Abstract
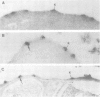
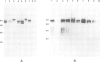
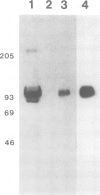
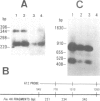
Plasmodium falciparum modifies the host erythrocyte's plasma membrane by the formation of electron-dense structures called knobs. We have produced monoclonal antibodies (McAbs) which specifically bind to the knobs in immunoelectron microscopic experiments with thin sections of parasitized erythrocytes. However, the McAbs fail to bind to the surface of live parasitized erythrocytes. Immunoblotting experiments with these McAbs show the antigen is localized to the erythrocyte plasma membrane. The antigen with which the McAbs react varies in mol. wt from 80 to 95 kd in different knob-producing isolates of P. falciparum and is absent in knobless variants. The McAbs react with the expressed product of a P. falciparum cDNA clone, thus demonstrating that the clone encodes part of this knob-associated protein. The sequence of the cDNA fragment partially overlaps a published cDNA sequence reported to encode the amino-terminal portion of the knob protein, and extends the predicted open reading frame by 190 amino acids. The carboxyl-terminal portion of the predicted amino acid sequence contains a highly charged stretch of approximately 100 amino acid residues. We suggest that this unusual, highly charged region participates in intermolecular salt bridging leading to dense packing of these molecules. This would create the electron-dense regions observed by electron microscopy and might also explain the insolubility of the knob-associated protein in the absence of strong ionic detergents or chaotropic agents.
Full text
PDF
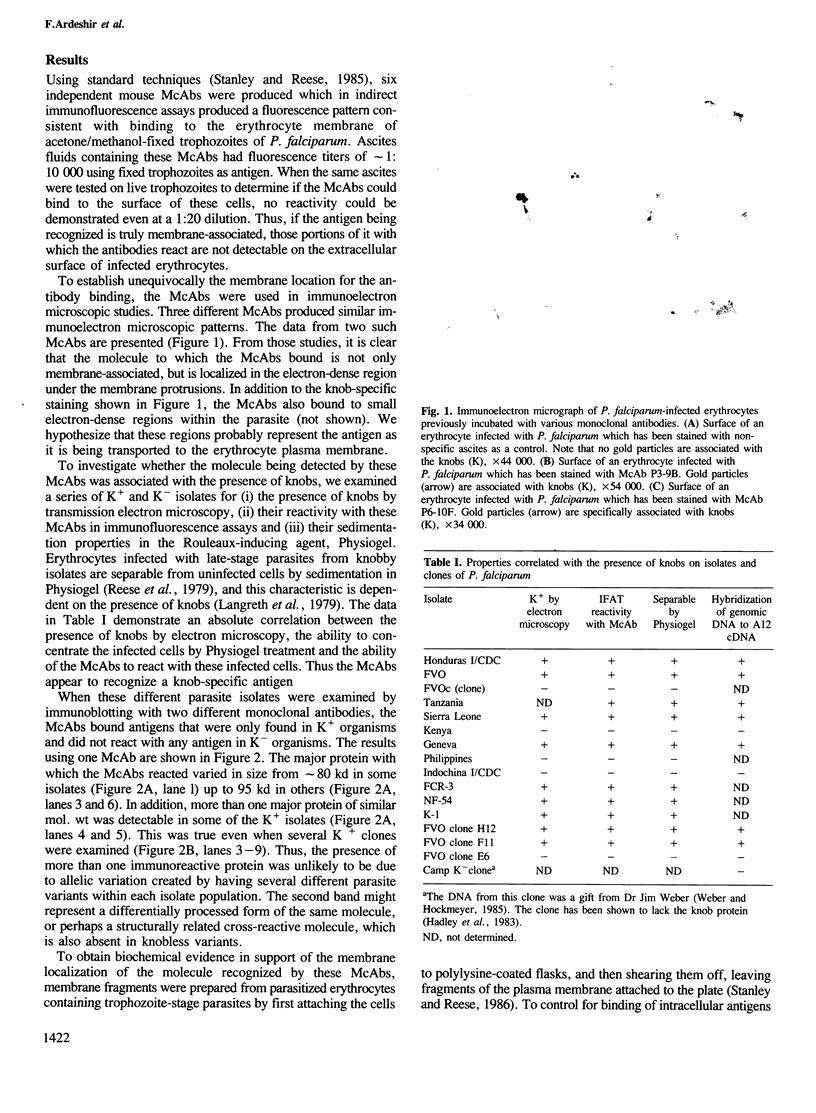

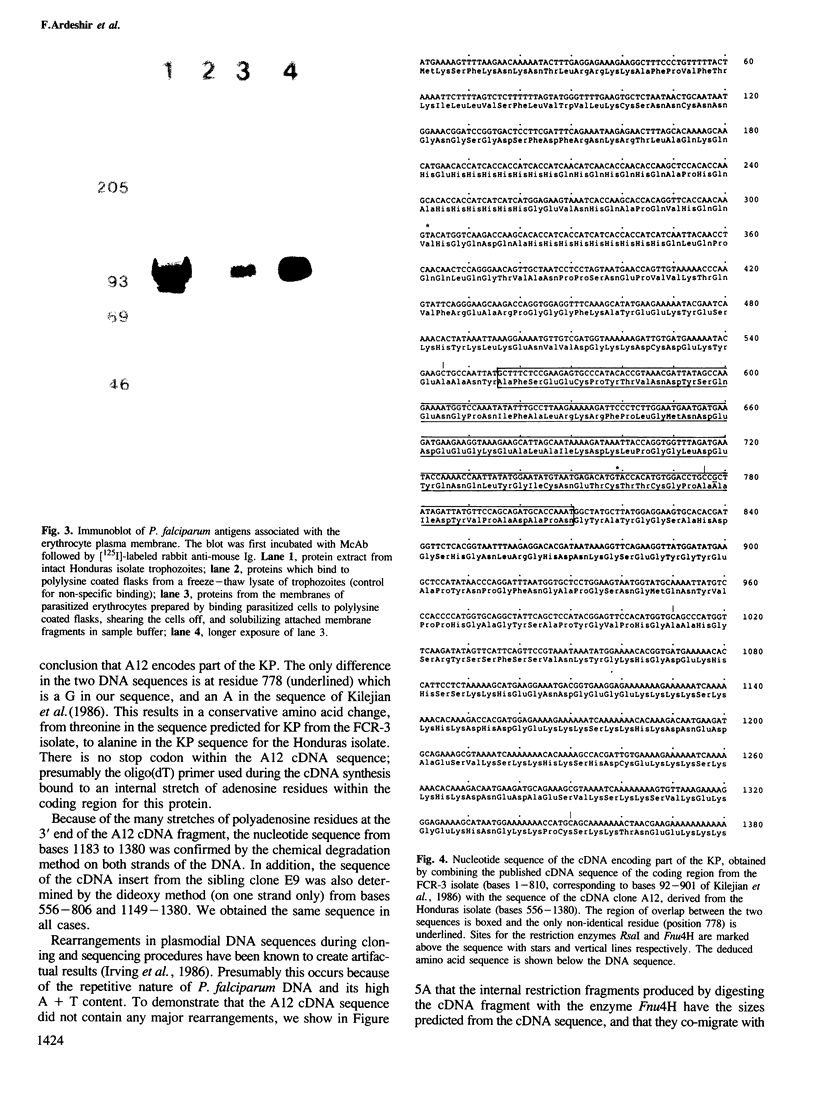
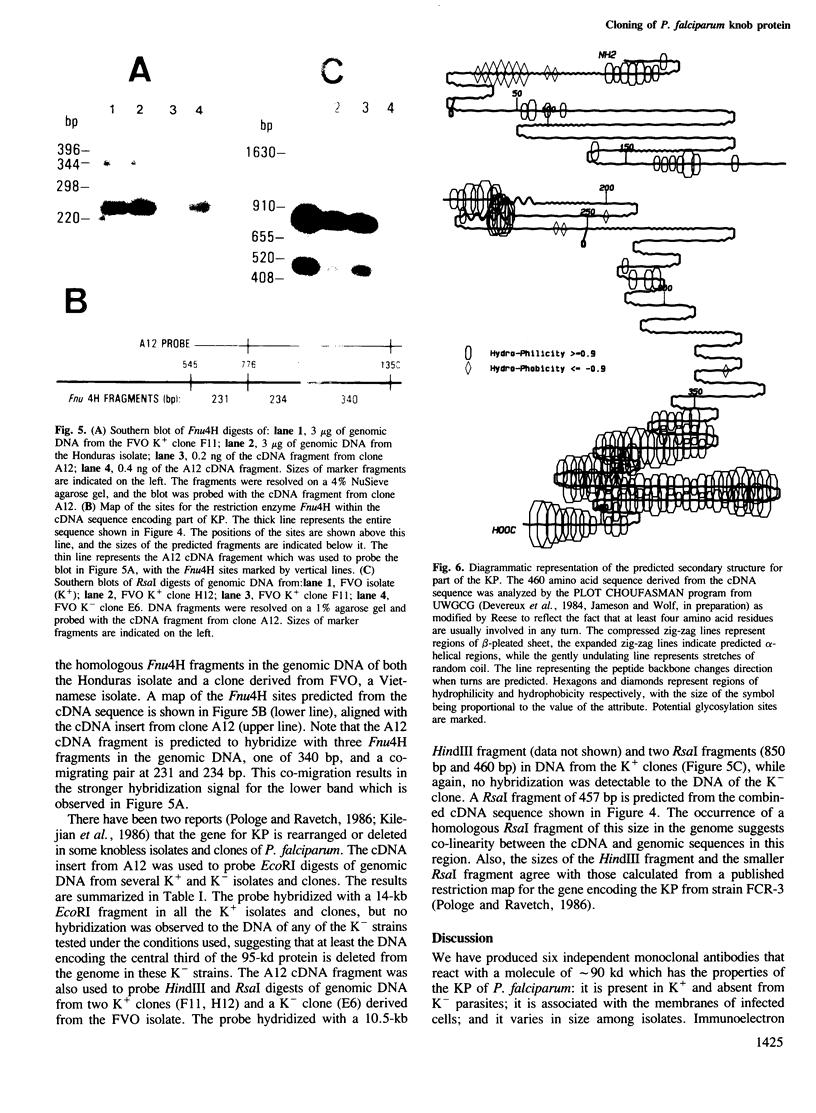


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Flint J. E., Reese R. T. Expression of Plasmodium falciparum surface antigens in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2518–2522. doi: 10.1073/pnas.82.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. The erythrocyte anion transport protein is contranslationally inserted into microsomes. Cell. 1982 Jan;28(1):23–31. doi: 10.1016/0092-8674(82)90371-3. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Kutner S., Krugliak M., Cabantchik Z. I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985 Mar;14(3):313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Gritzmacher C. A., Reese R. T. Reversal of knob formation on Plasmodium falciparum-infected erythrocytes. Science. 1984 Oct 5;226(4670):65–67. doi: 10.1126/science.6382613. [DOI] [PubMed] [Google Scholar]
- Hadley T. J., Leech J. H., Green T. J., Daniel W. A., Wahlgren M., Miller L. H., Howard R. J. A comparison of knobby (K+) and knobless (K-) parasites from two strains of Plasmodium falciparum. Mol Biochem Parasitol. 1983 Nov;9(3):271–278. doi: 10.1016/0166-6851(83)90102-0. [DOI] [PubMed] [Google Scholar]
- Irving D. O., Cross G. A., Feder R., Wallach M. Structure and organization of the histidine-rich protein gene of Plasmodium lophurae. Mol Biochem Parasitol. 1986 Feb;18(2):223–234. doi: 10.1016/0166-6851(86)90040-x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. Homology between a histidine-rich protein from Plasmodium lophurae and a protein associated with the knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. J Exp Med. 1980 Jun 1;151(6):1534–1538. doi: 10.1084/jem.151.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Sharma Y. D., Karoui H., Naslund L. Histidine-rich domain of the knob protein of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7938–7941. doi: 10.1073/pnas.83.20.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A. The biosynthesis of the knob protein and a 65 000 dalton histidine-rich polypeptide of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jun;12(2):185–194. doi: 10.1016/0166-6851(84)90134-8. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect Immun. 1985 Mar;47(3):760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T. Antigenicity of the infected-erythrocyte and merozoite surfaces in Falciparum malaria. J Exp Med. 1979 Nov 1;150(5):1241–1254. doi: 10.1084/jem.150.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T., Motyl M. R., Trager W. Plasmodium falciparum: loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol. 1979 Oct;48(2):213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Langreth S. G., Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull World Health Organ. 1979;57 (Suppl 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H. A., Mayes J. T., Cooper N. R., Reese R. T. Complement activation by the surface of Plasmodium falciparum infected erythrocytes. Mol Immunol. 1984 Feb;21(2):145–150. doi: 10.1016/0161-5890(84)90129-9. [DOI] [PubMed] [Google Scholar]
- Stanley H. A., Reese R. T. Monkey-derived monoclonal antibodies against Plasmodium falciparum. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6272–6275. doi: 10.1073/pnas.82.18.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H. A., Reese R. T. Plasmodium falciparum polypeptides associated with the infected erythrocyte plasma membrane. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6093–6097. doi: 10.1073/pnas.83.16.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. Time-course of synthesis, transport and incorporation of a protein identified in purified membranes of host erythrocytes infected with a knob-forming strain of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jul;12(3):337–350. doi: 10.1016/0166-6851(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Hockmeyer W. T. Structure of the circumsporozoite protein gene in 18 strains of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;15(3):305–316. doi: 10.1016/0166-6851(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Wilson R. J., Bates P. A., McCready S., Perler F., Qiang B. U. Nuclear and mitochondrial DNA of the primate malarial parasite Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Feb;14(2):199–209. doi: 10.1016/0166-6851(85)90038-6. [DOI] [PubMed] [Google Scholar]