Abstract
The wiring of synaptic connections in the developing mammalian brain is shaped by both intrinsic and extrinsic signals. One point where these regulatory pathways converge is via the sensory experience-dependent regulation of new gene transcription. Recent studies have elucidated a number of molecular mechanisms that allow nuclear transcription factors and chromatin regulatory proteins to encode aspects of specificity in experience-dependent synapse development. Here we review the evidence for the transcriptional mechanisms that sculpt activity-dependent aspects of synaptic connectivity during postnatal development and discuss how disruption of these processes is associated with aberrant brain development in autism and intellectual disability.
Keywords: activity-dependent synaptic plasticity, autism, chromatin, synapse development, transcription
the mammalian brain is a paragon of complexity. The average human brain contains tens of billions of neurons and hundreds of trillions of synaptic connections (DeFelipe 2010; Bargmann and Marder 2013). Yet, every day, legions of new brains are assembled from scratch in developing embryos, and for the most part they work as intended. As is true for all developmental programs, the fundamental instructions for wiring the brain are encoded in DNA. Those plans are executed in concert with signals from the environment that guide and refine the developmental process. Importantly, during the prenatal period both the intrinsic and extrinsic regulatory mechanisms that drive brain development are highly robust, such that—barring mutations in key regulatory genes—the total number of neurons, their relative position in the brain, and their primary connections are grossly similar between individual members of a species.
By contrast, in the period after birth a new variable comes into play in the form of sensory experience, which adds dynamic range to the final state of any given connectome. Activation of sensory neurons drives patterned neural activity in the cortex that initiates critical period stages of synapse development and maturation (Katz and Shatz 1996; Wiesel 1982). Neural activity regulates excitatory and inhibitory synapse development in this time window in large part by converging on the regulation of environmentally sensitive transcription factors that coordinate the expression of gene products required for synapse formation, elimination, and plasticity (Majdan and Shatz 2006; West and Greenberg 2011). Depending on the environment into which any given animal is born, the nature, timing, and extent of this sensory experience will differ, and thus the experience-dependent development of synapses will vary between these organisms as well. A likely evolutionary advantage of introducing uncertainty to the late stage of brain development is allowing adaptations in brain function to match an unpredictable outside world. However, the trade-off is the possibility that the process could go very wrong, leading to neurological or psychiatric disease.
A large number of transcription factors that promote both prenatal stages of brain patterning and postnatal stages of synapse development have been identified through gene expression profiling and molecular genetic studies (Chédotal and Richards 2010; Polleux et al. 2007; Ross et al. 2003). The identity and molecular genetic function of these early developmental transcription factors have been extensively reviewed elsewhere and will not be further detailed here. Instead, we focus on three areas of substantial recent growth in our understanding of the role of gene transcription in the experience-dependent development of synapses in the postnatal mammalian brain. In each case, although we profile only a subset of the many transcriptional regulators that contribute to synapse development, we use these stories to demonstrate major principles of the transcriptional mechanisms at play. First, we highlight new insights into the conundrum of synapse specificity, by reviewing studies that elucidate how transcriptional processes occurring in the nucleus of the neuron can promote the development of specific subsets of synapses in the cell periphery. Next, we discuss a series of recent studies showing how chromatin regulatory factors can serve as modulators of the postnatal experience-dependent stages of synapse development. Finally, we review emerging evidence that synapse dysfunction due to dysregulation of these transcriptional regulatory processes can lead to autism and intellectual disability.
Transcription Factors and Synapse Specificity
Transcription factors reside predominantly in the nucleus, where they are well poised to integrate signaling information originating anywhere within the cell. This integrative capacity of stimulus-regulated transcription factors allows them to transduce reception of tiny amounts of growth factors into binary cell survival/cell death decisions (Brunet et al. 2001) and to drive homeostatic plasticity by summating neural activity levels into global synaptic scaling responses (Ibata et al. 2008). However, the central location of transcription factors raises the question of how they can have a meaningful impact on local synaptic connectivity, where the specificity of connections is essential to brain function. Here we consider three examples of transcription factors that exemplify aspects of this specificity. These examples show that although nuclear transcription factors are unlikely to directly instruct the formation or elimination of individual synapses, they do encode specificity at the level of synapse type (e.g., excitatory, inhibitory) and subcellular synapse location (e.g., somatic, dendritic). Furthermore, by interacting functionally with local translational machinery they can have regulatory control over local activity-dependent synaptic plasticity.
CaRF: a context-specific regulator of Bdnf transcription and GABAergic synapse formation.
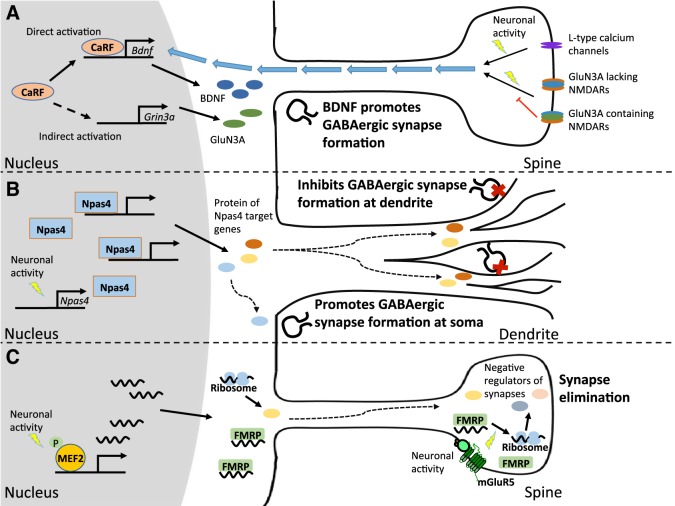
Calcium-response factor (CaRF) was initially discovered as a transcriptional activator of Brain-Derived Neurotrophic Factor (Bdnf), which it directly induces by binding to a 10-bp calcium response element (CaRE1) in the proximal region of Bdnf promoter IV (McDowell et al. 2010; Tao et al. 2002) (Fig. 1A). Expression of exon IV-containing forms of Bdnf is significantly reduced in the cortex of CaRF-knockout mice, demonstrating that CaRF contributes to transcriptional activation of Bdnf promoter IV in vivo (McDowell et al. 2010). Surprisingly, although the CaRE1 element is required for transcriptional induction of Bdnf upon membrane depolarization, and the transcriptional activity of CaRF is induced by the subsequent calcium influx, CaRF is not required for the induction of Bdnf transcription that is driven by this stimulus (McDowell et al. 2010; Tao et al. 2002). Instead, membrane depolarization-mediated activation of Bdnf promoter IV requires the transcription factor MEF2C, which binds CaRE1 independently of CaRF and acts together with CREB to drive the calcium-inducible component of Bdnf transcription (Hong et al. 2008; Lyons et al. 2012).
Fig. 1.
Mechanisms of specificity in the transcriptional regulation of synapses. A: calcium-response factor (CaRF) binds to Bdnf IV promoter and activates basal Bdnf transcription. It also inhibits activity-dependent Bdnf transcription via indirect activation of Grin3a expression, which encodes the NMDA receptor (NMDAR) subunit GluN3A. GluN3A inhibits NMDAR-induced Bdnf transcription without impairing the ability of L-type voltage-gated calcium channels to activate Bdnf transcription. B: Npas4 expression is induced rapidly by neuronal activity. Npas4 target genes promote GABAergic synapse formation at the soma while also inhibiting GABAergic synapse formation at the apical dendritic spines. C: neuronal activity induces MEF2-dependent gene transcription. FMRP traffics mRNAs from the nucleus to the synapses and represses local mRNA translation at the synapses until neuronal activation of mGluR5 receptors leads to target gene mRNA dissociation from FMRP. The ability of constitutively active MEF2-VP16 to drive synapse elimination is blocked in FMRP-knockout neurons, suggesting that these 2 pathways converge to locally regulate synapse pruning.
Given this context, it is intriguing that CaRF has recently been found to inhibit the induction of Bdnf promoter IV following activation of NMDA-type glutamate receptors (Lyons et al. 2016). Membrane depolarization promotes the activation of Bdnf transcription via the opening of L-type voltage-gated calcium channels (Tao et al. 1998). By contrast, treatment of neurons with the sodium channel inhibitor tetrodotoxin (TTX) silences action potentials and induces homeostatic synaptic plasticity such that upon TTX withdrawal there is rebound excitation, release of synaptic glutamate, activation of synaptic glutamate receptors, and the NMDA receptor (NMDAR)-dependent induction of transcription from genes including Bdnf promoter IV (Ghiretti et al. 2014; Turrigiano and Nelson 2004). After TTX withdrawal-induced activation of NMDARs, neurons lacking CaRF show significantly higher induction of exon IV-containing forms of Bdnf mRNA and BDNF protein compared with CaRF-expressing control neurons (Lyons et al. 2016). Unlike the direct activation of Bdnf transcription by CaRF, which requires binding of CaRF to Bdnf promoter IV, the CaRF-dependent inhibition of Bdnf transcription is proposed to occur through an indirect mechanism that involves CaRF-dependent induction of the unusual NMDAR subunit GluN3A. Incorporation of GluN3A into functional NMDARs with GluN1 and GluN2 subunits reduces receptor currents, including the influx of calcium (Das et al. 1998; Kehoe et al. 2013). Levels of GluN3A are reduced in the brains of CaRF-knockout mice, and restoration of GluN3A expression in CaRF-knockdown neurons restores Bdnf inducibility to wild-type levels (Lyons et al., 2016). Thus although CaRF is a direct activator of basal Bdnf transcription via its binding to Bdnf promoter elements, it acts as an indirect inhibitor of NMDAR-inducible Bdnf transcription by its ability to modulate the subunit composition of neuronal NMDARs (Fig. 1A).
The link between CaRF and GluN3A is important because it suggests a potential mechanism by which CaRF could regulate the timing of synapse development. Both CaRF and GluN3A are most highly expressed during the first two postnatal weeks of brain development, after which time their expression declines (McDowell et al. 2010; Sucher et al. 1995). This decline parallels the onset of the critical period for sensory-dependent synapse development in the cortex, and, notably, prolonging the expression of GluN3A inhibits critical period maturation of synapses in visual cortex, suggesting an essential function for the changing NMDAR composition in this process (Roberts et al. 2009). BDNF expression is strongly induced in sensory cortex during the critical period, and it drives critical period closure by promoting the formation of inhibitory GABAergic synapses (Huang et al. 1999). Consistent with a role for CaRF-dependent repression of NMDAR-inducible Bdnf expression in the regulation of GABAergic synapse development in vivo, adult CaRF-knockout mice show substantially increased synaptic expression of GABAergic synapse markers (McDowell et al. 2010). This appears to be a cell-autonomous effect of CaRF, because single-cell knockdown of CaRF in cultured mouse hippocampal neurons significantly enhances the formation of GABAergic synapses onto that neuron in a manner that can be rescued by simultaneous knockdown of BDNF (Lyons et al., 2016). Taken together, these data establish CaRF as a novel regulator of the formation of GABAergic synapses in the developing brain and suggest that, via its regulation of GluN3A, it could contribute to the timing of the synaptic changes that underlie critical period closure.
Npas4: an activity-dependent, cell type-specific master regulator of inhibitory/excitatory balance.
Npas4 belongs to the basic helix-loop-helix-Per-Arnt-Sim (bHLH-PAS) transcription factor family (Ooe et al. 2004), whose members share a bHLH DNA-binding domain, dual PAS domains, and a COOH-terminal activation domain (Partch and Gardner 2010). These transcription regulators dimerize or form complexes with transcriptional coactivators through their bHLH and PAS domains to regulate diverse functions including development, circadian rhythms, and cellular responses to hypoxia and environmental toxins (Gu et al. 2000; Kewley et al. 2004; Partch and Gardner 2010). Among the bHLH-PAS family, Npas4 is unique for its restricted expression in neurons and the fact that its expression is selectively induced in neurons after membrane depolarization-induced calcium influx (Lin et al. 2008). Indeed, even compared with other immediate-early gene transcription factors such as Fos, Npas4 is far more selective for calcium signaling cascades, as its expression is induced only by calcium influx through L-type voltage-gated calcium channels or NMDARs, but it is insensitive to the elevation of intracellular cAMP or the application of growth or neurotrophic factors (Lin et al. 2008). The mechanisms that confer this specificity upon Npas4 induction are not completely understood, although Npas4 seems to be a direct target of the activity-inducible transcription factor SRF (Kim et al. 2010; Kuzniewska et al. 2016).
Npas4 has been shown to play a prominent role in linking synaptic activity to inhibitory/excitatory synapse balance in a cell type- and subcellular-specific manner during brain development. The calcium-dependent induction of Npas4 was first discovered in excitatory glutamatergic neurons, and, interestingly, in these cells Npas4 was found to selectively promote the development of GABAergic synapses (Lin et al. 2008). Reduced numbers of GABAergic synapses were found in cultured hippocampal neurons when Npas4 was knocked down, whereas increased numbers of GABAergic synapses were found when Npas4 was overexpressed. In contrast, these manipulations did not affect glutamatergic synapses, indicating a selective role for Npas4 in inhibitory synapse development in these neurons. In addition to regulating the total number of inhibitory connections made during synapse formation, Npas4 appears to play an important role in the activity-dependent plasticity of GABAergic synapses in mature neural circuits. Npas4-knockout mice show decreased CA1 hippocampal neuron miniature inhibitory postsynaptic current (IPSC) frequency after exposure to an enriched environment compared with their wild-type littermates (Bloodgood et al. 2013), and Npas4 has also been found to underlie the activity-dependent increase in inhibition on newborn excitatory granule cells of the dentate gyrus as they integrate into the hippocampal circuitry (Sim et al. 2013). These studies together support a role for Npas4 in selectively upregulating inhibitory GABAergic synapse development in excitatory neurons following neuronal activity, suggesting that Npas4 diminishes future excitation and maintains homeostatic balance between inhibition and excitation.
Perhaps the most interesting and unexpected finding regarding the Npas4-dependent regulation of GABAergic synapses onto excitatory neurons is that there is subcellular specificity with respect to the effects of Npas4 manipulation on the synapses on the postsynaptic cell (Fig. 1B). Bloodgood et al. (2013) used the spatial organization of synaptic inputs onto distinct regions of pyramidal neurons in the hippocampus to reveal this specificity in vivo. The authors found that exposure of mice to an enriched environment induced expression of Npas4 in the hippocampus. After this exposure, when a stimulating electrode was placed in the pyramidal layer to activate somatic inhibitory synapses onto nearby pyramidal neurons, the authors saw relatively smaller evoked inhibitory currents in Npas4-knockout neurons compared with Npas4 wild-type neurons, which is consistent with the culture experiments showing that Npas4 promotes inhibitory synapse formation. By contrast, when the authors stimulated stratum radiatum to activate inhibitory synapses on the distal apical dendrites of these pyramidal neurons they found significantly larger evoked inhibitory currents in the knockout neuron compared with wild type, suggesting that Npas4 actually inhibits the formation of distal inhibitory synapses, presumably in the same neurons where it promotes somatic inhibition (Bloodgood et al. 2013). Given that different classes of inhibitory interneurons are known to synapse onto different subcellular domains of hippocampal pyramidal neurons (Freund and Buzsáki 1996), having distinct Npas4 regulation of these events is likely to be important for fine-tuning circuit plasticity in the hippocampus.
Excitatory neurons comprise the large majority of cells in the culture systems that are most often used to study activity-inducible transcription; thus until recently very little was known about the identity of activity-inducible genes in inhibitory neurons. To fill this gap in knowledge, Spiegel et al. (2014) cultured neurons from the embryonic medial ganglionic eminence, which gives rise to the GABAergic interneurons that populate the forebrain. Using this system, they discovered that Npas4 expression was indeed induced in inhibitory neurons by membrane depolarization and subsequent calcium influx, similar to its regulation in excitatory neurons. However, when the authors studied the effects of knocking out Npas4 on synapse formation, they found that, rather than affecting GABAergic synapses, the loss of Npas4 reduced glutamatergic input to inhibitory neurons while having no effect on the GABAergic synapses made onto these neurons (Spiegel et al. 2014). Thus, in contrast to its effects in excitatory neurons, activity-inducible Npas4 expression appears to upregulate glutamatergic synapses in inhibitory neurons to increase their inhibitory output. This suggests a more complex role than previously thought for Npas4 in mediating neuronal inhibition/excitation balance across multiple cell types in the heterogeneous neural circuits that comprise the cortex. Overall, these studies suggest that Npas4 functions as a key homeostatic factor in the cortex, depressing network excitability following neuronal activity via a negative feedback mechanism that is spread across multiple cell types and synapses.
How it is possible for Npas4 to promote opposite types of synapses in two different classes of neurons? The expression of distinct cell type-specific programs of Npas4-regulated genes in excitatory and inhibitory neurons may underlie the differential effects of Npas4 in these two neuron types (Spiegel et al. 2014). Using a genetic method to identify activity-regulated genes that are selectively induced in inhibitory vs. excitatory neurons, Spiegel et al. (2014) identified a set of inhibitory neuron-specific genes whose induction is impaired in the absence of Npas4. These Npas4-regulated inhibitory neuron genes include Kcna1, Frmpd3, and Nptx2. These gene products have been reported previously to be postsynaptic at glutamatergic synapses and therefore may contribute to the Npas4-dependent enhancement of glutamatergic synapse formation in inhibitory neurons. With respect to the Npas4-regulated genes that drive GABAergic synapse formation in excitatory neurons, one possible target is Bdnf. Bdnf is selectively induced by activity only in excitatory and not inhibitory neurons and is well known to promote GABAergic synapse development in the postnatal cortex. Npas4 binds to three regulatory elements within the Bdnf gene, and knocking down Bdnf partly rescues the effects of Npas4 overexpression on increased GABAergic synapse number and function in hippocampal neurons (Lin et al. 2008). Interestingly, BDNF expression is reduced in the hippocampus of Npas4-knockout mice, and disruption of BDNF function prevents Npas4-mediated increases in inhibition at the soma but does not affect Npas4’s effects at the dendrites, thus providing a potential explanation for the subcellular specificity of Npas4-mediated inhibitory synapse formation (Bloodgood et al. 2013). Taken together, these data support the idea that Npas4’s differential effects in these two cell types are due to distinct cell type-specific programs of Npas4-regulated gene expression. Further understanding of the distribution of Npas4 binding sites in different cell types by chromatin immunoprecipitation (ChIP) will help to elucidate our understanding of how broadly expressed transcription factors can generate cell type-specific biological consequences.
MEF2: activation, repression, transcription, translation—MEF2 does it all.
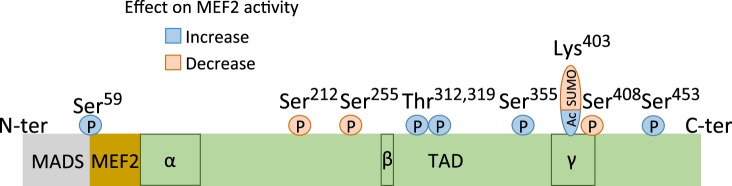
Initially identified for their role in muscle cell differentiation (Gossett et al. 1989), the myocyte enhancer factor 2 (MEF2) family of transcription factors comprises four members, MEF2A–D, that are now understood to have essential functions in multiple tissues including the CNS (Dietrich 2013; Pon and Marra 2016). In the brain the MEF2A, -C, and -D proteins are highly expressed in distinct, yet overlapping brain regions, both during neuronal development and in the adult (Leifer et al. 1993; Lyons et al. 1995). MEF2B is found at much lower levels and is not discussed further here. Importantly, the MEF2s are known to be targets of activity-dependent calcium signaling in neurons, where they have been studied for their roles in activity-dependent neuronal survival (Mao et al. 1999; Okamoto et al. 2000) as well as stimulus-regulated glutamatergic synapse formation and elimination (Flavell et al. 2006). The MEF2 proteins are subject to multiple, stimulus-regulated posttranslational modifications that are thought to regulate MEF2’s dual function as both a repressor and an activator (Fig. 2) (Lyons and West 2011; McKinsey et al. 2002). In resting neurons, the MEF2s are sumoylated and they function primarily as transcriptional repressors (Shalizi et al. 2006). After neuronal activity, calcium influx through NMDARs and L-type voltage-gated calcium channels results in calcineurin-mediated dephosphorylation, MAP kinase-dependent phosphorylation, and a switch from sumoylation to acetylation at specific residues on the MEF2s, all of which are associated with functional MEF2 activation (Flavell et al. 2006; Shalizi et al. 2006). Thus MEF2 transcription factors are ideally poised to be important regulators of stimulus-inducible effects on neuronal development and function.
Fig. 2.
Domain organization and posttranslational regulatory modifications of MEF2 family members in neurons. MEF2A, -C, and -D proteins all contain NH2-terminal MADS and MEF2 domains (gray and brown), which mediate DNA binding and dimerization, and a conserved transactivation domain (TAD, green). α, β, and γ indicate alternatively spliced exons. In neurons the MEF2s predominantly contain the α1 splice variant and are β+. The γ-domain is constitutively present in MEF2A and MEF2D but alternatively spliced in MEF2C. Figure shows posttranslational modifications (P, phosphorylation; SUMO, sumoylation; Ac, Acetylation) and their residue locations in human MEF2A. Whether the modifications function to enhance or repress MEF2 activity is shown above the diagram.
Both the transcriptional repressor and activator functions of the MEF2s have been implicated in synapse formation, with the accumulated evidence suggesting that the repressor forms regulate synapse formation and the activator forms promote synapse elimination. The repressor functions of the MEF2s have been studied in both the cerebellum and cortex, and repression by sumoylated MEF2A is particularly well characterized for its role in early cerebellar synapse development (Shalizi et al. 2006). In cerebellar granule neurons, sumoylated MEF2A enhances the formation of the mature claw morphology of granule neuron dendrites and knocking down MEF2A in cultured rat cerebellar slices impairs this dendritic differentiation. In cortical excitatory neurons, the repressor functions of MEF2C have similarly been shown to function in a cell-autonomous manner to regulate the development of glutamatergic synapses. Knocking out MEF2C in cortical neurons results in a mildly decreased frequency of miniature excitatory synaptic currents and significantly decreased dendritic spine density (Harrington et al. 2016). Spine density in the MEF2C-knockout neurons was rescued by expression of a fusion protein that links the DNA binding domain of MEF2C to the repressor domain of Engrailed (MEF2-En), indicating that the synapse-promoting effects of MEF2C in this context are mediated by its function as a transcriptional repressor. Interestingly, MEF2C also has a cell-autonomous effect on GABAergic synapses made onto cortical glutamatergic neurons, such that single-cell knockout of MEF2C is associated with increased amplitude and frequency of miniature inhibitory synaptic currents, and an increased number of GABAergic synapses, and this effect is again rescued by overexpression of MEF2C-En.
By contrast, the transcriptional activator functions of the MEF2 transcription factors have been implicated in the activity-dependent elimination of glutamatergic synapses in the hippocampus. Knocking down/out MEF2A, MEF2C, or MEF2D in hippocampal neurons was found to increase excitatory synapse number, which first suggested a role for these factors in restricting synapse number (Barbosa et al. 2008; Flavell et al. 2006). The increase in glutamatergic synapses seen in MEF2A/D-knockdown neurons can be reversed by expressing a fusion protein that combines the DNA binding domain of MEF2 and the transcriptional activation domain of the viral transcription factor VP16 (MEF2-VP16), implicating the transcriptional activator function of MEF2 in this negative-regulatory effect on synapses.
A further level of MEF2-driven synapse specificity was revealed in a recent paper that showed that MEF2C differentially regulates local vs. long-range excitatory synaptic inputs onto neocortical neurons (Rajkovich et al. 2017). The authors found that knocking out Mef2c in a sparse population of layer 2/3 neurons in mouse barrel cortex weakened local excitatory synaptic input while enhancing long-range transcolossal excitatory inputs onto the same cells. Thus even for the same type of synapses (excitatory) on a single type of neuron (also excitatory), MEF2C shows functional specificity in the direction of its regulatory effect depending on the source of the presynaptic input.
As MEF2s are sequence-specific, DNA-binding transcription factors, a key focus of investigation has been identifying MEF2 targets by a combination of gene expression profiling and MEF2 chromatin binding analyses. In RNA-seq data comparing transcriptional profiles from MEF2C conditional knockout and control cortex, >1,000 differentially expressed genes were identified (Harrington et al. 2016). Consistent with MEF2’s function as both an activator and a repressor, these were nearly evenly split between those that are upregulated and downregulated in the absence of MEF2C, and they were enriched for genes involved in neuron differentiation and development and synaptic transmission. As expected from their posttranslational regulation, the MEF2s also play an important role in the regulation of activity-inducible programs of gene expression, and a set of activity-inducible genes that are direct targets of MEF2 were identified in hippocampal neurons by coordinate microarray analysis and ChIP for MEF2D (Flavell et al. 2008). Interestingly, ChIP-Seq experiments in primary mouse cortical neurons have found that MEF2A and MEF2C bind not only gene promoters but also distal enhancer regions for genes involved in glutamatergic synapse transmission, drug addiction, axon guidance, and MAP kinase signaling pathways (Telese et al. 2015).
As in all cases where a transcription factor regulates synapses, it is natural to wonder how gene transcription in the nucleus of the neuron can be transduced into distal changes in synapse number in the periphery. Intriguingly, insights gleaned from studying a mouse model of fragile X syndrome (FXS), a genetic form of autism and mental retardation that occurs because of loss-of-function mutations in the Fmr1 gene (Abrahams and Geschwind 2008; Bassell and Warren 2008), have suggested that the Fragile X Mental Retardation Protein (FMRP) encoded by Fmr1 may link MEF2 in the nucleus and its effects at synapses (Fig. 1C). FMRP is an RNA binding protein that mediates RNA trafficking from the nucleus to synapses and regulates local mRNA translation at synapses. FMRP is required for MEF2-dependent synapse elimination because MEF2-VP16 overexpression fails to drive excitatory synapse elimination in the absence of FMRP expression (Pfeiffer et al. 2010).
An explanation for the requirement for FMRP in MEF2-dependent synaptic changes may be that FMRP regulates translation of MEF2-induced transcripts at synapses. In hippocampal neurons, MEF2 promotes excitatory synapse elimination by inducing degradation of postsynaptic density protein 95 (PSD-95) at the dendrites, a process requiring Mdm2-dependent ubiquitination and Pcdh10-dependent degradation of PSD-95. Pcdh10 is a target of both MEF2- and activity-dependent transcription and FMRP-dependent translational repression (Morrow et al. 2008; Tsai et al. 2012), and inhibition of Pcdh10 function inhibits MEF2-dependent synapse elimination. MEF2 and FMRP also cooperate to regulate the synaptic localization and activation of Mdm2. As a result of this dual regulatory circuit, even though Pcdh10 levels are elevated in Fmr1-knockout neurons, MEF2-dependent degradation of PSD-95 is blocked in the absence of FMRP because of a failure of PSD-95 at synapses to be ubiquitinated. Dendritic FMRP is also known to regulate the transport and translation of specific mRNAs in response to metabotropic glutamate receptor (mGluR) activation, and blocking dendritic mGluR5 activity also results in loss of MEF2-induced glutamatergic synapse elimination in cultured hippocampal neurons (Wilkerson et al. 2014). One potential mediator of synapse elimination in this context is the cytoskeletal protein Arc, which plays important roles in dendritic spine plasticity and glutamate receptor trafficking (Korb and Finkbeiner 2011). Arc transcription is induced in an activity- and MEF2-dependent manner, and it is required for MEF2 and dendritic mGluR5-induced synapse elimination (Wilkerson et al. 2014). Dendritic mGluR5 activation promotes local translation of Arc mRNA, and Arc is also a known translational target of FMRP in dendrites (Park et al. 2008). Thus through coordinate transcriptional and translational control of a program of synapse regulatory gene products, MEF2 and FMRP work together to shape synaptic activity-dependent changes in glutamatergic synapses.
Chromatin Regulation of Synapse Development
Sequence-specific transcription factors are the primary determinants of gene expression programs in cells because they directly mediate the recruitment of RNA polymerase II onto gene promoters. However, there are many more potential binding sites across the genome for any given transcription factor than are used, and it is the biochemical state and physical structure of DNA and its associated histone proteins, a complex called chromatin, that determine which of the potential sites are available to be bound (Sheffield et al. 2013). In this manner chromatin state functions to establish the potential range of transcription factor action in any given cell. Although chromatin structure is one step upstream from transcription factor binding, loss-of-function phenotypes for several chromatin regulatory factors have revealed important and specific roles for many of these proteins in synapse development. Here we review how studies of three different classes of chromatin regulatory factors have advanced insights into the distinct mechanisms by which chromatin can regulate synapses in the developing brain.
Methyl-CpG binding protein 2: non-CpG methylation matters, too.
Methyl-CpG-binding protein 2 (MeCP2) was first characterized as a factor purified from the brain that has the ability to bind single methylated CpG dinucleotides in DNA (Lewis et al. 1992). MeCP2 is the founding member of the 11-member methyl-DNA binding (MDB) domain family (Du et al. 2015), and it is ubiquitously expressed in both neuronal and nonneuronal tissues. However, expression levels of MeCP2 are higher in neurons than they are in other cell types even within the brain (Skene et al. 2010), and mutations that result in loss of MeCP2 function have a predominant effect on the function of neurons, causing the neurodevelopmental disorder Rett syndrome (RTT) (Amir et al. 1999). These data raise the possibility that understanding how MeCP2 regulates transcription may offer insights into how a global chromatin regulatory process like DNA methylation can selectively impact neuronal functions.
MeCP2 levels are low in neural precursors and rise as neurons mature, peaking around the time of synaptogenesis (Cohen et al. 2003). The biological requirement for MeCP2 tracks with the postnatal increase in expression, such that individuals affected by RTT exhibit largely normal development for the first 6–18 mo of life followed by a period of developmental regression when motor and cognitive milestones are lost (Hagberg et al. 1983). RTT patients exhibit acquired microcephaly, meaning they are born with brain sizes in the normal range but fall below the normal brain growth curve as they age (Hagberg et al. 2001). Neuropathological studies in brains from RTT patients found no evidence of neuronal death, but they did show a global reduction in the size of neurons and decreased dendritic spine density in the cortex and hippocampus, suggesting that the impaired postnatal brain growth in RTT patients was due to a failure of MeCP2-dependent synapse development (Armstrong 2005; Chapleau et al. 2009). Indeed, the findings of reduced neuronal size and decreased dendritic spine density are recapitulated in Mecp2-knockout neurons (Belichenko et al. 2009; Chen et al. 2001). Furthermore, hippocampal neurons from Mecp2-knockout mice show a reduced excitatory synaptic response whereas neurons from mice overexpressing MeCP2 display an enhanced synaptic response, and these differences in neurotransmission are primarily due to changes in the number of glutamatergic synapses (Chao et al. 2007). Taken together, these studies indicate that MeCP2 plays a critical role in synapse development and function and establish Mecp2-knockout mice as a model for discovering the molecular mechanisms of MeCP2-dependent effects on synapse development.
Because MeCP2 was isolated on the basis of its ability to bind methylated DNA and because methylation of gene promoters is associated with transcriptional repression, the first models of MeCP2 function in neurons proposed that it acted primarily at gene promoters to repress expression of specific genes (Chen et al. 2003; Martinowich et al. 2003). Indeed, MeCP2 has been found to recruit a multiprotein corepressor complex to gene promoters that includes histone deacetylases and methyltransferases to silence gene transcription (Fuks et al. 2003; Jones et al. 1998; Lyst et al. 2013; Nan et al. 1998). However, a subsequent microarray study of gene expression in the hypothalamus from Mecp2-knockout mice revealed that nearly as many genes were downregulated in the absence of MeCP2 as were upregulated, suggesting that MeCP2 might also act as a transcriptional activator (Chahrour et al. 2008). Furthermore, once genome-scale analyses of MeCP2 binding profiles emerged, they revealed widespread binding of MeCP2 across the genome, suggesting that MeCP2 might act to modulate the global chromatin state rather than functioning as a gene-specific regulator of either transcriptional activation or repression (Baker et al. 2013; Cohen et al. 2011; Skene et al. 2010).
Some resolution of these disparate findings has recently begun to emerge from the recent discovery that MeCP2 also binds non-CpG dinucleotides (primarily methyl-CpA) in neuronal DNA (Chen et al. 2015; Gabel et al. 2015; Guo et al. 2014). As an epigenetic mark on genomic DNA, cytosine methylation at non-CpG dinucleotides (i.e., CpA, CpC, and CpT, collectively called CpH) exhibits distinct regulation and function as compared with the more widely studied CpG methylation (Guo et al. 2014; Lister et al. 2013). Of particular relevance for understanding MeCP2 function in neurons, it was found that global methyl-CpG levels are stable throughout the life span in both neurons and glial cells, whereas methyl-CpH is very low in the fetal brain and then increases rapidly and selectively in neurons after birth (Lister et al. 2013). Methyl-CpH levels are then actively maintained by the action of DNA methyltransferase 3A (DNMT3A) in mature neurons, and the distribution of this mark over gene bodies is inversely proportional to the abundance of the associated mRNA transcript, suggesting that methyl-CpH inhibits transcription of expressed genes (Guo et al. 2014).
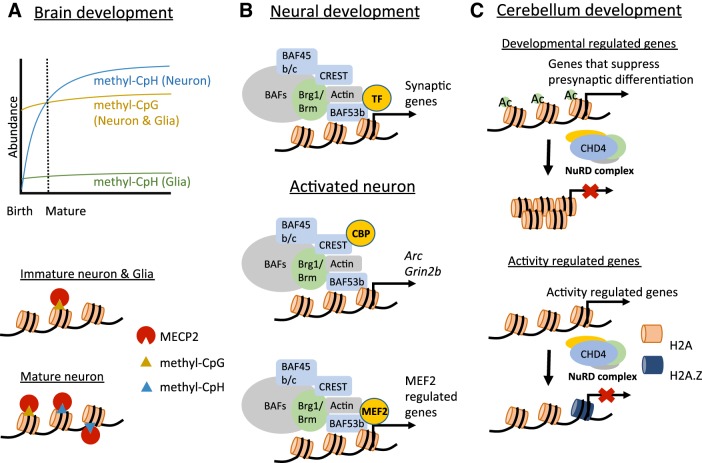
The timing of the onset of neurological symptoms in RTT parallels the time course of methyl-CpH accumulation in neurons, suggesting that MeCP2-dependent regulation of the genes marked by methyl-CpH in neurons might explain the synaptic developmental defects in RTT (Fig. 3A). Genes that are upregulated in Mecp2-knockout mice have higher MeCP2 binding and higher methyl-CpH levels over their gene bodies than unaffected genes (Chen et al. 2015; Gabel et al. 2015). Furthermore, the genes that rely most on MeCP2 and methyl-CpH for their proper regulation tend to be long genes (>100 kb) (Gabel et al. 2015; Sugino et al. 2014). These long genes contain the highest levels of methyl-CpA in neurons and are most sensitive to disruption of DNMT3A expression (Gabel et al. 2015). Interestingly, long genes are enriched for neuronal and synaptic functions, many are selectively expressed in the brain, and a number of these genes are found among the set of high-likelihood candidates for causing autism spectrum disorders (King et al. 2013). Taken together, these data suggest that although MeCP2 likely has many functions in transcriptional regulation including CpG-mediated gene repression and global effects on chromatin structure, its impact on synapse development may be most closely related to its ability to modulate methyl-CpA-dependent repression of long genes in maturing neurons of the postnatal brain. This model awaits in vivo testing in Mecp2-knockout mice to determine the degree to which correcting long gene expression can rescue synaptic and behavioral phenotypes of MeCP2 deficiency.
Fig. 3.
Chromatin regulatory factors in synapse development. A: neuronal-specific accumulation of methyl-CpH leads to a distinct MeCP2-regulated gene program in mature neurons. Top: during brain development methyl-CpH levels increase and accumulate selectively in mature neurons, whereas methyl-CpG levels are constant across development in both neurons and glia. Bottom: because MeCP2 binds to both methyl-CpG and methyl-CpH, the distinct high level of methyl-CpH in mature neurons leads to a neuron-specific developmental effect on MeCP2 regulated gene transcription. Diagrams are based on the data in Lister et al. (2013) and Chen et al. (2015). B: tissue-specific BAF subunits interact with distinct transcription factors or transcriptional coactivators, resulting in activation of different gene programs during neural development. BAF complexes regulate a group of synaptic genes and mediate synapse formation and maturation. In activated neurons, BAF complexes regulate Arc and Grin2b expression by interacting with CBP. BAF complexes also interact with MEF2C and are required for MEF2C-mediated synapse elimination. C: NuRD controls the timing of gene expression by turning genes off both on a developmental timescale and in response to transient neuronal activity. During cerebellar development, NuRD turns off genes that suppress presynaptic differentiation by histone deacetylation and inactivates neuronal activity-dependent gene transcription by replacing H2A with H2A.Z.
ATP-dependent chromatin remodelers.
ATP-dependent chromatin remodelers use the energy of ATP hydrolysis to alter nucleosome positions along genomic DNA or to exchange nucleosomes on chromatin. By moving nucleosomes into or out of transcription factor binding sites and clearing chromatin of nucleosomes marked with regulatory histone modifications, chromatin remodelers have the ability to modulate gene transcription. There are four families of ATP-dependent chromatin remodeling complexes defined by the ATPase they contain: 1) BAF, 2) INO80/SWR1, 3) ISWI, and 4) CHD (Hargreaves and Crabtree 2011). Recently several specific members of the BAF and CHD families have been shown to play important roles in synapse development, and those factors are reviewed here.
BAF complexes: specificity of function through subunit variation.
Brg1/Brm-associated factor (BAF) complexes are mammalian SWI/SNF-like, ATP-dependent chromatin-remodeling complexes that are comprised of an assembly of at least 15 subunits encoded by 29 genes. The core ATPase subunit of these ~2-MDa protein complexes can be either Brg1 (also known as SmarcA4) or Brm (also known as SmarcA2) (Kadoch and Crabtree 2015; Ronan et al. 2013; Vogel-Ciernia and Wood 2014). Some accessory subunits of the BAF complexes are tissue specific, and the particular subunit composition of a given BAF complex affects both its genome targeting and functions. For example, in neural progenitors BAF complexes contain primarily BAF45a and BAF53a, and in these cells BAF complexes are required for cell proliferation. By contrast, when progenitors exit the cell cycle to become postmitotic neurons, BAF45a and BAF53a are replaced by BAF45b, BAF45c, and BAF53b, and blocking this subunit switch inhibits neural differentiation (Lessard et al. 2007). Study of the functions and protein interactions of neural-specific subunits of the BAF complex has helped explain how these chromatin-remodeling complexes regulate neuronal and synaptic development.
In postmitotic neurons, the BAF complexes have been implicated as regulators of both dendrite outgrowth and synapse maturation. For example, neurons lacking Brm show increased numbers of mature, mushroom-shaped spines (Loe-Mie 2010) whereas neurons lacking Brg1 show increased numbers of immature, thin spines (Zhang et al. 2015), and Brg1 is required for activity-dependent dendritic outgrowth (Wu et al. 2007). Although BAF complexes can still assemble without the neural-specific subunit BAF53b, hippocampal neurons lacking BAF53b show defects similar to neurons lacking Brg1 including reduced activity-dependent dendritic outgrowth (Wu et al. 2007). Profiling gene expression in mice with heterozygous knockout of BAF53b revealed altered expression of genes involved in actin cytoskeletal remodeling and postsynaptic density, suggesting that the BA53b-mediated recruitment of the BAF complex to these genes may play an important role in regulation of synapse functions (Vogel-Ciernia et al. 2013). ChIP studies also show that BAF53b is required for the recruitment of BAF complexes to the promoter region of genes involved in dendritic outgrowth (Wu et al. 2007). Taken together, these studies indicate that BAF complexes regulate genes that are important for synaptic functions and suggest that neural-specific subunits are important for targeting the complexes to their genes.
How do distinct BAF complexes achieve functional biological specificity? It is well established that BAF complexes are recruited to target genes via their interaction with sequence-specific transcription factors, and if these interactions differ between cell types or conditions, then the function of the BAF complex will differ as well (Cosma et al. 1999). For example, under basal conditions in neural progenitors Brg1 interacts with the transcription factor Gli3 and functions as a transcriptional repressor to suppress the expression of Shh target genes (Zhan et al. 2011). However, after exposure to Shh Brg1 interacts with the transcription factor Gli1 and is required for Shh-induced gene activation. In postmitotic neurons, BAF complex subunit-specific interactions with transcription factors and other chromatin regulators may define the specific function of BAF complexes in synapse development (Fig. 3B). For example, the neural-specific BAF subunit CREST has been shown to regulate dendrite development via its interactions with the transcriptional coactivator and histone acetyltransferase CBP (Aizawa et al. 2004). BAF complexes containing CREST are found bound to the promoters of genes related to synapse functions including Arc and Grin2b, where CREST is required to mediate their expression (Qiu and Ghosh 2008). One key transcription factor that has been shown to link BAF complexes to synapse development is MEF2C. Gene expression profiling from a recent study revealed a significant overlap between neuronal activity-induced genes that are disrupted in the absence of Brg1 and genes that are targets of regulation by MEF2. Knocking out MEF2C was shown to impair the activity-dependent recruitment of Brg1 to gene promoters, and, conversely, knocking out Brg1 impaired the ability of MEF2C overexpression to drive synapse elimination (Zhang et al. 2015). These data suggest that Brg1 functions as a coactivator of the MEF2C-dependent transcriptional program that mediates excitatory synapse elimination. Taken together, these studies suggest that identifying and selectively disrupting specific BAF subunit-transcription factor interactions offers a strong opportunity to dissect the many roles of BAF complexes in synapse development.
CHD4: timing transcriptional repression.
Whereas the BAF complexes illustrate how subunit specificity can allow a chromatin remodeler to differentially control distinct stages in the process of synapse formation, recent findings about the CHD family chromatin remodeler CHD4 have provided new insights into regulation of gene expression on distinct timescales during synapse development.
The ATPases of the CHD family are characterized by the presence of a chromodomain in addition to the conserved DEAD/H-related ATPase domain. CHD4 assembles with DNA binding proteins and histone deacetylases into a large protein complex called the nucleosome-remodeling and histone deacetylase (NuRD) complex (Hargreaves and Crabtree 2011). NuRD functions as a transcriptional repressor and has canonically been suggested to play an opposing role to the gene activation mediated by BAF complexes in regulation of gene transcription (Ho and Crabtree 2010). Although NuRD is well established to play a critical role in the differentiation of embryonic stem cells, far less is known about functions of NuRD in postmitotic tissue. However, a recent study showed that knocking out CHD4 in granule neurons in the cerebellar cortex results in a reduction in the density of synapses and neurotransmission between parallel fibers and Purkinje cell dendrites, suggesting that the NuRD complex promotes synaptogenesis (Yamada et al. 2014). Genomewide chromatin state profiling in the cerebellum of mice lacking CHD4 revealed that in the absence of NuRD a number of gene promoters fail to undergo developmentally regulated, NuRD-dependent repression. Expression of these NuRD target genes, which include Nhlh1, Elavl2, and Cplx3, is highly developmentally regulated in wild-type mice, such that expression peaks at postnatal day 6 and then decreases to very low levels in the adult brain. In the absence of CHD4, expression of the NuRD target genes remains high. Because in vivo RNAi knockdown reveals that many of these genes suppress presynaptic differentiation in the developing cerebellar cortex, these findings suggest that NuRD-dependent gene repression mediates the timing of proper synapse development by releasing the suppression of presynaptic differentiation in granule neurons during neural development (Fig. 3C).
Interestingly, in addition to its role in gene repression on a developmental timescale, CHD4-dependent repression has also been found to act much more rapidly to regulate the dynamics of neural activity-dependent gene expression in neurons (Yang et al. 2016). Surprisingly, given its known functions in gene repression, genomewide CHD4 binding and chromatin state profiling revealed that CHD4 occupies the promoters of most actively transcribed genes in the mouse cerebellum including the classic activity-inducible genes Bdnf, Fos, and Npas4. Conditional knockout mice lacking CHD4 in cerebellar granule neurons show a prolonged time course of expression after the induction of activity-regulated genes is triggered, and this gene expression change is associated with defective activity-dependent granule neuron dendrite pruning. A clue to the mechanism of CHD4’s action in neuronal activity-regulated gene transcription came when the authors observed that binding of the histone variant H2A.Z at the promoters of activity-regulated genes was reduced in CHD4-knockout granule neurons. In yeast, the homolog of H2A.Z is enriched at the promoters of genes when they are in their inactive state but is rapidly lost from gene promoters when transcription is induced, which suggested that H2A.Z might poise genes in the off state for their rapid activation (Zhang et al. 2005). However, in the absence of CHD4, H2A.Z is depleted from activity-inducible gene promoters, and, rather than affecting induction, knockdown of H2A.Z phenocopies the prolonged time course of activity-induced transcription seen in CHD4-knockout neurons. (Yang et al. 2016) Thus these data suggest a new model in which CHD4-dependent recruitment of H2A.Z to activity-inducible gene promoters in neurons controls the timing of transcriptional inactivation, perhaps by serving to facilitate recruitment of repressors to promoters as has been observed for H2A.Z in embryonic stem cells (Hu et al. 2013). In sum, these data reveal two distinct functions for CHD4 in cerebellar synapse regulation—one of which relies on the classic function of the NuRD complex in developmental gene silencing and the other of which is mediated by interactions between CHD4 and the histone variant H2A.Z to control the inactivation kinetics of stimulus-inducible genes. Future understanding of the CHD4-dependent mechanisms that differentiate its function in these two processes will enhance our understanding of the timescales of gene transcription that contribute to synapse formation and refinement in the developing CNS.
Transcriptional Dysregulation of Synapses in Autism and Intellectual Disability
The Human Genome Project was key to discovering single-gene mutations that cause brain disorders, such as those in CHD7 in CHARGE syndrome, TCF4 in Pitt-Hopkins syndrome, MECP2 in Rett syndrome, GTF2I in Williams syndrome, and PHF6 in Börjeson-Forssman-Lehman syndrome (Amir et al. 1999; Jahani-Asl et al. 2016; Morris et al. 2003; Zentner et al. 2010; Zweier et al. 2007). Now substantial improvements in genetic methodologies, including increases in the speed and decreases in the cost of genome sequencing, are driving a revolution in our understanding of the genetic underpinnings of neurodevelopmental disorders by giving a more nuanced understanding of the types of gene variants that may predispose individuals to complex disorders including intellectual disability (ID) and autism spectrum disorders (ASDs) (Sanders 2015). By compiling the data derived from multiple large patient cohorts and building statistical tools to mine these data comprehensively, there has been a significant expansion in our knowledge of the genes that can be linked to these brain disorders. Not surprisingly, one major category of gene function linked to neurodevelopmental disorders including both ASDs and ID are genes involved in synapse function, supporting the well-established idea that it is dysregulation of brain wiring that underlies the nature of these diseases. However, it is perhaps somewhat surprising that a second major category of genes implicated in ASDs and ID are those involved in transcriptional regulation (Bourgeron 2015; De Rubeis et al. 2014; Najmabadi et al. 2011; Ronan et al. 2013). Although a comprehensive understanding of how transcriptional dysregulation contributes to ASDs and ID remains elusive, the sheer number of genetic variants in transcriptional regulators that have been associated with neurodevelopmental disorders in recent large sequencing studies (summarized in Table 1) suggests the physiological relevance of studying this process (Deciphering Developmental Disorders Study 2017; Stessman et al. 2017; Yuen et al. 2017).
Table 1.
Genes encoding transcriptional regulatory proteins that have de novo mutations achieving statistical significance in large-scale sequencing screens of ASDs and other developmental disorders
| ADNP | CHD4 | FOXP1 | KMT2C | NR3C2 | SMARCA2 |
| AHDC1 | CHD8 | GATAD2B | MECP2 | NSD1 | SMARCC2 |
| ARID1B | CNOT3 | HDAC8 | MED13 | PAX5 | SMC1A |
| ASH1L | CREBBP | KANSL1 | MED13L | PHF3 | SUV420H1 |
| ASXL1 | CTCF | KAT6A | MEF2C | PURA | TBL1XR1 |
| ASXL3 | DNMT3A | KAT6B | MLL3 | SATB2 | TBR1 |
| AUTS2 | EHMT1 | KDM5B | MSL3 | SET | TCF20 |
| CHD2 | EP300 | KDM6A | MYT1L | SETD5 | TCF4 |
| CHD2 | FOXG1 | KMT2A | NFIX | SMAD4 | TCF7L2 |
Given the evidence linking transcription factors and chromatin regulators with synapse development, it is likely that mechanistic insights into the pathophysiology of these disorders will come from studying how disease-associated mutations in transcriptional regulators affects the programs of gene transcription required for synapse formation. Here we review two recent examples of these findings and discuss both the advances and the remaining challenges for understanding the links between transcription and synapse abnormalities in disease.
CHD8: de novo mutations confer risk to sporadic ASDs.
The most reproducible genetic association with transcriptional regulation to arise from autism exome sequencing studies to date is the finding that de novo mutations in the chromodomain family nucleosome remodeling protein CHD8 are associated with increased risk for sporadic ASDs (Iossifov et al. 2014; Neale et al. 2012; O’Roak et al. 2012b; Talkowski et al. 2012). After its identification as a high-confidence hit in two large GWAS studies, focused rescreening of additional patient samples led to the estimation that CHD8 is one of a set of just six genes (CHD8, DYRK1A, GRIN2B, TBR1, PTEN, and TBL1XR1) that contribute to as many as 1% of sporadic ASDs (Iossifov et al. 2014; O’Roak et al. 2012a). Phenotypically, those with CHD8 mutations were found to have significantly larger head circumference relative to patients lacking these mutations (O’Roak et al. 2012a). Interestingly, in the same cohort patients with DYRK1A mutations had significantly reduced head circumference, suggesting that distinct genetics underlie the range of ASD presentations. The association between CHD8 mutations and macrocephaly was validated in a second targeted resequencing study that focused on a cohort of patients with a broader range of developmental delay phenotypes (Iossifov et al. 2014). In addition to increased head circumference, this study broadened the putative CHD8 phenotype to include distinct facial characteristics and gastrointestinal complaints. Importantly, in a zebrafish model chd8 knockdown during development was shown to cause both macrocephaly and impaired gastrointestinal motility due to reduced colonization of the gastrointestinal tract by enteric neurons (Iossifov et al. 2014), suggesting a causative association between loss of CHD8 and these phenotypic features.
To identify gene targets of CHD8 that may explain its function in brain development, several groups have conducted genomewide ChIP and/or transcriptome sequencing studies from CHD8 mutant cells in culture (Cotney et al. 2015; Sugathan et al. 2014; Wang et al. 2015). In human iPSC-derived neural progenitor cells, CHD8 was found to be broadly distributed across the genome and bound near ~5,000 genes that were enriched for functions in chromatin modifications and transcriptional regulation. Reducing CHD8 expression led to both up- and downregulation of a large set of genes, with the downregulated set enriched for neuronal annotations such as cell adhesion, synapse, and axon guidance (Sugathan et al. 2014). Most of the genes that showed differential regulation in the CHD8-knockdown cells were not direct targets of CHD8 binding, leading the authors to suggest a model in which CHD8 sits atop a program of transcriptional regulation that subsequently feeds forward to coordinate neuronal differentiation.
New insights into the basic biology of CHD8 have come from the analysis of CHD8 loss-of-function mouse models (Durak et al. 2016; Katayama et al. 2016; Platt et al. 2017). Durak et al. (2016) focused on the functions of CHD8 in neural progenitors by knocking down expression of CHD8 in vivo with in utero electroporation. This controlled, cell type-specific approach allowed the authors to demonstrate that loss of CHD8 in progenitors leads to precocious neurogenesis due to loss of a cell type-specific action of CHD8 as an activator of genes in the Wnt signaling pathway. By contrast, Katayama et al. (2016) and Platt et al. (2017) mimicked the messier but more physiologically relevant haploinsufficiency of human CHD8 mutations by generating constitutive heterozygous CHD8-knockout mice. All three studies found that reducing expression of CHD8 in the developing brain was associated with ASD-like behaviors, such as impaired sociability and social novelty in three-chamber interaction tasks. Both approaches also revealed disrupted expression of neuronal differentiation genes, validating the function of CHD8 as a modulator of neuronal development in vivo. However, only the haploinsufficient models mirrored the macrocephaly that is seen in human patients with CHD8 mutations (Katayama et al. 2016). Thus despite the gross behavioral similarities between the models, these distinct observations raise the possibility that the ultimate phenotype of CHD8 mutation may depend on both cell-autonomous and cell-nonautonomous effects on brain function, and they highlight the importance of using multiple kinds of loss-of-function models for gaining insight into the complex etiologies of disorders like ASDs and ID.
MEF2C haploinsufficiency: a human-specific requirement for MEF2C in brain development?
MEF2C haploinsufficiency was relatively recently recognized as a cause of syndromic mental retardation that is characterized by severe intellectual disability, lack of speech, hypotonia, stereotypic movements, and epilepsy. Using comparative genomic hybridization to genome oligonucleotide arrays, two clinical groups discovered overlapping microdeletions on chromosome 5q14.3 in small cohorts of unrelated patients (Le Meur et al. 2010; Nowakowska et al. 2010). Although the microdeletions in these patients ranged from ~150 kb to 8.8 Mb, the minimal common deleted region encompassed only MEF2C, suggesting loss of MEF2C function as the common cause of the consistent phenotype. A number of additional patients have been reported with deletions that disrupt MEF2C who show highly similar presentations, suggesting that MEF2C haploinsufficiency is a discrete clinical entity (Novara et al. 2010; Paciorkowski et al. 2013; Rocha et al. 2016). Finally, in the strongest case for the causative association of MEF2 haploinsufficiency with the clinical syndrome, one patient with a similar phenotype has been characterized to have a de novo point mutation within exon 8 of the MEF2C gene that is predicted to result in a premature stop codon (Le Meur et al. 2010).
Despite the common clinical phenotype, the underlying brain pathophysiology of MEF2 haploinsufficiency syndrome remains unknown. Patients with 5q14.3 have variable morphological abnormalities of the brain that include mild thinning of the corpus callosum and delay of white matter myelination, but no detailed pathological findings have been reported from patients. Often, as we described above for CHD8, hypotheses of pathophysiological mechanism emerge from studies of model organisms. As discussed above, neuron- or brain-specific conditional MEF2C-knockout mice show changes in synapse formation (Barbosa et al. 2008; Harrington et al. 2016). Furthermore, these mouse models show deficits in learning and memory (Barbosa et al. 2008) and cortical development (Li et al. 2008) and deficits in behaviors relevant to ASDs and ID such as changes in ultrasonic vocalizations, deficits in social novelty, reduced sucrose preference, locomotor hyperactivity, and impaired fear conditioning (Harrington et al. 2016). However, heterozygous conditional deletion of MEF2C either in excitatory forebrain neurons (Harrington et al. 2016) or throughout the brain (Barbosa et al. 2008) fails to disrupt performance on cognitive and social tasks compared with wild-type littermates. A more faithful model of the human genetic condition is found in mice heterozygous for germline deletion of Mef2c, and although these were described upon generation as showing no discernible phenotype (Lin et al. 1997), they have not yet been subjected to detailed neural and behavioral phenotyping. Thus the relevance of the synaptic mechanisms underlying behavioral abnormalities in the brains of conditional MEF2C-knockout mice for understanding the human haploinsufficiency syndrome remains to be fully determined.
A further point of interest with respect to understanding MEF2C haploinsufficiency syndrome is a recent paper that suggests MEF2C may have human-specific targets. Specifically, MEF2C was discovered to bind to an evolutionarily acquired MEF2-responsive element regulating the gene encoding the secreted protein osteocrin (OSTN) by ChIP from primary fetal human neurons (Ataman et al. 2016). This MEF2C binding enhancer element emerged in the primate lineage, where it is required for the primate-specific neuronal activity-inducible transcription of OSTN. Whether osteocrin expression is affected in patients with MEF2C haploinsufficiency syndrome is not known; however, knockdown of osteocrin disturbs dendrite development in cultured human iPSC-derived neurons, suggesting that it could be part of a human-specific mechanism of synapse development. These data raise the possibility that MEF2C could play species-specific roles in synapse formation by virtue of its differential recruitment to evolutionarily adapted enhancers. Further development of methods for examining transcription factor function in human neurons will advance our understanding of this and other similar effects of regulatory element variation on species-specific aspects of brain function and dysfunction.
Conclusions
The data reviewed here reveal the degree to which transcription factors and chromatin regulators can have complex and selective effects on synapse development. The function of sequence-specific binding transcription factors is ultimately controlled by the distribution and accessibility of their binding sites in the genome, which determines their potential target genes. ChIP-seq and RNA-seq studies have begun to reveal the expression profiles controlled by synapse regulatory transcription factors and chromatin regulators, giving new insights into the molecular mechanisms of synapse formation, elimination, and maintenance. Interestingly, evidence that mRNAs regulated by the transcription factor MEF2 are transported to synapses by the RNA binding protein FMRP and can be subject to local translation suggests a new model for how the action of a transcription factor in the nucleus can be directed to specific sets of synapses in the periphery. Understanding the extent to which other RNA trafficking proteins contribute to the synaptic actions of transcription factors will be an interesting area of exploration for the future.
The advances in next-generation sequencing methods that emerged from the completion of the human genome drove many of the recent advances in our understanding of transcription factors in brain development and disease. We are still feeling the impact of these methods—for example, exome sequencing is still in its relative infancy and the majority of cases of sporadic ASDs and ID are without a defined genetic cause. With the cost of sequencing continuing to fall and whole genome sequencing becoming an increasing reality at scale, it is reasonable to expect that new mutations including noncoding mutations will emerge that show association with brain disorders, offering new clues to the etiology of these diseases. As we discussed above for CHD8 and MEF2C, one way the clarification of disease genetics can improve outcomes for patients is by offering diagnostic criteria for predicting the course of the clinical syndrome. Furthermore, the development of model systems that permit investigations into the pathophysiology of the disorder can in ideal scenarios suggest potential treatment options. For example the loss-of-function mutations in MeCP2 that cause Rett syndrome in girls were found to disrupt expression of the neuronal growth factor IGF-1 in the developing brain of a mouse model (Tropea et al. 2009). Because IGF-1 was already FDA approved for treatment of certain endocrine disorders, this rapidly led to a clinical trial for IGF-1 replacement therapy in Rett syndrome (Khwaja et al. 2014). Although at the time of this writing the outcome of the larger trial remains unknown, the simple fact that a neurodevelopmental disorder could potentially be treatable offers great hope to the families of the children with these disorders. Finally, in the future gene editing strategies using new developments in TALEs or Cas9/CRISPR (Long et al. 2016; Nelson et al. 2016; Tabebordbar et al. 2016) might have the potential to fully correct these genetic disorders or their transcriptional consequences, in the most desirable outcomes of all.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-098804.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.-F.C. and A.S.Z. prepared figures; L.-F.C., A.S.Z., and A.E.W. drafted manuscript; L.-F.C., A.S.Z., and A.E.W. edited and revised manuscript; L.-F.C., A.S.Z., and A.E.W. approved final version of manuscript.
REFERENCES
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet 9: 341–355, 2008. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science 303: 197–202, 2004. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188, 1999. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. Neuropathology of Rett syndrome. J Child Neurol 20: 747–753, 2005. doi: 10.1177/08830738050200082401. [DOI] [PubMed] [Google Scholar]
- Ataman B, Boulting GL, Harmin DA, Yang MG, Baker-Salisbury M, Yap EL, Malik AN, Mei K, Rubin AA, Spiegel I, Durresi E, Sharma N, Hu LS, Pletikos M, Griffith EC, Partlow JN, Stevens CR, Adli M, Chahrour M, Sestan N, Walsh CA, Berezovskii VK, Livingstone MS, Greenberg ME. Evolution of Osteocrin as an activity-regulated factor in the primate brain. Nature 539: 242–247, 2016. doi: 10.1038/nature20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Chen L, Wilkins AD, Yu P, Lichtarge O, Zoghbi HY. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell 152: 984–996, 2013. doi: 10.1016/j.cell.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci USA 105: 9391–9396, 2008. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Marder E. From the connectome to brain function. Nat Methods 10: 483–490, 2013. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60: 201–214, 2008. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, Francke U. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol 514: 240–258, 2009. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 503: 121–125, 2013. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 16: 551–563, 2015. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21: 952–965, 2001. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229, 2008. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 56: 58–65, 2007. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis 35: 219–233, 2009. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A, Richards LJ. Wiring the brain: the biology of neuronal guidance. Cold Spring Harb Perspect Biol 2: a001917, 2010. doi: 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen K, Lavery LA, Baker SA, Shaw CA, Li W, Zoghbi HY. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc Natl Acad Sci USA 112: 5509–5514, 2015. doi: 10.1073/pnas.1505909112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27: 327–331, 2001. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302: 885–889, 2003. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cohen DR, Matarazzo V, Palmer AM, Tu Y, Jeon OH, Pevsner J, Ronnett GV. Expression of MeCP2 in olfactory receptor neurons is developmentally regulated and occurs before synaptogenesis. Mol Cell Neurosci 22: 417–429, 2003. doi: 10.1016/S1044-7431(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 72: 72–85, 2011. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma MP, Tanaka T, Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97: 299–311, 1999. doi: 10.1016/S0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, Liu W, Klei L, Lei J, Yin J, Reilly SK, Tebbenkamp AT, Bichsel C, Pletikos M, Sestan N, Roeder K, State MW, Devlin B, Noonan JP. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun 6: 6404, 2015. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393: 377–381, 1998. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimäki T, Lin CF, Ma’ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnström K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Rüther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515: 209–215, 2014. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature 542: 433–438, 2017. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. From the connectome to the synaptome: an epic love story. Science 330: 1198–1201, 2010. doi: 10.1126/science.1193378. [DOI] [PubMed] [Google Scholar]
- Dietrich JB. The MEF2 family and the brain: from molecules to memory. Cell Tissue Res 352: 179–190, 2013. doi: 10.1007/s00441-013-1565-2. [DOI] [PubMed] [Google Scholar]
- Du Q, Luu PL, Stirzaker C, Clark SJ. Methyl-CpG-binding domain proteins: readers of the epigenome. Epigenomics 7: 1051–1073, 2015. doi: 10.2217/epi.15.39. [DOI] [PubMed] [Google Scholar]
- Durak O, Gao F, Kaeser-Woo YJ, Rueda R, Martorell AJ, Nott A, Liu CY, Watson LA, Tsai LH. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat Neurosci 19: 1477–1488, 2016. doi: 10.1038/nn.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 311: 1008–1012, 2006. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60: 1022–1038, 2008. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem 278: 4035–4040, 2003. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- Gabel HW, Kinde B, Stroud H, Gilbert CS, Harmin DA, Kastan NR, Hemberg M, Ebert DH, Greenberg ME. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature 522: 89–93, 2015. doi: 10.1038/nature14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiretti AE, Moore AR, Brenner RG, Chen LF, West AE, Lau NC, Van Hooser SD, Paradis S. Rem2 is an activity-dependent negative regulator of dendritic complexity in vivo. J Neurosci 34: 392–407, 2014. doi: 10.1523/JNEUROSCI.1328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett LA, Kelvin DJ, Sternberg EA, Olson EN. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol 9: 5022–5033, 1989. doi: 10.1128/MCB.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol 40: 519–561, 2000. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- Guo JU, Su Y, Shin JH, Shin J, Li H, Xie B, Zhong C, Hu S, Le T, Fan G, Zhu H, Chang Q, Gao Y, Ming GL, Song H. Distribution, recognition and regulation of non-CpG methylation in the adult mammalian brain. Nat Neurosci 17: 215–222, 2014. doi: 10.1038/nn.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol 14: 471–479, 1983. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hagberg G, Stenbom Y, Engerström IW. Head growth in Rett syndrome. Brain Dev 23, Suppl 1: S227–S229, 2001. doi: 10.1016/S0387-7604(01)00375-8. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res 21: 396–420, 2011. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AJ, Raissi A, Rajkovich K, Berto S, Kumar J, Molinaro G, Raduazzo J, Guo Y, Loerwald K, Konopka G, Huber KM, Cowan CW. MEF2C regulates cortical inhibitory and excitatory synapses and behaviors relevant to neurodevelopmental disorders. eLife 5: e20059, 2016. doi: 10.7554/eLife.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature 463: 474–484, 2010. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 60: 610–624, 2008. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Cui K, Northrup D, Liu C, Wang C, Tang Q, Ge K, Levens D, Crane-Robinson C, Zhao K. H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12: 180–192, 2013. doi: 10.1016/j.stem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755, 1999. doi: 10.1016/S0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826, 2008. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, Smith JD, Paeper B, Nickerson DA, Dea J, Dong S, Gonzalez LE, Mandell JD, Mane SM, Murtha MT, Sullivan CA, Walker MF, Waqar Z, Wei L, Willsey AJ, Yamrom B, Lee YH, Grabowska E, Dalkic E, Wang Z, Marks S, Andrews P, Leotta A, Kendall J, Hakker I, Rosenbaum J, Ma B, Rodgers L, Troge J, Narzisi G, Yoon S, Schatz MC, Ye K, McCombie WR, Shendure J, Eichler EE, State MW, Wigler M. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515: 216–221, 2014. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani-Asl A, Cheng C, Zhang C, Bonni A. Pathogenesis of Börjeson-Forssman-Lehmann syndrome: insights from PHF6 function. Neurobiol Dis 96: 227–235, 2016. doi: 10.1016/j.nbd.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19: 187–191, 1998. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci Adv 1: e1500447, 2015. doi: 10.1126/sciadv.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Nishiyama M, Shoji H, Ohkawa Y, Kawamura A, Sato T, Suyama M, Takumi T, Miyakawa T, Nakayama KI. CHD8 haploinsufficiency results in autistic-like phenotypes in mice. Nature 537: 675–679, 2016. doi: 10.1038/nature19357. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138, 1996. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kehoe LA, Bernardinelli Y, Muller D. GluN3A: an NMDA receptor subunit with exquisite properties and functions. Neural Plast 2013: 145387, 2013. doi: 10.1155/2013/145387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol 36: 189–204, 2004. doi: 10.1016/S1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Khwaja OS, Ho E, Barnes KV, O’Leary HM, Pereira LM, Finkelstein Y, Nelson CA 3rd, Vogel-Farley V, DeGregorio G, Holm IA, Khatwa U, Kapur K, Alexander ME, Finnegan DM, Cantwell NG, Walco AC, Rappaport L, Gregas M, Fichorova RN, Shannon MW, Sur M, Kaufmann WE. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc Natl Acad Sci USA 111: 4596–4601, 2014. doi: 10.1073/pnas.1311141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME. Widespread transcription at neuronal activity-regulated enhancers. Nature 465: 182–187, 2010. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IF, Yandava CN, Mabb AM, Hsiao JS, Huang HS, Pearson BL, Calabrese JM, Starmer J, Parker JS, Magnuson T, Chamberlain SJ, Philpot BD, Zylka MJ. Topoisomerases facilitate transcription of long genes linked to autism. Nature 501: 58–62, 2013. doi: 10.1038/nature12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci 34: 591–598, 2011. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniewska B, Nader K, Dabrowski M, Kaczmarek L, Kalita K. Adult deletion of SRF increases epileptogenesis and decreases activity-induced gene expression. Mol Neurobiol 53: 1478–1493, 2016. doi: 10.1007/s12035-014-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, Auvin S, Boucher C, Kerckaert JP, David V, Manouvrier-Hanu S, Saugier-Veber P, Frébourg T, Dubourg C, Andrieux J, Bonneau D. MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Genet 47: 22–29, 2010. doi: 10.1136/jmg.2009.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer D, Krainc D, Yu YT, McDermott J, Breitbart RE, Heng J, Neve RL, Kosofsky B, Nadal-Ginard B, Lipton SA. MEF2C, a MADS/MEF2-family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci USA 90: 1546–1550, 1993. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 55: 201–215, 2007. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69: 905–914, 1992. doi: 10.1016/0092-8674(92)90610-O. [DOI] [PubMed] [Google Scholar]
- Li H, Radford JC, Ragusa MJ, Shea KL, McKercher SR, Zaremba JD, Soussou W, Nie Z, Kang YJ, Nakanishi N, Okamoto S, Roberts AJ, Schwarz JJ, Lipton SA. Transcription factor MEF2C influences neural stem/progenitor cell differentiation and maturation in vivo. Proc Natl Acad Sci USA 105: 9397–9402, 2008. doi: 10.1073/pnas.0802876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276: 1404–1407, 1997. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455: 1198–1204, 2008. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science 341: 1237905, 2013. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe-Mie Y, Lepagnol-Bestel AM, Maussion G, Doron-Faigenboim A, Imbeaud S, Delacroix H, Aggerbeck L, Pupko T, Gorwood P, Simonneau M, Moalic JM. SMARCA2 and other genome-wide supported schizophrenia-associated genes: regulation by REST/NRSF, network organization and primate-specific evolution. Hum Mol Genet 19: 2841–2857. doi: 10.1093/hmg/ddq184. [DOI] [PubMed] [Google Scholar]
- Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, Olson EN. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351: 400–403, 2016. doi: 10.1126/science.aad5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci 15: 5727–5738, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, Chen LF, Deng JV, Finn C, Pfenning AR, Sabhlok A, Wilson KM, West AE. The transcription factor calcium-response factor limits NMDA receptor-dependent transcription in the developing brain. J Neurochem 137: 164–176, 2016. doi: 10.1111/jnc.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, Schwarz CM, West AE. Members of the myocyte enhancer factor 2 transcription factor family differentially regulate Bdnf transcription in response to neuronal depolarization. J Neurosci 32: 12780–12785, 2012. doi: 10.1523/JNEUROSCI.0534-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, West AE. Mechanisms of specificity in neuronal activity-regulated gene transcription. Prog Neurobiol 94: 259–295, 2011. doi: 10.1016/j.pneurobio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyst MJ, Ekiert R, Ebert DH, Merusi C, Nowak J, Selfridge J, Guy J, Kastan NR, Robinson ND, de Lima Alves F, Rappsilber J, Greenberg ME, Bird A. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat Neurosci 16: 898–902, 2013. doi: 10.1038/nn.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci 9: 650–659, 2006. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286: 785–790, 1999. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302: 890–893, 2003. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- McDowell KA, Hutchinson AN, Wong-Goodrich SJ, Presby MM, Su D, Rodriguiz RM, Law KC, Williams CL, Wetsel WC, West AE. Reduced cortical BDNF expression and aberrant memory in Carf knock-out mice. J Neurosci 30: 7453–7465, 2010. doi: 10.1523/JNEUROSCI.3997-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci 27: 40–47, 2002. doi: 10.1016/S0968-0004(01)02031-X. [DOI] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, Sommer A, Moore CA, Hopkin RJ, Spallone PA, Keating MT, Osborne L, Kimberley KW, Stock AD. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A 123A: 45–59, 2003. doi: 10.1002/ajmg.a.20496. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science 321: 218–223, 2008. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F, Haas S, Jamali P, Zecha A, Mohseni M, Püttmann L, Vahid LN, Jensen C, Moheb LA, Bienek M, Larti F, Mueller I, Weissmann R, Darvish H, Wrogemann K, Hadavi V, Lipkowitz B, Esmaeeli-Nieh S, Wieczorek D, Kariminejad R, Firouzabadi SG, Cohen M, Fattahi Z, Rost I, Mojahedi F, Hertzberg C, Dehghan A, Rajab A, Banavandi MJ, Hoffer J, Falah M, Musante L, Kalscheuer V, Ullmann R, Kuss AW, Tzschach A, Kahrizi K, Ropers HH. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478: 57–63, 2011. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389, 1998. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH Jr, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485: 242–245, 2012. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, Asokan A, Zhang F, Duan D, Gersbach CA. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351: 403–407, 2016. doi: 10.1126/science.aad5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novara F, Beri S, Giorda R, Ortibus E, Nageshappa S, Darra F, Dalla Bernardina B, Zuffardi O, Van Esch H. Refining the phenotype associated with MEF2C haploinsufficiency. Clin Genet 78: 471–477, 2010. doi: 10.1111/j.1399-0004.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- Nowakowska BA, Obersztyn E, Szymańska K, Bekiesińska-Figatowska M, Xia Z, Ricks CB, Bocian E, Stockton DW, Szczałuba K, Nawara M, Patel A, Scott DA, Cheung SW, Bohan TP, Stankiewicz P. Severe mental retardation, seizures, and hypotonia due to deletions of MEF2C. Am J Med Genet B Neuropsychiatr Genet 153B: 1042–1051, 2010. doi: 10.1002/ajmg.b.31071. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, Munson J, Hiatt JB, Turner EH, Levy R, O’Day DR, Krumm N, Coe BP, Martin BK, Borenstein E, Nickerson DA, Mefford HC, Doherty D, Akey JM, Bernier R, Eichler EE, Shendure J. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338: 1619–1622, 2012a. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485: 246–250, 2012b. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA 97: 7561–7566, 2000. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooe N, Saito K, Mikami N, Nakatuka I, Kaneko H. Identification of a novel basic helix-loop-helix-PAS factor, NXF, reveals a Sim2 competitive, positive regulatory role in dendritic-cytoskeleton modulator drebrin gene expression. Mol Cell Biol 24: 608–616, 2004. doi: 10.1128/MCB.24.2.608-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorkowski AR, Traylor RN, Rosenfeld JA, Hoover JM, Harris CJ, Winter S, Lacassie Y, Bialer M, Lamb AN, Schultz RA, Berry-Kravis E, Porter BE, Falk M, Venkat A, Vanzo RJ, Cohen JS, Fatemi A, Dobyns WB, Shaffer LG, Ballif BC, Marsh ED. MEF2C haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways. Neurogenetics 14: 99–111, 2013. doi: 10.1007/s10048-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron 59: 70–83, 2008. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Gardner KH. Coactivator recruitment: a new role for PAS domains in transcriptional regulation by the bHLH-PAS family. J Cell Physiol 223: 553–557, 2010. doi: 10.1002/jcp.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Zang T, Wilkerson JR, Taniguchi M, Maksimova MA, Smith LN, Cowan CW, Huber KM. Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66: 191–197, 2010. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, Zhou Y, Slaymaker IM, Shetty AS, Weisbach NR, Kim JA, Sharma J, Desai M, Sood S, Kempton HR, Crabtree GR, Feng G, Zhang F. Chd8 mutation leads to autistic-like behaviors and impaired striatal circuits. Cell Rep 19: 335–350, 2017. doi: 10.1016/j.celrep.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci 8: 331–340, 2007. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- Pon JR, Marra MA. MEF2 transcription factors: developmental regulators and emerging cancer genes. Oncotarget 7: 2297–2312, 2016. doi: 10.18632/oncotarget.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Ghosh A. A calcium-dependent switch in a CREST-BRG1 complex regulates activity-dependent gene expression. Neuron 60: 775–787, 2008. doi: 10.1016/j.neuron.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovich KE, Loerwald KW, Hale CF, Hess CT, Gibson JR, Huber KM. Experience-dependent and differential regulation of local and long-range excitatory neocortical circuits by postsynaptic Mef2c. Neuron 93: 48–56, 2017. doi: 10.1016/j.neuron.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Díez-García J, Rodriguiz RM, López IP, Luján R, Martínez-Turrillas R, Picó E, Henson MA, Bernardo DR, Jarrett TM, Clendeninn DJ, López-Mascaraque L, Feng G, Lo DC, Wesseling JF, Wetsel WC, Philpot BD, Pérez-Otaño I. Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron 63: 342–356, 2009. doi: 10.1016/j.neuron.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha H, Sampaio M, Rocha R, Fernandes S, Leão M. MEF2C haploinsufficiency syndrome: report of a new MEF2C mutation and review. Eur J Med Genet 59: 478–482, 2016. doi: 10.1016/j.ejmg.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet 14: 347–359, 2013. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron 39: 13–25, 2003. doi: 10.1016/S0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Sanders SJ. First glimpses of the neurobiology of autism spectrum disorder. Curr Opin Genet Dev 33: 80–92, 2015. doi: 10.1016/j.gde.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Shalizi A, Gaudillière B, Yuan Z, Stegmüller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science 311: 1012–1017, 2006. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Sheffield NC, Thurman RE, Song L, Safi A, Stamatoyannopoulos JA, Lenhard B, Crawford GE, Furey TS. Patterns of regulatory activity across diverse human cell types predict tissue identity, transcription factor binding, and long-range interactions. Genome Res 23: 777–788, 2013. doi: 10.1101/gr.152140.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Antolin S, Lin CW, Lin Y, Lois C. Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J Neurosci 33: 7928–7940, 2013. doi: 10.1523/JNEUROSCI.1571-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell 37: 457–468, 2010. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, Harmin DA, Greenberg ME. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 157: 1216–1229, 2014. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman HA, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, Kvarnung M, Gerdts J, Trinh S, Cosemans N, Vives L, Lin J, Turner TN, Santen G, Ruivenkamp C, Kriek M, van Haeringen A, Aten E, Friend K, Liebelt J, Barnett C, Haan E, Shaw M, Gecz J, Anderlid BM, Nordgren A, Lindstrand A, Schwartz C, Kooy RF, Vandeweyer G, Helsmoortel C, Romano C, Alberti A, Vinci M, Avola E, Giusto S, Courchesne E, Pramparo T, Pierce K, Nalabolu S, Amaral DG, Scheffer IE, Delatycki MB, Lockhart PJ, Hormozdiari F, Harich B, Castells-Nobau A, Xia K, Peeters H, Nordenskjöld M, Schenck A, Bernier RA, Eichler EE. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet 49: 515–526, 2017. doi: 10.1038/ng.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci 15: 6509–6520, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugathan A, Biagioli M, Golzio C, Erdin S, Blumenthal I, Manavalan P, Ragavendran A, Brand H, Lucente D, Miles J, Sheridan SD, Stortchevoi A, Kellis M, Haggarty SJ, Katsanis N, Gusella JF, Talkowski ME. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc Natl Acad Sci USA 111: E4468–E4477, 2014. doi: 10.1073/pnas.1405266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Okaty BW, Arnson HA, Kato S, Dani VS, Nelson SB. Cell-type-specific repression by methyl-CpG-binding protein 2 is biased toward long genes. J Neurosci 34: 12877–12883, 2014. doi: 10.1523/JNEUROSCI.2674-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabebordbar M, Zhu K, Cheng JK, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, Cong L, Zhang F, Vandenberghe LH, Church GM, Wagers AJ. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351: 407–411, 2016. doi: 10.1126/science.aad5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Rosenfeld JA, Blumenthal I, Pillalamarri V, Chiang C, Heilbut A, Ernst C, Hanscom C, Rossin E, Lindgren AM, Pereira S, Ruderfer D, Kirby A, Ripke S, Harris DJ, Lee JH, Ha K, Kim HG, Solomon BD, Gropman AL, Lucente D, Sims K, Ohsumi TK, Borowsky ML, Loranger S, Quade B, Lage K, Miles J, Wu BL, Shen Y, Neale B, Shaffer LG, Daly MJ, Morton CC, Gusella JF. Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell 149: 525–537, 2012. doi: 10.1016/j.cell.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709–726, 1998. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron 33: 383–395, 2002. doi: 10.1016/S0896-6273(01)00561-X. [DOI] [PubMed] [Google Scholar]
- Telese F, Ma Q, Perez PM, Notani D, Oh S, Li W, Comoletti D, Ohgi KA, Taylor H, Rosenfeld MG. LRP8-Reelin-regulated neuronal enhancer signature underlying learning and memory formation. Neuron 86: 696–710, 2015. doi: 10.1016/j.neuron.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA 106: 2029–2034, 2009. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai NP, Wilkerson JR, Guo W, Maksimova MA, DeMartino GN, Cowan CW, Huber KM. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 151: 1581–1594, 2012. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Matheos DP, Barrett RM, Kramár EA, Azzawi S, Chen Y, Magnan CN, Zeller M, Sylvain A, Haettig J, Jia Y, Tran A, Dang R, Post RJ, Chabrier M, Babayan AH, Wu JI, Crabtree GR, Baldi P, Baram TZ, Lynch G, Wood MA. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci 16: 552–561, 2013. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Wood MA. Neuron-specific chromatin remodeling: a missing link in epigenetic mechanisms underlying synaptic plasticity, memory, and intellectual disability disorders. Neuropharmacology 80: 18–27, 2014. doi: 10.1016/j.neuropharm.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Lin M, Pedrosa E, Hrabovsky A, Zhang Z, Guo W, Lachman HM, Zheng D. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Mol Autism 6: 55, 2015. doi: 10.1186/s13229-015-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol 3: a005744, 2011. doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature 299: 583–591, 1982. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wilkerson JR, Tsai NP, Maksimova MA, Wu H, Cabalo NP, Loerwald KW, Dictenberg JB, Gibson JR, Huber KM. A role for dendritic mGluR5-mediated local translation of Arc/Arg3.1 in MEF2-dependent synapse elimination. Cell Reports 7: 1589–1600, 2014. doi: 10.1016/j.celrep.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron 56: 94–108, 2007. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yang Y, Hemberg M, Yoshida T, Cho HY, Murphy JP, Fioravante D, Regehr WG, Gygi SP, Georgopoulos K, Bonni A. Promoter decommissioning by the NuRD chromatin remodeling complex triggers synaptic connectivity in the mammalian brain. Neuron 83: 122–134, 2014. doi: 10.1016/j.neuron.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yamada T, Hill KK, Hemberg M, Reddy NC, Cho HY, Guthrie AN, Oldenborg A, Heiney SA, Ohmae S, Medina JF, Holy TE, Bonni A. Chromatin remodeling inactivates activity genes and regulates neural coding. Science 353: 300–305, 2016. doi: 10.1126/science.aad4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen RK, Merico D, Bookman M, L Howe J, Thiruvahindrapuram B, Patel RV, Whitney J, Deflaux N, Bingham J, Wang Z, Pellecchia G, Buchanan JA, Walker S, Marshall CR, Uddin M, Zarrei M, Deneault E, D’Abate L, Chan AJ, Koyanagi S, Paton T, Pereira SL, Hoang N, Engchuan W, Higginbotham EJ, Ho K, Lamoureux S, Li W, MacDonald JR, Nalpathamkalam T, Sung WW, Tsoi FJ, Wei J, Xu L, Tasse AM, Kirby E, Van Etten W, Twigger S, Roberts W, Drmic I, Jilderda S, Modi BM, Kellam B, Szego M, Cytrynbaum C, Weksberg R, Zwaigenbaum L, Woodbury-Smith M, Brian J, Senman L, Iaboni A, Doyle-Thomas K, Thompson A, Chrysler C, Leef J, Savion-Lemieux T, Smith IM, Liu X, Nicolson R, Seifer V, Fedele A, Cook EH, Dager S, Estes A, Gallagher L, Malow BA, Parr JR, Spence SJ, Vorstman J, Frey BJ, Robinson JT, Strug LJ, Fernandez BA, Elsabbagh M, Carter MT, Hallmayer J, Knoppers BM, Anagnostou E, Szatmari P, Ring RH, Glazer D, Pletcher MT, Scherer SW. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci 20: 602–611, 2017. doi: 10.1038/nn.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A 152A: 674–686, 2010. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Shi X, Zhang Z, Chen Y, Wu JI. Dual role of Brg chromatin remodeling factor in Sonic hedgehog signaling during neural development. Proc Natl Acad Sci USA 108: 12758–12763, 2011. doi: 10.1073/pnas.1018510108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Roberts DN, Cairns BR. Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123: 219–231, 2005. doi: 10.1016/j.cell.2005.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cao M, Chang CW, Wang C, Shi X, Zhan X, Birnbaum SG, Bezprozvanny I, Huber KM, Wu JI. Autism-associated chromatin regulator Brg1/SmarcA4 is required for synapse development and myocyte enhancer factor 2-mediated synapse remodeling. Mol Cell Biol 36: 70–83, 2015. doi: 10.1128/MCB.0s0534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, Reardon W, Saraiva J, Cabral A, Gohring I, Devriendt K, de Ravel T, Bijlsma EK, Hennekam RC, Orrico A, Cohen M, Dreweke A, Reis A, Nurnberg P, Rauch A. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome). Am J Hum Genet 80: 994–1001, 2007. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]