Blasts exposures often produce hearing difficulties. Although cochlear damage typically occurs, the downstream effects on central auditory processing are less clear. Moreover, outcomes were compared between individuals exposed to the blast pressure wave vs. those who experienced the blast noise without the pressure wave. It was found that a single blast exposure produced changes at all stages of the ascending auditory path at least 4 wk postblast, whereas blast noise alone produced largely transient changes.
Keywords: blast, TBI, hearing loss, auditory brain stem responses, envelope following responses
Abstract
Hearing difficulties are the most commonly reported disabilities among veterans. Blast exposures during explosive events likely play a role, given their propensity to directly damage both peripheral (PAS) and central auditory system (CAS) components. Postblast PAS pathophysiology has been well documented in both clinical case reports and laboratory investigations. In contrast, blast-induced CAS dysfunction remains understudied but has been hypothesized to contribute to an array of common veteran behavioral complaints, including learning, memory, communication, and emotional regulation. This investigation compared the effects of acute blast and nonblast acoustic impulse trauma in adult male Sprague-Dawley rats. An array of audiometric tests were utilized, including distortion product otoacoustic emissions (DPOAE), auditory brain stem responses (ABR), middle latency responses (MLR), and envelope following responses (EFRs). Generally, more severe and persistent postinjury central auditory processing (CAP) deficits were observed in blast-exposed animals throughout the auditory neuraxis, spanning from the cochlea to the cortex. DPOAE and ABR results captured cochlear and auditory nerve/brain stem deficits, respectively. EFRs demonstrated temporal processing impairments suggestive of functional damage to regions in the auditory brain stem and the inferior colliculus. MLRs captured thalamocortical transmission and cortical activation impairments. Taken together, the results suggest blast-induced CAS dysfunction may play a complementary pathophysiological role to maladaptive neuroplasticity of PAS origin. Even mild blasts can produce lasting hearing impairments that can be assessed with noninvasive electrophysiology, allowing these measurements to serve as simple, effective diagnostics.
NEW & NOTEWORTHY Blasts exposures often produce hearing difficulties. Although cochlear damage typically occurs, the downstream effects on central auditory processing are less clear. Moreover, outcomes were compared between individuals exposed to the blast pressure wave vs. those who experienced the blast noise without the pressure wave. It was found that a single blast exposure produced changes at all stages of the ascending auditory path at least 4 wk postblast, whereas blast noise alone produced largely transient changes.
with increases in explosive device usage for modern conflict, blast injuries have unfortunately gained notoriety as warfare’s hallmark injury (Rosenfeld et al. 2013). Advances in military protective equipment have increased the survival rate of individuals exposed to blast, permitting exposure to higher intensity shock waves before mortality thresholds are reached (Bass et al. 2008, 2012; Rafaels et al. 2011). As a result of increased blast survivability, new organ systems have emerged as being vulnerable to blast, particularly the brain and auditory system. Even before the advent of modern protective equipment, damage to the ear historically has been the most commonly reported consequence of blast exposure (Sharpnack et al. 1991). Among veterans with service-connected disabilities, tinnitus and hearing loss are the two most prevalent conditions (U.S. Department of Veterans Affairs 2014). Given the recently discovered vulnerability of the brain to blast (Rafaels et al. 2011; Rosenfeld et al. 2013; Song et al. 2015; Walls et al. 2016) and known susceptibility of the peripheral auditory system (DePalma et al. 2005; Sharpnack et al. 1991), it is likely that repeated exposure to blast events incites significant damage at all levels of the auditory pathway and is a major factor in the high prevalence of hearing impairment in veterans.
Traditionally, postblast hearing impairment has mostly been attributed to peripheral damage to the eardrum, middle ear, and inner ear (Kerr 1980). Eardrum rupture was once used to identify blast-injured individuals but was determined to be a poor diagnostic indicator due to a high rate of false negatives (DePalma et al. 2005; Ritenour et al. 2008). Recent clinical (Berger et al. 1997; Bressler et al. 2017; Cave et al. 2007; Cohen et al. 2002; Gallun et al. 2012; Lew et al. 2009; Ritenour et al. 2008; Saunders et al. 2015) and laboratory (Cho et al. 2013b; Du et al. 2013; Ewert et al. 2012; Luo et al. 2014a, 2014b; Mahmood et al. 2014; Mao et al. 2012; Patterson and Hamernik 1997) investigations have reported that both the peripheral (middle and inner ear) and central (brain stem and brain) auditory systems (PAS and CAS, respectively) are vulnerable to blast injuries, even at relatively mild intensities that could be encountered by a large proportion of personnel. Such knowledge warrants deeper understanding of the pathology of posttrauma auditory dysfunction, particularly deficits that stem from CAS damage, an area with little research effort to date. Furthermore, given the specialized signaling and energetic demands necessary to permit the exquisite temporal precision (Joris et al. 2004) and sound source localization (Brand et al. 2002; Grothe et al. 2010) of the auditory system, assessments of auditory processing may act as a sensitive biomarker more generally for central nervous system (CNS) function. Finally, no effective therapies for auditory damage have been established, leading to a great deal of ongoing suffering for active military personnel, veterans, and civilians exposed to blast events.
In addition to observations of tympanic membrane (eardrum) rupture in combat (DePalma et al. 2005), clinical reports have consistently documented lasting hearing impairment and tinnitus in veterans (Cave et al. 2007; Cohen et al. 2002; Ritenour et al. 2008; Saunders et al. 2015). Recent laboratory investigations have demonstrated the capacity of blast injury to damage both inner (IHC) and outer hair cells (OHC) in the cochlea (Cho et al. 2013b; Ewert et al. 2012; Patterson and Hamernik 1997), which could lead to permanently elevated thresholds. However, other studies have demonstrated that sound exposure leading to slight temporary shifts in thresholds can still lead to lasting auditory and perceptual deficits. In support of this notion, it was recently reported for humans exposed to blasts that behavioral deficits are common despite relatively small threshold shifts and little change in auditory brain stem responses (Bressler et al. 2017; Gallun et al. 2012; Saunders et al. 2015). In these cases, cochlear ultrastructure is relatively intact, but the number of synapses between hair cells and auditory nerve (AN) fibers is greatly reduced (Cho et al. 2013b). This suggests that many of the military personnel exposed to blast but deemed within the normal range by threshold testing may in fact have hearing impairment, stemming from compromised processing or perception, that could impact their performance in military operations and their return to civilian life (Bressler et al. 2017; Gallun et al. 2012; Lew et al. 2009; Saunders et al. 2015). Furthermore, PAS assessments alone may inadequately capture the immediate and lasting effects of blast exposure on the auditory system and behaviors that depend on its intact function.
To date, few controlled laboratory studies of postblast CAS pathophysiology have been performed. Some clinical reports have suggested that, over the long term, the principal lasting injuries to the auditory system may reside centrally rather than peripherally (Bressler et al. 2017; Gallun et al. 2012; Saunders et al. 2015). In rats exposed to blast injury, there have been reports of neuronal spontaneous hyperactivity in brain stem auditory nuclei (Luo et al. 2014a, 2014b). Additionally, diffusion tensor imaging postblast in rats showed changes in the inferior colliculus (IC) and auditory thalamus, but not the auditory cortex (Mao et al. 2012), suggesting the subcortical CAS may be particularly vulnerable to blast injury. Despite these findings, the pathophysiological mechanisms of blast injury to the auditory system and respective contributions of damage to the PAS vs. CAS remain unclear. This knowledge gap precludes targeted development of innovative diagnostic techniques, therapies, and protective technologies, although some therapies have been attempted with mixed results (Du et al. 2013; Ewert et al. 2012; Mahmood et al. 2014). Moreover, if CAS deficits can serve as sensitive biomarkers for more general CNS neuronal function, they have the major advantage of being easily accessible for diagnostics. It has been widely demonstrated by our group and others that even mild blast exposures can diffusely injure the brain (Budde et al. 2013; Cernak et al. 2001; Cho et al. 2013a; Garman et al. 2011; Readnower et al. 2010; Walls et al. 2016), a finding consistent with the few existing reports of postblast CAS dysfunction (Gallun et al. 2012; Luo et al. 2014a, 2014b). In particular, our recent work has revealed that rapid, heterogeneous intracranial deformation during mild blast can deform and ultimately damage the brain via subsequent changes in oxidative stress levels, neuroinflammation, and blood-brain barrier permeability (BBB), possibly initiating mechanisms of altered CAS function (Song et al. 2015; Walls et al. 2016).
We have conducted a preliminary investigation into PAS and CAS dysfunction by examining differences between the effects of two different forms of acute acoustic trauma (AAT): a single mild blast exposure (shock wave + noise) and a single impulse noise-only acoustic trauma. Multiple measures of auditory processing were performed, including distortion product otoacoustic emissions (DPOAE), auditory brain stem responses (ABRs), middle latency responses (MLRs), and envelope following responses (EFRs). Collectively, the results of these tests suggest that postblast functional impairments are present in the CAS for the processing of both simple (clicks and pure tones) and temporally modulated sounds throughout the auditory neuraxis. We demonstrate with the data presented in this article that auditory evoked potential recordings possess strong prospects for future diagnostic utility in identifying blast-injured individuals that are otherwise asymptomatic, a common clinical presentation among the >50% of blast-induced traumatic brain injuries (bTBI) classified as mild in severity (Galarneau et al. 2008; Tanielian and Jaycox 2008). Furthermore, evoked potentials may aid in understanding basic pathophysiological mechanisms of neuronal dysfunction following blast injuries.
MATERIALS AND METHODS
Subjects
Sprague-Dawley male rats (3–4 mo) were used in this study. The animals were assigned into two groups randomly: noise (n = 8) and blast (n = 10). Noise animals were exposed only to blast noise, whereas blast animals were exposed to both blast noise and shock wave injury (described below). All the animals were kept and raised in relatively quiet and standard laboratory animal housing conditions. All protocols were approved by the Purdue Animal Care and Use Committee (PACUC no. 1111000280).
Blast Exposure
Animals were anesthetized by ketamine-xylazine cocktail injected intraperitoneally (80 and 10 mg/kg, respectively). Absence of eye-blink and paw-withdrawal reflexes was ensured before experiments proceeded. After depth of anesthesia was verified, animals were placed on a platform beneath an open-ended shock tube to be exposed to the blast event, as described in our prior publications (Song et al. 2015; Walls et al. 2016). Briefly, a custom Plexiglas housing was used for body protection to simulate protective effects of military body armor (Rafaels et al. 2011). A stereotaxic head frame with bite bar and ear bars (Kopf Instruments) was utilized to fix the head in place and prevent blast wind-induced head acceleration. For the blast group, each rat’s head was positioned beneath the open end of the shock tube such that the dorsum of the skull was the incident surface exposed to a composite blast (shock wave + blast wind). The exposure conditions were consistent with those described in our prior publications, having a recorded pressure profile with a near-instantaneous rise to peak pressure followed by overpressure and underpressure periods, as follows: side-on (static) 150-kPa maximum overpressure, 1.25-ms overpressure duration, and 20-kPa minimum underpressure; face-on (dynamic) 160-kPa maximum overpressure, 1.75-ms overpressure duration, and 5-kPa minimum underpressure. These exposure conditions have been validated to correspond to a mild severity in this model, lacking acute motor and memory deficits or evidence of intracranial hemorrhage while presenting evidence of subclinical elevations in oxidative stress, BBB permeability, and neuroinflammatory activity (Walls et al. 2016). Millimeter-scale point deformations in the brain have also been observed in this model (Song et al. 2015). For the noise group, the animals were placed in a location equidistant from the source of the blast but out of the path of the shock wave so as to only be exposed to loud sound from the blast event. Tympanic membrane integrity was verified for all animals after injury using a surgical microscope. One blast animal was excluded due to bilateral tympanic membrane perforation.
Auditory Evoked Potential Recordings
Auditory functions and thresholds of all animals were assessed neurophysiologically at time points of preexposure (baseline), 2 wk postexposure, and 1 mo postexposure. The measurements that were used in this study include DPOAEs, ABRs, MLRs, and EFRs.
DPOAEs.
DPOAEs are commonly used in clinics and in research to test the biomechanical gain functions of OHC in the cochlea (Shaffer et al. 2003). DPOAEs are elicited by the presentation of two simultaneous tones at a ratio of ~1.2 to the cochlea. Electromotility driven by prestin (a protein located on OHC wall; Dallos et al. 2008; Liberman et al. 2004) and mechanoelectrical transduction of stereocilia above the hair cells (Avan et al. 2013; Kennedy et al. 2005) are believed to be the two main active processes that produce DPOAE responses. All DPOAEs were performed in a 9-ft. × 9-ft. double-walled acoustic chamber (Industrial Acoustics Corporation) using standard techniques similar to those reported in Lai and Bartlett (2015). For anesthetization, animals were treated with 4% isoflurane via inhalation in an induction chamber. They were then transferred to the manifold and maintained with 1.8–2% isoflurane on a water-circulated warming blanket (Kent Scientific) to keep body temperature at 37°C throughout the duration of the recording session. Stimulus presentation and recordings were performed using BioSig (Tucker Davis Technologies, TDT) in the acoustic chamber. An earpiece (Etymotic-10B), which contained a miniature low-noise microphone and two sound delivery tubes, was inserted into the right ear canal of animals. The two sound delivery tubes connected the earpiece to the sound sources, which were two multifunction closed field speakers (TDT) that delivered pure tones to the ear canal. The two speakers presented sounds while the microphone recorded DPOAEs from the ear canal simultaneously. The output of the microphone was delivered as an input to a TDT RZ5 system that converted the recorded responses from analog to digital. Each response is an average response of 100 stimulus sweeps presented continuously to the animals. DPOAE input/output functions using two pure tones (f1 and f2, f2/f1 = 1.2) centering at 2, 4, 8, or 12 kHz were tested in all animals and in all recording sessions. For 2 and 4 kHz, the intensity of f1 was varied from 40 to 85 dB SPL in 5-dB steps. For 8 and 12 kHz, the intensity of f1 was varied from 35 to 75 dB SPL in 5-dB steps. The intensity of f2 was set at 10 dB SPL below that of f1. Data were processed with high-pass (HP) and low-pass (LP) filters using cutoff frequencies (fc) of 80 and 13,200 Hz, respectively, before analysis.
ABRs.
Hearing thresholds of 2, 4, 8, 12, 16, and 32 kHz were estimated in all animals via ABRs. ABRs were performed after DPOAEs. While animals were under 1.8–2% isoflurane, needle electrodes (Ambu) were inserted subdermally. The channel 1 positive electrode was placed along the midline of the head (midsagittal) oriented Fz to Cz. The channel 2 positive electrode was positioned C3 to C4 along the interaural line. The negative/inverting electrode (used with positive electrodes for both channels 1 and 2) was placed under the mastoid of the right ear ipsilateral to the inserted earpiece and speaker. A ground electrode was placed in the nape of the neck. These configurations are consistent with prior publications from our laboratory (Lai and Bartlett 2015; Parthasarathy and Bartlett 2011, 2012; Parthasarathy et al. 2014). After electrode placement, electrode impedances were confirmed to be <1 kΩ using a low-impedance amplifier (RA4LI; TDT). Animals were subsequently sedated by intramuscular injection of 0.2 mg/kg dexmedetomidine (Domitor). To avoid anesthetic effects on neural responses, isoflurane was taken off after injection. Because anesthetic effects take ~10 min to wear off (data not shown), ABRs were performed 15 min after cessation of isoflurane. Dexmedetomidine is an α-adrenergic agonist that acts as a sedative and an analgesic (Ter-Mikaelian et al. 2007). It decreases motivation but preserves behavioral and neural responses in rodents (Ruotsalainen et al. 1997). After injection, animals still respond to pain and acoustic stimuli but are immobile for ~3 h of recording period.
By using a point directly in front of the animals’ face as the reference for 0° azimuth, the stimulus was presented free-field to the right ear (90° azimuth) of animals from a calibrated speaker (Bowers and Wilkins) from a distance of 115 cm. The speaker was directly facing the right ear. Rectangular clicks (0.1-ms duration) and tone pips (2-ms duration, 0.5-ms cos2 rise-fall time) varying in sound levels from 95 to 5 dB peak SPL (pSPL) in 5-dB steps were used in ABR recording. The frequencies of tone pips were varied from 1 to 32 kHz in one-octave steps, and 12 kHz was included to improve resolution near the most sensitive portion of the animal’s audiogram (Parthasarathy et al. 2014). All stimuli were presented in alternating polarity at 26.6 per second. A 20-ms acquisition window (0–20 ms) was used, and each ABR was an average of a total of 1,500 repetitions for each sound level (750 at each polarity). Data were processed with HP (fc = 30 Hz) and LP (fc = 3,000 Hz) filters before analysis. The ABR threshold was defined as the minimum sound level that produced a distinct ABR waveform. The ABR amplitudes of waves I, IV, and V from channel 2 were estimated as the amplitude of the wave from the baseline (a cursor was placed at the average of the noise floor on the waveform before the cochlear microphonic, and another cursor was placed at the peak to estimate the wave amplitude) in BioSig.
MLRs.
MLRs were recorded using short click and tone stimuli presented at a slower rate (4/s vs. 26.6/s in ABRs) and with a recording window of longer duration (100 vs. 20 ms in ABRs). Increasing the interstimulus interval and recording window duration to 100 ms provides sufficient time for the stimulus-evoked neural responses to occur in and be recorded from the auditory midbrain, thalamus, and cortex (Barth and Di 1991; Di and Barth 1992; McGee and Kraus 1996; Phillips et al. 2011; Šuta et al. 2011) while still capturing responses from ABR brain stem generators in parallel. As such, early components (<10 ms) of the waveform collected under the MLR acquisition settings correspond to responses from ABR generator regions, whereas later responses correspond to the aforementioned more central generators in the thalamus and cortex. Only MLRs recorded from the interaural line (channel 2) were analyzed. Rectangular clicks (0.1 ms) and brief 8-kHz tones (2 ms) of alternating polarity were used in MLR recording. Stimuli were presented at 4/s, and 1,500 repetitions were collected over an acquisition time window of 100 ms to obtain an average response. The sound levels of clicks were set at 80 and 70 dB pSPL, whereas the intensity of brief 8-kHz tones was 80 dB pSPL. Data were processed with HP (fc = 300 Hz) and LP (fc = 3,000 Hz) filters before analysis.
EFRs.
EFRs were recorded during the same recording session as ABRs and MLRs, using the same electrodes and techniques similar to those of Parthasarathy et al. (2014). Two channels were used to record EFRs in this study because they were sensitive to a complementary range of amplitude modulation frequencies (AMFs) (Parthasarathy and Bartlett, 2012). Channel 1 (vertical configuration) is more sensitive to higher AMFs (90–2,048 Hz), whereas channel 2 (horizontal configuration) is more sensitive to lower AMFs (8–90 Hz). Simultaneous recording of EFRs from these two channels aids in analyzing a wider range of AMFs as well as identifying temporal processing deficits at various auditory subcortical regions. Stimuli used for EFRs were sinusoidally amplitude-modulated (SAM) 8-kHz tone carriers at various AMFs and 100% modulation depth with stimulus duration of 200 ms. The carrier frequency was set at 8 kHz because frequencies of 6–16 kHz span the most sensitive hearing region of rats (Parthasarathy et al. 2014) and 8 kHz is approximately at the center (in octave) of this region. Frequency of 8 kHz is near the most sensitive region of normal rat audiogram (Parthasarathy et al. 2014). The acquisition window was 300 ms long, and each response was an average of 200 repetitions. The modulation frequency of the stimulus was varied from 16 to 2,048 Hz in 0.5-octave steps. SAM 8-kHz stimuli were presented at 80 dB SPL. Before EFR amplitude analysis, data were passed through LP and HP filters. The HP filters were applied differentially to AMFs 16–24 Hz (fc = 12 Hz), 32–64 Hz (fc = 30 Hz), and ≥90 Hz (fc = 80 Hz). The LP filter was applied uniformly across data for all AMFs (fc = 3,000 Hz). The amplitudes measured at the stimulus modulation frequencies after a fast-Fourier transform (FFT) of time-domain response waveforms were used as a measure of phase-locking for multigroup comparison. Swaminathan and Heinz (2012) demonstrated that speech sound recognition depends on envelope cues <64 Hz in quiet and in noise, as well as temporal fine-structure cues (64–300 Hz). Only envelope responses were assessed for the 90- to 2,048-Hz AMFs used in the present study, which cover the entirety of the reported behaviorally relevant range (Swaminathan and Heinz 2012).
Statistics
All statistics for DPOAE, ABR, and EFR measurements utilized a three-way or two-way repeated-measures ANOVA test (α = 0.01) to check the significance of each main effect and interaction. To improve efficiency of presentation and facilitate ease of data interpretation, a two-panel style is utilized to illustrate the majority of the post hoc Tukey’s least squares mean (LSM) results, specifically for the primary outcome metric of interest: comparison of means for the group × time point interaction. In such figures, one panel illustrates the data sets via standard line plots or bar graphs, whereas the other panel incorporates color maps to describe statistical results. A sample panel showing such maps with an accompanying key is illustrated in Fig. 1, right.
Fig. 1.
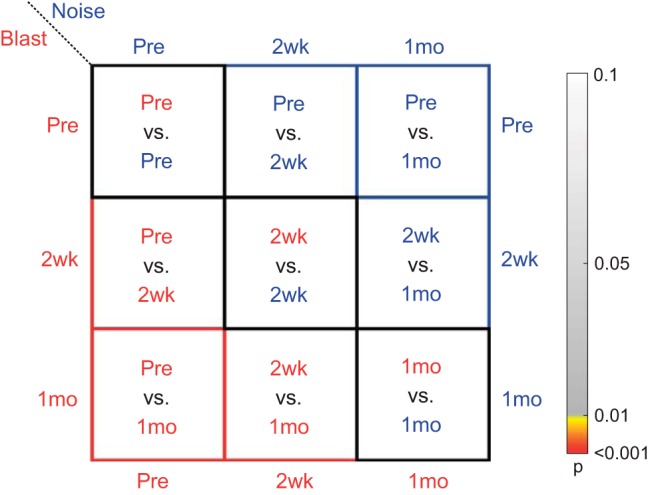
Statistics color map key. A color map is used to illustrate P values for data. Grayscale colors indicate nonsignificant effect (P > 0.01). “Hot” colors (yellow to red) indicate significant effect (P < 0.01), with red indicating values <0.001. Boxes outlined in black are between-group comparisons at each time point (blast vs. noise). Red and blue boxes indicate within-group comparisons at different time points for blast (red) and noise (blue), respectively. Pre, preexposure to blast or noise; 2wk, 2 wk after exposure; 1mo, 1 mo after exposure.
Between-group comparisons (blast vs. noise) at a given time point (preexposure, 2 wk, or 1 mo) are shown in the black-outlined boxes along the diagonal. Within-group comparisons are designated by the colored-border boxes, with red corresponding to blast and blue corresponding to noise groups. The comparisons in each box are documented explicitly in Fig. 1. The color of each box corresponds to the P value for the comparison of interest, with grayscale values representing nonsignificant results (P > 0.01) and “hot” values (yellow to red) representing statistically significant results (P < 0.01). Red indicates P < 0.001 (close to 0). Directly in the text, P values, F statistics, degrees of freedom (reported as df1, df2 subscripts on F statistics), and effect size () are reported.
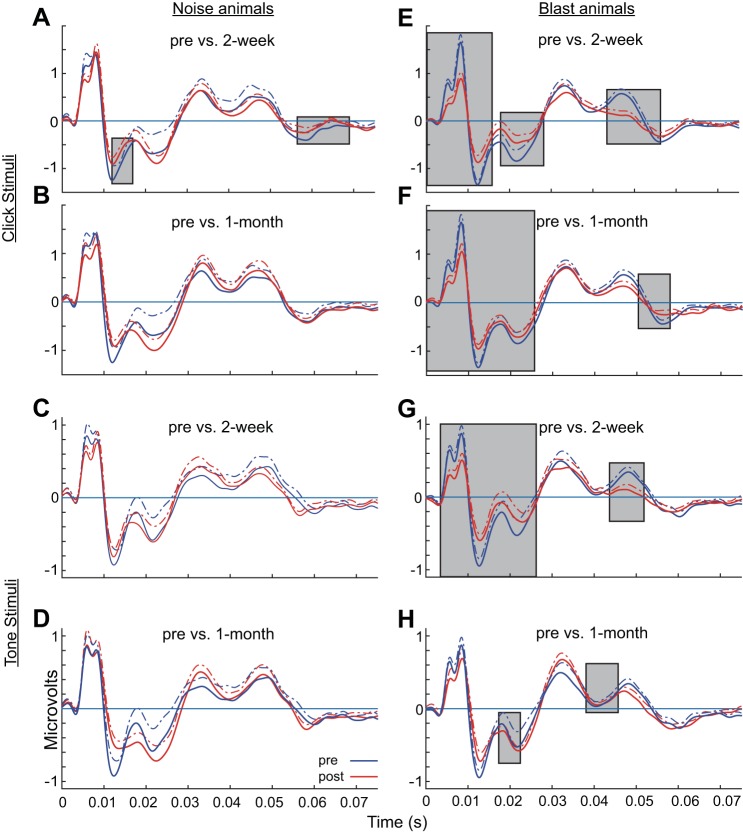
For MLR statistics, a 5-ms moving window (1-ms steps) of absolute amplitude was used to assess differences between preinjury (blue) and postinjury (red) conditions at 2 wk and 1 mo postexposure using the Wilcoxon rank-sum test. Only when two or more consecutive windows have P values <0.05 is the window considered significant. Gray boxes in Fig. 9 indicate time windows in which significant differences were observed between pre- and postinjury waveforms.
Fig. 9.
Click and tone MLRs captured more apparent thalamic and cortical deficits in blast than noise animals. MLRs were elicited by clicks (A, B, E, F) or brief 8-kHz tones (C, D, G, H) at 80 dB pSPL. Minimal pre- vs. postinjury differences were observed in noise animals (A–D). Pre- vs. postinjury differences were significant in blast animals (E–H) for the early wave components corresponding to ABR generator regions (<10 ms) as well as two later MLR components (15–25 ms and 35–48 ms). Changes in the later MLR components observed in blast animals corresponded to altered thalamic, thalamocortical, and cortical activation. Gray boxes indicate time windows in which significant differences were observed between the pre- and postinjury waveforms, based on comparison of 5-ms moving averages (1-ms steps) and using the Wilcoxon rank-sum test (P < 0.05). Data are means (solid lines) and means + SE (dashed lines).
RESULTS
DPOAEs
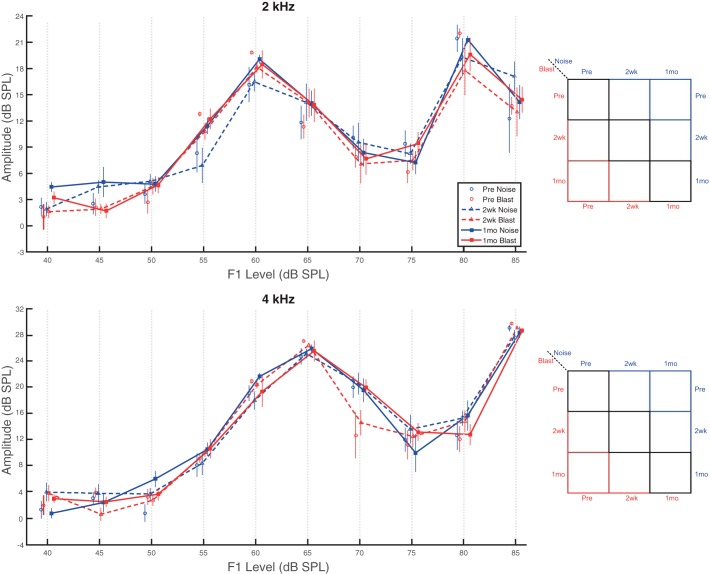
DPOAE recordings, which assess the integrity of OHC function (Shaffer et al. 2003), demonstrated frequency-dependent changes in input/output functions (I/O) after blast or noise exposure. Neither blast nor noise animals demonstrated significantly different I/O function amplitudes at 2 wk or 1 mo after injury for pure tones at 2 or 4 kHz (Fig. 2). Main effects of group (2 kHz: F1,12 = 0.43, P = 0.525, = 0.03; 4 kHz: F1,12 = 0.27, P = 0.611, = 0.02) and time point (2 kHz: F2,24 = 2.09, P = 0.146, = 0.15; 4 kHz: F2,24 = 1.98, P = 0.160, = 0.14) were not significant. Unsurprisingly, a significant main effect of sound level was observed (2 kHz: F9,108 = 104.22, P < 0.0001, = 0.90; 4 kHz: F9,108 = 267.73, P < 0.0001, = 0.96). No significant interaction effects were present.
Fig. 2.
No impairments were observed for lower frequency DPOAEs. DPOAEs at 2 and 4 kHz indicated similar pre- and postinjury I/O function generation for both blast and noise animals. No significant effects of group (blast vs. noise) or time point (Pre vs. 2wk vs. 1mo) or group × time point interaction were present for 2-kHz (top) or 4-kHz (bottom) pure tones. Data are means ± SE.
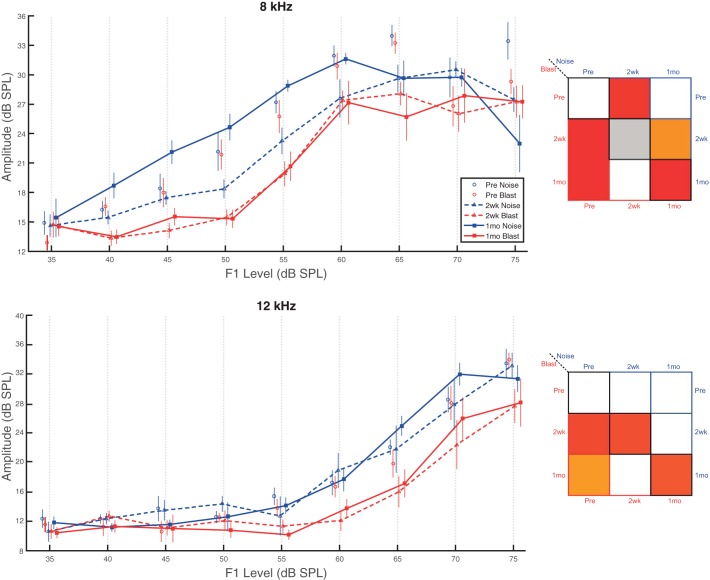
Conversely, both blast and noise animal I/O functions were altered postinjury for 8-kHz pure tones (Fig. 3, top). Significant main effects of group (F1,12 = 12.33, P = 0.004, = 0.51) and time point (F2,24 = 20.10, P < 0.0001, = 0.63) were observed. Unsurprisingly, a significant main effect of sound level was also observed (F8,96 = 120.54, P < 0.0001, = 0.91). A significant time point × sound level interaction effect was also present (F16,192 = 2.72, P < 0.0006, = 0.18). Blast animal I/O functions were significantly reduced (worse) to a similar degree compared with preexposure recordings at both 2 wk and 1 mo postinjury. Noise animals, however, only demonstrated significant reductions (worse) at 2 wk postinjury. At 1 mo postinjury, significant I/O function amplitude differences were observed between groups. Blast group amplitudes were significantly lower (worse) than noise group amplitudes, which returned to statistical equivalence with noise preinjury recordings by the 1-mo time point.
Fig. 3.
Blast animals demonstrated more severe and persistent deficits on higher frequency DPOAEs. DPOAEs at 8 and 12 kHz revealed group- and time point-dependent I/O function differences. Top, pure tones set at 8 kHz. Both blast and noise animals demonstrated significant differences at 2 wk postinjury from preinjury recordings. At 1 mo postinjury, only blast group recordings were significantly lower (worse) than preinjury, at which point between-group differences also emerged. Bottom, pure tones set at 12 kHz. Only blast group animals demonstrated significant reductions (worse) compared with preinjury recordings at both the 2-wk and 1-mo postinjury time points. Between-group differences were also present at both 2 wk and 1 mo with blast group results lower (worse) than the noise group. Data are means ± SE.
For 12-kHz pure tones (Fig. 3, bottom), only blast group DPOAE I/O amplitudes were significantly different from preexposure recordings at 2 wk and 1 mo postinjury, showing reduced (worse) amplitudes at both time points. Noise group recordings did not demonstrate any time-dependent changes. Significant main effects of group (F1,12 = 12.08, P = 0.005, = 0.50), but not time point (F2,24 = 3.55, P = 0.045, = 0.23), were observed. Consistent with this observation, significant differences between groups were observed at both 2 wk and 1 mo postinjury. Unsurprisingly, a significant main effect of sound level was observed (F8,96 = 124.00, P < 0.0001, = 0.91). No significant interaction effects were present. Overall, our DPOAE results are consistent with the previous report that more apical, lower frequency OHCs (2 and 4 kHz) are damaged by blast exposure to a lesser degree than more basal, higher frequency OHCs (8 and 12 kHz) (Cho et al. 2013b).
ABR Thresholds
Click ABR recordings captured significant threshold differences for blast, but not noise, animals at both postinjury time points compared with preexposure recordings (Fig. 4). Preliminary testing showed that both blast and noise groups had significant threshold shifts of >50 dB at 48 h postexposure (not shown), making it very difficult to evoke any measurable response in most animals. Thresholds for blast group animals remained significantly elevated (worse) at both 2 wk and 1 mo postinjury, whereas noise group animals did not demonstrate any observable changes in click thresholds (Fig. 4). Significant main effects of group (F1,16 = 30.50, P < 0.0001, = 0.66) and time point (F2,28 = 13.16, P < 0.0001, = 0.48) were observed, in addition to a significant group × time point interaction effect (F2,28 = 7.55, P = 0.002, = 0.35).
Fig. 4.
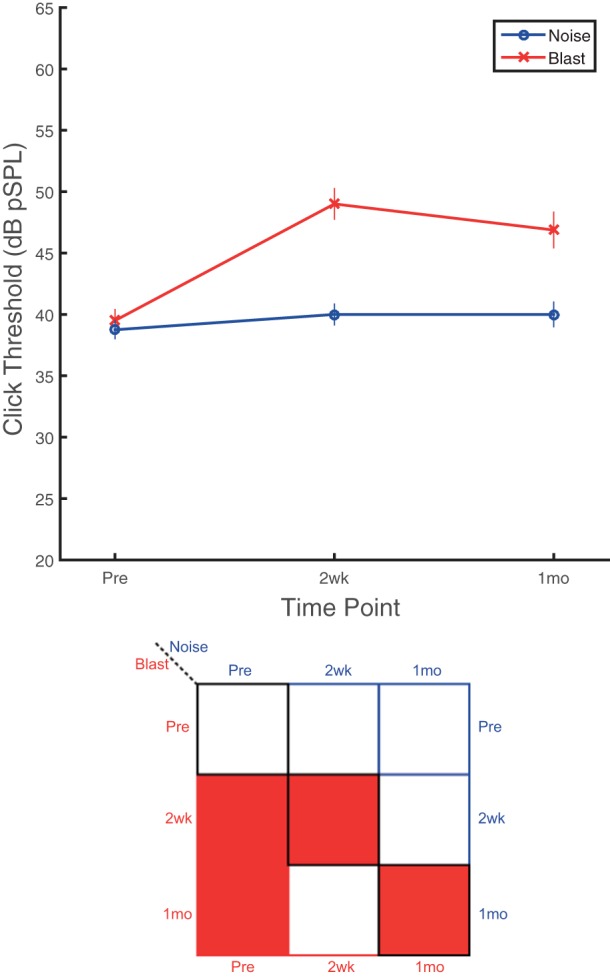
Blast animals experienced sustained alterations in click ABR thresholds for at least 1 mo, whereas noise animals appear unaffected. Click ABR threshold changes indicate a significant group effect, with blast group thresholds significantly higher (worse) than the noise group. A time point effect was also present for the blast group with both 2-wk and 1-mo postexposure thresholds significantly higher (worse) than preinjury recordings. Noise group animals did not exhibit any time-dependent significant alterations in click ABR thresholds. Blast thresholds were significantly higher (worse) than noise thresholds at both postinjury time points. Data are means ± SE.
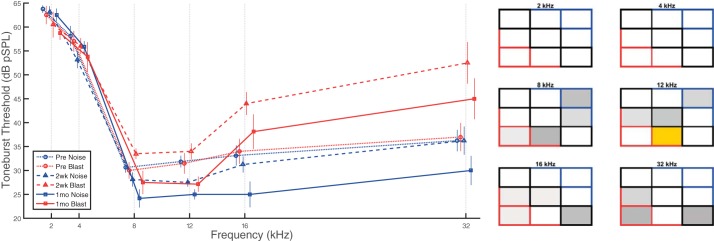
Tone ABR threshold shifts did not demonstrate consistent statistically significant effects for either blast or noise group animals at any of the frequencies tested between 2 and 32 kHz at any postinjury time point (Fig. 5). Unsurprisingly, a significant main effect of frequency was observed (F5,80 = 69.26, P < 0.0001, = 0.81), but main effects of group (F1,16 = 1.66, P = 0.215, = 0.09) and time point (F2,28 = 2.55, P = 0.096, = 0.15), as well as all interaction effects, were nonsignificant. However, some informative trends were observed. High-frequency recordings (≥8kHz) suggest potentially differential postinjury trends between groups, with blast animals having slightly elevated (worse) thresholds (5–10 dB), whereas noise animals had lower (better) thresholds, compared with preexposure recordings. In blast animals, only 12-kHz hearing thresholds were significantly different between 2 wk and 1 mo postinjury, where hearing thresholds recovered at post 1 mo and became lower (better), but not significantly, than at preexposure. In addition, there were 10- to 15-dB increases (worse) in mean thresholds for frequencies ≥16 kHz 2 wk postblast. Higher frequency thresholds returned closer to preinjury levels by 1 mo postblast (5- to 10-dB threshold increases: worse than preinjury but better than 2 wk postinjury).
Fig. 5.
Tone ABR thresholds demonstrated trends of threshold increases (worse) for blast and decreases (better) for noise animals at high frequencies. No consistent statistically significant effects were observed for tone threshold measurements, except at 12 kHz. However, blast group recordings show trends of threshold elevation (worse) for higher frequencies at both postinjury time points. Noise group threshold changes were minimal at 2 wk postinjury, but recordings at 1 mo demonstrated a trend of decreased (better) thresholds for tones ≥8kHz. Data are means ± SE.
ABR Wave Amplitudes
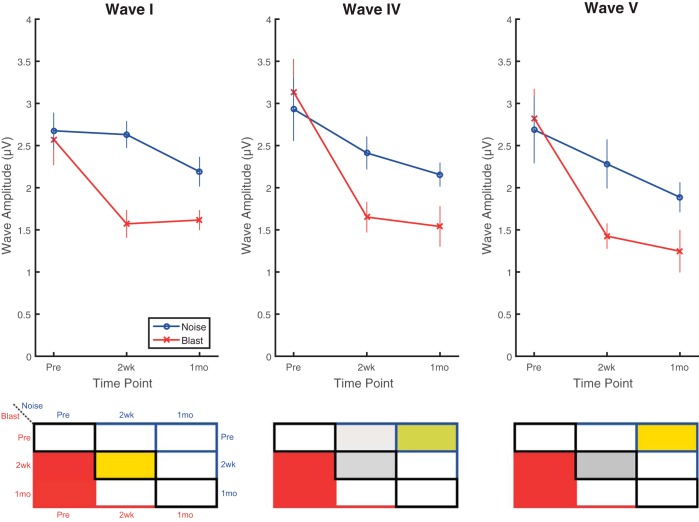
ABR wave amplitudes were assessed for waves I, IV, and V in response to click stimuli at 80 dB pSPL (Fig. 6) and 30 dB sensation level (SL) (Fig. 7). For the measurements at 80 dB pSPL (Fig. 6), significant main effects of time point (F2,24 = 48.48, P < 0.0001, = 0.80), but not group (F1,12 = 2.74, P < 0.124, = 0.18) or wave (F2,24 = 2.52, P = 0.101, = 0.17), were observed. A significant group × time point interaction effect was observed, however (F2,24 = 10.25, P < 0.0006, = 0.46). At time points 2 wk and 1 mo postinjury, recordings were significantly lower (worse) than preexposure recordings for blast animals for all waves. For noise animals, significant reductions (worse) were present for waves IV and V only at 1 mo postinjury compared with preexposure recordings. These imply that injury-induced reduction (worse) of wave amplitudes happened at different rates in blast and noise animals. The only significant difference between groups was observed at 2 wk postinjury for wave I.
Fig. 6.
click ABRs presented at 80 dB pSPL revealed reduced (worse) wave I, IV, and V amplitudes postinjury to a greater degree in blast animals. Blast animals demonstrated significant wave I (top), IV (middle), and V (bottom) amplitude decreases (worse) at both 2 wk and 1 mo postinjury compared with preinjury recordings. Waves IV and V were significantly decreased in noise animals, but only at the 1-mo postinjury time point. Wave I recordings captured significant differences between blast and noise groups at 2 wk postinjury, showing lower (worse) amplitudes in the blast group. Wave IV and V amplitudes demonstrated nonsignificant trends of difference, again with blast group having lower (worse) amplitudes. Data are means ± SE.
Fig. 7.
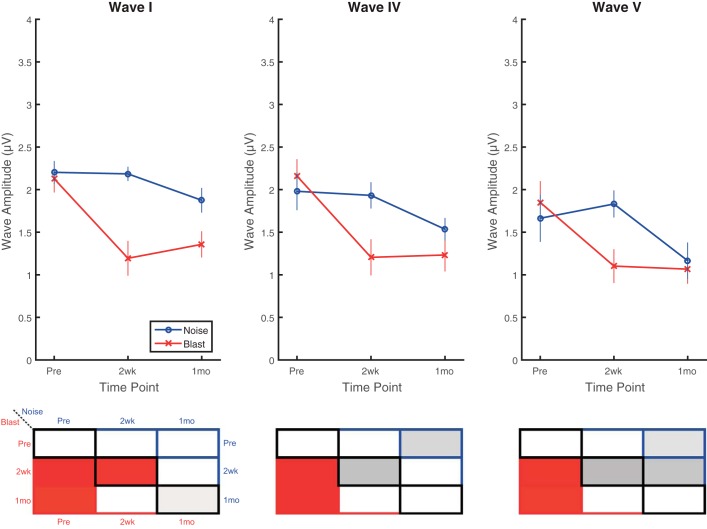
Postinjury 30-dB SL click ABRs revealed wave I, IV, and V amplitude reductions (worse) in blast but not noise animals. Blast animals demonstrated significant decreases (worse) in wave I (top), IV (middle), and V (bottom) amplitudes at both 2 wk and 1 mo postinjury compared with preinjury recordings. No significant pre- vs. postinjury changes were observed for noise animals. Wave I recordings captured the only significant difference between blast and noise groups at 2 wk postinjury, although wave IV and V amplitudes demonstrated trends of difference. Data are means ± SE.
To compensate for changes in threshold induced by blast, measurements were also made at 30 dB above each animal’s individual threshold for clicks. For the 30 dB SL measurements (Fig. 7), significant main effects of time point (F2,28 = 22.66, P < 0.0001, = 0.62) and wave (F2,32 = 8.33, P = 0.001, = 0.34), but not group (F1,16 = 3.94, P = 0.065, = 0.20), were observed. Additionally, a significant group × time point interaction effect was observed (F2,28 = 9.36, P < 0.0008, = 0.40). For time points 2 wk and 1 mo postinjury, amplitudes were significantly lower (worse) than preexposure in blast animals for all waves. For noise animals, no significant differences were observed between pre- and postinjury recordings. Consistent with the 80-dB pSPL measurements, the only significant difference between groups was observed at the 2-wk postinjury time point for wave I, but trends of between-group differences were present for all waves at the 2-wk time point.
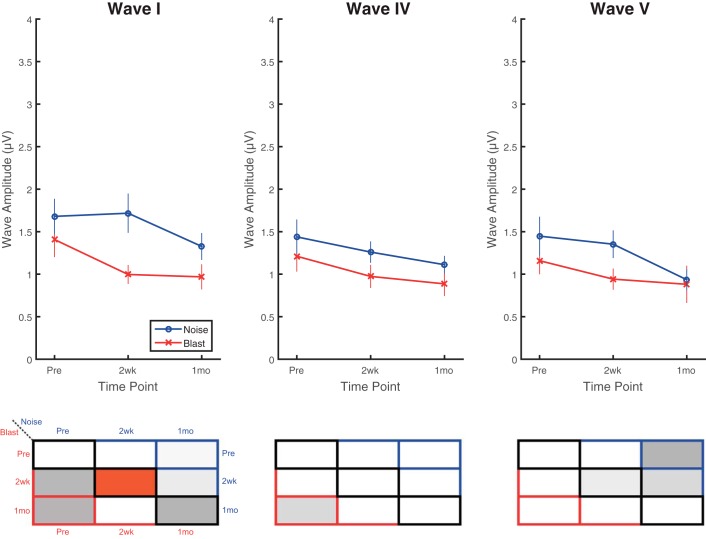
Tone ABR wave I, IV, and V amplitudes were also assessed in response to brief 8-kHz pure tones at 80 dB pSPL (Fig. 8). Significant main effects were observed for time point (F2,25 = 12.00, P = 0.0002, = 0.49) and wave (F2,32 = 6.54, P = 0.004, = 0.29), but not group (F1,16 = 3.86, P = 0.067, = 0.19). No significant interaction effects were observed. Trends of decrease (worse) were observed for blast animals at 2 wk that sustained to 1 mo postinjury and for noise animals at 1 mo, but none of the pre- vs. postinjury comparisons were significant for either group. Wave I amplitudes revealed a significant difference between blast and noise groups at the 2-wk time point, where wave I amplitudes were significantly lower for blast than for noise animals. Similar to the threshold measurements, pure tones, at least those at 8 kHz, seem to produce less consistent or dramatic reductions in ABR amplitudes postinjury compared with broadband clicks. On all click and tone ABRs, wave latencies were unchanged in all groups, at all time points, and for all ABR waves (not shown).
Fig. 8.
Deficits observed on tone-burst ABRs were less apparent than on click ABRs. Significant differences were observed using 80-dB pSPL, 8-kHz tone-burst ABRs between blast and noise groups at 2 wk postinjury with similar overall trends to click ABR results. Blast animals demonstrated trends of decrease (worse) in 2-wk postinjury wave I (top), IV (middle), and V (bottom) amplitudes that appeared to flatten and sustain to 1 mo postinjury, but changes were not significant compared with preinjury results. Trends in wave amplitude decreases (worse) in noise animals that occurred between 2 wk and 1 mo postinjury were also nonsignificant. Wave I amplitudes captured the only significant difference between blast and noise groups at 2 wk postinjury. Data are means ± SE.
MLRs
MLRs were measured using click or 8-kHz clicks and tones at 80 dB pSPL, similarly to the ABRs. They were presented much more slowly (4/s MLR vs. 26.6/s ABR) and measured over longer time windows (ranging from 10 to 50 ms) to track the impulse response to more central structures including the thalamocortical pathway. MLR grand-average responses of noise animals (Fig. 9, A–D) vs. blast animals (Fig. 9, E–H) are shown. A 5-ms moving window (1-ms steps) of absolute amplitude was used to assess differences between preinjury (blue) and postinjury (red) conditions at 2 wk and 1 mo postexposure. Gray boxes in Fig. 9 indicate time windows in which significant differences were observed between pre- and postinjury waveforms. Perhaps because of the slower presentation rate, pre- and postexposure differences were minimal for noise-exposed animals. For the noise group (Fig. 9, A–D), slight differences were apparent with click stimuli at 2 wk postinjury, mainly in the 10- to 15-ms time window (Fig. 9A) corresponding to IC and thalamic excitation in rodents and humans (Barth and Di 1991; Kraus and McGee 1992; Kraus et al. 1992; McGee et al. 1991; Phillips et al. 2011). These differences were no longer significant by 1 mo postinjury. Slight differences were noted in the 10- to 20-ms time window for other comparisons but did not reach statistical significance with the number of animals for comparison (n = 6–8 for noise animals). In contrast, there were clear significant differences in the ABR components of the waveform (<10 ms) under this slower stimulus presentation rate (4/s) in blast animals (Fig. 9, E–G), similar to results shown in Figs. 6–8 at a faster presentation rate (26.6/s). Longer latency peaks corresponding to thalamocortical transmission and cortical activation (Barth and Di 1991; Brett et al. 1996; Kraus and McGee 1992; Kraus et al. 1992; McGee et al. 1991) also demonstrated significant differences in the blast group (Fig. 9, E–H). There were significant differences in the 15- to 25-ms window for both time points and both stimuli for blast animals, corresponding to thalamocortical transmission (Di and Barth 1992). Finally, there were two late peaks in the MLR, at ~35- and 48-ms latencies. The second peak, corresponding to auditory cortical activation (Barth and Di 1991; Di and Barth 1992), was affected in blast animals for both stimuli and time points, but this was not the case for noise animals.
EFRs
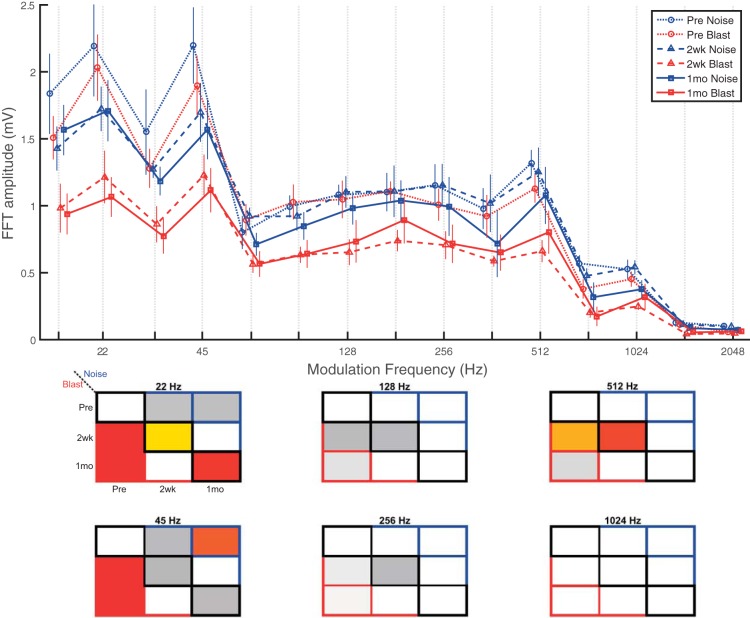
EFRs were recorded using an 8-kHz tone (SAM) carrier with sinusoidal amplitude modulation at 100% modulation depth. Fifteen amplitude modulation frequencies (AMFs) ranging from 16 to 2,048 Hz in half-octave steps were tested in each recording session. All results are plotted in Fig. 10, top, but for ease of statistical visualization, 6 representative AMFs were chosen to represent the range of AMFs tested (22, 45, 128, 256, 512, and 1,024 Hz) in Fig. 10, bottom. These AMFs have been tested in previous EFR studies in aging animals (Parthasarathy and Bartlett 2011, 2012; Parthasarathy et al. 2010, 2014).
Fig. 10.
EFRs captured central subcortical processing impairments in the IC with possible cortical contribution. SAM EFRs at 8 kHz and 80 dB SPL showed significantly decreased (worse) EFR amplitudes at lower AMFs (≤64 Hz), corresponding to IC and possibly cortex, at 2 wk and 1 mo postinjury to a greater degree in blast than noise animals. Mid-range AMF EFRs (90–512 Hz) showed trends of decrease (worse) for blast but not noise animals, whereas higher AMF EFRs (>512 Hz) showed minimal changes in both groups, suggesting more peripheral EFR remained intact. Data are means ± SE.
For EFRs of SAM at 80 dB SPL (Fig. 10), significant main effects of group (F1,12 = 15.59, P = 0.002, = 0.56), time point (F2,23 = 36.23, P < 0.0001, = 0.76), and, unsurprisingly, frequency (F14,168 = 81.00, P < 0.0001, = 0.87) were observed. A significant group × time point interaction effect was also observed (F2,23 = 7.38, P = 0.003, = 0.39). At both 2 wk and 1 mo, the main effects indicate significantly lower EFR amplitudes in blast compared with noise animals. Lower modulation frequency recordings (≤64 Hz) at 2 wk and 1 mo in blast animals revealed widespread significant EFR amplitude reductions (worse) compared with preexposure recordings. Noise animals demonstrated trends of slight decrease (worse) for lower modulation frequency EFR amplitudes, but only a few were significant (e.g., 45 Hz pre vs. 1 mo). Blast EFR amplitudes were reduced (worse) compared with noise EFR amplitudes at lower modulation frequencies (significant at 22 and 32 Hz; trend at 16, 45, and 64 Hz) for both 2-wk and 1-mo postinjury time points. Middle modulation frequency recordings (90–512 Hz) demonstrated trends of decrease (worse) for blast animals, but not for noise animals, for 2-wk and 1-mo postinjury time points compared with preinjury recordings. Some of the observed decreases were statistically significant (e.g., 512 Hz). Noise animals did not demonstrate trends or significant changes at either 2 wk or 1 mo postinjury compared with preinjury recordings. Neither group demonstrated trends or significant differences for higher modulation frequencies (≥724 Hz).
DISCUSSION
The auditory system is dually susceptible to blast via a combined injury to both the PAS and CAS. The presented results detail our investigation of PAS and CAS function after exposure to a single blast (shock wave + noise) or noise-only acoustic trauma event. We hypothesized the dual insult intrinsic to the nature of blast exposure would cause a greater degree of hearing impairment in blast animals than in noise animals that would be detectable and differentiable with noninvasive auditory tests. This phenomenon was observable in our results, which widely demonstrated blast animals having more severe, persistent auditory deficits in postinjury PAS and CAS assessments compared with noise animals. The findings from the present study extend previous findings and are largely consistent with them. Previous animal studies have found hearing threshold increases (worse), impairments in gap detection, and compromised prepulse inhibition of startle following blast exposure, primarily for higher frequency sounds (Luo et al. 2014a; Mahmood et al. 2014). These changes were associated with OHC loss (Cho et al. 2013b; Ewert et al. 2012) and loss of hair cell/spiral ganglion synapses for IHCs and OHCs (Cho et al. 2013b). Those observations are consistent with the reduced (worse) DPOAE amplitudes for higher frequencies (Fig. 3) and lasting reduction (worse) of wave I amplitudes (Figs. 6 and 7). At the single-unit level, both the dorsal cochlear nuclei (DCN) and the IC have been reported to undergo postblast increases in spontaneous activity for units sensitive to higher frequencies (Luo et al. 2014a, 2014b), which could desynchronize responses and compromise wave IV and V of the ABR response, similar to our findings (Figs. 6–8).
Notably, the impairments we observed were elicited by a relatively mild, single blast exposure, compared with more intense exposures in previous studies (Cho et al. 2013b; Du et al. 2013; Luo et al. 2014a, 2014b; Mahmood et al. 2014). Furthermore, most of the acoustic stimuli utilized in this and prior studies, specifically clicks and pure tones in the absence of background noise, are relatively simple in terms of CAP complexity needed for appropriate signal decomposition and response. It is possible and likely that more complex stimuli such as amplitude-modulated sounds and speech sounds, particularly with the addition of background noise, would reveal greater deficits. EFR results from this investigation and prior studies in aging support this notion (Anderson et al. 2012, 2013). Our results capturing significant postblast impairments in CAP of temporally modulated sounds (Fig. 10) is an important step forward in this regard. The results of this investigation offer broad implications, summarized in Fig. 11, toward the scope of blast-induced auditory processing impairments that may impact battlefield performance, quality of life, and societal reintegration, and future laboratory investigations of blast injury.
Fig. 11.
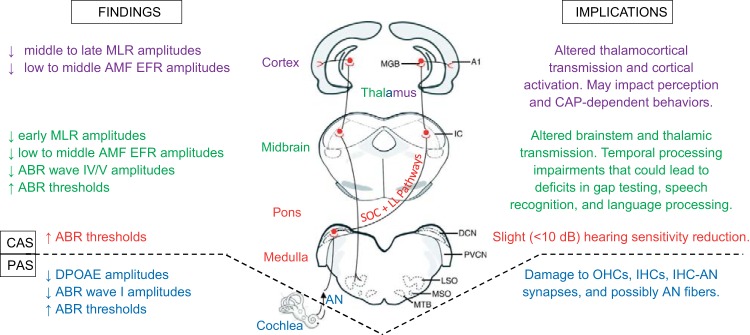
Summary schematic of blast-induced auditory pathophysiological findings and implications from the cochlea to the cortex. Auditory system drawing was adapted with permission from Caspary et al. (2008). A1, primary auditory cortex; ABR, auditory brain stem recording; AMF, amplitude modulation frequency; AN, auditory nerve; DCN, dorsal cochlear nucleus; DPOAE, distortion product otoacoustic emission; EFR, envelope following response; IC, inferior colliculus; IHC, inner hair cell; LL, lateral lemniscus; MGB, medial geniculate body; MLR, middle latency recording; OHC, outer hair cell; SOC, superior olivary complex.
Mild Blast Effects on the Peripheral Auditory System
DPOAE amplitudes and OHC integrity.
The DPOAEs demonstrate differential injury effects on OHC performance dependent on both frequency (and thus basilar membrane locus) and injury type (blast vs. noise), as shown in Figs. 2 and 3. Among the tested stimuli, noise-only injury appeared to cause selective damage to OHCs at the 8-kHz region, whereas blast exposure incited injury to OHCs at both 8 and 12 kHz. This suggests shock wave exposure indeed causes additional detriment to the PAS compared with conventional noise-induced acute acoustic trauma, even when the tympanic membrane remains structurally intact as in this model. A reduction in DPOAE amplitude indicates the possibility of either OHC death (complete loss of OHC) or OHC dysfunction (OHCs are present but have lost their function; Chen et al. 2009; Kemp 1978). DPOAE results from a prior study indicated that the overall cochlear anatomy was normal but that complete OHC loss was discovered in the cochlear base (Cho et al. 2013b), which corresponds to higher frequency regions. This finding is similar to other reports describing blast-induced hearing loss in rats (Ewert et al. 2012) as well as to our studies in which DPOAEs were affected at 8 and 12 kHz, but not at 2 or 4 kHz (Figs. 2 and 3). We thus infer that blast-induced OHC dysfunction and/or death existed primarily at higher frequency regions in blast animals, consistent with previous reports (Cho et al. 2013b; Ewert et al. 2012). This may be a common feature of shock wave exposure independent of differing blast conditions between investigatory teams. For stimuli at or above 8 kHz, blast and noise pathological responses appear to diverge between 2 wk and 1 mo postinjury. With respect to preinjury I/O functions, noise animals became more sensitive (better) at 1 mo, whereas blast animals were persistently impaired, suggesting underlying differences in pathophysiology and capacity for functional recovery between single-event blast and noise-only acoustic trauma (Fig. 3). The findings of Luo et al. (2014a, 2014b) agree with the notion that the pathological basis of blast- and noise-induced auditory processing deficits are fundamentally different.
Auditory nerve-evoked potential thresholds and response amplitudes.
Damage to OHC integrity naturally suggests downstream effects on ABR thresholds and wave amplitudes may be present. ABRs contain waveform features that correspond to specific generators within the auditory neuraxis. Evoked potential responses from the distal, extracranial fibers of the AN are represented in ABR wave I, where significant amplitude reductions were observed at 80 dB pSPL and 30 dB SL in blast animals at all postinjury time points (Figs. 4 and 5). Additionally, our results indeed indicated significant, but small (<10 dB), persistent threshold elevations (worse) in response to 0.1-ms broadband click stimuli (with similar trends for higher frequency tone stimuli) at both 2 wk and 1 mo postinjury for blast, but not noise, animals (Fig. 4). Threshold shifts <10 dB are less than those reported by many animal and human studies of aging or nonblast acoustic trauma (Henderson et al. 1994; Parthasarathy et al. 2014; Van Campen et al. 2002) and could be asymptomatic/subclinical. This observation is consistent with recent clinical reports of minimal changes in hearing sensitivity after blast in humans, despite the presence of behavioral alterations (Bressler et al. 2017; Gallun et al. 2012; Saunders et al. 2015).
Taken in the context of DPOAE findings, the high-frequency threshold shifts in the audiograms suggest noise-only acoustic trauma principally impacts OHCs. Conversely, blast exposure demonstrates capacity to disrupt OHC and IHC integrity, synaptic coupling of IHCs to AN fibers, and perhaps even an overall reduction in AN fibers (Cho et al. 2013b). In higher frequency tone data, a trend of diverging responses between groups was observed similar to that for the DPOAEs: the blast group demonstrated threshold increases (worse), whereas decreases were observed in the noise group (better). This could indicate a larger role for peripheral sensitization in the pathophysiological response to noise-only compared with blast-induced acoustic trauma, again suggesting OHC and OHC+IHC damage in the noise and blast groups, respectively. Alternatively, as previously suggested by Ewert et al. (2012), it is possible that the heightened severity of blast trauma overwhelms the repair and remodeling capacity of the PAS, precluding development of peripheral compensatory recovery mechanisms. The persistence of blast animals’ significant postinjury 80-dB pSPL click wave I amplitude reductions with SL matching (30 dB SL) supports our hypothesis regarding the likely blast-induced functional disruption of synaptic connections between IHCs and AN fibers, for which some histologic evidence has been previously reported (Cho et al. 2013b), in addition to possible reduction of AN fibers. Conversely, SL-matched ABRs for the noise group did not demonstrate significant differences from preinjury levels, supporting the assertion that single-event noise-only peripheral auditory deficits are primarily related to compromised integrity and function of OHCs, but not IHCs.
Mild Blast Effects on the Central Auditory System
Click and tone processing impairments.
Longer latency waveforms in the click- or brief pure tone-evoked potential waveform correspond to the midbrain and brainstem auditory regions. The primary generators of ABR waves IV and V in rats are a combination of afferent inputs to the inferior colliculus (IC) including the superior olivary complex (SOC), lateral lemniscus (LL), and associated projections in the auditory midbrain (Blatchley et al. 1987; Chen and Chen 1991). Significant postinjury click wave IV and V amplitude reductions (worse) were present in both blast and noise groups at 80 dB pSPL, but only in the blast group at 30 dB SL (Figs. 6 and 7). In contrast, 8-kHz tones at 80 dB pSPL did not reveal significant postinjury differences in wave IV or V amplitudes for either group (Fig. 8). The consistency of postblast click threshold elevations (worse) and wave amplitude reductions (worse) could be a reflection of a click stimulus’s broad coverage across a wide range of frequencies, particularly its inclusion of higher frequency regions. The applied click stimuli induce broadband cochlear excitation encompassing all tested tone frequencies in addition to higher frequencies beyond 32 kHz. The click stimuli’s broad inclusion of numerous higher frequency regions, where more PAS damage was observed in this study (Figs. 3 and 5) and reported previously (Cho et al. 2013b; Ewert et al. 2012), is likely an asset for diagnostic purposes. The observed higher frequency (16 and 32 kHz) tone threshold increases echo this notion in that, although somewhat variable, they were consistently elevated (worse) at all postinjury time points (Fig. 5). In contrast, lower frequency (2–12 kHz) tone ABRs captured mixed, inconsistent, or a lack of postinjury threshold alterations (Fig. 5).
On the basis of the threshold and wave amplitude results, we suggest broadband click and higher frequency (≥12 kHz) tone stimuli may be the most reliable simple stimuli for capturing postblast ABR threshold shifts and wave amplitude alterations, particularly for wave IV and V generators. Clicks may prove especially useful given 1) their inclusion of higher frequency regions where more obvious changes were also observed in the tone results, and 2) evidence, although speculative, supporting the notion that blast-induced CAS injuries are likely multifocal rather than diffuse (Budde et al. 2013; Cernak et al. 2001; Cho et al. 2013a; Garman et al. 2011; Readnower et al. 2010; Song et al. 2015; Walls et al. 2016). As such, after a single-injury exposure, regions corresponding to some frequencies could be injured enough to elicit measureable ABR deficits while other nearby areas retain relatively normal function. This type of multifocal impairment likely differs on a subject-to-subject basis (even in the laboratory, albeit to a lesser degree than in the clinic) due to variability between blast exposures. The broad frequency coverage of clicks could help capture such heterogeneous, multifocal injuries. In the same vein, narrowing a test’s frequency coverage to a specific frequency (i.e., using a pure tone stimulus) may be less likely to capture deficits in an individual subject.
The lack of major alterations in waves IV and V of the 8-kHz, 80-dB pSPL tone ABR were surprising (Fig. 8) given the significant reductions in DPOAE I/O function amplitude (Fig. 3) and wave I amplitude (Fig. 8) at 8 kHz. Neuroplastic remodeling within CAS ABR generator regions is a reasonable hypothesis that could help explain this phenomenon. Indeed, neuroplastic changes in brain stem auditory nuclei have been widely reported in the hours, days, and weeks following acoustic trauma (Manzoor et al. 2013; Mulders and Robertson 2009, 2011, 2013; Mulders et al. 2011; Robertson et al. 2013). In the 2 wk following injury, brain stem auditory evoked potential generators could remodel to preserve core CAP functions near the best hearing frequencies and compensate for compromised PAS integrity. Alternatively, it is possible that relatively simple stimuli such as pure tones near the rats’ best hearing frequency were simply not sufficient to reveal substantial wave IV and V deficits but that wave I measurements were sensitive enough to detect changes. Of note, wave I measurements are also being pursued as diagnostic tools for “hidden hearing loss” in humans, due to their high sensitivity and potential for objective evaluation (Plack et al. 2016). It is also possible that a larger sample size is needed to observe effects for tone measurements in waves IV and V, due to higher variability in more central generator responses.
Cho et al. (2013b) reported increased ABR thresholds across all tested frequencies immediately following injury and incomplete recovery of ABR thresholds in animals injured with blast pressures (123 and 181 kPa), similar to the findings of our study. They also reported least recovery of function at the highest and lowest frequencies. These findings are partially consistent with our ABR results, which captured incomplete recovery of ABR thresholds at higher frequencies (≥16 kHz) at 1 mo postblast but no threshold shift at lower frequencies (≤8 kHz) in blast animals. Prior blast studies have reported ABR thresholds, but not wave amplitudes or latencies. This study is the first that reports blast effects on amplitudes of ABR wave I, IV, and V elicited by clicks and brief tones (latencies were unaffected). As such, we are the first to directly demonstrate electrophysiological evidence of CAP dysfunction following a single blast exposure that was significantly worse than deficits observed following a single noise-only acoustic trauma. Overall, the ABR wave deficits suggest altered signal processing between the AN and inputs to the IC.
Moving more centrally along the auditory neuraxis, MLR click and tone assessments provided a window into noise- and blast-induced alterations in thalamic, thalamocortical, and cortical processing. The slower presentation rate and longer analysis window for the MLR permit analysis of later responses and more central regions of the auditory hierarchy. Whereas minimal changes in the MLR were observed for noise exposure alone, blast exposure incited lasting waveform amplitude changes in the latency windows corresponding to thalamocortical and cortical generators (Fig. 9), based on lesion studies in rats and other mammals (Barth and Di 1991; Brett et al. 1996; Di and Barth 1992; Kraus et al. 1992; Kraus and McGee 1992; Phillips et al. 2011; Šuta et al. 2011). Whereas Mao et al. (2012) previously demonstrated region-specific blast-related damage using diffusion tensor imaging (DTI) in the IC and auditory thalamus of rats, but not the auditory cortex, cortex sparing can likely be attributed to nonequivalent injury mechanics from differing exposure conditions. Another DTI investigation found predominantly cortical changes in DTI results after blast exposure, including the auditory cortex (Budde et al. 2013). It is unclear from our work alone whether damage to auditory thalamic and cortical function results directly from the physical insult of blast, delayed secondary biological processes, or a combination of both, but our physiological data are sufficiently consistent with these anatomical data to strongly suggest that both subcortical and cortical auditory structures are susceptible to blast and result in functional deficits. As has been demonstrated in ototoxicity, noise-induced hearing loss, and aging studies, these deficits can propagate more centrally via maladaptive plasticity to result in deficits in higher auditory regions (in this case the cortex), even when those regions were not physically damaged (Mulders et al. 2011; Mulders and Robertson 2011; Salvi et al. 2000). In addition to PAS and brain stem blunted hearing sensitivity and general dysfunction, thalamus-cortex and cortex-cortex signaling alterations after blast (whether direct or secondary) are important to recognize as possible etiologic contributors toward eventual changes in perception and behavior, as have been observed by postblast impairments in prepulse inhibition acoustic startle tests (Elder et al. 2012; Luo et al. 2014b; Mao et al. 2012). In veterans exposed to blast, cortical impairments have been directly implicated as a source of lasting impairment in an auditory attentional tasks, because peripheral sensory deficits could not explain the deficits (Bressler et al. 2017). Collectively, the DPOAE, ABR, and MLR data strongly suggest blast exposure compromises normal electrophysiological function, even to relatively simple stimuli such as clicks and pure tones, at all levels of the auditory neuraxis ranging from the organ of Corti to the auditory cortex. Given the simplicity of the tested stimuli, it is possible and likely, as our EFR results demonstrate, that more challenging processing tasks could elicit more dramatic CAP deficits.
Temporal modulation processing impairments.
ABRs, evoked by click or brief tone stimuli, provide phasic (onset) information regarding hearing thresholds, neural synchronization and activation, and neural signal transmission along the auditory pathway (Chen and Chen 1991; Hall 2006; Rowe 1981; Thompson et al. 2001). However, human speech and other complex sounds are longer in duration (ten to hundreds of milliseconds) and are rapidly modulated in amplitude as well as frequency over time (Rosen 1992). To gain information about sustained responses and temporal modulation processing of the central auditory pathway, EFRs are useful because they reflect the faithfulness of the auditory subcortices in encoding and phase-locking to the modulation envelope of complex sounds (Aiken and Picton 2008a, 2008b; Clinard et al. 2010; Cunningham et al. 2001; Parthasarathy and Bartlett 2011; Picton et al. 2003). In fact, EFRs are the summed synchronized responses (spiking activity and synaptic potentials) of populations of neurons in the auditory brain stem and midbrain (Chandrasekaran and Kraus 2010; Herdman et al. 2002; Kuwada et al. 2002; Parthasarathy and Bartlett 2012; Picton et al. 2003). From more central (cortex) to more peripheral (auditory nerve) anatomical locations, each contributor to the EFR signal principally encodes increasingly higher AMFs (Gaese and Ostwald 1995; Paolini et al. 2001; Rees and Møller 1983, 1987). This phenomenon has been observed across numerous species and has been well summarized by Joris et al. (2004).
Blast group EFR deficits were observed primarily in the lower range of AMFs tested (≤64 Hz) without major differences at higher AMFs (corresponding to lower generator regions), suggesting the IC may be the primary source of blast-induced temporal processing deficits in this study (Fig. 10). Additionally, ABR waves IV and V (inputs to the IC) for the pure, unmodulated carrier tone (8 kHz, 80 dB SPL) did not capture significant differences from preinjury recordings in either amplitude (Fig. 8) or latency (data not shown). These results support the notion that alterations within the IC itself, not preceding conduction or envelope coding failures, were the principal etiologic contributors to the observed reduction in EFR amplitudes. However, potential contributions of thalamocortical damage cannot be ruled out (Fig. 9). Deficits suggesting IC dysfunction are consistent with our prior assertion from the MLR data that subcortical auditory structures may be particularly vulnerable to blast, as was reported previously via DTI abnormalities in the IC and MGB (Mao et al. 2012). Furthermore, both the DCN and IC have been reported to demonstrate spontaneous hyperactivity after blast exposure in rats; however, only the IC sustained long-term hyperactivity (Luo et al. 2014a, 2014b).
CAP deficits in the auditory midbrain were more readily observable in 8-kHz SAM EFRs (Fig. 10, AMFs ≤64 Hz) than pure tone 8-kHz ABRs (Fig. 8, waves IV and V), reinforcing our assertion that complex stimuli may be more sensitive to CAP deficits after mild blast exposure than clicks and pure tones. More broadly, the lower AMF deficits in SAM EFRs but not pure tone ABRs imply that CAS neuroplasticity after blast exposure can compensate to some degree, perhaps enough to pass as “within normal limits” on simple click and pure tone threshold or ABR tests. However, more complex stimuli may have higher sensitivity for detection of “silent” subclinical (asymptomatic/nonperceived) CAS dysfunction. Indeed, perceptual and behavioral deficits have been reported in blast-exposed veterans in absence of major threshold or ABR abnormalities (Bressler et al. 2017; Gallun et al. 2012). In rats, deficits on postblast gap testing, a behavioral test dependent on temporal processing, have also been widely reported despite inconsistent mechanism-related observations regarding the presence of tinnitus, DPOAE threshold changes, or other potential etiologic contributors (Luo et al. 2014a, 2014b; Mahmood et al. 2014; Mao et al. 2012). Our observations of postblast EFR deficits in the IC and altered thalamocortical MLRs could help explain previous reports of impaired gap test performance in blast-exposed animals despite inconsistent PAS observations (Luo et al. 2014a, 2014b; Mahmood et al. 2014; Mao et al. 2012). Impairments in midbrain temporal processing and thalamocortical transmission could certainly impact a subject’s performance in a gap testing paradigm by disrupting the ability to perceive and respond appropriately to the stimuli. Such deficits could result from progressive centralization of compensatory remodeling in the auditory system after acoustic trauma (Mulders et al. 2011; Mulders and Robertson 2011), direct blast-induced damage to CAS structures (Budde et al. 2013; Cernak et al. 2001; Cho et al. 2013a; Garman et al. 2011; Readnower et al. 2010; Walls et al. 2016), or (most likely) a combination of both pathophysiological processes. Of note, a clinical investigation of blast-exposed veterans did not find consistent evidence of subcortical EFR deficits (although small amplitude reductions were present), and cortical networks were implicated as principally responsible for auditory attentional task impairments (Bressler et al. 2017). Although this is somewhat in contrast to our EFR findings, it is likely due to the high variability in number and type of blast exposures in uncontrolled battlefield environments as well as the wide range of auditory brain stem responses in human subjects, underscoring the importance of laboratory investigations to supplement clinical findings.
Implications for Blast-Exposed Individuals and Future Laboratory Studies
Study of blast injury to the brain is a young field, having gained significant popularity and funding opportunities stemming from military operations in Middle Eastern conflicts over the last two decades. Despite significant effort, mild injuries remain elusive from both a diagnostic and treatment perspective, in large part due to lack of obvious symptoms and unremarkable imaging findings (Centers for Disease Control and Prevention 2013). As demonstrated in this study, the exquisite sensitivity of the auditory system to even mild bTBI highlights it as a promising avenue for development of new diagnostic tests. Our results suggest numerous rapid, noninvasive auditory evoked potential assessments (ABRs, MLRs, and EFRs) appear sensitive to CAP changes after blast exposure. As such, auditory evoked potentials may present a promising avenue for future investigation in both animal and human studies.
Beyond diagnosis, the intertwined nature of auditory processing with critical activities for optimal battlefield performance and for successful civilian reentry opens new avenues for research that may benefit blast-injured individuals. Heightened understanding of how blast-induced CAP alterations affect learning, memory, communication, and emotional responses could play an important role in shaping new therapies ranging from devices to drugs to psychotherapy, which have been reported to have reduced efficacy in the veteran population (Institute of Medicine 2014). Hearing and auditory processing are an integral part of daily life, both on the battlefield and upon societal reintegration, which underscores the importance of protecting, rather than restoring, both PAS and CAS function of current and future service personnel. To this end, the military has begun rollout of the Tactical Communication and Protective System (TCAPS), a damped “smart” earplug designed to protect the PAS from impulse noise-induced hearing impairment and enhance battlefield communication (Casali et al. 2009; Clasing and Casali 2014). Further measures will be needed to confer protection against direct TBI-induced (with or without blast) CAS damage in the brain and brain stem, as highlighted in this study.
There are many functional networks in the brain that, to complete certain tasks or respond to stimuli appropriately, are intertwined with auditory processing. Many of these networks are of interest in bTBI laboratory investigations. Postblast learning, memory, fear, and emotional alterations have emerged as high-interest topics because of the elevated reporting frequency of such deficits among the veteran population (Carlson et al. 2010; Elder et al. 2012; Goldstein et al. 2012; Maguen et al. 2012; Tanielian and Jaycox 2008). In the laboratory setting, rodent behavior to assess such changes is often studied in one of many paradigms in which a set of stimuli, often acoustic, correspond to subsequent events that rats can be trained to perform or respond to. This phenomenon is known as cued operant conditioning and is commonly used in working memory tasks or fear conditioning paradigms (Goosens and Maren 2001; Heldt et al. 2014; Sakurai 1994). Based on the findings of this study and with the understanding that auditory processing is often a critical component for successful completion of these paradigms, it is prudent to avoid using stimuli for which auditory processing impairments could confound interpretation of paradigm performance. If nonauditory brain regions are the primary investigatory target(s), nonacoustic alternatives such as light, odor, or tactile stimuli may be more suitable operant task discriminants in the context of mild bTBI.
Conclusions
Taken together, the data from this study strongly suggest blast exposure compromises normal auditory processing functions at all levels of the auditory neuraxis ranging from the organ of Corti to the auditory cortex (Fig. 11) to a greater degree than nonblast acoustic trauma. We observed particular difficulty in the processing of temporally modulated sounds, suggesting blast-induced CAP deficits may become more dramatic with increasingly complex processing tasks such as sound localization, speech and nonspeech sound recognition in noise, or language processing. With this study we reinforce Luo et al.’s (2014a, 2014b) assertion that acute blast exposure and noise exposure incite fundamentally different pathologies that may require distinct interventions to prevent or treat resultant auditory processing impairments. The contributions of primary (mechanical) and secondary (biological/biochemical) damage should be elucidated. Although the prior work of us and others in blast-induced TBI would suggest a multifocal primary and secondary injury to numerous brain and brain stem CAS regions (Budde et al. 2013; Cernak et al. 2001; Cho et al. 2013a; Garman et al. 2011; Readnower et al. 2010; Song et al. 2015; Walls et al. 2016), further research in this model using biochemical, immunohistochemical, and in vivo imaging techniques is warranted to directly expound on the biophysical basis of the observed deficits. Specifically, efforts should be targeted toward discovering the initial anatomical contributor(s) to postblast auditory processing deficits and documenting pathological progression throughout the auditory neuraxis. These efforts may help disentangle the mechanisms and time course of the differential contributions of 1) direct blast-induced damage to the CAS and 2) the progressive centralization of secondary maladaptive plasticity (stemming from PAS dysfunction) that is known to occur after AAT (Mulders and Robertson 2011). If the root mechanisms linking the peripheral and central physical insults to electrophysiological deficits and subsequent behavioral changes are better understood, there exists great opportunity for well-directed development of protective and therapeutic innovations.
GRANTS
This work was supported in part by Indiana State Department of Health Grant 204200 (to R. Shi), National Institutes of Health Grants NS073636 and 1 R21 NS090244-01 (to R. Shi) and R01 DC011580 (to E. Bartlett), and Indiana CTSI Collaboration in Biomedical Translational Research Pilot Program Grant RR025761 (to R. Shi).
DISCLOSURES
R. Shi is a cofounder of Neuro Vigor, a company developing novel drug treatments and diagnostic approaches for neurodegenerative diseases and neurotrauma.
AUTHOR CONTRIBUTIONS
N.R. and J.L. performed experiments; N.R., J.L., and E.L.B. analyzed data; N.R., J.L., R.S., and E.L.B. interpreted results of experiments; N.R., J.L., and E.L.B. prepared figures; N.R., J.L., and E.L.B. drafted manuscript; N.R., J.L., R.S., and E.L.B. edited and revised manuscript; N.R., J.L., R.S., and E.L.B. approved final version of manuscript; R.S. and E.L.B. conceived and designed research.
ACKNOWLEDGMENTS
We gratefully acknowledge Gavin Kuziel and Jordan Addison for assistance in collecting electrophysiological data. In addition, we thank the Purdue University Department of Statistics for consultation regarding appropriate statistical methods.
Present address of R. Shi: 625 Harrison St., West Lafayette, IN 47907 (e-mail: riyi@purdue.edu).
REFERENCES
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res 245: 35–47, 2008a. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW. Human cortical responses to the speech envelope. Ear Hear 29: 139–157, 2008b. doi: 10.1097/AUD.0b013e31816453dc. [DOI] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci 32: 14156–14164, 2012. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Auditory brainstem response to complex sounds predicts self-reported speech-in-noise performance. J Speech Lang Hear Res 56: 31–43, 2013. doi: 10.1044/1092-4388(2012/12-0043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avan P, Büki B, Petit C. Auditory distortions: origins and functions. Physiol Rev 93: 1563–1619, 2013. doi: 10.1152/physrev.00029.2012. [DOI] [PubMed] [Google Scholar]
- Barth DS, Di S. The functional anatomy of middle latency auditory evoked potentials. Brain Res 565: 109–115, 1991. doi: 10.1016/0006-8993(91)91741-I. [DOI] [PubMed] [Google Scholar]
- Bass CR, Panzer MB, Rafaels KA, Wood G, Shridharani J, Capehart B. Brain injuries from blast. Ann Biomed Eng 40: 185–202, 2012. doi: 10.1007/s10439-011-0424-0. [DOI] [PubMed] [Google Scholar]
- Bass CR, Rafaels KA, Salzar RS. Pulmonary injury risk assessment for short-duration blasts. J Trauma 65: 604–615, 2008. doi: 10.1097/TA.0b013e3181454ab4. [DOI] [PubMed] [Google Scholar]
- Berger G, Finkelstein Y, Avraham S, Himmelfarb M. Patterns of hearing loss in non-explosive blast injury of the ear. J Laryngol Otol 111: 1137–1141, 1997. doi: 10.1017/S0022215100139544. [DOI] [PubMed] [Google Scholar]
- Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Brain Res 32: 75–84, 1987. doi: 10.1016/0165-3806(87)90140-4. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature 417: 543–547, 2002. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Bressler S, Goldberg H, Shinn-Cunningham B. Sensory coding and cognitive processing of sound in veterans with blast exposure. Hear Res 349: 98–110, 2017. doi: 10.1016/j.heares.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett B, Krishnan G, Barth DS. The effects of subcortical lesions on evoked potentials and spontaneous high frequency (gamma-band) oscillating potentials in rat auditory cortex. Brain Res 721: 155–166, 1996. doi: 10.1016/0006-8993(96)00168-0. [DOI] [PubMed] [Google Scholar]
- Budde MD, Shah A, McCrea M, Cullinan WE, Pintar FA, Stemper BD. Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Front Neurol 4: 154, 2013. doi: 10.3389/fneur.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress 23: 17–24, 2010. doi: 10.1002/jts.20483. [DOI] [PubMed] [Google Scholar]
- Casali JG, Ahroon WA, Lancaster JA. A field investigation of hearing protection and hearing enhancement in one device: for soldiers whose ears and lives depend upon it. Noise Health 11: 69–90, 2009. doi: 10.4103/1463-1741.48564. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211: 1781–1791, 2008. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: clinical update from the global war on terror. Mil Med 172: 726–730, 2007. doi: 10.7205/MILMED.172.7.726. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Blast Injuries: Fact Sheets for Professionals. Atlanta, GA: National Center for Injury Prevention and Control, 2013. [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma 50: 695–706, 2001. doi: 10.1097/00005373-200104000-00017. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology 47: 236–246, 2010. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-D, Li M, Tanaka C, Bielefeld EC, Hu B-H, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res 248: 39–47, 2009. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Chen T-J, Chen S-S. Generator study of brainstem auditory evoked potentials by a radiofrequency lesion method in rats. Exp Brain Res 85: 537–542, 1991. doi: 10.1007/BF00231737. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Sajja VS, Vandevord PJ, Lee YW. Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience 253: 9–20, 2013a. doi: 10.1016/j.neuroscience.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Cho S-I, Gao SS, Xia A, Wang R, Salles FT, Raphael PD, Abaya H, Wachtel J, Baek J, Jacobs D, Rasband MN, Oghalai JS. Mechanisms of hearing loss after blast injury to the ear. PLoS One 8: e67618, 2013b. doi: 10.1371/journal.pone.0067618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasing JE, Casali JG. Warfighter auditory situation awareness: effects of augmented hearing protection/enhancement devices and TCAPS for military ground combat applications. Int J Audiol 53, Suppl 2: S43–S52, 2014. doi: 10.3109/14992027.2013.860489. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res 264: 48–55, 2010. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JT, Ziv G, Bloom J, Zikk D, Rapoport Y, Himmelfarb MZ. Blast injury of the ear in a confined space explosion: auditory and vestibular evaluation. Isr Med Assoc J 4: 559–562, 2002. [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol 112: 758–767, 2001. doi: 10.1016/S1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58: 333–339, 2008. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. N Engl J Med 352: 1335–1342, 2005. doi: 10.1056/NEJMra042083. [DOI] [PubMed] [Google Scholar]
- Di S, Barth DS. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol 68: 425–431, 1992. [DOI] [PubMed] [Google Scholar]
- Du X, Ewert DL, Cheng W, West MB, Lu J, Li W, Floyd RA, Kopke RD. Effects of antioxidant treatment on blast-induced brain injury. PLoS One 8: e80138, 2013. doi: 10.1371/journal.pone.0080138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Dorr NP, De Gasperi R, Gama Sosa MA, Shaughness MC, Maudlin-Jeronimo E, Hall AA, McCarron RM, Ahlers ST. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma 29: 2564–2575, 2012. doi: 10.1089/neu.2012.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert DL, Lu J, Li W, Du X, Floyd R, Kopke R. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res 285: 29–39, 2012. doi: 10.1016/j.heares.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Gaese BH, Ostwald J. Temporal coding of amplitude and frequency modulation in the rat auditory cortex. Eur J Neurosci 7: 438–450, 1995. doi: 10.1111/j.1460-9568.1995.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Galarneau MR, Woodruff SI, Dye JL, Mohrle CR, Wade AL. Traumatic brain injury during Operation Iraqi Freedom: findings from the United States Navy-Marine Corps Combat Trauma Registry. J Neurosurg 108: 950–957, 2008. doi: 10.3171/JNS/2008/108/5/0950. [DOI] [PubMed] [Google Scholar]
- Gallun FJ, Diedesch AC, Kubli LR, Walden TC, Folmer RL, Lewis MS, McDermott DJ, Fausti SA, Leek MR. Performance on tests of central auditory processing by individuals exposed to high-intensity blasts. J Rehabil Res Dev 49: 1005–1025, 2012. doi: 10.1682/JRRD.2012.03.0038. [DOI] [PubMed] [Google Scholar]
- Garman RH, Jenkins LW, Switzer RC III, Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE, Clark RS, Bayir H, Kagan V, Jackson EK, Kochanek PM. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma 28: 947–959, 2011. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4: 134ra60, 2012. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8: 148–155, 2001. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Hall J. Handbook of Auditory Evoked Responses. Boston: Allyn & Bacon, 2006. [Google Scholar]
- Heldt SA, Elberger AJ, Deng Y, Guley NH, Del Mar N, Rogers J, Choi GW, Ferrell J, Rex TS, Honig MG, Reiner A. A novel closed-head model of mild traumatic brain injury caused by primary overpressure blast to the cranium produces sustained emotional deficits in mice. Front Neurol 5: 2, 2014. doi: 10.3389/fneur.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D, Spongr V, Subramaniam M, Campo P. Anatomical effects of impact noise. Hear Res 76: 101–117, 1994. doi: 10.1016/0378-5955(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Picton TW, Stapells DR. Place specificity of multiple auditory steady-state responses. J Acoust Soc Am 112: 1569–1582, 2002. doi: 10.1121/1.1506367. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: National Academies Press, 2014. [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev 84: 541–577, 2004. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kemp D. The evoked cochlear mechanical response and the auditory microstructure-evidence for a new element in cochlear mechanics. Scand Audiol Suppl 9: 35–47, 1979. [PubMed] [Google Scholar]
- Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433: 880–883, 2005. doi: 10.1038/nature03367. [DOI] [PubMed] [Google Scholar]
- Kerr AG. Trauma and the temporal bone. The effects of blast on the ear. J Laryngol Otol 94: 107–110, 1980. doi: 10.1017/S0022215100088538. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T. Electrophysiology of the human auditory system. In: The Mammalian Auditory Pathway: Neurophysiology, edited by Popper AN and Fay RR. New York: Springer, 1992, p. 335–403. doi: 10.1007/978-1-4612-2838-7_6. [DOI] [Google Scholar]
- Kraus N, McGee T, Littman T, Nicol T. Reticular formation influences on primary and non-primary auditory pathways as reflected by the middle latency response. Brain Res 587: 186–194, 1992. doi: 10.1016/0006-8993(92)90996-M. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol 13: 188–204, 2002. [PubMed] [Google Scholar]
- Lai J, Bartlett EL. Age-related shifts in distortion product otoacoustic emissions peak-ratios and amplitude modulation spectra. Hear Res 327: 186–198, 2015. doi: 10.1016/j.heares.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew HL, Garvert DW, Pogoda TK, Hsu PT, Devine JM, White DK, Myers PJ, Goodrich GL. Auditory and visual impairments in patients with blast-related traumatic brain injury: effect of dual sensory impairment on Functional Independence Measure. J Rehabil Res Dev 46: 819–826, 2009. doi: 10.1682/JRRD.2008.09.0129. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Zuo J, Guinan JJ Jr. Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea? J Acoust Soc Am 116: 1649–1655, 2004. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Pace E, Zhang X, Zhang J. Blast-induced tinnitus and spontaneous activity changes in the rat inferior colliculus. Neurosci Lett 580: 47–51, 2014a. doi: 10.1016/j.neulet.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Luo H, Pace E, Zhang X, Zhang J. Blast-Induced tinnitus and spontaneous firing changes in the rat dorsal cochlear nucleus. J Neurosci Res 92: 1466–1477, 2014b. doi: 10.1002/jnr.23424. [DOI] [PubMed] [Google Scholar]
- Maguen S, Lau KM, Madden E, Seal K. Relationship of screen-based symptoms for mild traumatic brain injury and mental health problems in Iraq and Afghanistan veterans: distinct or overlapping symptoms? J Rehabil Res Dev 49: 1115–1126, 2012. doi: 10.1682/JRRD.2011.02.0015. [DOI] [PubMed] [Google Scholar]
- Mahmood G, Mei Z, Hojjat H, Pace E, Kallakuri S, Zhang JS. Therapeutic effect of sildenafil on blast-induced tinnitus and auditory impairment. Neuroscience 269: 367–382, 2014. doi: 10.1016/j.neuroscience.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, Kaltenbach JA. Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res 295: 114–123, 2013. doi: 10.1016/j.heares.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JC, Pace E, Pierozynski P, Kou Z, Shen Y, VandeVord P, Haacke EM, Zhang X, Zhang J. Blast-induced tinnitus and hearing loss in rats: behavioral and imaging assays. J Neurotrauma 29: 430–444, 2012. doi: 10.1089/neu.2011.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T, Kraus N. Auditory development reflected by middle latency response. Ear Hear 17: 419–429, 1996. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- McGee T, Kraus N, Comperatore C, Nicol T. Subcortical and cortical components of the MLR generating system. Brain Res 544: 211–220, 1991. doi: 10.1016/0006-8993(91)90056-2. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Ding D, Salvi R, Robertson D. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol 519: 2637–2647, 2011. doi: 10.1002/cne.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience 164: 733–746, 2009. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Progressive centralization of midbrain hyperactivity after acoustic trauma. Neuroscience 192: 753–760, 2011. doi: 10.1016/j.neuroscience.2011.06.046. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Development of hyperactivity after acoustic trauma in the guinea pig inferior colliculus. Hear Res 298: 104–108, 2013. doi: 10.1016/j.heares.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear Res 159: 101–116, 2001. doi: 10.1016/S0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E. Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear Res 289: 52–62, 2012. doi: 10.1016/j.heares.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience 192: 619–630, 2011. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Front Aging Neurosci 2: 152, 2010. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JAL, Hopkins C, Bartlett EL. Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol 15: 649–661, 2014. doi: 10.1007/s10162-014-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JH Jr, Hamernik RP. Blast overpressure induced structural and functional changes in the auditory system. Toxicology 121: 29–40, 1997. doi: 10.1016/S0300-483X(97)03653-6. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Schei JL, Meighan PC, Rector DM. State-dependent changes in cortical gain control as measured by auditory evoked responses to varying intensity stimuli. Sleep 34: 1527–1537, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses: respuestas auditivas de estado estable en humanos. Int J Audiol 42: 177–219, 2003. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Léger A, Prendergast G, Kluk K, Guest H, Munro KJ. Toward a diagnostic test for hidden hearing loss. Trends Hear 20: 1-9, 2016. doi: 10.1177/2331216516657466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaels K, Bass CR, Salzar RS, Panzer MB, Woods W, Feldman S, Cummings T, Capehart B. Survival risk assessment for primary blast exposures to the head. J Neurotrauma 28: 2319–2328, 2011. doi: 10.1089/neu.2009.1207. [DOI] [PubMed] [Google Scholar]
- Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res 88: 3530–3539, 2010. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A, Møller AR. Responses of neurons in the inferior colliculus of the rat to AM and FM tones. Hear Res 10: 301–330, 1983. doi: 10.1016/0378-5955(83)90095-3. [DOI] [PubMed] [Google Scholar]
- Rees A, Møller AR. Stimulus properties influencing the responses of inferior colliculus neurons to amplitude-modulated sounds. Hear Res 27: 129–143, 1987. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Ritenour AE, Wickley A, Ritenour JS, Kriete BR, Blackbourne LH, Holcomb JB, Wade CE. Tympanic membrane perforation and hearing loss from blast overpressure in Operation Enduring Freedom and Operation Iraqi Freedom wounded. J Trauma 64, Suppl: S174–S178, 2008. doi: 10.1097/TA.0b013e318160773e. [DOI] [PubMed] [Google Scholar]
- Robertson D, Bester C, Vogler D, Mulders WH. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res 295: 124–129, 2013. doi: 10.1016/j.heares.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci 336: 367–373, 1992. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol 12: 882–893, 2013. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- Rowe MJ., 3rd The brainstem auditory evoked response in neurological disease: a review. Ear Hear 2: 41–51, 1981. doi: 10.1097/00003446-198101000-00008. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen S, Haapalinna A, Riekkinen PJ Sr, Sirviö J. Dexmedetomidine reduces response tendency, but not accuracy of rats in attention and short-term memory tasks. Pharmacol Biochem Behav 56: 31–40, 1997. doi: 10.1016/S0091-3057(96)00151-7. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. J Neurosci 14: 2606–2623, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res 147: 261–274, 2000. doi: 10.1016/S0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Saunders GH, Frederick MT, Arnold M, Silverman S, Chisolm TH, Myers P. Auditory difficulties in blast-exposed Veterans with clinically normal hearing. J Rehabil Res Dev 52: 343–360, 2015. doi: 10.1682/JRRD.2014.11.0275. [DOI] [PubMed] [Google Scholar]
- Shaffer LA, Withnell RH, Dhar S, Lilly DJ, Goodman SS, Harmon KM. Sources and mechanisms of DPOAE generation: implications for the prediction of auditory sensitivity. Ear Hear 24: 367–379, 2003. doi: 10.1097/01.AUD.0000090439.16438.9F. [DOI] [PubMed] [Google Scholar]
- Sharpnack DD, Johnson AJ, Phillips Y. The pathology of primary blast injury. In: Textbook of Military Medicine. Part 1: Welfare, Weaponry and the Casualty. Conventional Warfare, Ballistic, Blast and Burn Injuries, edited by Zaitchuk R and Bellamy RF. Washington, DC: U.S. Government Printing Office, 1991, vol. 5, p. 271–294. [Google Scholar]
- Song S, Race NS, Kim A, Zhang T, Shi R, Ziaie B. A wireless intracranial brain deformation sensing system for blast-induced traumatic brain injury. Sci Rep 5: 16959, 2015. doi: 10.1038/srep16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šuta D, Rybalko N, Pelánová J, Popelář J, Syka J. Age-related changes in auditory temporal processing in the rat. Exp Gerontol 46: 739–746, 2011. doi: 10.1016/j.exger.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Swaminathan J, Heinz MG. Psychophysiological analyses demonstrate the importance of neural envelope coding for speech perception in noise. J Neurosci 32: 1747–1756, 2012. doi: 10.1523/JNEUROSCI.4493-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian TL, Jaycox L, editors. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation, 2008. [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci 27: 6091–6102, 2007. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening: summary of evidence. JAMA 286: 2000–2010, 2001. doi: 10.1001/jama.286.16.2000. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs. Veterans Benefits Administration Annual Benefits Report Fiscal Year 2014 (Online). http://www.benefits.va.gov/REPORTS/abr/ABR_FY_14.asp.
- Van Campen LE, Murphy WJ, Franks JR, Mathias PI, Toraason MA. Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res 164: 29–38, 2002. doi: 10.1016/S0378-5955(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Walls MK, Race N, Zheng L, Vega-Alvarez SM, Acosta G, Park J, Shi R. Structural and biochemical abnormalities in the absence of acute deficits in mild primary blast-induced head trauma. J Neurosurg 124: 675–686, 2016. doi: 10.3171/2015.1.JNS141571. [DOI] [PMC free article] [PubMed] [Google Scholar]