Abstract
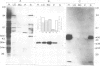
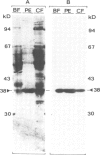
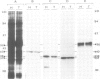
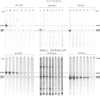
Glycosomes, the microbodies of Trypanosoma brucei, contain a number of enzymes involved in glucose and glycerol metabolism. The biogenesis of three of these enzymes has been studied. Aldolase, D-glyceraldehyde-3-phosphate dehydrogenase and NAD-linked glycerol-3-phosphate dehydrogenase are all synthesized in the cytosol on free rather than on membrane-bound polysomes. In vitro, as well as in vivo, these polypeptides are synthesized at their mature size, and no evidence was found for any processing upon entry into the glycosomes. Continuous and pulse-chase labelling experiments with procyclic trypomastigotes revealed that the enzymes have a half-life in the cytosol of approximately 3 min or less, and then turn over rapidly in the glycosomes, with half-lives as short as 30 min.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bienen E. J., Hammadi E., Hill G. C. Trypanosoma brucei: biochemical and morphological changes during in vitro transformation of bloodstream- to procyclic-trypomastigotes. Exp Parasitol. 1981 Jun;51(3):408–417. doi: 10.1016/0014-4894(81)90128-4. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. Structure and regulated expression of genes encoding fructose biphosphate aldolase in Trypanosoma brucei. EMBO J. 1985 Nov;4(11):2997–3003. doi: 10.1002/j.1460-2075.1985.tb04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Misset O., Edwards S. W., Opperdoes F. R. A comparison of the glycosomes (microbodies) isolated from Trypanosoma brucei bloodstream form and cultured procyclic trypomastigotes. Mol Biochem Parasitol. 1984 May;12(1):25–35. doi: 10.1016/0166-6851(84)90041-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel B. E., Samson S., Wu J., Hirschberg R., Yarbrough L. R. Tubulin genes of the African trypanosome Trypanosoma brucei rhodesiense:nucleotide sequence of a 3.7-kb fragment containing genes for alpha and beta tubulins. Gene. 1985;35(3):237–248. doi: 10.1016/0378-1119(85)90002-2. [DOI] [PubMed] [Google Scholar]
- Kindl H. The biosynthesis of microbodies (peroxisomes, glyoxysomes). Int Rev Cytol. 1982;80:193–229. doi: 10.1016/s0074-7696(08)60370-8. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Robbi M., Fujiki Y., Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. Ann N Y Acad Sci. 1982;386:285–300. doi: 10.1111/j.1749-6632.1982.tb21423.x. [DOI] [PubMed] [Google Scholar]
- Mechler B., Rabbitts T. H. Membrane-bound ribosomes of myeloma cells. IV. mRNA complexity of free and membrane-bound polysomes. J Cell Biol. 1981 Jan;88(1):29–36. doi: 10.1083/jcb.88.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels P. A., Poliszczak A., Osinga K. A., Misset O., Van Beeumen J., Wierenga R. K., Borst P., Opperdoes F. R. Two tandemly linked identical genes code for the glycosomal glyceraldehyde-phosphate dehydrogenase in Trypanosoma brucei. EMBO J. 1986 May;5(5):1049–1056. doi: 10.1002/j.1460-2075.1986.tb04321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Paterson B. M., Ricciardi R. P., Cohen L., Roberts B. E. Methods utilizing cell-free protein-synthesizing systems for the identification of recombinant DNA molecules. Methods Enzymol. 1983;101:650–674. doi: 10.1016/0076-6879(83)01046-0. [DOI] [PubMed] [Google Scholar]
- Misset O., Bos O. J., Opperdoes F. R. Glycolytic enzymes of Trypanosoma brucei. Simultaneous purification, intraglycosomal concentrations and physical properties. Eur J Biochem. 1986 Jun 2;157(2):441–453. doi: 10.1111/j.1432-1033.1986.tb09687.x. [DOI] [PubMed] [Google Scholar]
- Misset O., Opperdoes F. R. Simultaneous purification of hexokinase, class-I fructose-bisphosphate aldolase, triosephosphate isomerase and phosphoglycerate kinase from Trypanosoma brucei. Eur J Biochem. 1984 Nov 2;144(3):475–483. doi: 10.1111/j.1432-1033.1984.tb08490.x. [DOI] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Opperdoes F. R., Baudhuin P., Coppens I., De Roe C., Edwards S. W., Weijers P. J., Misset O. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J Cell Biol. 1984 Apr;98(4):1178–1184. doi: 10.1083/jcb.98.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes F. R. Biochemical peculiarities of trypanosomes, African and South American. Br Med Bull. 1985 Apr;41(2):130–136. doi: 10.1093/oxfordjournals.bmb.a072039. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Markoŝ A., Steiger R. F. Localization of malate dehydrogenase, adenylate kinase and glycolytic enzymes in glycosomes and the threonine pathway in the mitochondrion of cultured procyclic trypomastigotes of Trypanosoma brucei. Mol Biochem Parasitol. 1981 Dec 31;4(5-6):291–309. doi: 10.1016/0166-6851(81)90062-1. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., Swinkels B. W., Gibson W. C., Borst P., Veeneman G. H., Van Boom J. H., Michels P. A., Opperdoes F. R. Topogenesis of microbody enzymes: a sequence comparison of the genes for the glycosomal (microbody) and cytosolic phosphoglycerate kinases of Trypanosoma brucei. EMBO J. 1985 Dec 30;4(13B):3811–3817. doi: 10.1002/j.1460-2075.1985.tb04152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Czichos J., Stock U., Nonnengaesser C. Repression of glycoprotein synthesis and release of surface coat during transformation of Trypanosoma brucei. EMBO J. 1983;2(10):1721–1728. doi: 10.1002/j.1460-2075.1983.tb01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Leighton F., De Duve C. The synthesis and turnover of rat liver peroxisomes. II. Turnover of peroxisome proteins. J Cell Biol. 1969 May;41(2):536–546. doi: 10.1083/jcb.41.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger R. F., Opperdoes F. R., Bontemps J. Subcellular fractionation of Trypanosoma brucei bloodstream forms with special reference to hydrolases. Eur J Biochem. 1980 Mar;105(1):163–175. doi: 10.1111/j.1432-1033.1980.tb04486.x. [DOI] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Stone J., Dairman W., Udenfriend S. Amino acid analysis with fluorescamine at the picomole level. Arch Biochem Biophys. 1973 Mar;155(1):202–212. doi: 10.1016/s0003-9861(73)80022-0. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Gibson W. C., Osinga K. A., Kramer R., Veeneman G. H., van Boom J. H., Borst P. Characterization of the gene for the microbody (glycosomal) triosephosphate isomerase of Trypanosoma brucei. EMBO J. 1986 Jun;5(6):1291–1298. doi: 10.1002/j.1460-2075.1986.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Swinkels B., Michels P. A., Osinga K., Misset O., Van Beeumen J., Gibson W. C., Postma J. P., Borst P., Opperdoes F. R. Common elements on the surface of glycolytic enzymes from Trypanosoma brucei may serve as topogenic signals for import into glycosomes. EMBO J. 1987 Jan;6(1):215–221. doi: 10.1002/j.1460-2075.1987.tb04741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]