Mild-to-moderate hearing loss in one ear and essentially normal hearing in the other triggers cortical reorganization that is different in the two hemispheres. Asymmetry of cochlea sensitivities does not simply propagate to the two auditory cortices in mirror-image fashion. The resulting anisomorphic cortical reorganization may be a neurophysiological basis of clinical deficits in asymmetric hearing loss, such as difficulty with hearing in noise, impaired spatial hearing, and accelerated decline of the poorer ear.
Keywords: auditory cortex, frequency map alignment, interaural latency, plasticity, spike timing-dependent plasticity
Abstract
Acoustic trauma or inner ear disease may predominantly injure one ear, causing asymmetric sensorineural hearing loss (SNHL). While characteristic frequency (CF) map plasticity of primary auditory cortex (AI) contralateral to the injured ear has been detailed, there is no study that also evaluates ipsilateral AI to compare cortical reorganization across both hemispheres. We assess whether the normal isomorphic mirror-image relationship between the two hemispheres is maintained or disrupted in mild-to-moderate asymmetric SNHL of adult squirrel monkeys. At week 24 after induction of acoustic injury to the right ear, functional organization of the two hemispheres differs in direction and magnitude of interaural CF difference, percentage of recording sites with spectrally nonoverlapping binaural activation, and the concurrence of peripheral and central activation thresholds. The emergence of this anisomorphic cortical reorganization of the two hemispheres is replicated by simulation based on spike timing-dependent plasticity, where 1) AI input from the contralateral ear is dominant, 2) reestablishment of relatively shorter contralateral ear input timing drives reorganization, and 3) only AI contralateral to the injured ear undergoes major realignment of interaural frequency maps that evolve over months. Asymmetric SNHL disrupts isomorphic organization between the two hemispheres and results in relative local hemispheric autonomy, potentially impairing performance of tasks that require binaural input alignment or interhemispheric processing.
NEW & NOTEWORTHY Mild-to-moderate hearing loss in one ear and essentially normal hearing in the other triggers cortical reorganization that is different in the two hemispheres. Asymmetry of cochlea sensitivities does not simply propagate to the two auditory cortices in mirror-image fashion. The resulting anisomorphic cortical reorganization may be a neurophysiological basis of clinical deficits in asymmetric hearing loss, such as difficulty with hearing in noise, impaired spatial hearing, and accelerated decline of the poorer ear.
directional acoustic trauma, endolymphatic hydrops, and sudden hearing loss are some common clinical entities that cause permanent asymmetric sensorineural hearing loss (SNHL), terminating the usual delivery of matched sound inputs to neurons in primary auditory cortex (AI) of each hemisphere. In normal hearing, alignment of both cochlea sensitivities to tonal stimuli enforces isomorphic mirror-image functional organization across the two cortical hemispheres (Cheung 2005; Philibert et al. 2005; Recanzone et al. 1999). In asymmetric SNHL, interhemispheric organization may reorganize to an altered isomorphic form that reflects change in peripheral threshold profiles or to an anisomorphic form that expresses clear differences between the two hemispheres, disrupting symmetry of functional organization. Research to define interhemispheric functional relationships in asymmetric SNHL of adult subjects may uncover neural correlates for some of its differentiated clinical consequences, such as poorer speech recognition in noise on dichotic testing (Vannson et al. 2015), more severe spatial hearing disability (Noble and Gatehouse 2004), and higher predictive value of tinnitus laterality to the poorer ear with greater interaural threshold asymmetry (Tsai et al. 2012).
In normal-hearing mammals, the ear that provides contralateral sound input to a particular hemisphere necessarily provides ipsilateral sound input to the other, and vice versa. Characteristic frequency (CF) measured from AI neurons of either hemisphere is tightly matched for tones delivered from the two ears (Cheung et al. 2009). Consequently, the interaural CF map has nearly unity correlation. Other interhemispheric relationships of frequency-domain maps, namely, spatial extent, direction and slope of the frequency gradient, and local distortions in frequency representation (Cheung 2005; Philibert et al. 2005; Recanzone et al. 1999), also demonstrate close registration of input from the two ears. We refer to frequency map symmetry across the two hemispheres as cortical isomorphism.
Deviation of the interaural CF map from near unity has been the principal metric to assess for cortical plastic change in asymmetric SNHL. However, published accounts of cortical reorganization have reported solely on AI contralateral to the injured ear or “contralateral AI” (Irvine et al. 2006; Noreña et al. 2003; Rajan et al. 1993; Robertson and Irvine 1989; Schwaber et al. 1993). By documenting ipsilateral AI reorganization after acoustic injury and contrasting results with contralateral AI changes at the same time point, we can broaden our understanding of how a paired sensory system triggered into asymmetry may impact interhemispheric functional organization. A related investigation on contralateral AI plasticity in adult squirrel monkeys with similar mild-to-moderate asymmetric SNHL provides context to this study. Contralateral AI reorganization evolves over at least several months (Cheung et al. 2009). Acoustic overexposure directed at the right ear affects interaural CF maps of AI contralateral to the injured, dominant ear. There are profound distortions in the neighborhood of the 1-kHz overstimulation lesion extending to week 12. Thereafter, in the interval between week 12 and week 24, contralateral AI interaural CF and threshold maps come into realignment. It is hypothesized that delayed plasticity of cortical representation of sounds arriving from the normal but nondominant ear may be mediating this realignment, as its associated cortical thresholds are unexpectedly elevated at week 24.
The present study seeks to advance knowledge of the interhemispheric functional relationship between AI ipsilateral and AI contralateral to the injured ear at week 24 after induction of asymmetric SNHL in adult squirrel monkeys. Right and left auditory cortices of barbiturate-anesthetized animals are mapped, and microelectrode-derived frequency-response areas (FRAs) to tonal stimulation are captured. Two contrasting outcomes of asymmetric SNHL are considered: 1) cortical isomorphism is maintained, evidenced by hemispheric alignment of interaural frequency maps, and 2) cortical isomorphism is disrupted and anisomorphism is created, evidenced by interaural alignment differences of frequency inputs for the two hemispheres. We evaluate these two possibilities experimentally and theoretically by reconstructing electrophysiological response maps and performing network simulations based on experimental observations.
MATERIALS AND METHODS
Hearing loss and recording procedures.
Asymmetric SNHL was induced in young adult squirrel monkeys (Saimiri sciureus) in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committees at the Department of Veterans Affairs Medical Center and the University of California, San Francisco. General anesthesia was maintained with an inhalation mixture of isoflurane-nitrous oxide-oxygen (2:48:50%). The airway was protected, the cardiovascular system was supported with normal saline with 1.5% dextrose and 20 mEq KCl delivered at 6–8 ml·kg−1·h−1, and core temperature was maintained at ~38°C.
The left ear was protected by placement of an occlusive foam earplug (E-A-R Classic; Aearo, Indianapolis, IN) into the external auditory canal. Sound attenuation conferred by the E-A-R insert was rated to be 23 dB at 1 kHz by the manufacturer and the National Acoustics Laboratory (Sydney, Australia). The left external meatus was occluded further by packing the concha cavum with mineral oil-soaked cotton pledgets, which provided unknown incremental sound attenuation. SNHL induction directed at the right ear was performed in a sound-attenuating chamber (IAC, Bronx, NY) fitted with a video surveillance system. Eardrums appeared normal before and after procedures. A sine wave oscillator (General Radio 1396-A; General Radio, West Concord, MA) generated the continuous 1-kHz signal used for acoustic overstimulation at 136 dB SPL for 3 h. A power amplifier (MA2400; Crown Audio, Elkhart, IN) conditioned the 1-kHz input signal for sound delivery through a high-performance midrange driver (M4; Community Professional Loudspeakers, Chester, PA) that was positioned 5–6 cm from the tragus, along the interaural line. The intensity level of the 1-kHz tone was measured at the right lateral ear canal with a sound meter (Brüel and Kjær 2209; Brüel and Kjær, Norcross, GA).
Twenty-four weeks after hearing loss induction, tone bursts (linear rise/fall 1 ms, total duration 17 ms, interstimulus interval 35 ms; 0.5, 1, 2, 3, 4, 6, and 8 kHz) were used to determine peripheral auditory brain stem response (ABR) thresholds under pentobarbital anesthesia. Thereafter, tone bursts (linear rise/fall 3 ms, total duration 50 ms, interstimulus interval 400 ms; 675 presented in pseudorandom order from 2.5 to 77.5 dB SPL in 5-dB steps centered on the neuron’s estimated CF) were presented to derive cortical FRA tuning curves. Sound stimuli were generated by a microprocessor (TMS32010, 16-bit analog-to-digital converter at 120 kHz; Texas Instruments, Dallas, TX). A single STAX-54 headphone (Sokolich 1981) enclosed in a small chamber that was connected into the external auditory canal via a sealed tube delivered stimuli for ABR recordings. Two STAX-54 headphones delivered stimuli to the two ears for AI mapping procedures. Frequency response of the sound delivery system was measured with a sound meter (Brüel and Kjær 2209; Brüel and Kjær) and a waveform analyzer (General Radio 1521-B; General Radio) and determined to be flat within 6 dB up to 14 kHz. The frequency response rolled off at 10 dB/octave above 14 kHz. The two headphones were calibrated before and after cortical recording experiments and were within ±2 dB from 0.5 to 16 kHz measured at 1-octave intervals.
ABR waveforms were recorded at the time of electrophysiological brain mapping procedures to construct tone-specific audiograms (Cheung et al. 2009). Silver wire electrodes were placed at the retroauricular sulci of the two pinnae (active and ground) and at the skull vertex (indifferent). Two hundred to four hundred repetitions of each frequency were delivered for triggered waveform averaging. Response waveforms were band-pass filtered from 0.3 to 3 kHz, amplified, and digitized within a 15-ms window. ABR thresholds were determined interactively for specific frequencies by two individuals, an ABR threshold evaluator and an electrophysiological experimenter. Neither was blinded to the condition of the animal or the ear stimulated. The evaluator made threshold determinations, while the experimenter increased and decreased sound pressure levels in 2- to 5-dB steps that were unknown to the evaluator. ABR thresholds were determined visually at the time of ABR waveform collection by notation of the lowest sound level at which the ABR wave I complex became indistinguishable from background noise.
Electrophysiological brain mapping procedures in squirrel monkeys were performed under pentobarbital anesthesia. After craniotomy, the brain was kept moist under a layer of silicone oil. A magnified video image of the recording zone was stored in a microcomputer for documenting electrode positions. Multiunit recordings at depths 550–850 µm, corresponding to cortical layers III and IV, were performed with Parylene-coated tungsten microelectrodes (FHC, Bowdoinham, ME) with 1–2 MΩ impedance at 1 kHz. Within a vertical column, the electrode position where the neuronal cluster responded to both right and left ear stimulation was chosen whenever possible. Only one site was studied in detail per penetration. On occasion, cortical neurons were activated exclusively by either right or left ear stimulus presentation despite a thorough search along the targeted depth range. In those cases, a comparison of responses between ipsilateral and contralateral stimulation was not possible. Seventy to eighty percent of penetrations yielded a binaurally excited response in both normal and hearing loss animals. Action potentials were isolated from background noise with an online window discriminator (DIS-1; BAK, Mount Airy, MD) with a threshold set to twice the amplitude of background noise. The arrival times of discriminated spikes that occurred within 50 ms after each tone burst onset were recorded digitally.
Data analysis.
From each excitatory FRA (Schreiner and Mendelson 1990), CF, minimum threshold (threshold), bandwidth 10 dB above threshold (BW10), bandwidth 20 dB above threshold (BW20), and minimum response latency (latency) were extracted. CF was defined as the frequency of the quietest tone that evoked a response, threshold as the sound intensity level of the quietest tone burst that marked CF, bandwidth of the FRA as the octave distance spanned by the lower and upper frequencies of the excitatory tuning curve at BW10 and BW20, and latency as the asymptotic minimum of first spike arrival times about CF for all stimulus intensity levels. Data analysis was restricted to contralaterally determined CFs between 0.6 kHz and 7 kHz for all monkeys to standardize group comparisons.
CF and threshold data for hearing loss monkeys SM15 and SM23 (left AI mapping at week 24) and normal control monkeys SM64 and SM82 (right AI and left AI mapping, respectively) have been reported in part in a prior publication (Cheung et al. 2009), but they are included in this article to facilitate direct comparisons of interhemispheric AI response maps. It should be noted that those cases underwent additional analyses for this study, including peripheral-to-cortical threshold asymmetry concurrence, bandwidth overlap with CF from the other ear (see below), and latency. Temporal evolution of interaural latency difference, not previously published, in left AI at week 3 (SM60), week 6 (SM17 and SM75), and week 12 (SM02 and SM87) was added from other squirrel monkeys with injured right ear asymmetric SNHL to display the time course of recovery.
Neuronal clusters recorded at the majority of recording sites responded to both right ear and left ear inputs. At a particular recording site, the interaural differences for threshold, latency, and CF were computed by subtracting contralaterally determined from ipsilaterally determined values. Interaural CF alignment was calculated with the following formula: CF difference (octaves) = log2(CFcontra/CFipsi). The determination of statistically significant CF difference between the two ears was based on an empirical CF distribution in normal squirrel monkeys (Cheung et al. 2009). At the α = 0.025 level, the upper tail cutoff was 0.63 octave and the lower tail cutoff was −0.27 octave. Descriptive statistics (mean, median, 1st quartile, 3rd quartile, and standard deviation) summarized data distributions. The t-, Wilcoxon rank sum, χ2-, and Fisher exact tests were used to evaluate for differences between groups.
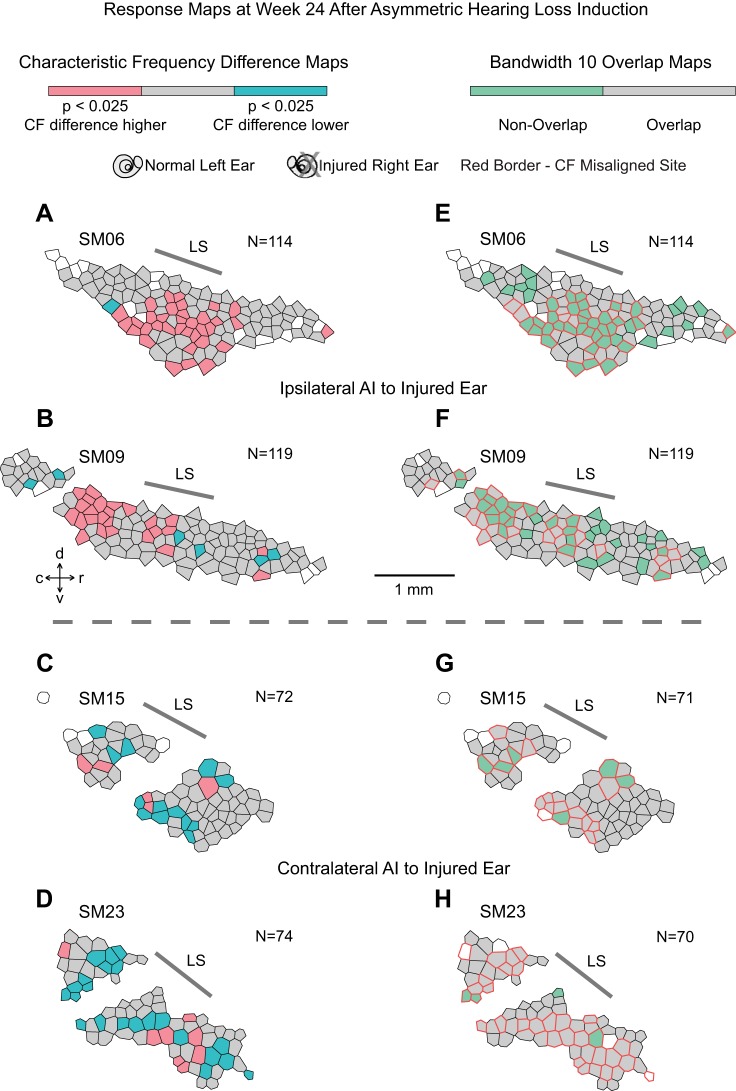
Frequency-response maps were reconstructed with the Voronoi-Dirichlet tessellation technique (Cheung et al. 2001; Philibert et al. 2005). Each recording site was assigned a polygon. The bounded area of each polygon was determined by minimizing the cumulative perimeter of all polygons with an optimization algorithm (MATLAB; The MathWorks, Natick, MA). Smaller polygons reflected areas of denser sampling, whereas larger polygons reflected areas of sparser sampling. Composite “characteristic frequency difference maps” highlighted recording sites with significant interaural CF difference and used the following color scheme: 1) gray—interaural CF difference not significant (P > 0.05; see Fig. 4B in Cheung et al. 2009), 2) pink—CFcontra > CFipsi or CF difference higher (P < 0.025), 3) aqua blue—CFcontra < CFipsi or CF difference lower (P < 0.025), and 4) white—absent ipsilateral or contralateral response. AI ipsilateral to the injured ear is referred to as “ipsilateral AI,” and AI contralateral to the injured ear is referred to as “contralateral AI.”
Fig. 4.
Characteristic frequency (CF) difference maps and bandwidth 10 overlap maps in primary auditory cortex (AI). Orientation of AI maps contralateral to the injured ear has been vertically reflected to facilitate comparisons. A–D: maps show that clustering of CFs from contralateral ear input is higher (CFcontra > CFipsi, pink) in ipsilateral AI but lower (CFcontra < CFipsi, aqua blue) in contralateral AI. E–H: BW10 overlap maps show a higher proportion of spectrally nonoverlapping binaural activation sites (nonoverlap, light green) in ipsilateral AI, encompassing both CF-aligned and CF-misaligned sites (red border). Map discontinuities reflect coursing veins. Gray (overlap), interaural CF difference not significant or BW10 overlaps with CF from the other ear; white, absent ipsilateral or contralateral response. C, contralateral; I, ipsilateral; LS, lateral sulcus; d, dorsal; v, ventral; c, caudal; r, rostral; N, count.
Composite “bandwidth 10 overlap maps” marked recording sites with spectrally nonoverlapping binaural activation to narrow band sounds. At recording sites that responded to both ears, the BW10 value derived from one ear was computed and overlap with CF from the other ear was assessed. The intersection of overlap maps from the two ears gave rise to composite maps that deployed the following color scheme: 1) gray—overlap of either right ear or left ear BW10 span with CF derived from the opposite ear, 2) light green—nonoverlap of both BW10 spans with CFs derived from opposite ears, 3) red border—CF misalignment to higher or lower CF (redrawn from “characteristic frequency difference maps”) for that particular recording site, and 4) white—absent ipsilateral or contralateral response.
Concurrence of interaural peripheral ABR and cortical neuronal threshold asymmetries was assessed for the four experimental monkeys with asymmetric SNHL. Peripheral ABR threshold data from 12 normal-hearing control squirrel monkeys at all seven test frequencies in both ears showed the following tone-specific right ear minus left ear threshold difference statistics: mean = −0.9 dB and SD = 6.2 dB, or 1 SD interval = [−7.1, 5.3]. Cortical threshold data from two normal-hearing monkeys for CFs between 0.6 and 7 kHz showed the following contralateral ear minus ipsilateral ear threshold difference statistics: mean = −1.9 dB and SD = 10.6 dB, or 1 SD interval = [−12.5, 8.7]. To enable comparisons with peripheral ABR data at specific frequencies, cortical threshold data were aggregated into discrete frequency bands at 0.5 kHz (<1.0 kHz), 1.0 kHz (1.01–2.0 kHz), 2.0 kHz (2.01–3.0 kHz), 3.0 kHz (3.01–4.0 kHz), 4.0 kHz (4.01–6.0 kHz), and 6.0 kHz (>6.01 kHz). Concurrence between interaural peripheral and cortical threshold asymmetries was determined by applying the following criteria to the three domains of ABR right minus left threshold asymmetries: 1) no peripheral asymmetry (absolute difference less than or equal to 7 dB)—concurrence if cortical threshold asymmetry was within the 1 SD interval or between −12.5 dB and 8.7 dB; 2) poorer ear was right (difference greater than 7 dB)—concurrence if cortical threshold asymmetry > 8.7 dB, and 3) poorer ear was left (difference less than −7 dB)—concurrence if cortical threshold asymmetry was less than −12.5 dB.
Simulations.
In our simulations, each cell was modeled as a conductance-based, leaky integrate-and-fire neuron, with time constant τ = 20 ms and resting membrane potential V0 = –60 mV (Vogels et al. 2011). Every time the membrane potential crossed the spiking threshold Vt = –50 mV, the neuron fired an action potential. The membrane potential was reset to V0, where it remained clamped for the duration of the refractory period, trefr = 3 ms. Finally, we used a membrane resistance of 100 MΩ, where gleak = 10 nS. The reversal potential was VR = 0 mV.
As a conductance-based model the membrane voltage was computed as
and the conductance was computed as
After each presynaptic action potential, the postsynaptic conductance increased as g → g + Δg, with Δg = g0 × w, where w was the appropriate weight between the presynaptic and postsynaptic neurons; w was either fixed or plastic. The integration step was 0.5 ms.
For excitatory spike timing-dependent plasticity (STDP), we simulated a standard asymmetric form of synaptic plasticity. Long-term potentiation (LTP) was induced as a function of the timing between pre- and postsynaptic spikes (Δt) when a postsynaptic spike occurred after a presynaptic spike (Caporale and Dan 2008). Conversely, long-term depression (LTD) was induced when a presynaptic spike followed a postsynaptic spike. To calculate the changes of synaptic weights at each spike, an activity variable x was assigned to each neuron; x increased by 1 after each spike: x → x + 1 and decayed as τSTDP , with τSTDP = 20 ms.
Thus the synaptic weight wij from neuron j to neuron i was updated as
with the learning rate α = 0.002 and the maximum synaptic weight wmax = 100.
The model consisted of one postsynaptic neuron and five populations of presynaptic neurons, each representing a different frequency channel. Each population had 102 neurons, 60 of which were activated by the contralateral ear and 42 by the ipsilateral ear.
Each presynaptic neuron was stimulated by a pink noise process (Np) characterized with a power spectrum inversely proportional to f2 and obtained as the integral of a white noise signal. We rectified Np by setting negative values to 0 and normalized Np to ensure postsynaptic firing rates between 10 and 50 Hz. The normalized and rectified pink noise was then a varying parameter of a Poisson distribution. At every time point, each presynaptic neuron received a number of spikes corresponding to independent samples to that Poisson distribution.
Initial binaural delays were chosen as equally likely values from a Gaussian distribution of mean μ = 0 ms and variance σ2 = 1 ms. For each simulation, the delay increases by 2 ms (by 0.5-ms increments) during the “week 12” condition to reach delays centered around μ = 2 ms at the beginning of the “week 24” condition. The pink noise trace was shifted by this delay value, with positive delays indicating that the contralateral population was activated first.
Each of the five presynaptic populations was activated sequentially and separately for 15 s for each trial. For a single trial the five populations would receive inputs generated from the same pink noise trace. Each trial would begin with synaptic weights w initialized to 1.0. At the end of each trial, new g0 values were calculated for each synapse as g0 × w.
Initially, g0 values were set such that the postsynaptic neuron was tuned to the third input population. Specifically, g0 was first set to be 0.8 for all inputs from channels 1 and 5, 1.4 for channels 2 and 4, and 4.0 for channel 3.
Then, we “deafened” either the ipsilateral or contralateral ear by reducing the g0 values for the corresponding input:
The model was then run for 75 trials with increasing delays between the ipsilateral and contralateral inputs to obtain the “week 12” condition. From this result, the model was run again with delays centered around 2 ms for 75 trials to obtain the “week 24” condition.
RESULTS
Interaural CF alignment.
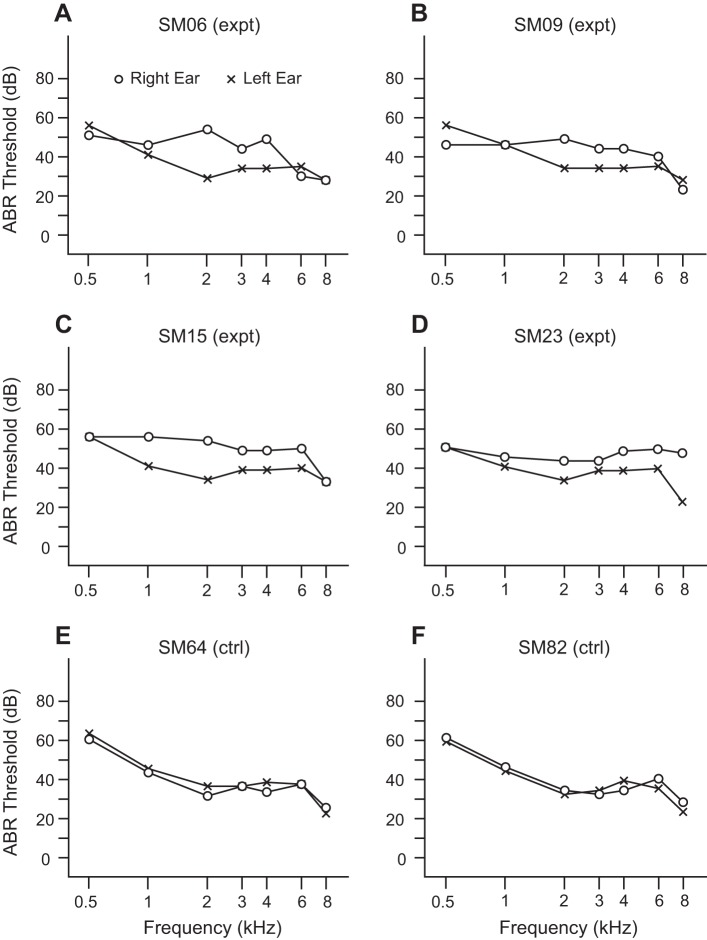
The right ear of experimental monkeys has mild-to-moderate SNHL from acoustic overstimulation, while the left ear is essentially normal. To simplify tracking of the two ears and the two hemispheres in experimental monkeys, we adopt the following descriptive nomenclature: right ear = injured ear; left ear = normal ear; right AI = ipsilateral AI; left AI = contralateral AI. ABR tone-specific audiograms in all four experimental monkeys (SM06 and SM09: ipsilateral AI mapped; SM15 and SM23: contralateral AI mapped) show interaural asymmetry in the middle frequencies (Fig. 1, A–D). With the exception of SM23 at higher (≥6 kHz) frequencies, experimental monkeys have nearly the same configuration of interaural asymmetry. The absolute level of asymmetry has a mean = 10.9 dB and SD = 6.1 dB between 1 kHz and 4 kHz. Control monkeys (SM64: right AI mapped; SM82: left AI mapped) confirm symmetry of interaural ABR thresholds (Fig. 1, E and F) across all frequencies.
Fig. 1.
Auditory brain stem (ABR) tone-specific audiograms in experimental and control squirrel monkeys. The right ear is injured by acoustic overstimulation at 1 kHz in all experimental monkeys. Asymmetric sensorineural hearing loss is most consistently centered by the middle frequencies, expressed between 1 kHz and 4 kHz. A–D: 4 experimental (expt) monkeys. E and F: 2 control (ctrl) monkeys.
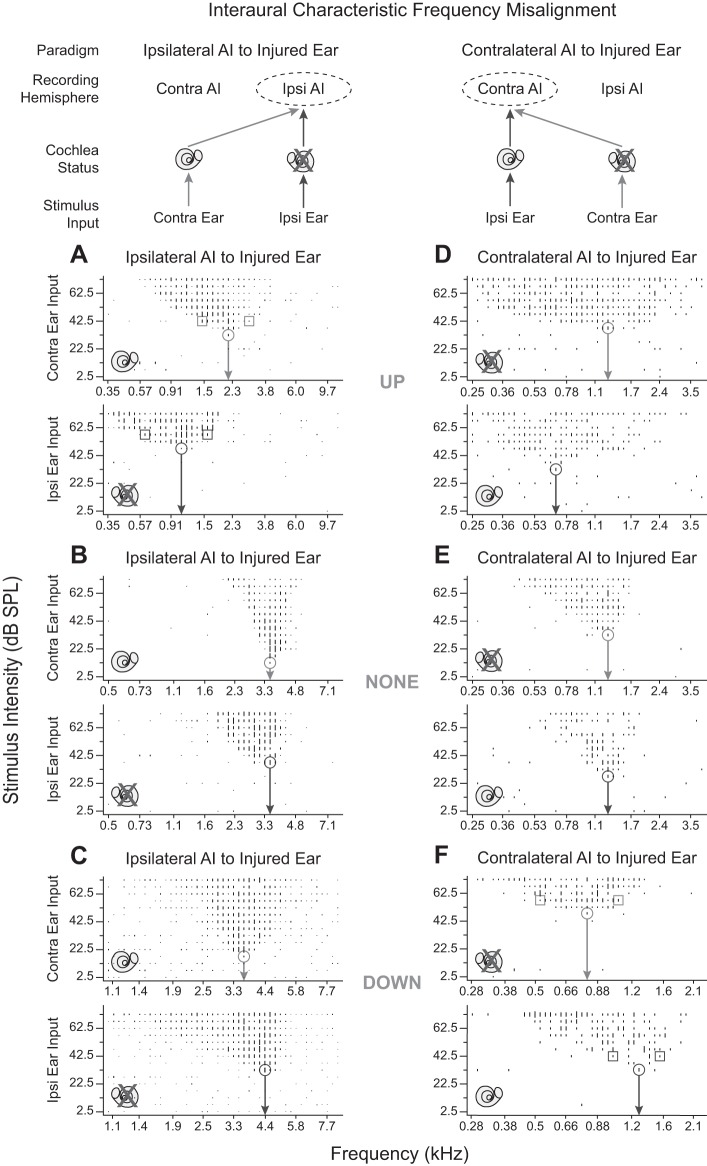
Examples of different types of interaural CF and threshold misalignments are demonstrated in Fig. 2, where the top row summarizes cochlea status of contralateral and ipsilateral ear inputs to each AI. Frequency tuning curves are presented for both hemispheres (Fig. 2) to clarify data exposition of the main observation at week 24 after asymmetric SNHL induction. Compared with the injured ear, the normal ear has a lower threshold in ipsilateral AI (Fig. 2, A–C) but nearly the same elevated threshold in contralateral AI (Fig. 2, D and E). Along this vein, the normal ear has a narrower BW10 in both hemispheres (Fig. 2, A and F). CF of the dominant ear to the hemisphere (top subplot in each panel) can be shifted either up (Fig. 2, A and D) or down (Fig. 2, C and F) or not shifted (none; Fig. 2, B and E) relative to the nondominant ear.
Fig. 2.
Frequency tuning curves (FTC) from the 2 ears illustrate 3 classes (UP, NONE, and DOWN) of interaural characteristic frequency (CF, arrow) misalignment in primary auditory cortex (AI) ipsilateral and contralateral to the injured right ear (X crossout). Top: summary of cochlea status of contralateral (contra) and ipsilateral (ipsi) ear inputs to each AI. A–F, top subplot: FTC from contra ear input. Bottom subplot: FTC from ipsi ear input. Bottom subplot CF is the reference value for CF misalignment classification. A–C and D–F show data from AI ipsi and AI contra to the injured ear, respectively. A and F illustrate nonoverlap of tuning curve bandwidth 10 dB above threshold (BW10) with CF derived from the other ear. Input ear is specified at bottom left of subplots. Circle marks CF; square marks upper and lower frequency edges of BW10.
CF values for input from the two ears at week 24 are markedly different between the two hemispheres. Ipsilateral AI (Fig. 3A) shows the combined interaural CF distribution of SM06 and SM09 to deviate noticeably above the upper limit of the normal 95% confidence interval (CI) between 0.6 kHz and 2 kHz. Contralateral AI (Fig. 3B) shows the combined interaural CF map of SM15 and SM23 to fall mostly within the upper boundary of the normal 95% CI and to deviate just below the lower boundary. The upper (0.63 octaves) and lower (−0.27 octaves) dashed diagonal lines in Fig. 3, A and B, delimit boundaries for significant (α/2 = 0.025) interaural CF difference based on an empirical distribution in normal monkeys (Cheung et al. 2009). To enable plotting of the normal 95% CI in identical positions for the two hemispheres, CFs from the ear contralateral to the recorded hemisphere are plotted on the x-axis.
Fig. 3.
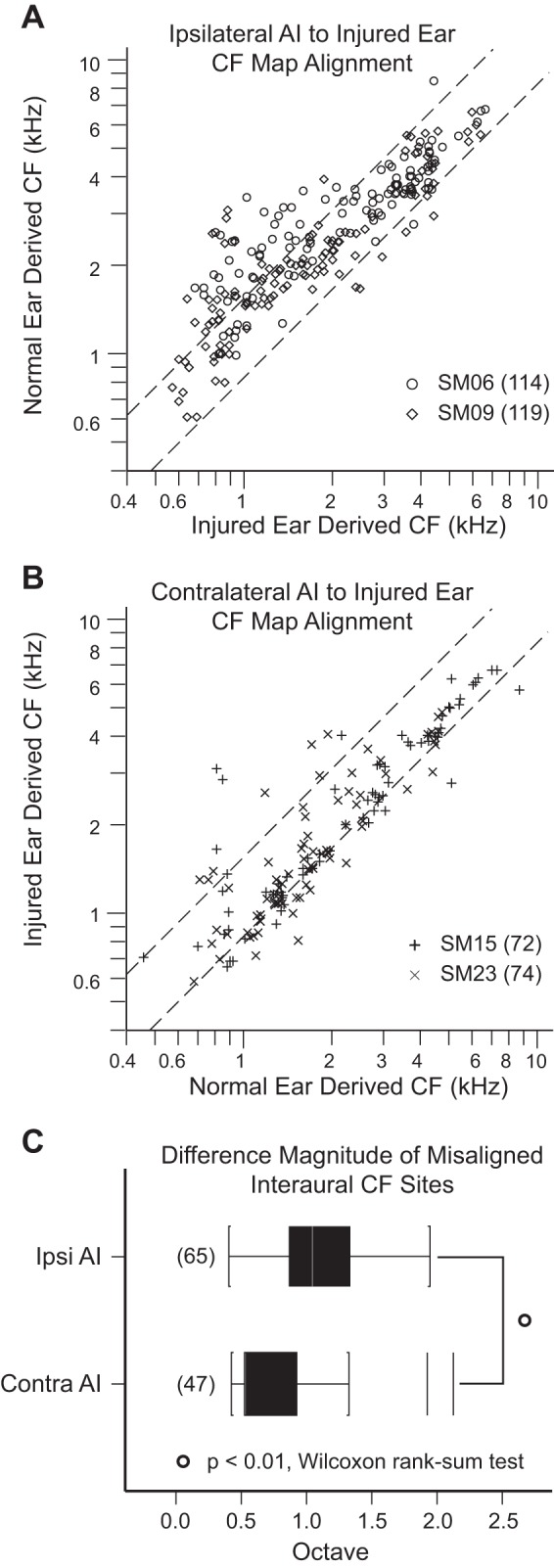
Interaural characteristic frequency (CF) alignment. Right is the injured ear. A and B: scatterplots of CF derived from normal ear vs. injured ear input obtained in AI ipsilateral and AI contralateral to the injured, right ear. Data were collected 24 wk after acoustic overstimulation. Dashed lines represent the 95% confidence interval of CF alignment in normal animals (Cheung et al. 2009). Data in ipsilateral AI (A) and contralateral AI (B) fall above and below limits of the 95% confidence interval (upper bound: 0.63 octaves; lower bound; −0.27 octaves), indicating cortical misalignment of the frequency input from the 2 ears. C: ipsilateral AI absolute CF difference magnitude for sites above and below the 95% confidence interval is nearly twice the CF difference in contralateral AI (P < 0.01, Wilcoxon rank sum test). CFs from the ear contralateral to the recorded hemisphere are plotted on x-axis. Counts of cortical recording sites are in parentheses. Ipsilateral AI refers to AI ipsilateral to the injured ear. Contralateral AI refers to AI contralateral to the injured ear.
The predominant direction of interaural CF misalignment is up in ipsilateral AI but down in contralateral AI. CF misalignments in the up and down directions are shown in detail (Table 1) by segmenting CF into octave bands that range from <0.5 kHz to >8.0 kHz. CF misalignment contributions from all four experimental monkeys are grouped by hemisphere. In both auditory cortices, the majority of CF-misaligned sites are found in frequency bands between 1 and 4 kHz, encompassing the main acoustic overexposure-induced elevations in ABR thresholds. While the proportion of sites that fall outside the 95% CI is ~30% in both hemispheres, ipsilateral AI has a higher proportion of CF-misaligned sites in the up direction (26% up vs. 3% down; Table 1) compared with contralateral AI, which has a higher proportion of CF-misaligned sites in the down direction (9% up vs. 24% down, P < 0.01, χ2-test).
Table 1.
Characteristic frequency misalignment direction in ipsilateral AI and contralateral AI, referenced to injured right ear
| Ipsilateral AI CF Misalignment |
Contralateral AI CF Misalignment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency Band | Monkey | Up | Down | None | N | Monkey | Up | Down | None | N |
| <0.5 kHz | ||||||||||
| SM06 | 0 | 0 | 0 | 0 | SM15 | 0 | 0 | 0 | 0 | |
| SM09 | 0 | 0 | 0 | 0 | SM23 | 0 | 0 | 0 | 0 | |
| 0.5–1 kHz | ||||||||||
| SM06 | 0 | 0 | 2 | 2 | SM15 | 1 | 6 | 3 | 10 | |
| SM09 | 1 | 0 | 11 | 12 | SM23 | 0 | 7 | 8 | 15 | |
| 1–2 kHz | ||||||||||
| SM06 | 11 | 0 | 11 | 22 | SM15 | 1 | 2 | 18 | 21 | |
| SM09 | 16 | 2 | 31 | 49 | SM23 | 3 | 12 | 20 | 35 | |
| 2–4 kHz | ||||||||||
| SM06 | 23 | 1 | 43 | 67 | SM15 | 2 | 4 | 18 | 24 | |
| SM09 | 7 | 4 | 30 | 41 | SM23 | 4 | 3 | 11 | 18 | |
| 4–8 kHz | ||||||||||
| SM06 | 0 | 0 | 22 | 22 | SM15 | 1 | 1 | 15 | 17 | |
| SM09 | 1 | 0 | 16 | 17 | SM23 | 1 | 0 | 5 | 6 | |
| >8 kHz | ||||||||||
| SM06 | 1 | 0 | 0 | 1 | SM15 | 0 | 0 | 0 | 0 | |
| SM09 | 0 | 0 | 0 | 0 | SM23 | 0 | 0 | 0 | 0 | |
| All bands | ||||||||||
| Total | 60 | 7 | 166 | 233 | 13 | 35 | 98 | 146 | ||
| Percentage | 25.8 | 3.0 | 71.2 | 100 | 8.9 | 24.0 | 67.1 | 100 | ||
In both cortices at week 24, the majority of characteristic frequency (CF) misalignment sites are found in frequency bands between 1 and 4 kHz. CF difference = log[(CF contralateral ear stimulation)/CF ipsilateral ear stimulation)]/log(2). Up criterion: CF difference > 0.63. Down criterion: CF difference < −0.27. Ipsilateral AI (up: 25.8%, down: 3.0%) differs from contralateral AI (up: 8.9%, down: 24.0%) most notably in the dominant direction of CF misalignment (P < 0.01, χ2-test). N, count.
The magnitude (octave) of interaural CF difference falling outside the normal CI (~30% of all recoded sites) is larger in ipsilateral AI. Tukey box plots of absolute CF difference values outside the 95% CI for ipsilateral AI and contralateral AI are displayed in Fig. 3C. The magnitude of interaural CF difference in ipsilateral AI (mean = 0.98, median = 0.92, 1st quartile = 0.74, 3rd quartile = 1.21) is ~50% larger compared with contralateral AI (mean = 0.60, median = 0.40, 1st quartile = 0.40, 3rd quartile = 0.75) (P < 0.01, Wilcoxon rank sum test). In summary, AI frequency organization in the two hemispheres differs in predominant direction and magnitude of interaural CF difference at week 24 after acoustic overstimulation.
Composite characteristic frequency difference maps.
AI frequency map changes are spatially clustered. Composite interaural CF difference maps are shown in Fig. 4, left. Contralateral AI has been vertically reflected to facilitate comparisons. Map discontinuities indicate areas inaccessible for sampling because of cortical veins that traverse isofrequency contours.
Ipsilateral AI CF difference maps (Fig. 4, A and B) show large clusters of sites that have higher CF differences (pink polygons). They are positioned centrally in SM06 and SM09. Contralateral AI CF difference maps (Fig. 4, C and D) show mostly clusters that have lower CF differences (aqua blue polygons). They are contiguous but somewhat spatially disparate in SM15 and SM23. Overall, spatial organization of CF difference maps illustrates qualitative clustering of penetration sites that fall outside the 95% CI of interaural CF alignment (P < 0.05, empirical distribution in normal monkeys).
Spectrally nonoverlapping binaural activation.
Perfect alignment of CF and tuning curve shape of inputs from the two ears would provide binaural, nearly simultaneous driven input to a cortical neuron. Relative shifts in the tuning curves from the two ears can result in instances when only one ear drives the neuron (spectrally nonoverlapping binaural activation) because the stimulus is outside the tuning curve of the other ear. Here interaural receptive field overlap is used to identify those sites. Overlap is assessed by querying whether tuning curve bandwidth span at 10 dB and 20 dB above threshold derived from one ear intersects with the CF derived from the other ear (e.g., Fig. 2, A and F). Ipsilateral AI has a higher percentage of sites with spectrally nonoverlapping binaural activation to narrow-band sounds for both BW10 (Table 2; 35.6% vs. 7.1% P < 0.01, χ2-test) and BW20 (Table 3; 16.3% vs. 1.4%, P < 0.01, χ2-test). As CF misalignment may covary with spectrally nonoverlapping binaural activation, Tables 2 and 3 stratify this factor. CF-misaligned sites in ipsilateral AI BW10 and BW20, and contralateral AI BW10 have higher rates of spectrally nonoverlapping binaural activation compared with CF-aligned sites (Tables 2 and 3; P < 0.01, Fisher exact test). AI spectrally nonoverlapping binaural activation rates are different in the two hemispheres, where CF misalignment is a key covariate.
Table 2.
Spectrally nonoverlapping binaural activation (BW10) of ipsilateral AI and contralateral AI, referenced to injured right ear
| Ear Integration |
Percentage |
||||
|---|---|---|---|---|---|
| Nonoverlap | Overlap | Nonoverlap | Overlap | N | |
| Ipsilateral AI BW10 | |||||
| CF aligned | |||||
| SM06 | 14 | 64 | 17.9 | 82.1 | 78 |
| SM09 | 17 | 71 | 19.3 | 80.7 | 88 |
| Subtotal | 31 | 135 | 18.7 | 81.3 | 166 |
| CF misaligned | |||||
| SM06 | 33 | 3 | 91.7 | 8.3 | 36 |
| SM09 | 19 | 12 | 61.3 | 38.7 | 31 |
| Subtotal | 52 | 15 | 77.6 | 22.4 | 67 |
| Both CF categories | |||||
| Total | 83 | 150 | 35.6 | 64.4 | 233 |
| Contralateral AI BW10 | |||||
| CF aligned | |||||
| SM15 | 0 | 54 | 0.0 | 100.0 | 54 |
| SM23 | 1 | 41 | 2.4 | 97.6 | 42 |
| Subtotal | 1 | 95 | 1.0 | 99.0 | 96 |
| CF misaligned | |||||
| SM15 | 6 | 11 | 35.3 | 64.7 | 17 |
| SM23 | 3 | 25 | 10.7 | 89.3 | 28 |
| Subtotal | 9 | 36 | 20.0 | 80.0 | 45 |
| Both CF categories | |||||
| Total | 10 | 131 | 7.1 | 92.9 | 141 |
Nonoverlap binaural activation criterion for bandwidth 10 (BW10) at week 24: frequency tuning curve receptive field span at 10 dB above threshold derived from stimulation of either ear does not overlap with characteristic frequency (CF) derived from stimulation of the other ear. BW10 in ipsilateral AI has a higher rate of spectrally nonoverlapping binaural activation compared with contralateral AI (P < 0.01, χ2-test). CF-misaligned sites in both hemispheres have higher rates of nonoverlap activation (P < 0.01, Fisher exact test). N, count.
Table 3.
Spectrally nonoverlapping binaural activation at bandwidth 20 dB (BW20) of ipsilateral AI and contralateral AI, referenced to injured right ear
| Ear Integration |
Percentage |
||||
|---|---|---|---|---|---|
| Nonoverlap | Overlap | Nonoverlap | Overlap | N | |
| Ipsilateral AI BW20 | |||||
| CF aligned | |||||
| SM06 | 7 | 71 | 9.0 | 91.0 | 78 |
| SM09 | 6 | 82 | 6.8 | 93.2 | 88 |
| Subtotal | 13 | 153 | 7.8 | 92.2 | 166 |
| CF misaligned | |||||
| SM06 | 18 | 18 | 50.0 | 50.0 | 36 |
| SM09 | 7 | 24 | 22.6 | 77.4 | 31 |
| Subtotal | 25 | 42 | 37.3 | 62.7 | 67 |
| Both CF categories | |||||
| Total | 38 | 195 | 16.3 | 83.7 | 233 |
| Contralateral AI BW20 | |||||
| CF aligned | |||||
| SM15 | 0 | 54 | 0.0 | 100.0 | 54 |
| SM23 | 0 | 42 | 0.0 | 100.0 | 42 |
| Subtotal | 0 | 96 | 0.0 | 100.0 | 96 |
| CF misaligned | |||||
| SM15 | 2 | 15 | 11.8 | 88.2 | 17 |
| SM23 | 0 | 28 | 0.0 | 100.0 | 28 |
| Subtotal | 2 | 43 | 4.4 | 95.6 | 45 |
| Both CF categories | |||||
| Total | 2 | 139 | 1.4 | 98.6 | 141 |
At week 24 BW20 in ipsilateral AI has a higher rate of spectrally nonoverlapping binaural activation compared with contralateral AI (P < 0.01, χ2-test). CF misaligned sites in ipsilateral AI have higher rates of nonoverlap activation (P < 0.01, Fisher exact test). Relative to BW10, monaural activation rates have decreased, as expected. Convention same as in Table 2. N, count.
The possibility that right ear (injured) bandwidth difference between the two hemispheres may confound interaural receptive field overlap analysis is considered. BW10 and BW20 of the four experimental monkeys with asymmetric SNHL are evaluated by ipsilateral AI and contralateral AI and left ear vs. right ear stimulation for BW10 and BW20 (Table 4). Control data from normal monkeys show no difference between ipsilaterally and contralaterally derived bandwidths (Table 5). The injured ear has broader cortical neuronal bandwidths irrespective of hemisphere (Fig. 5; P < 0.01, t-test), and interhemispheric differences are not significant (Table 4; P > 0.05, t-test). Therefore, differences in spectrally nonoverlapping binaural activation rates between the two hemispheres are not confounded by variations in bandwidth span derived from the injured ear.
Table 4.
Bandwidths derived from right and left ears of experimental and control monkeys
| Mean (SD) | N | |
|---|---|---|
| Ipsilateral BW10 | ||
| Control | 0.74 (0.38) | 224 |
| Normal left ear (expt) | 0.72 (0.35) | 141 |
| Injured right ear (expt) | 0.92 (0.32) | 230 |
| Ipsilateral BW20 | ||
| Control | 1.01 (0.54) | 224 |
| Normal left ear (expt) | 1.00 (0.47) | 140 |
| Injured right ear (expt) | 1.28 (0.46) | 220 |
| Contralateral BW10 | ||
| Control | 0.77 (0.44) | 224 |
| Normal left ear (expt) | 0.84 (0.36) | 232 |
| Injured right ear (expt) | 0.96 (0.40) | 140 |
| Contralateral BW20 | ||
| Control | 1.14 (0.61) | 224 |
| Normal left ear (expt) | 1.14 (0.49) | 230 |
| Injured right ear (expt) | 1.36 (0.56) | 149 |
Bandwidth 10 (BW10) and bandwidth 20 (BW20) dB derived from the injured ear of experimental (expt) monkeys at week 24 are broader in both hemispheres (all P < 0.01, t-test). Injured ear interhemispheric comparisons for BW10 and BW20 show no significant differences (P > 0.05, t-test). N, count.
Table 5.
Control ipsilateral and contralateral bandwidths
| Mean (SD) | N | |
|---|---|---|
| Ipsilateral BW10 | ||
| SM64 (right ear, right AI) | 0.70 (0.41) | 92 |
| SM82 (left ear, left AI) | 0.76 (0.36) | 132 |
| Pooled | 0.74 (0.38) | 224 |
| Ipsilateral BW20 | ||
| SM64 (right ear, right AI) | 1.00 (0.59) | 92 |
| SM82 (left ear, left AI) | 1.03 (0.51) | 132 |
| Pooled | 1.01 (0.54) | 224 |
| Contralateral BW10 | ||
| SM64 (left ear, right AI) | 0.74 (0.46) | 92 |
| SM82 (right ear, left AI) | 0.80 (0.42) | 132 |
| Pooled | 0.77 (0.44) | 224 |
| Contralateral BW20 | ||
| SM64 (left ear, right AI) | 1.08 (0.64) | 92 |
| SM82 (right ear, left AI) | 1.18 (0.59) | 132 |
| Pooled | 1.14 (0.61) | 224 |
All pairwise comparisons are not significant (all P > 0.20, t-test). Pooled data used as reference values for subsequent comparisons. N, count.
Fig. 5.
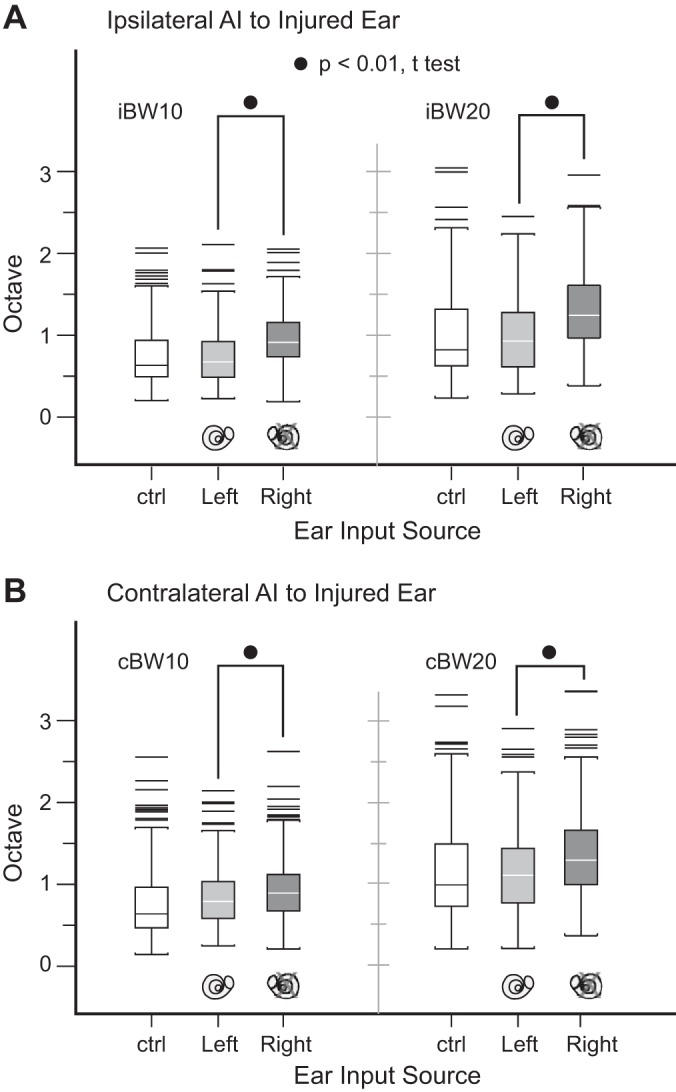
Bandwidth sizes at 10 dB (BW10) and 20 dB (BW20) above threshold in both hemispheres, contrasting injured and normal ears. The injured right ear has broader cortical neuronal bandwidths compared with the normal left ear for all conditions (all P values < 0.01). AI, primary auditory cortex; i, ipsilateral; c, contralateral; ctrl, control monkey data for reference.
Composite bandwidth 10 overlap maps.
Ipsilateral AI and contralateral AI BW10 overlap maps exhibit spatial clustering of sites (Fig. 4, E–H) with nonoverlap (light green polygons) and overlap (gray polygons) of inputs from the two ears; the two hemispheres differ in covariation of nonoverlap sites with CF misalignment (polygons with red borders). Ipsilateral AI shows nonoverlap clusters that are positioned in both CF-misaligned and CF-aligned locations. This spatial pattern is distinct from contralateral AI, where nonoverlap patches are positioned almost exclusively in CF-misaligned locations. The qualitative clustering of spectrally nonoverlapping binaural activation sites in contralateral AI restricted to CF-misaligned locations is a consequence of the smaller magnitude of CF misalignment in this hemisphere (Fig. 3C).
Concurrence of peripheral and cortical threshold asymmetries.
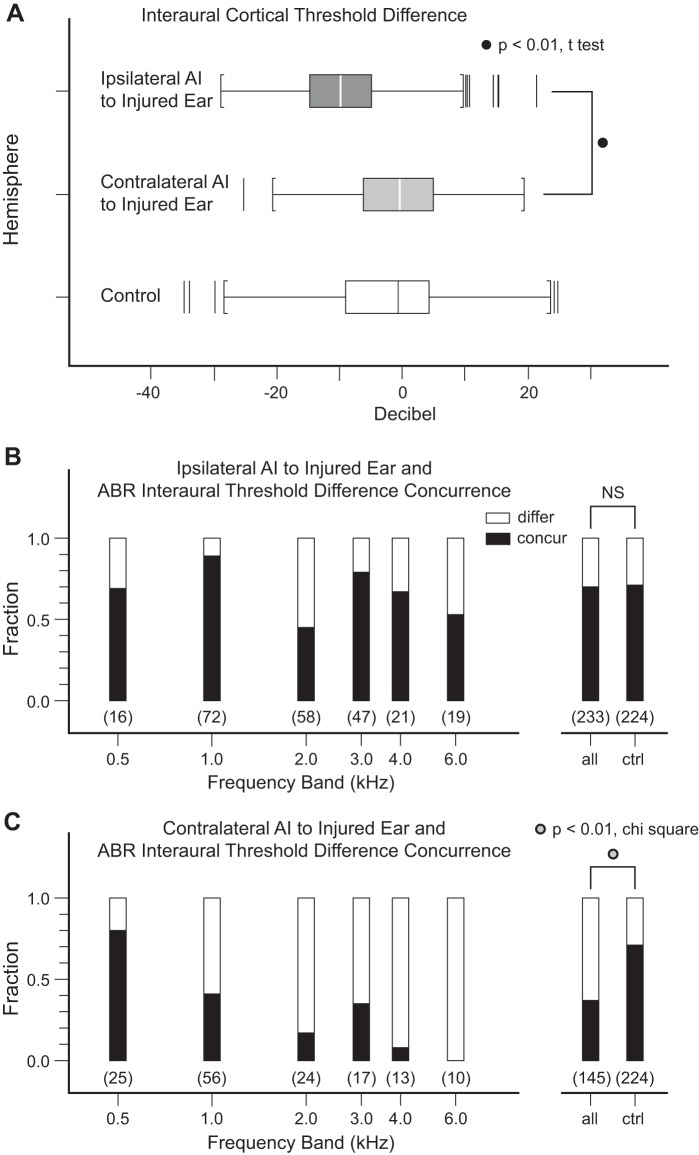
The extent to which interaural cortical neuronal threshold asymmetries reflect peripheral ABR threshold asymmetries is evaluated next. Interaural threshold difference in ipsilateral AI (normal ear minus injured ear) is much greater compared with contralateral AI (injured ear minus normal ear) [Fig. 6A; mean (SD) dB, −9.4 (9.0) vs. −0.7 (8.5), P < 0.01, t-test]. By contrast, thresholds in contralateral AI and control AI data are indistinguishable [−0.7 (8.5) vs. −1.9 (10.6), P > 0.20, t-test]. Ipsilateral AI interaural threshold asymmetry faithfully reflects peripheral asymmetric hearing loss induced in the right ear, but contralateral AI does not.
Fig. 6.
Interaural cortical threshold differences and concurrence rates of peripheral and cortical threshold difference profiles. A: ipsilateral AI threshold differences are larger compared with contralateral AI (P < 0.01, t-test). Threshold difference distribution in control monkeys is displayed for reference (bottom). B and C: ipsilateral AI interaural threshold difference profile reflects peripheral asymmetric hearing loss (~70%), a concurrence rate similar to control data. In contrast, contralateral AI and peripheral interaural threshold difference profiles have a much lower concurrence rate (P < 0.01, χ2-test). Counts are in parentheses. ctrl, Control; NS, not significant.
Peripheral-to-cortical interaural threshold asymmetry concurrence profiles are analyzed in finer detail by segmenting data into six frequency bands from 0.5 kHz and 6.0 kHz in both hemispheres. Segmented frequency band data are in general agreement with consolidated data (Fig. 6, B and C). The control concurrence rate of 70% in normal monkeys is derived from data pooled across both hemispheres. Ipsilateral AI and control concurrence rates (69% vs. 70%, P > 0.20, χ2-test) are nearly the same. However, contralateral AI and control concurrence rates are different (37% vs. 70%, P < 0.01, χ2-test). Whereas cortical interaural threshold asymmetries reflect peripheral ABR threshold asymmetries in ipsilateral AI to a level expected of normal monkeys, there is no such mirror-image counterpart in contralateral AI.
Interaural latency differences.
A temporal feature of isomorphic response maps is earlier spike arrival times from the dominant, contralateral ear. For a particular AI hemisphere, the minimum latency of first spike arrival times centered about CF in normal-hearing control monkeys is different for the two ears. Contralateral ear latency is shorter (Fig. 7) by ~2 ms for either hemisphere. In the case of asymmetric mild-to-moderate SNHL, the injured right ears of experimental monkeys have higher peripheral thresholds between 1 kHz and 4 kHz (Fig. 1, A–D), which may impact minimum latency. The cortical minimum latency consequences of asymmetric peripheral inputs are analyzed for both hemispheres.
Fig. 7.
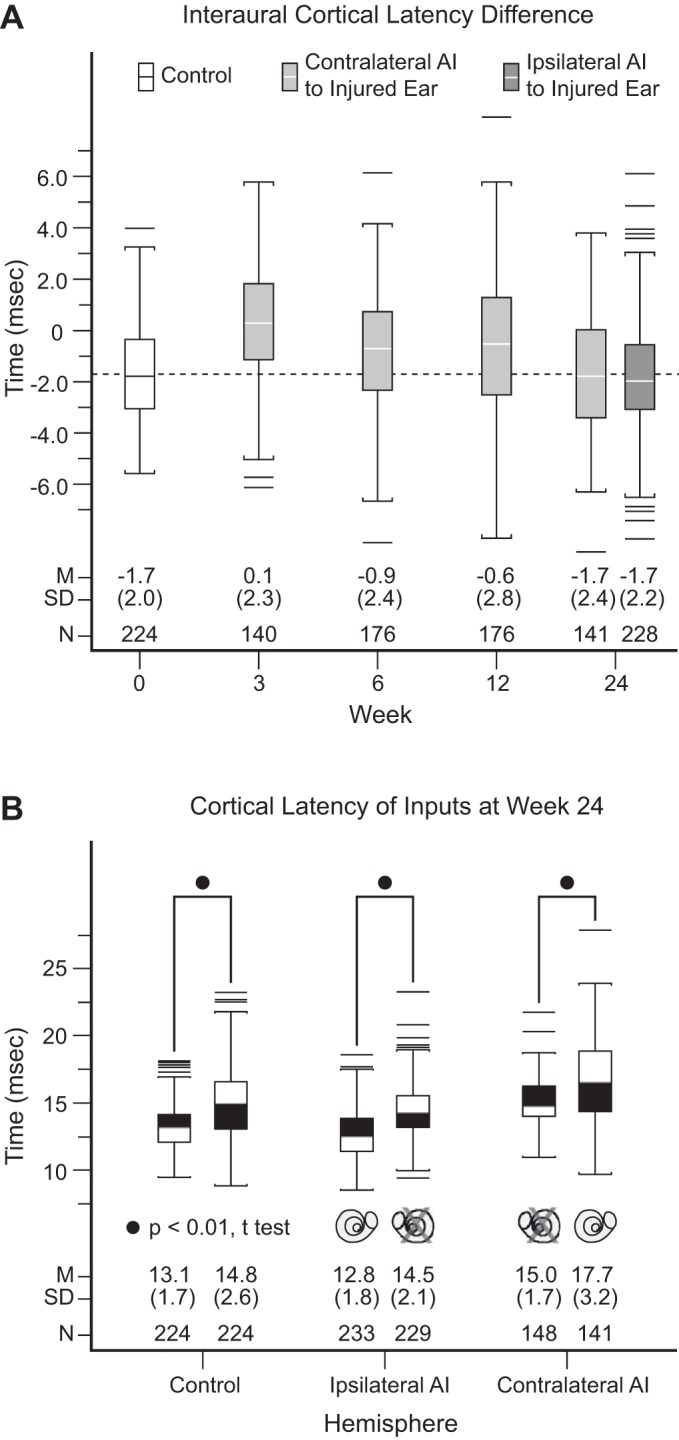
Cortical latency profiles in both hemispheres. A: interaural cortical latency difference. Both ipsilateral AI and contralateral AI at week 24 after acoustic overstimulation in experimental monkeys and normal monkeys have indistinguishable interaural latency difference (contralateral minus ipsilateral) values, all at −1.7 ms. In contralateral AI, there is temporal evolution of interaural latency difference values, which progress from 0.1 ms at week 3 to −0.9 ms at week 6 to −0.6 ms at week 12. Dashed line highlights latency difference value at −1.7 ms. B: cortical latency of inputs at week 24. In both control (left) and experimental (center and right) monkeys, contralateral ear input latencies are shorter than ipsilateral latencies (all P values < 0.01, t-test). Using control data as the comparator, pairwise comparisons of ear input latencies are indistinguishable (all P values > 0.05) from ipsilateral AI but significantly different from contralateral AI (all P values < 0.01, t-test). Normal ear input latencies to contralateral AI are unexpectedly elevated at week 24 to reestablish the normal interaural latency relationship of −1.7 ms. M, mean; SD, standard deviation; N, count.
After hearing loss induction and through weeks 6 and 12, contralateral AI in experimental monkeys is relatively weakly driven by the injured ear, a condition imposed by higher peripheral and cortical thresholds. However, by week 24 the two ears activate contralateral AI at indistinguishable cortical thresholds. Late elevation of normal ear cortical thresholds between weeks 12 and 24 closes the interaural threshold difference gap (Figs. 6 and 7 in Cheung et al. 2009). Interaural CF realignment occurs in parallel with closure of interaural cortical threshold differences. That is, initial contralateral AI interaural CF misalignment at weeks 6 and 12 evolves into CF realignment by week 24 (Fig. 4 in Cheung et al. 2009). With this background, related interaural cortical latency differences at weeks 3, 6, 12, and 24 in contralateral AI and at week 24 in ipsilateral AI are assessed.
In control monkeys, the interaural mean (SD) cortical latency difference (contralateral ear minus ipsilateral ear) is −1.7 (2.0) ms (Fig. 7A). Contralateral AI latency differences in experimental monkeys shortly after SNHL are very small [0.1 (2.3) ms]. This is compatible with the notion of delayed input from the injured ear for this hemisphere. Latency of the dominant, but now injured ear nearly matches the naturally longer latency from the nondominant, normal ear. Latency differences then evolve from the highly abnormal value of 0.1 (2.3) ms at week 3 to the normal value of −1.7 (2.4) ms at week 24 (see Fig. 6A for difference values at intermediate time points). There is a significant effect of time after SNHL induction on contralateral AI interaural latency difference for the four time points [F(3,629) = 13.3, P < 0.01, 1-way ANOVA]. This reestablishment of a normal latency difference for the hemisphere contralateral to the injured ear appears to parallel CF and threshold differences between the two inputs of this hemisphere. While the dominant input latency at week 24 (Fig. 7B) to this hemisphere is longer compared with control monkeys [15.0 (1.7) vs. 13.1 (1.7), P < 0.01, t-test], the nondominant input latency is commensurately longer [17.7 (3.2) vs. 14.8 (2.6), P < 0.01, t-test] to maintain a normal interaural latency relationship where the dominant ear leads the nondominant ear.
Ipsilateral AI latency difference is also at the normal value of −1.7 (2.2) at week 24. This is not surprising since the dominant, short-latency input to this hemisphere is from the normal, left ear, which has a latency that is indistinguishable from control monkeys [12.8 (1.8) vs. 13.1 (1.7), P = 0.07, t-test]. The latency of the nondominant input at week 24 (Fig. 7B) is also indistinguishable from control monkeys [14.8 (2.6) vs. 14.5 (2.1), P = 0.18, t-test]. Normal interaural latency differences at week 24 for both hemispheres is made possible by cortical timing readjustment that results in abnormally long latency for the nondominant, normal ear in contralateral AI.
It appears that restoration of a normal interaural latency relationship between the two ears is an important feature that may drive the observed frequency map realignment in contralateral AI. The latency relationship between normal and injured ears in ipsilateral AI is similar for monkeys with and without asymmetric SNHL, without perturbation to contralateral ear dominance. Six months after acoustic injury, isomorphic interaural latency difference is conserved in mild-to-moderate asymmetric SNHL in both hemispheres.
Model of anisomorphism emergence.
A network model is constructed to gain insight into how anisomorphism may emerge in asymmetric mild-to-moderate SNHL. Specifically, we simulate the effects of STDP of excitatory inputs in AI (Froemke 2015), examining response strength and timing after shorter and longer periods of deafening to ask whether this minimal model could capture the major experimental results in Figs. 4 and 7. As the input arrival timing differences between the ipsilateral and contralateral ears are on the order of milliseconds, STDP is a reasonable candidate plasticity mechanism in these simulations.
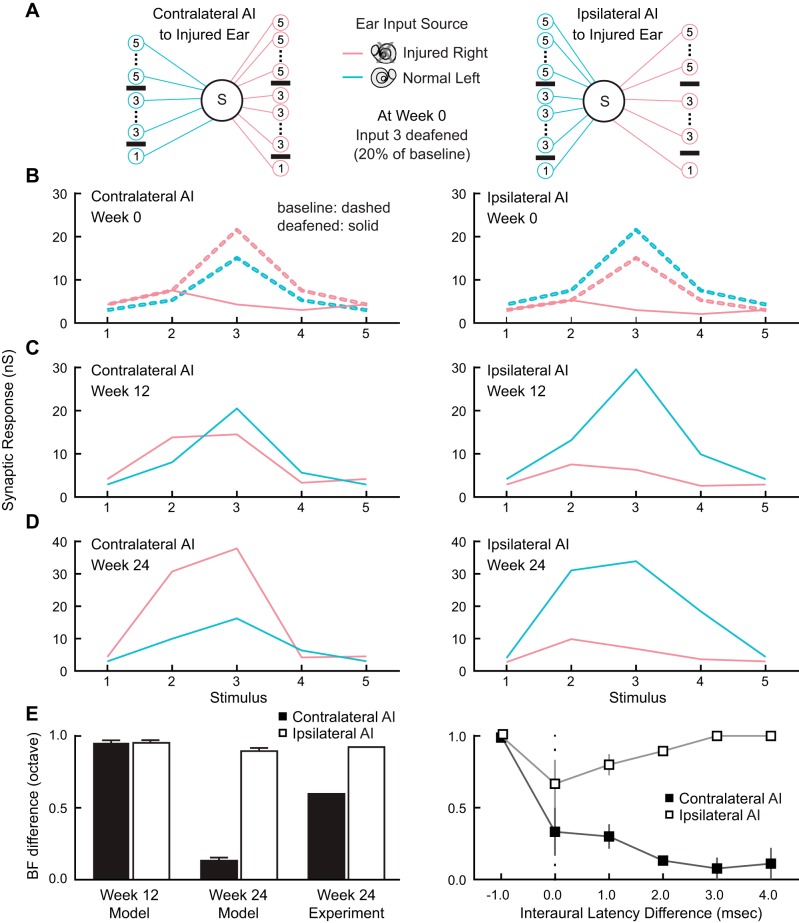
The network model consists of single postsynaptic neurons in both contralateral AI and ipsilateral AI, each receiving five channels of contralateral ear input and five channels of ipsilateral ear input, representing different sound frequencies (Fig. 8A). The ipsilateral ear population is 30% smaller than the number of contralateral ear inputs (60 presynaptic contralateral neurons vs. 42 presynaptic ipsilateral neurons, for each of the 5 frequency channels), reflecting dominance of the contralateral ear (Reale and Kettner 1986). Each presynaptic neuron is stimulated with a rectified pink noise process, set to ensure baseline postsynaptic responses of 10–50 Hz depending on the frequency. Synaptic weights of the third (central) input channel are set at approximately four times the magnitude of inputs from channels 1 and 5, and about twice as large as inputs from channels 2 and 4, to initialize baseline frequency tuning of postsynaptic neurons. Inputs from the ipsilateral, nondominant ear are delayed relative to the contralateral, with binaural delays chosen as equally likely values from a Gaussian distribution of mean µ = 2 ms and variance σ2 = 2 ms, consistent with experimental data from normal-hearing and week 24 monkeys (Fig. 8, A and B). Simulated auditory input is then shifted by this delay value, with positive delays indicating that the contralateral population is activated first. At baseline conditions, there is no plasticity in the model, reflecting general stability of adult AI receptive fields under passive stimulation conditions (Dorrn et al. 2010). We specifically query whether change in relative latency between injured and normal ear inputs observed experimentally (Fig. 8B) can account for the differential changes in tuning curves observed in contralateral AI and ipsilateral AI over 12 and 24 wk.
Fig. 8.
Simulation by spike timing-dependent plasticity (STDP) of excitatory inputs in mild-to-moderate asymmetric sensorineural hearing loss illustrates anisomorphism emergence. A: model network architecture. Contralateral AI and ipsilateral AI (left ear, blue; right ear, red) are modeled separately, as a single postsynaptic neuron receiving a set of 5 input channels representing different sound frequencies from the 2 ears. For all channels there are 30% fewer inputs from the ipsilateral ear. We simulate frequency tuning curve profiles for both hemispheres over 4 phases: baseline before hearing loss induction, week 0 immediately upon deafening (inputs from channels 3 and 4 selectively weakened and delays between ipsilateral and contralateral inputs set to 0 ms), week 12 after deafening (with STDP), and week 24 after hearing loss onset (with STDP, after ipsilateral delays reset to values ranging between −1 and 5 ms). B: baseline (dashed lines) and week 0 tuning curves. At baseline, ipsilateral ear responses are weaker but tuning is aligned to contralateral ear responses [best frequency (BF) difference = 0]. Deafening is simulated by setting right ear (red) responses of channel 3 to 20% of original value and channel 4 to 40% of original value. C: to simulate the experimental outcomes at week 12, inputs are modified according to a conventional STDP learning rule, leading to partial recovery of right ear (red) responses in contralateral AI while left ear (blue) responses in ipsilateral AI are enhanced. One example run of the model is shown. D: to simulate experimental outcomes after 24 wk, ipsilateral ear input timing is altered relative to contralateral arrival times. One example run of the model is shown (same run as in C), where the ipsilateral ear delay is 2 ms relative to contralateral input. In contralateral AI, frequency tuning is realigned for the 2 ears, whereas frequency tuning curves remain misaligned in ipsilateral AI. E, left: BF difference in contralateral AI and ipsilateral AI. Model results at week 24 capture the difference in tuning observed experimentally [see Fig. 3C; mean CF difference (octave): contralateral AI = 0.60, ipsilateral AI = 0.98]. Right: different runs of the model varying interaural latency difference over the range −1 to 5 ms at week 24. This factor is the key determinant of changes to BF difference.
Our model has four phases: 1) hearing is normal and symmetric at baseline (Fig. 8B), before asymmetric hearing loss induction in the right ear; 2) deafening simulation of the right ear at “week 0” (Fig. 8B); 3) adjusting the input delays to be equivalent (mean relative delay of 0 ms between ipsilateral and contralateral inputs) to examine consequences of excitatory STDP at “week 12” (Fig. 8C); and 4) exploring how tuning curves recover when the delay between contralateral and ipsilateral inputs returns to being normally delayed at “week 24” (Fig. 8D), with contralateral inputs preceding again relative to ipsilateral inputs.
In asymmetric SNHL, contralateral AI best frequency inputs are modified because the contralateral input initially has relatively longer latency and decreased activity strength. Inputs from the two ears arrive at similar times, triggering STDP mechanisms that balance both synaptic LTP and LTD (Caporale and Dan 2008). Latency differences distributed around 0 ms between contralateral and ipsilateral depolarization can strengthen as well as weaken synaptic strengths of the inputs, preventing immediate realignment of frequency tuning in the two ears at week 12 (Fig. 8C). However, at week 24, frequency maps realign with overlap of receptive fields derived from the two ears (Fig. 8D). Hemispheric functional organization differences emerge and a new state of cortical anisomorphism settles in place at week 24 (Fig. 8E, left). This is caused by reestablishment of earlier arrival of dominant, contralateral inputs that shifts the relative spike timing, leading to STDP that biases ipsilateral synapses toward selectivity of contralateral tuning. On the other hand, ipsilateral AI receives dominant inputs that remain earlier than nondominant inputs from the injured ear. The level of plasticity in this hemisphere is low, and cortical representation of the inputs from the two ears continues to be misaligned. The simulation supports the notion that return to a normal interaural latency relationship between the two ears is predominantly responsible for interaural best frequency realignment occurring in contralateral AI but not ipsilateral AI (Fig. 8E, right).
DISCUSSION
Mild-to-moderate asymmetric SNHL in adult monkeys triggers anisomorphic cortical reorganization, manifested by hemispheric differences in predominant direction and magnitude of CF misalignment, occurrence rate of spectrally nonoverlapping binaural activation for narrow-band sounds in both CF-aligned and CF-misaligned sites, and concurrence rate of peripheral and central threshold asymmetry profiles. While those interhemispheric mirror-image relationships have been disrupted, there is conservation of relatively shorter minimum latency for contralateral ear input.
We refer to changes in ipsilateral cortex following mild-to-moderate asymmetric SNHL as “cortical reorganization.” This term primarily refers to the observed changes in organization of binaural convergence of frequency tuning. It does not imply that the organizational changes are a result of a gradual change, as observed in contralateral AI (Cheung et al. 2009). When ipsilateral AI and contralateral AI patterns of reorganization are compared, the state of ipsilateral AI reorganization at week 24 corresponds closely to contralateral AI at week 6, suggesting that ipsilateral AI reorganization arrests after initial changes. However, we cannot exclude intermediate or longer-term duration changes to ipsilateral AI organization without further studies.
Published accounts of mammalian asymmetric hearing loss have focused on developmental aspects. Those studies indicate that binaural processing may be particularly labile in development, following early induction of asymmetric hearing loss (Keating and King 2013; Popescu and Polley 2010). Best frequency and threshold differences between inputs from the two ears have been observed 2 wk after imposition of brief, reversible conductive hearing loss in one ear during the critical period (Polley et al. 2013). However, longer-term adjustments in the adult animal were not studied. Monaural deprivation during the critical period of rats enhances responsiveness to inputs from the normal, ipsilateral ear in the hemisphere contralateral to the reduced input (Popescu and Polley 2010). Similar changes attributed to activity-dependent sensory end-organ dominance have been observed in the developing visual and somatosensory systems (Fox 1992; Mioche and Singer 1989). Our results reveal that organizational changes between the two hemispheres in adult animals differ from this notion of ear input dominance adjustment. Dominance of the injured ear for input to the contralateral hemisphere is not overtly changed in mild-to-moderate hearing loss because the ipsilateral input readjusts itself to match the weaker, spectrally shifted contralateral input (Cheung et al. 2009). Dominance of the normal ear to the contralateral hemisphere (ipsilateral AI to the injured ear) is maintained and perhaps strengthened because of weaker input from the injured ear.
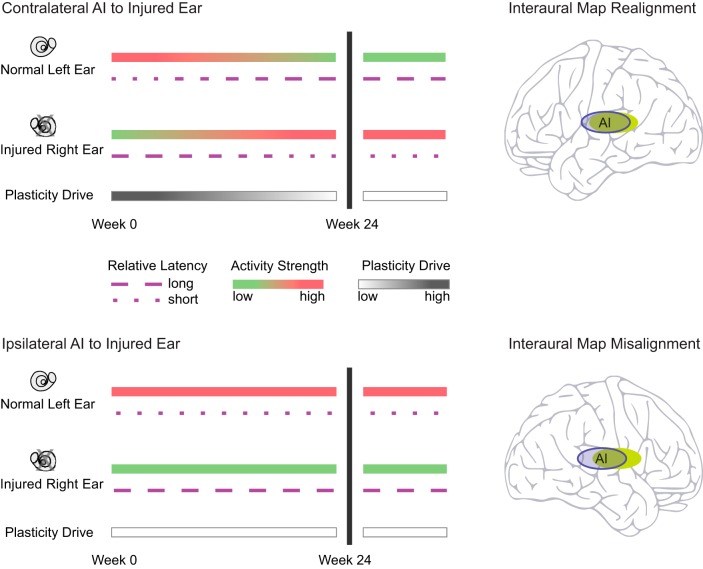
Factors that drive postcritical period plasticity in the two hemispheres are illustrated in Fig. 9. In contralateral AI, the dominant injured right ear has higher peripheral thresholds after acoustic overstimulation, resulting in relatively low activity strength. However, spike arrival times from the injured ear are no longer earlier than the normal, ipsilateral input and the expected interaural latency relationship has been broken. With this disruption to interaural timing, plasticity drive becomes high and contralateral AI reorganizes representation of inputs from both ears. There is realignment of interaural frequency maps and reduction of interaural threshold differences. By effectively deafening the normal ear cortically, high activity strength from the injured ear and synergistic, spectrally matched binaural processing are reestablished. By week 24, the normal interaural latency difference is restored and plasticity drive becomes low.
Fig. 9.
Summary of factors that drive cortical reorganization in mild-to-moderate asymmetric hearing loss. Top: contralateral AI is driven to plastic change, despite relatively low activity strength of the injured right ear secondary to higher thresholds. The key factor driving cortical reorganization is abnormal interaural latency. By week 24, interaural frequency maps come into realignment, interaural latency difference is restored to the normal value, and plasticity drive becomes low. Bottom: ipsilateral AI is in a low plasticity drive state as the normal left ear has relatively lower thresholds after acoustic injury to the right ear and normal interaural latency is not perturbed. By week 24, interaural frequency maps persist in misalignment. Anisomorphic cortical reorganization in mild-to-moderate asymmetric SNHL has stabilized.
In ipsilateral AI, the dominant normal left ear has relatively lower peripheral thresholds after acoustic overstimulation of the right ear and continues to have high activity strength. As normal interaural timing relationship is unaltered, plasticity drive remains low. Interaural CF misalignment persists, where the difference magnitude in ipsilateral AI at week 24 (mean = 0.98 octaves) is comparable to contralateral AI at week 6 (mean = 0.96 octaves, see Fig. 7 in Cheung et al. 2009). Furthermore, ipsilateral AI nonoverlap activation rate for narrow-band sounds is higher compared with contralateral AI. Assuming that a recovery interval of 24 wk is suitably long to observe emergence of plastic change in ipsilateral AI, anisomorphic cortical reorganization in mild-to-moderate asymmetric SNHL has stabilized.
Cortical anisomorphism may result from timing-based local autonomous hemispheric reorganization governed by STDP, where input from bilateral ascending pathways from both cochleae shape the process. Corticocortical connectivity between the two hemispheres is mediated in part by the commissural system. Interhemispheric callosal connections are highly topographic and modular in AI, with considerable connectional reciprocity (Code and Winer 1986). Nonetheless, the role of interhemispheric connectivity in maintenance of registration between cortical response maps remains obscure.
We focused on STDP as a synaptic mechanism for cortical plasticity, given that the timing of ipsilateral and contralateral input arrival to AI is on the timescale of milliseconds. Rather subtle changes in spike timing may have profound consequences for induction of LTP or LTD under STDP-like learning rules (Froemke 2015). There is considerable evidence for STDP in primary sensory cortex (Caporale and Dan 2008; Celikel et al. 2004; Froemke 2015; Markram et al. 2012; Young et al. 2007), including ferret AI in vivo (Dahmen et al. 2008) and mouse auditory cortex in brain slices (D’amour and Froemke 2015). Of course, there are many forms of long-term synaptic plasticity that have been identified in cortical circuits, and precisely paired pre- and postsynaptic action potentials are not necessarily required for induction of LTP or LTD (Froemke 2015; Lisman and Spruston 2010). Instead, our model is proof of concept that local plasticity induced by changes in ipsilateral and contralateral activity arrival times could in principle account for the physiological changes documented here. More data are required, especially about the temporal dynamics of ipsilateral changes, to refine the model and to derive and assess potential predictions about postinsult plasticity.
Physiological studies have shown diverse effects of callosal input on contralateral cortical neurons, ranging from mostly excitatory contributions (Anton-Erxleben and Carrasco 2013; Mitani and Shimokouchi 1985) to more mixed facilitatory and suppressive effects (Kitzes and Doherty 1994; Rock and Apicella 2015). However, those observations were topographically unspecific with regard to internal organizational aspects, such as tonotopic organization.
Interhemispheric auditory processing can be interrupted by corpus callosum division, a historical treatment modality for intractable seizures. Patients who underwent this intervention showed deficits in dichotic consonant-vowel (Springer and Gazzaniga 1975) and digits (Milner et al. 1968; Musiek and Wilson 1979; Sparks et al. 1970) testing. In an adult who was treated by callosotomy, there was a distinct asymmetry for monaural stimulation but not for binaural inputs (Paiement et al. 2011). Human fMRI studies have shown differences in the two hemispheres with respect to processing of binaural vs. monaural inputs, with the left hemisphere favoring contralateral ear input and the right hemisphere responding in a more balanced fashion to inputs from the two ears (Magezi and Krumbholz 2010; Stecker et al. 2015).
Interhemispheric cooperation in the visual system contributes to performance of higher-level tasks (Santhouse et al. 2002). Decline in cognitive ability with aging, Alzheimer disease, schizophrenia, and dyslexia are often accompanied by structural changes of the callosal system (Endrass et al. 2002; Hynd et al. 1995; Narr et al. 2000; Peters and Sethares 2002). This suggests that appropriate functional hemispheric alignment via callosal connectivity may contribute to maintaining full perceptual and cognitive capacity (Hackett and Phillips 2011). Whether and when those interhemispheric connections come into play after asymmetric SNHL remains unclear.
Clinical implications of cortical anisomorphism.
In clinical studies of patients with asymmetric SNHL, the most profound impairments are extraction of signal from noise and spatial hearing (Douglas et al. 2007; Noble and Gatehouse 2004). Auditory rehabilitation by amplification can improve hearing performance, along with change in brain stem electrophysiological responses (Munro et al. 2007) and functional organization of auditory cortex (Thai-Van et al. 2010). Interestingly, monaural amplification in symmetric SNHL results in more than expected divergence between aided and unaided ears (Silman et al. 1993), and withholding amplification to the poorer ear in asymmetric SNHL is associated with its accelerated decline in word recognition scores (Silverman et al. 2006). While those studies and others suggest that hearing aid rehabilitation can improve function and mitigate further degradation, the neurophysiological underpinnings of rehabilitation-based cortical plastic change are unknown. Based on this referential study, evaluating lateralized hemispheric function and corticocortical processing may uncover neural correlates of performance gains from sound amplification therapy for asymmetric SNHL.
Conclusions.
In mild-to-moderate asymmetric hearing loss, the cortical hemisphere contralateral but not ipsilateral to the injured ear undergoes realignment of interaural frequency maps. This anisomorphic cortical reorganization stabilizes with reestablishment of earlier input arrival times from the injured ear to the contralateral hemisphere by week 24 after injury. Realignment of frequency maps in contralateral AI enables input convergence from the two ears for binaural processing at the expense of rendering the normal ear partially deaf through elevated cortical thresholds. Permanent misalignment of frequency maps in ipsilateral AI preserves sensitivity to soft sounds for efficient signal detection at the expense of binaural processing. The emergence of these functional trade-offs can be modeled as consequences of STDP. Anisomorphic cortical reorganization resulting in relative local hemispheric autonomy may be a neurophysiological basis of impaired performance of auditory tasks that require interhemispheric processing.
GRANTS
This work was supported by Department of Veterans Affairs Medical Research Merit Review (to S. W. Cheung), the National Institutes of Health (DC-02260 and MH-77970 to C. E. Schreiner and DC-012557 to R. C. Froemke), the Coleman Memorial Fund, Hearing Research, Incorporated, and the Steven Bauer Research Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.W.C. conceived and designed research; S.W.C., C.A.A., and E.R.L. performed experiments; S.W.C., E.R.L., and R.C.F. analyzed data; S.W.C., C.A.A., E.R.L., R.C.F., and C.E.S. interpreted results of experiments; S.W.C. prepared figures; S.W.C. drafted manuscript; S.W.C., R.C.F., and C.E.S. edited and revised manuscript; S.W.C., C.A.A., E.R.L., R.C.F., and C.E.S. approved final version of manuscript.
REFERENCES
- Anton-Erxleben K, Carrasco M. Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence. Nat Rev Neurosci 14: 188–200, 2013. doi: 10.1038/nrn3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 31: 25–46, 2008. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci 7: 534–541, 2004. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SW. Frequency map variations in squirrel monkey primary auditory cortex. Laryngoscope 115: 1136–1144, 2005. doi: 10.1097/01.MLG.0000165369.65046.CD. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bedenbaugh PH, Nagarajan SS, Schreiner CE. Functional organization of squirrel monkey primary auditory cortex: responses to pure tones. J Neurophysiol 85: 1732–1749, 2001. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bonham BH, Schreiner CE, Godey B, Copenhaver DA. Realignment of interaural cortical maps in asymmetric hearing loss. J Neurosci 29: 7065–7078, 2009. doi: 10.1523/JNEUROSCI.6072-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code RA, Winer JA. Columnar organization and reciprocity of commissural connections in cat primary auditory cortex (AI). Hear Res 23: 205–222, 1986. doi: 10.1016/0378-5955(86)90110-3. [DOI] [PubMed] [Google Scholar]
- D’amour JA, Froemke RC. Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron 86: 514–528, 2015. doi: 10.1016/j.neuron.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci 28: 13629–13639, 2008. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature 465: 932–936, 2010. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Yeung P, Daudia A, Gatehouse S, O’Donoghue GM. Spatial hearing disability after acoustic neuroma removal. Laryngoscope 117: 1648–1651, 2007. doi: 10.1097/MLG.0b013e3180caa162. [DOI] [PubMed] [Google Scholar]
- Endrass T, Mohr B, Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neurosci Lett 320: 57–60, 2002. doi: 10.1016/S0304-3940(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci 12: 1826–1838, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC. Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci 38: 195–219, 2015. doi: 10.1146/annurev-neuro-071714-034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Phillips DP. The commissural auditory system. In: The Auditory Cortex, edited by Winer JA, Schreiner CE. New York: Springer, 2011, p. 117–132. doi: 10.1007/978-1-4419-0074-6_5 [DOI] [Google Scholar]
- Hynd GW, Hall J, Novey ES, Eliopulos D, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M. Dyslexia and corpus callosum morphology. Arch Neurol 52: 32–38, 1995. doi: 10.1001/archneur.1995.00540250036010. [DOI] [PubMed] [Google Scholar]
- Irvine DR, Fallon JB, Kamke MR. Plasticity in the adult central auditory system. Acoust Aust 34: 13–17, 2006. [PMC free article] [PubMed] [Google Scholar]
- Keating P, King AJ. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci 7: 123, 2013. doi: 10.3389/fnsys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzes LM, Doherty D. Influence of callosal activity on units in the auditory cortex of ferret (Mustela putorius). J Neurophysiol 71: 1740–1751, 1994. [DOI] [PubMed] [Google Scholar]
- Lisman J, Spruston N. Questions about STDP as a general model of synaptic plasticity. Front Synaptic Neurosci 2: 140, 2010. doi: 10.3389/fnsyn.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magezi DA, Krumbholz K. Evidence for opponent-channel coding of interaural time differences in human auditory cortex. J Neurophysiol 104: 1997–2007, 2010. doi: 10.1152/jn.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gerstner W, Sjöström PJ. Spike-timing-dependent plasticity: a comprehensive overview. Front Synaptic Neurosci 4: 2, 2012. doi: 10.3389/fnsyn.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Taylor L, Sperry RW. Lateralized suppression of dichotically presented digits after commissural section in man. Science 161: 184–186, 1968. doi: 10.1126/science.161.3837.184. [DOI] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol 62: 185–197, 1989. [DOI] [PubMed] [Google Scholar]
- Mitani A, Shimokouchi M. Neuronal connections in the primary auditory cortex: an electrophysiological study in the cat. J Comp Neurol 235: 417–429, 1985. doi: 10.1002/cne.902350402. [DOI] [PubMed] [Google Scholar]
- Munro KJ, Walker AJ, Purdy SC. Evidence for adaptive plasticity in elderly monaural hearing aid users. Neuroreport 18: 1237–1240, 2007. doi: 10.1097/WNR.0b013e32822025f4. [DOI] [PubMed] [Google Scholar]
- Musiek FE, Wilson DH. SSW and dichotic digit results pre- and post-commissurotomy: a case report. J Speech Hear Disord 44: 528–533, 1979. doi: 10.1044/jshd.4404.528. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Cannestra AF, Toga AW. Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex 10: 40–49, 2000. doi: 10.1093/cercor/10.1.40. [DOI] [PubMed] [Google Scholar]
- Noble W, Gatehouse S. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int J Audiol 43: 100–114, 2004. doi: 10.1080/14992020400050015. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol 90: 2387–2401, 2003. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Paiement P, Champoux F, Lassonde M, Mensour B, Leroux JM, Bacon BA, Lepore F. Functional reorganization of the auditory pathways following late callosotomy. Neurocase 17: 440–446, 2011. doi: 10.1080/13554794.2010.532142. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442: 277–291, 2002. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Philibert B, Beitel RE, Nagarajan SS, Bonham BH, Schreiner CE, Cheung SW. Functional organization and hemispheric comparison of primary auditory cortex in the common marmoset (Callithrix jacchus). J Comp Neurol 487: 391–406, 2005. doi: 10.1002/cne.20581. [DOI] [PubMed] [Google Scholar]
- Polley DB, Thompson JH, Guo W. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat Commun 4: 2547, 2013. doi: 10.1038/ncomms3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron 65: 718–731, 2010. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Irvine DR, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol 338: 17–49, 1993. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Reale RA, Kettner RE. Topography of binaural organization in primary auditory cortex of the cat: effects of changing interaural intensity. J Neurophysiol 56: 663–682, 1986. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol 415: 460–481, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol 282: 456–471, 1989. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Rock C, Apicella AJ. Callosal projections drive neuronal-specific responses in the mouse auditory cortex. J Neurosci 35: 6703–6713, 2015. doi: 10.1523/JNEUROSCI.5049-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhouse AM, Ffytche DH, Howard RJ, Williams SC, Stewart AL, Rooney M, Wyatt JS, Rifkin L, Murray RM. The functional significance of perinatal corpus callosum damage: an fMRI study in young adults. Brain 125: 1782–1792, 2002. doi: 10.1093/brain/awf174. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Mendelson JR. Functional topography of cat primary auditory cortex: distribution of integrated excitation. J Neurophysiol 64: 1442–1459, 1990. [DOI] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J Otol 14: 252–258, 1993. [PubMed] [Google Scholar]
- Silman S, Silverman CA, Emmer MB, Gelfand SA. Effects of prolonged lack of amplification on speech-recognition performance: preliminary findings. J Rehabil Res Dev 30: 326–332, 1993. [PubMed] [Google Scholar]
- Silverman CA, Silman S, Emmer MB, Schoepflin JR, Lutolf JJ. Auditory deprivation in adults with asymmetric, sensorineural hearing impairment. J Am Acad Audiol 17: 747–762, 2006. doi: 10.3766/jaaa.17.10.6. [DOI] [PubMed] [Google Scholar]
- Sokolich WG, inventor and assignee. Closed sound delivery system. US Patent 4251686A, February 17, 1981.
- Sparks R, Goodglass H, Nickel B. Ipsilateral versus contralateral extinction in dichotic listening resulting from hemisphere lesions. Cortex 6: 249–260, 1970. doi: 10.1016/S0010-9452(70)80014-4. [DOI] [PubMed] [Google Scholar]
- Springer SP, Gazzaniga MS. Dichotic testing of partial and complete split brain subjects. Neuropsychologia 13: 341–346, 1975. doi: 10.1016/0028-3932(75)90011-1. [DOI] [PubMed] [Google Scholar]
- Stecker GC, McLaughlin SA, Higgins NC. Monaural and binaural contributions to interaural-level-difference sensitivity in human auditory cortex. Neuroimage 120: 456–466, 2015. doi: 10.1016/j.neuroimage.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai-Van H, Veuillet E, Norena A, Guiraud J, Collet L. Plasticity of tonotopic maps in humans: influence of hearing loss, hearing aids and cochlear implants. Acta Otolaryngol 130: 333–337, 2010. doi: 10.3109/00016480903258024. [DOI] [PubMed] [Google Scholar]
- Tsai BS, Sweetow RW, Cheung SW. Audiometric asymmetry and tinnitus laterality. Laryngoscope 122: 1148–1153, 2012. doi: 10.1002/lary.23242. [DOI] [PubMed] [Google Scholar]
- Vannson N, James C, Fraysse B, Strelnikov K, Barone P, Deguine O, Marx M. Quality of life and auditory performance in adults with asymmetric hearing loss. Audiol Neurootol 20, Suppl 1: 38–43, 2015. doi: 10.1159/000380746. [DOI] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334: 1569–1573, 2011. doi: 10.1126/science.1211095. [DOI] [PubMed] [Google Scholar]
- Young JM, Waleszczyk WJ, Wang C, Calford MB, Dreher B, Obermayer K. Cortical reorganization consistent with spike timing-but not correlation-dependent plasticity. Nat Neurosci 10: 887–895, 2007. doi: 10.1038/nn1913. [DOI] [PubMed] [Google Scholar]