 2014. doi: 10.1109/TNSRE.2014.2308997. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain 113: 103–120, 1990. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hübsch T, Du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T, Fancellu T. Scale for the assessment and rating of ataxia. Neurology 66: 1717–1720, 2006. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15: 657–680, 1987. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Seaman MA, Serlin RC. Equivalence confidence intervals for two-group comparisons of means. Psychol Methods 3: 403–411, 1998. doi: 10.1037/1082-989X.3.4.403. [DOI] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34: 721–733, 2010. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: e179, 2006. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 17: 37–53, 2011. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Taig E, Küper M, Theysohn N, Timmann D, Donchin O. Deficient use of visual information in estimating hand position in cerebellar patients. J Neurosci 32: 16274–16284, 2012. doi: 10.1523/JNEUROSCI.1153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature 407: 742–747, 2000. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmann D, Konczak J, Ilg W, Donchin O, Hermsdörfer J, Gizewski ER, Schoch B. Current advances in lesion-symptom mapping of the human cerebellum. Neuroscience 162: 836–851, 2009. doi: 10.1016/j.neuroscience.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B; The Ataxia Neuropharmacology Committee of the World Federation of Neurology . International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci 145: 205–211, 1997. doi: 10.1016/S0022-510X(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tseng Y, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba-Shimizu K, Rothwell JC, Iwata M, Kanazawa I. Suppression of motor cortical excitability by electrical stimulation over the cerebellum in ataxia. Ann Neurol 36: 90–96, 1994. doi: 10.1002/ana.410360117. [DOI] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127: 1031–1048, 2016. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol 73: 10–15, 2013. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]