Widespread bilateral activation of both sides of the cerebrum and cerebellum are demonstrated on functional MRI after motor recovery of a completely nonfunctional left hand that began 23 yr after a severe stroke. This suggests that the generally accepted window of recovery beyond which further therapy is not indicated should be entirely reconsidered. Physiotherapy and new modalities in development might be indicated long after a stroke.
Keywords: delayed, functional MRI, motor, recovery, stroke
Abstract
It is widely believed that most stroke recovery occurs within 6 mo, with little benefit of physiotherapy or other modalities beyond 1 yr. We report a remarkable case of stroke recovery beginning 23 yr after a severe stroke due to embolization from the innominate artery and subclavian artery, resulting from compression of the right subclavian artery by a cervical rib. The patient had a large right frontoparietal infarction with severe left hemiparesis and a totally nonfunctional spastic left hand. He experienced some recovery of hand function that began 23 yr after the stroke, 1 yr after he took up regular swimming. As a result, intensive physiotherapy was initiated, with repetitive large muscle movement and a spring-loaded mechanical orthosis that provides resistance to finger flexors and supports finger extensors. Within 2 yr, he could pick up coins with the previously useless left hand. Functional MRI studies document widespread distribution of the recovery in both hemispheres. This case provides impetus not only to more intensive and prolonged physiotherapy, but also to treatment with emerging modalities such as stem cell therapy and exosome and microRNA therapies.
NEW & NOTEWORTHY Widespread bilateral activation of both sides of the cerebrum and cerebellum are demonstrated on functional MRI after motor recovery of a completely nonfunctional left hand that began 23 yr after a severe stroke. This suggests that the generally accepted window of recovery beyond which further therapy is not indicated should be entirely reconsidered. Physiotherapy and new modalities in development might be indicated long after a stroke.
most stroke survivors experience some degree of functional recovery. The extent and the time course of stroke recovery are variable, depending on such factors as size and location of the infarction and the quantity and quality of rehabilitation interventions (Langhorne et al. 2011). In most patients, even in pediatric stroke, most motor recovery is achieved within 6 mo, without significant improvement thereafter (Kim et al. 2009; Langhorne et al. 2011). In consequence, most stroke patients do not receive physiotherapy after this supposed therapeutic window and are not always encouraged to train their affected limbs. In a recent review in Physiology, Jones and Adkins (2015) said, “Motor system stroke instigates a dramatic and widespread reorganization of the connectivity of surviving neurons, involving extensive axonal sprouting, dendritic remodeling, and synapse formation in either hemisphere. This is linked with reorganization of motor and sensory cortical maps, and bilateral changes in neural activity and functional connectivity patterns.” They emphasized early changes after stroke.
We report a patient that exemplifies the widespread reorganization described by Jones and Adkins (2015) but with a remarkable delay between the initial stroke and recovery. The patient had a severe stroke due to compression of the right subclavian artery by a cervical rib and experienced significant recovery of left-hand function beginning 23 yr after the stroke. The widespread distribution of cerebral reorganization in both hemispheres was shown by functional MRI.
The planned study was submitted to the Western University Human Research Ethics Board, which exempts case reports from formal review; a letter of exemption was issued to the authors and submitted to the Editor.
Case report.
In December 1979, a 15-yr-old, right-handed boy getting off a train in Toronto, Canada, carried a heavy suitcase the entire way by the right hand, along the length of the train, the train platform, downstairs, through a tunnel, upstairs, and across a wide plaza to where his parents awaited him. He hefted the suitcase up into the trunk of his father's car. He got into the back seat, and ~30 s later he keeled over unconscious. A cervical rib had compressed his right subclavian artery long enough to cause thrombosis back into the innominate artery, with carotid and vertebrobasilar embolization. Initially, a stroke was not suspected.
Several days later, he was transferred from a Toronto hospital to Victoria Hospital in London, Ontario (closer to his home). He had regained consciousness but was drowsy with dense left hemiplegia and neglect. There was a large right frontoparietal infarction. The right hand was ischemic; it was blue and cold with poor capillary reflux. Angiography, performed 4 days after the event, revealed occlusion of the right subclavian artery just beyond the site where the clavicle crossed the cervical rib, complete thrombosis of the subclavian artery back to the innominate artery, and complete occlusion of the right internal carotid artery near the petrous bone. Filling defects thought to be emboli were seen in the right middle cerebral, right vertebral, and right anterior mammary artery. He was anticoagulated because of the ischemia of the right arm, which recovered spontaneously via collaterals.
He gradually recovered some use of the left leg, shoulder, and elbow. In April 1980, 4 mo after the stroke, he was able to walk without support and went back to high school. The left hand was spastic and nonfunctional, with no movement for 23 yr.
In 2001, on advice related to his obesity, he took up swimming on a regular basis. A year later, he reported initial movements of his left fingers. In 2009, he began intensive physiotherapy using a spring-loaded mechanical orthosis (the SaeboGlove) that provides resistance to finger flexors and supports finger extensors. Recovery has been progressing over the intervening years, to the point that he can hold objects in his left hand and even pick up coins and paperclips.
To study neural activity associated with flexion of his right and left hands, we performed functional magnetic resonance imaging at 3 T at the Robarts Research Institute, London, Ontario, Canada.
METHODS
Magnetic resonance imaging.
To study neural activity associated with flexion of his right and left fingers, we performed structural and functional magnetic resonance imaging (fMRI) on a 3-T Siemens Magnetom Trio with syngo MR B17 software (Siemens Medizintechnik, Erlangen, Germany) at the Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, Ontario, Canada, on December 6, 2011. Relaxation time T1-weighted anatomic images of the brain were acquired using a 3-dimensional magnetization-prepared rapid acquisition gradient-echo (MP-RAGE) sequence with the following parameters: voxel size 1 × 1 × 1 mm3, field of view (FOV) = 256 × 256 mm, 192 sagittal slices, slice thickness 1 mm, repetition time (TR) = 2,300 ms, echo time (TE) = 2.98 ms, inversion time (TI) = 900 ms, flip angle = 9°, integrated parallel imaging techniques (iPAT) using an acceleration factor of 2, acquisition time 6:26 min. For optimal visualization of the ischemic lesion, a T2-weighted fluid-attenuated inversion recovery (FLAIR) sequence was acquired with the following parameters: voxel size 0.46875 × 0.46875 × 3 mm3, FOV = 202 × 240 mm, 38 axial slices, slice thickness 3 mm, TR = 8,800 ms, TE = 89 ms, TI = 2,476.3 ms, flip angle = 130°, no acceleration, acquisition time 4:08 min.
For the first fMRI experiment, the patient was asked to open and close his affected left hand repeatedly as much as possible, approximately one flexion per second. The closure of the affected hand was incomplete; the patient could not fully extend his fingers and could not touch his palm with the tips of his fingers. The distance between finger tips in maximum flexion and maximum extension was around 2–3 cm. For the second experiment, the patient was required to perform similar flexion and extension movements of the unaffected right hand regarding movement amplitude and velocity. Hand movements were studied using a block design experiment. For each hand, 6 blocks of 20-s movement alternated with 7 blocks of 20-s rest (no hand movement). To investigate neural activity during hand movements, a T2*-weighted blood oxygenation level-dependent (BOLD) echo-planar imaging sequence was chosen (voxel size 3 × 3 × 3 mm3, FOV = 240 × 240 mm, 45 axial slices, slice thickness = 3 mm, TR = 2,500 ms, TE = 30 ms, flip angle = 90°, iPAT factor = 2, 104 volumes, time of acquisition: 4:29 min per run).
fMRI data analysis.
Processing of fMRI data was carried out using FMRI Expert Analysis Tool (FEAT) version 5.98, part of FMRIB Software Library (FSL; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The following prestatistics processing was applied: motion correction using MCFLIRT; nonbrain removal using BET; spatial smoothing using a Gaussian kernel with full-width at half-maximum of 5 mm; grand-mean intensity normalization of the entire four-dimensional data set by a single multiplicative factor; and high-pass temporal filtering (Gaussian-weighted least-squares straight-line fitting). A linear model was then fitted to the fMRI data. Time-series statistical analysis was performed using FILM with local autocorrelation correction. Z-statistic images were thresholded using clusters determined by z > 3.1 and a (corrected) cluster significance threshold of P = 0.001. Linear registration of functional images to high-resolution T1- and T2-weighted images was carried out using FLIRT. For the overlay of the T2-weighted image and the z-statistical activation maps, MRIcroGL was used (https://www.nitrc.org/projects/mricrogl/).
Functional testing.
The Chedoke-McMaster Stroke Assessment (CMSA) Impairment Inventory for the upper-extremity arm and hand was performed on April 11, 2017, and compared with previous results. Each dimension of the CMSA Impairment Inventory is measured on a seven-point scale with each point corresponding to the seven stages of motor recovery similar to the Brunnstrom Recovery Stages where 1 is no voluntary movement and 7 is full recovery (Gowland et al. 1993). The CMSA Arm and Hand Impairment Inventory and the Fugl-Meyer Assessment shoulder, elbow, forearm, wrist, and hand scale have excellent convergent validity (r = 0.95; Gowland et al. 1993).
RESULTS
Results are presented in Table 1. On the Modified Ashworth Scale, the patient had increased tone only in his wrist flexors. On the Box and Block Test, a test of manual dexterity, he managed 10 blocks in 60 s. He did report using his left hand on a daily basis for bathing and dressing, but the contribution of effort is not the same as his unaffected limb.
Table 1.
Results of impairment and functional testing
| Area Tested | December 1979,a Stroke Onset | December 2004,b 25 yr After Stroke | March 2005,c 25.3 yr After Stroke | May 2007,d 27.5 yr After Stroke | April 2017,e 37.4 yr After Stroke |
|---|---|---|---|---|---|
| Left arm | 1 | 1 | 3 | 3 | 5 |
| Left hand | 1 | 1 | 3 | 3 | 4 |
Based on patient report and the neurological examination by J. D. Spence.
Entered interdisciplinary outpatient rehabilitation program.
Discharged from outpatient rehabilitation program.
Discharged from a 2nd outpatient rehabilitation program.
After treatment in an intensive functional electrical stimulation outpatient program.
Functional magnetic resonance imaging.
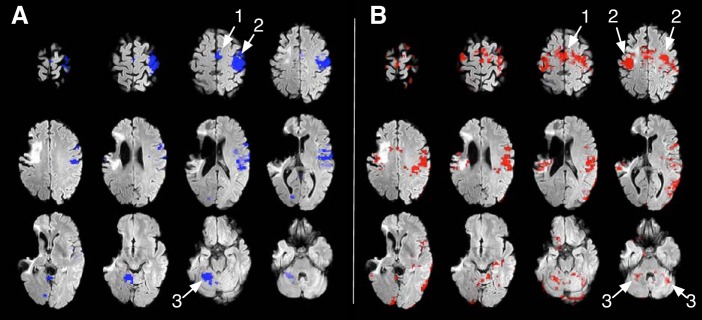
Flexing the fingers of his unaffected right hand was associated with circumscribed activity in the contralateral sensorimotor cortex as well as the ipsilateral supplementary motor area and cerebellum (Fig. 1A). By contrast, flexing his affected left fingers was associated with extensive activity in both hemispheres and both sides of the cerebellum (Fig. 1B).
Fig. 1.
Functional magnetic resonance imaging of movements of the unaffected (A) and affected (B) fingers. Areas active during finger movement are thresholded at z = 3.1–12 and superimposed on a T2-weighted structural FLAIR image showing the infarction in the distribution of the right middle cerebral artery. Movement of the unaffected fingers of the right arm (A) is associated with neural activation in the contralateral motor area (1), contralateral sensorimotor cortex (2), and the contralateral supplementary motor cortex and cerebellar hemisphere (3). Movement of the affected fingers of the left hand (B) is associated with widely distributed neural activation in both supplementary motor areas (Smith et al. 2004), sensorimotor cortex (Jenkinson et al. 2002) and cerebellar hemispheres (Smith 2002). The brain images are shown in radiological convention (left side of the image shows right hemisphere).
DISCUSSION
Our patient highlights a rare but serious complication of thoracic outlet syndrome caused by a cervical rib. A cervical rib can be found in ~1% of women and 0.4% of men and may cause brachial neuropathy and compression of the subclavian artery (Brewin et al. 2009). Pressure on the subclavian artery may cause a poststenotic dilatation with thrombosis and embolization into the affected arm and embolization into the ipsilateral cerebral circulation (Lee and Hines 2007).
His case also emphasizes that patients may experience clinically significant recovery of motor function many years after a large hemispheric stroke, should they undertake intensive and constant physiotherapy (Luft et al. 2004; McCombe Waller et al. 2014; Teasell et al. 2012). The unusual late recovery of finger movements in our patient may have been initiated by the use of proximal, bilaterally innervated muscles during swimming (Kim et al. 2009). Use of shoulder muscles, paired with sensory stimulation of the affected hand in water, may have facilitated neural reorganization of bilateral cortical sensorimotor areas, as demonstrated by fMRI during finger movement (Jones and Adkins 2015). The combination of general hemispheric activation occurring with exercise (swimming) and unilateral task-specific rehabilitation training may be responsible for new general and task-specific fMRI activations seen in this individual (Luft et al. 2004; McCombe Waller et al. 2014). Whether swimming, therapy, and/or daily life activities were the contributors to the reported bilateral activation patterns cannot be determined.
Although the mechanism of the arterial thrombosis was unusual, in essence this was a large embolic infarction, and the experience of this patient should be generalizable to large embolic ischemic strokes in young persons. The ischemia of the right arm was mild and recovered completely within days and so seems unlikely to have influenced the course of events. It seems likely that elderly patients might experience less recovery.
The current trend in stroke rehabilitation thinking is that initial motor recovery of the upper-extremity impairment occurs in a fixed proportion (70%) to the initial severity or baseline motor functioning deficit, assuming the corticospinal tract is intact and this recovery occurs within the 1st 3 mo (Winters et al. 2015). This is spontaneous neurological recovery and is thought to be largely independent of any rehabilitation. Three-dimensional kinematics, which look at how recovery occurs, find that after this any recovery associated with rehab is compensatory in nature or adaptive and that changes in the central nervous system (brain reorganization) simply reflect learning how to move differently, using remaining neural networks to relearn how to move (Hara 2015); it is, however, not the same as before (even though it may look similar), and this is the functional improvement. What is unusual about this case was the improvement in his impairment level as measured by the CMSA Impairment Inventory of the Upper Extremity. The widely distributed neural activation shown on functional MRI suggests that the recovery was based on discovering or relearning how to use existing neural networks, which is why the movements are still slow and awkward, although they reflect a dramatic improvement.
Our patient’s unexpected recovery of hand function, beginning 23 yr after his ischemic stroke, and continuing to improve over years of intensive physiotherapy, emphasizes that hope for further recovery should not necessarily be abandoned after 1 or 2 yr of recovery. It may be an impetus to study further with emerging modalities such as stem cell, exosome, and microRNA therapies (Chopp and Zhang 2015). Indeed, Steinberg et al. (2016) gave some indication of delayed recovery with mesenchymal stem cells in patients with stable chronic ischemic strokes. Stem cells were administered between 6 and 60 mo after the stroke. The markedly delayed recovery in our patient and the widespread recruitment of bilateral areas of the brain, shown in Fig. 1, indicate the potential for much greater stroke recovery than is generally assumed.
DISCLOSURES
P. Sörös received lecture fees from Boehringer Ingelheim. R. Teasell receives research support from Allergan, makers of botulinum toxin, but does not administer botulinum toxin. D. F. Hanley received grants from National Institutes of Health (NIH) and Genentech and fees for legal testimony. J. D. Spence in the past 2 years received grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada and lecture honoraria/consulting fees from Bayer and Bristol-Myers Squibb and has performed contract research with Pfizer, Bayer, Bristol-Myers Squibb, Acasti Pharma, POM Wonderful, CVRx, American Gastroenterological Association, and Gore. He is an officer and shareholder of Vascularis Inc.
AUTHOR CONTRIBUTIONS
D.F.H. and J.D.S. conceived and designed research; P.S. and R.T. performed experiments; P.S. and R.T. analyzed data; P.S., R.T., D.F.H., and J.D.S. interpreted results of experiments; P.S. prepared figures; J.D.S. drafted manuscript; P.S., R.T., D.F.H., and J.D.S. edited and revised manuscript; P.S., R.T., D.F.H., and J.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joe Gati, Associate Director of the Centre for Functional Imaging and Metabolic Mapping, Robarts Research Institute, Western University, London, Canada, for help with the functional MRI study.
REFERENCES
- Brewin J, Hill M, Ellis H. The prevalence of cervical ribs in a London population. Clin Anat 22: 331–336, 2009. doi: 10.1002/ca.20774. [DOI] [PubMed] [Google Scholar]
- Chopp M, Zhang ZG. Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs 20: 523–526, 2015. doi: 10.1517/14728214.2015.1061993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Vanspall B, Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 24: 58–63, 1993. doi: 10.1161/01.STR.24.1.58. [DOI] [PubMed] [Google Scholar]
- Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch 82: 4–13, 2015. doi: 10.1272/jnms.82.4. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jones TA, Adkins DL. Motor system reorganization after stroke: stimulating and training toward perfection. Physiology (Bethesda) 30: 358–370, 2015. doi: 10.1152/physiol.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CT, Han J, Kim H. Pediatric stroke recovery: a descriptive analysis. Arch Phys Med Rehabil 90: 657–662, 2009. doi: 10.1016/j.apmr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 377: 1693–1702, 2011. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- Lee TS, Hines GL. Cerebral embolic stroke and arm ischemia in a teenager with arterial thoracic outlet syndrome: a case report. Vasc Endovascular Surg 41: 254–257, 2007. doi: 10.1177/1538574407299780. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA 292: 1853–1861, 2004. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J, Jenkins T, Magder LS, Hanley DF, Goldberg A, Luft AR. Sequencing bilateral and unilateral task-oriented training versus task oriented training alone to improve arm function in individuals with chronic stroke. BMC Neurol 14: 236, 2014. doi: 10.1186/s12883-014-0236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, Suppl 1: S208–S219, 2004. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a Phase 1/2a study. Stroke 47: 1817–1824, 2016. doi: 10.1161/STROKEAHA.116.012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasell R, Mehta S, Pereira S, McIntyre A, Janzen S, Allen L, Lobo L, Viana R. Time to rethink long-term rehabilitation management of stroke patients. Top Stroke Rehabil 19: 457–462, 2012. doi: 10.1310/tsr1906-457. [DOI] [PubMed] [Google Scholar]
- Winters C, van Wegen EE, Daffertshofer A, Kwakkel G. Generalizability of the proportional recovery model for the upper extremity after an ischemic stroke. Neurorehabil Neural Repair 29: 614–622, 2015. doi: 10.1177/1545968314562115. [DOI] [PubMed] [Google Scholar]