Abstract
Free radicals in tobacco smoke are thought to be an important cause of smoking-induced diseases, yet the variation in free radical exposure to smokers from different brands of commercially available cigarettes is unknown. We measured the levels of highly reactive gas-phase and stable particulate-phase radicals in mainstream cigarette smoke by electron paramagnetic resonance (EPR) spectroscopy with and without the spin-trapping agent phenyl-N-tert-butylnitrone (PBN), respectively, in 27 popular US cigarettes and the 3R4F research cigarette, machine-smoked according to the FTC protocol. We find a 12-fold variation in the levels of gas-phase radicals (1.2 to 14 nmol per cigarette) and a 2-fold variation in the amounts of particulate-phase radicals (44 to 96 pmol per cigarette) across the range of cigarette brands. Gas and particulate-phase radicals were highly correlated across brands (ρ=0.62, p<0.001). Both radicals were correlated with TPM (gas-phase: ρ=0.38, p=0.04; particulate-phase: ρ=0.44, p=0.02) and ventilation (gas- and tar-phase: ρ= −0.58, p=0.001), with ventilation explaining nearly 30% of the variation in radical levels across brands. Overall, our findings of significant brand variation in free radical delivery under standardized machine-smoked conditions suggest that the use of certain brands of cigarettes may be associated with greater levels of oxidative stress in smokers.
Graphical abstract
INTRODUCTION
Cigarette smoke is a harmful mixture of more than 7000 chemicals generated by a combination of combustion, pyrolysis and distillation with temperature reaching up to 950°C under both oxygen-rich as well as oxygen-depleted conditions.1, 2 It has been known for almost seven decades that free radicals are generated in high concentrations as a result of complex reactions in mainstream smoke.3–7 Due to the presence of an unpaired electron, radicals are unstable, highly reactive and redox active. The inhaled free radicals, in turn, can cause significant oxidative damage to major biological macromolecules (DNA, lipids, protein and carbohydrates) and trigger the dysregulation of redox signaling pathways impacting numerous critical cellular functions.8–10 The resulting oxidative damage and stress has been implicated in the initiation and propagation of many chronic and degenerative diseases in smokers including cancer,11–13 cardiovascular diseases,12, 14–16 neurodegeneration,17 and lung diseases.12, 18, 19
Elegant work by Pryor and colleagues in the 1980s characterized cigarette smoke radicals into two types with distinct redox and chemical properties that partition into gas and particulate phases.5, 6, 20, 21 The gas-phase of the cigarette smoke contains oxygen- and carbon-centered radicals that are very short-lived and highly reactive. These radicals can penetrate the upper respiratory tract and propagate their damaging effects despite their relatively short half-lives and limited diffusion times.8 In contrast to the gas-phase radicals, the particulate-phase contains radical species such as semiquinone, which are relatively longer-lived and can persist in some cases up to several months. Exposure to these “Environmentally Persistent Free Radicals” (EPFRs) can occur directly in the smoker or indirectly in the passively exposed individual and can penetrate deep within the respiratory tract.
Research on the exposure and biological impact of free radicals, especially gas-phase radicals, has been hampered by their extremely short half-lives (milliseconds) and high reactivity making measurements difficult. Consequently, little is known regarding the levels and variation of free radical exposure in smokers and the impact that differences in exposure may have on health status. Our main goal was to systematically quantify free radicals produced from commercial cigarettes available in the US market as a means of estimating the range of tobacco-derived free radical exposure in smokers.
Numerous techniques have been used to measure free radicals in tobacco smoke yielding widely different results, and few attempts have been made to standardize these methodologies.22–29 Thus, as part of this research, we developed a standardized protocol for the reproducible and quantitative determination of free radicals in mainstream smoke using electron paramagnetic resonance (EPR) spectroscopy. EPR is a sensitive and selective technique for directly detecting and quantitating free radicals in complex mixtures such as cigarette smoke.30 In the current investigation, mainstream smoke from cigarettes was generated under the Federal Trade Commission (FTC) regimen, and both highly reactive gas-phase radicals and stable particulate-phase radicals were measured by EPR.
This is the first study to assess and rank free radical yields across commercially available cigarettes. Using these data, direct exposure of smokers to harmful free radicals can be assessed. These data can have important public health and regulatory implications given the importance of free radical exposure in the development of cigarette-induced diseases.
MATERIALS AND METHODS
Materials
The 3R4F research cigarette was obtained from the University of Kentucky (Lexington, Kentucky, USA). All the commercial cigarettes were purchased locally (Hershey, PA): 26 King size (85 mm) and Virginia Slims Gold (100 mm). The cigarette brand varieties were selected based on their market share or unique characteristics. Marlboro, Newport, Pall Mall and Camel were selected for their popularity, with variants of these brands comprising an estimated total US market share of 66%.31 Parliament and American Spirit exemplify the types of unique characteristics such as recessed filters and “additive free” formulation, respectively. The cigarettes were stored in their original packaging long term at −20°C in airtight plastic bags. Analytical grade chemicals: nitrone spin trap phenyl-N-tert-butylnitrone (PBN), tert-butylbenzene, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL), heptadecane from Sigma-Aldrich (St. Louis, MO, USA), Suprasil® EPR tubes (4mm o.d;Wilmad-Labglass,Vineland, NJ, USA), Schlenk line (Chemglass Life Sciences,Vineland, NJ, USA) and Cambridge filters pads (Performance Systematix Inc.,Grand Rapids, MI, USA) were used as supplied.
Mainstream Smoke Generation
The cigarettes were conditioned for testing by removing them from cold storage and placing them in a constant humidity chamber (60% relative humidity, 22°C), for at least 48 hours before smoking. Mainstream smoke was generated by using a 30-port smoking machine (Jaeger-Baumgartner, CSM JB2080). Cigarettes were smoked on the machine under the FTC smoking parameters: 35 mL puff volume, 2 second puff duration, and 60 second puff interval. For each replicate, five cigarettes were simultaneously smoked to provide for a strong EPR signal required for quantitative analysis. Mainstream smoke was separated into particulate-phase and gas-phase by passing through a Cambridge filters pad (CFP).
Analysis of Particulate-phase Radicals
A CFP, located down-stream of the smoke machine pump, was used to trap the particulate-phase radicals and was subsequently stored at −80°C in an airtight plastic bag until further analysis. EPR spectra were obtained by direct insertion of the CFP into the cavity of a Bruker eScan R spectrometer (Bruker-Biospin, Billerica, MA, USA) operating in X-band. The EPR parameters were as follows: microwave frequency, 9.7 GHz; modulation frequency, 86.0 kHz; microwave power, 6.00 mW; scan range, 50 G; modulation amplitude, 1.10 G; sweep time, 5.243 s; time constant, 10.240 ms; and conversion time, 10.240 ms. All measurements were carried out at room temperature (22 ± 1°C). Spin concentrations were determined by integration of the area under the curve of the EPR signal using WinEPR software (version 0.98, National Institute of Environmental Health Sciences, National Institutes of Health, USA). Standardized concentrations of TEMPOL in methanol or a blank methanol solution pipetted onto CFP were used to quantify the spin concentrations of cigarette particulate-phase radicals.
Analysis of Gas-phase Radicals
The gas-phase of mainstream smoke was passed through an impinger located down-stream of the pump and the CFP. Trapping of gas-phase radicals was accomplished using 4 mL ice-cold tert-butylbenzene and 0.05 M PBN. After smoking, aliquots of the PBN solution were immediately frozen in liquid nitrogen and stored at −80°C until further analysis. Upon analysis, PBN solutions were thawed at room temperature, and 400 μl aliquots were placed into EPR tubes and deoxygenated using three freeze-pump-thaw cycles with a Schlenk line, as previously described.25 Briefly, samples were frozen using liquid nitrogen, a vacuum was subsequently applied allowing trapped air to escape and gaseous argon was then introduced to provide an inert atmosphere before the sample was re-frozen. Samples were subjected to a total of three freeze-vacuum pump-thaw-argon cycles (2 minute each). The EPR spectra derived from PBN-radical adducts were measured using the following EPR parameters: microwave frequency, 9.7 GHz; modulation frequency, 86.0 kHz; microwave power, 6.00 mW; scan range, 60 G; modulation amplitude, 1.10 G; sweep time, 41.94 s; time constant, 81.92 ms; and conversion time, 81.92 ms. All measurements were carried out at room temperature (21.5 ± 0.5°C). Spin concentrations were determined by peak to peak height of the EPR signal using WinEPR software and comparison with standardized solutions of TEMPO in tert-butylbenzene. The standard TEMPO solution was not subjected to freeze-pump-thaw cycles, a common practice in the field.
Determination of Total Particulate Matter and filter ventilation of the Cigarettes
Total particulate matter (TPM) was determined by weighing the CFPs.32 Five replicates of total filter ventilation (%) of the cigarette were measured at the Centers For Disease Control and Prevention (CDC), Atlanta, USA as described in a previous study.33
Statistical Analysis
We sub-grouped the cigarette brands (Table 1) on the basis of two common cigarette design features; 1) flavor (regular vs. menthol) and 2) filter ventilation. In some analyses, cigarette brands were categorized according to filter ventilation tertiles (low, mid, and high). All analyses were performed using R statistical package 3.3.34 Summary statistics and boxplots are presented for major variables. The association between different variables is estimated and tested using robust Spearman correlations. Linear regression was used to determine the significance of the ventilation and flavor group effects. Tukey contrasts were used to evaluate all pairwise comparisons of means within the ventilation categories.
Table 1.
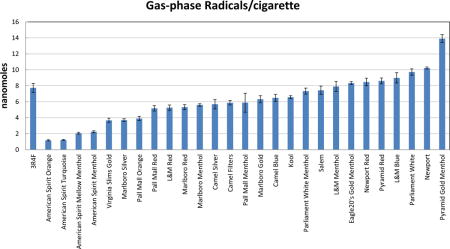
Cigarette brand identification information with UPC, listing of flavor, filter ventilation and their yields of gas-phase and particulate-phase radicals.
| Brands | UPC | Flavora | Filter Ventilation (%)b | Gas-Phase Radicalsc (nmol/cigarette) |
Particulate-Phase Radicalsd (pmol/cigarette) |
|---|---|---|---|---|---|
| 3R4F | N/A | R | 38 | 7.72 ± 2.22 | 63.87 ± 13.66 |
| American Spirit Mellow Menthol | 047995855055 | M | 39.2 | 2.06 ± 0.53 | 63.10 ± 9.47 |
| American Spirit Menthol | 047995855222 | M | 13.4 | 2.23 ± 0.63 | 64.22 ± 2.67 |
| American Spirit Orange | 047995855239 | R | 56.3 | 1.17 ± 0.28 | 44.08 ± 6.22 |
| American Spirit Turquoise | 047995855246 | R | 28.9 | 1.21 ± 0.15 | 52.72 ± 2.88 |
| Camel Blue | 01230739 | R | 32.3 | 6.49 ± 2.20 | 54.17 ± 11.38 |
| Camel Filters | 01230331 | R | 29.5 | 5.86 ± 0.84 | 60.33 ± 1.92 |
| Camel Silver | 012300200158 | R | 50.1 | 5.69 ± 1.78 | 64.04 ± 1.89 |
| Eagle20’s Gold Menthol | 01117307 | M | 10.6 | 8.33 ± 0.94 | 95.59 ± 16.76 |
| Kool | 02725228 | M | 29.6 | 6.58 ± 0.90 | 60.72 ± 9.29 |
| L&M Blue | 028200310026 | R | 16.4 | 8.99 ± 2.59 | 75.50 ± 9.61 |
| L&M Menthol | 028200310323 | M | 21.8 | 7.90 ± 0.91 | 60.38 ± 8.35 |
| L&M Red | 028200309822 | R | 19.4 | 5.26 ± 1.34 | 70.79 ± 12.14 |
| Marlboro Gold | 02838423 | R | 26.4 | 6.31 ± 1.74 | 65.71 ± 13.99 |
| Marlboro Menthol | 02836124 | M | 18.9 | 5.60 ± 0.61 | 67.03 ± 11.49 |
| Marlboro Red | 02835727 | R | 15.4 | 5.32 ± 1.26 | 59.53 ± 11.81 |
| Marlboro Silver | 02847722 | R | 45.9 | 3.70 ± 0.87 | 52.59 ± 7.92 |
| Newport | 02657512 | M | 2.4 | 10.2 ± 0.39 | 65.70 ± 5.07 |
| Newport Red | 02665618 | R | 3.5 | 8.48 ± 1.88 | 66.81 ± 5.09 |
| Pall Mall Menthol | 02785929 | M | 43.3 | 5.87 ± 1.25 | 60.00 ± 14.63 |
| Pall Mall Orange | 02717520 | R | 57.9 | 3.90 ± 1.34 | 56.87 ± 5.88 |
| Pall Mall Red | 02785123 | R | 36.2 | 5.17 ± 1.33 | 56.12 ± 21.44 |
| Parliament White | 02878421 | R | 31.8 | 9.71 ± 1.60 | 74.17 ± 9.75 |
| Parliament White Menthol | 02878722 | M | 26.1 | 7.32 ± 2.28 | 76.28 ± 3.21 |
| Pyramid Gold Menthol | 01149403 | M | 10 | 13.9 ± 1.97 | 78.98 ± 9.75 |
| Pyramid Red | 01149209 | R | 12.1 | 8.61 ± 1.85 | 74.11 ± 10.66 |
| Salem | 01232038 | M | 18.5 | 7.44 ± 2.06 | 68.36 ± 9.17 |
| Virginia Slims Gold | 02873329 | R | 41 | 3.67 ± 0.78 | 73.06 ± 3.52 |
Note: All cigarettes were from king-sizes hard packs (85mm), except 3R4F (soft pack) and Virginia Slims Gold (100s).
R = Regular; M = Menthol
Values are mean (n=5)
Values are mean ± SD (n=3–7)
Values are mean ± SD (n=2–4)
RESULTS
Standardized EPR Assessment of Tobacco Smoke Free Radicals
Standardized protocols for measurements of free radicals were developed using 3R4F research cigarettes. EPR analysis of smoke radicals requires the separation of whole smoke into its constituent gas and particulate-phases by use of Cambridge filters. Due to the lack of stability and short half-live of the gas-phase radicals in mainstream smoke, spin trapping was required prior to EPR analysis of the resulting radical adducts. For this purpose, PBN was used as the spin trap based on its ability to form adducts with a broad range of different radicals.30 Since the time between sample collection and EPR measurement was a critical factor, all analyses were performed within 15 minutes. The organic solvent for radical collection can influence trapping efficiency and stability. While benzene is commonly used, we selected to use tert-butylbenzene because it provided identical results as benzene but is far less toxic than benzene and is less subject to vaporization than other organic solvents. Since oxygen can interfere with the EPR signal, optimal results were obtained when deoxygenation was performed prior to EPR measurement.
For quantitative EPR, the use of a reference standard is required. Previous papers have used numerous reference radical standards interchangeably including diphenyl-β-pictryl hydrazil, (DPPH), TEMPO, and TEMPOL for tobacco smoke radical quantitation, very often providing few details about conditions such as the solvent used.5, 22, 35 In our studies, TEMPO in tert-butylbenzene was selected for use with gas-phase trapped radicals based on its higher degree of sensitivity upon EPR analysis. In contrast, particulate-phase radicals are not trapped, but rather collected and analyzed directly on the CFP. Since CFP emit a small background EPR signal (Figure 1B), measurement blanks were constructed by addition of solvent directly on to filter pads followed by evaporation. For these latter analyses, TEMPOL was selected as the reference standard based on its greater stability and higher sensitivity on CFP.
Figure 1.
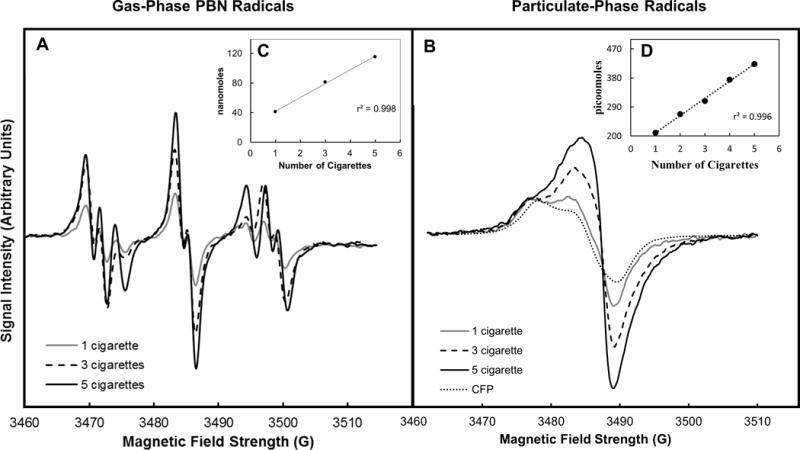
Representative EPR spectra for (A) gas-phase PBN radical adducts and (B) particulate-phase radicals for the 3R4F reference cigarette and blank CFP are shown. The concentrations of both gas-phase radicals (C) and particulate-phase radicals (D) are linearly dependent on the number of cigarettes smoked.
Utilizing optimized conditions for the collection and analysis of mainstream smoke radicals, representative EPR spectra for both gas-phase PBN radical adducts and particulate-phase stable radicals for the 3R4F reference cigarette are provided in Figure 1. The concentration of both gas-phase radicals and particulate-phase radicals was linearly dependent on the number of cigarettes smoked (R2= 0.998 and 0.996 for gas-phase and particulate-phase radicals, respectively). Results demonstrate that mainstream smoke generated from 3R4F cigarettes contained on average 8 ± 2 nmol (5 × 1015 spins, n=6) of gas-phase radicals and 64 ± 13 pmol (4 × 1013 spins, n=3) of particulate-phase radicals per cigarette, both in the ranges reported previously.5, 22 Overall, these methods provided highly reproducible results for both gas-phase radicals (3.6% precision) and particulate-phase radicals (1.7% precision).
Gas and Particulate phase Radicals in Mainstream Smoke from Popular US Cigarette Brands
Our standardized method for quantitation of tobacco smoke radicals was then used in assessing and ranking free radical yields from 27 popular commercial cigarette brands with diverse characteristics currently in the US market. As shown in Table 1, the gas-phase radical yields under the FTC smoking regimen varied widely (12-fold) among the cigarettes, ranging from 1.2 to 14 nmol per cigarette. Meanwhile, the particulate-phase radical yields varied 2-fold among the cigarettes brands under the FTC smoking regimen ranging from 44 to 96 pmol per cigarette (Table 1).
We examined whether free radical levels in the gas-phase were associated with those in the particulate-phase (Table 2). While levels of variation between products were substantially lower in the particulate-phase, overall, these levels were highly correlated to gas-phase radicals (Spearman coefficient (ρ)=0.62, p=0.0006). Correlational analyses were also performed to examine potential associations between free radicals and both TPM and filter ventilation (Table 2). Significant negative correlations were observed between filter ventilation and both gas-phase (ρ=−0.58, p=0.001) and particulate-phase radicals (ρ=−0.58, p=0.001). Significant associations were also observed between TPM and both gas-phase (ρ=0.38, p=0.04) and particulate-phase radicals (ρ=0.44, p=0.02).
Table 2.
Spearman’s rank correlation of mainstream smoke free radicals for the 27 brands of cigarettes.
| Gas-phase radicals | Tar-phase radicals | |
|---|---|---|
| Tar-phase radicals | 0.617 (0.0006) |
|
| Filter ventilation | −0.586 (0.001) |
−0.582 (0.001) |
| TPM | 0.380 (0.04) |
0.438 (0.02) |
Note: Values are Spearman’s Rank correlation coefficients (ρ) with corresponding p-values in parentheses.
To examine some potential factors that can affect radical production, the impact of common cigarette design features (flavor and filter ventilation) on gas and particulate phase radicals was examined. Analysis of gas-phase radicals based on filter ventilation tertiles indicated that highly ventilated cigarettes tended to produce significantly fewer gas-phase radicals compared to the low and medium ventilated cigarettes (Figure 2A, p<0.05). Meanwhile, particulate-phase radical production was significantly lower for highly ventilated cigarettes compared to those with low ventilation (Figure 2C, p<0.05). Overall, approximately 30% of the variation in both gas-phase (r2=0.34, p=0.001) and particulate-phase (r2=0.31, p=0.001) radicals was explained by differences in ventilation. Both gas-phase (Figure 2B, p=0.24) and particulate-phase radicals (Figure 2D, p=0.09) were not significantly different between regular and menthol brands.
Figure 2.
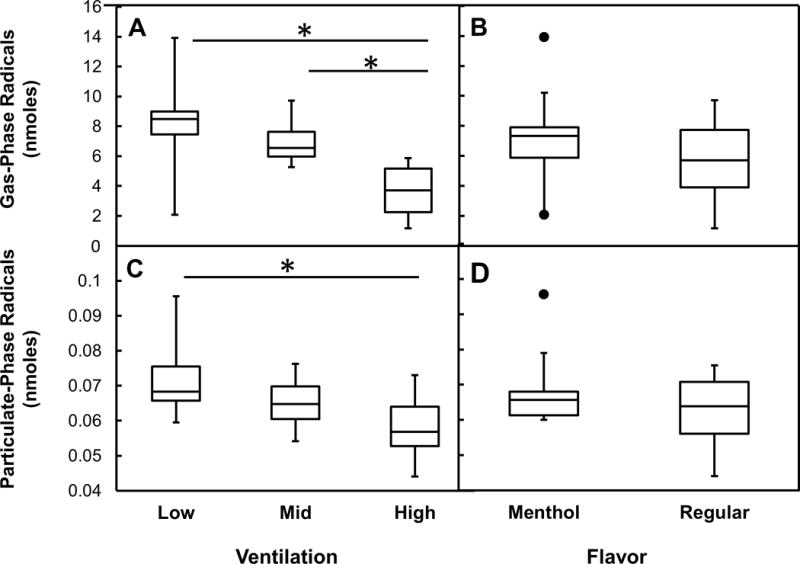
Box-plots of free radicals yields comparing brands by cigarette strength and flavor. Box-plots of gas-phase radicals (A) cigarette filter ventilation and (B) cigarette flavor (regular and menthol). Box-plots of particulate-phase (C) filter ventilation and (D) cigarette flavor (regular and menthol). *p ≤ 0.05 between groups.
DISCUSSION
While mainstream tobacco smoke contains over 7,000 chemicals, much of the damage is thought to result from the 93 carcinogens and respiratory, cardiovascular and other toxicants on the FDA’s list of hazardous and potentially hazardous chemicals (HPHC’s). However risk assessment models based on these smoke constituents underestimate the actual incidences of many smoking induced diseases.36–39 Some of the limitations of the models are due to the complexity and incomplete characterization of smoke constituents and lack of human exposure data. Free radicals are not included in the FDA’s HPHC list or in most risk models but may account, in part, for the gap between assessment models and disease incidences. Damage resulting from free radical exposure has long been known to have major negative health consequences, and, as clearly delineated in the 2010 Surgeon General’s Report, oxidative stress/damage resulting from tobacco free radical exposure was identified as one of the major causes of tobacco induced diseases.40 While direct experimental data linking exposure to free radicals with specific health outcomes are limited due to the difficulties in their measurement, direct evidence for free radical induced harm has been observed for some relevant outcomes. Diminished elastase activity due to the oxidation of alpha-1-proteinase inhibitor was observed after exposure to both gas and particulate-phase radicals, an effect likely linked to the onset of emphysema.41 Free radicals have also been shown to cause single-strand breaks in DNA.13, 42, 43 Cigarette tar fractions that had a strong EPR signal also reduced oxygen to hydrogen peroxide and nicked DNA.43 Inhaled free radicals from cigarette smoke can set off a cycle of secondary radical production resulting from reaction with other chemicals in smoke, including polycyclic aromatic hydrocarbons (PAHs), transition metals, heavy metals and aldehydes,44–46 and by evoking inflammatory responses, which include the generation of endogenous reactive oxygen/nitrogen species.18 Overall, the chronic exposure of a smoker to such high levels of oxidative stress/damage is commonly accepted as an important factor in the etiology of numerous diseases in the smokers47, such as cancer, atherosclerosis, COPD and emphysema.
Here we report for the first time that the levels of free radicals in mainstream smoke can vary substantially by cigarette brand. As observed previously,5, 22 in our study both highly reactive gas-phase radicals and more stable particulate-phase radicals were abundant in smoke from all brands tested with single cigarettes producing as much as 16 nmol (8 × 1015 spins) of highly reactive radicals and 114 pmol (5 × 1013 spins) of more stable radicals. However, when different brands were tested, a wide variation in radical production was observed: 12-fold for gas-phase radicals and 2-fold for particulate-phase radicals. The brands tested represent a sampling of 27 of the most commonly used or unique products out of the 260 different brand families identified on the market between 2002 and 2011.48 We believe that the differences in free radical production by brand may be a critical factor resulting in differences in exposure and potentially risk for tobacco-related diseases, particularly when one considers the impact of these differences when expressed over the lifetime of a smoker (e.g., 20 cigarettes per day for 40 years). The importance of examining both types of radicals is due to differences in their likely mechanisms of action since gas-phase radicals are highly reactive and can cause extensive oxidative damage and acute responses in the lung and upper digestive tract and whereas particulate-phase radicals are more stable and can penetrate deeper in the body and lead to more systemic consequences. However, to date, there are no studies that distinguish and rank the damage induced by the two types of smoke radicals. Further studies that examine the toxicological implications of both types of radials are desperately needed prior to development of regulations.
In the present study, we focused primarily on the measurement of total radicals without specifically identifying individual radical species and developed the methodology accordingly. To this end, of the many available spin traps, PBN was selected for use based upon its reactivity with a wide range of radical types, high level of stability of resulting radical adducts, and high trapping efficiency. Indeed, PBN trapping is a standard technique for measurement of tobacco smoke radicals that has been accepted and used for decades.5, 22 It is possible that there are radicals in tobacco smoke that we were unable to detect based upon limited reactivity with PBN or extreme short life span, but we expect these to be minimal. In the current studies, the specific identities of the individual radicals could not be determined due to the resolution of the hyperfine coupling constants obtained with or without PBN. Speciation of radicals is also complicated by their short half-life and high reactivity leading to a high degree of variation based upon reactions occurring in the smoking machine tubing and on the CFP.49, 50 It is possible that in addition to differences in total radicals produced, the specific radical species may differ by cigarette brand as may the toxicologic impact of each species. Previous investigations have suggested that cigarette smoke likely contains more than 35 different species49 with 18 carbon-centered radical species having been identified by HPLC-MS/MS in mainstream whole smoke.28, 29, 50 The unique chemistry of individual radicals requires further evaluation to help clarify the impact they may have on a smoker.
Filter ventilation is a significant variable differentiating the different cigarettes and allows mixing of the air with smoke, thus reducing the temperature of combustion and also diluting the smoke.51, 52 The degree of ventilation in the cigarette filter design has been responsible for confusion regarding the potential risk of different brands and sub-brands among the consumer due to the common practice of blocking the ventilation holes in the cigarette filters. When we tested these products on the FTC smoking regimen (without blocking filter ventilation), we found that highly ventilated cigarettes produce lower numbers of free radicals likely due to the dilution of the tobacco smoke. However, based on the common practice of blocking the ventilation holes, smokers of these highly ventilated products will not necessarily be exposed to reduced levels of radicals.
Menthol is the only marketed characterizing flavor in cigarettes to enhance taste and has been shown to alter smoking behavior.53 Menthol cigarettes account for approximately 25 percent of all cigarette sales in the U.S.54 Particular interest in menthol as a potential additional risk factor for tobacco related illnesses has been debated over the past decade.55–59 In order to determine if free radical formation may be related to menthol concentrations, we compared levels of radical production in menthol versus regular cigarettes. Overall, no significant difference in amounts of either gas or particulate-phase radicals was observed, suggesting that menthol does not impact radical generation during the combustion process.
Interestingly, we find that variation in filter ventilation across the brands explains about 30% of the brand to brand variation in free radical levels. Overall, the nature of the observed variation in radical generation after adjusting for ventilation is not known but may depend on other cigarette characteristics, such as rod length and circumference, filter length and type, tobacco weight and blend, and addition of tobacco additives. Combustion of different tobacco blends, such as burley, oriental and bright, show variations in the free radical production with yields of gas-phase radicals highest in burley > oriental > bright and particulate-phase radicals highest in oriental > burley > bright.7, 29 The pyrolysis temperature also has been shown to affect radical yields.60 The particulate-phase radical yield is temperature dependent, increasing from 0.8 × 1015 spins/gram tobacco at 240°C to 3.5–4.0 × 1015 spins/gram tobacco at 450–510°C, and is inversely dependent on the oxygen concentration.7 Meanwhile, oxidation of the tobacco is required for formation of gas-phase radicals. In our study, the fact that gas-phase radical yields are highly correlated to particulate-phase yields suggest that they are produced from similar tobacco constituents and thermal decomposition processes. Further, we find that both phases of radicals were correlated with total particulate matter (TPM). Since the majority of TPM constituents result from the incomplete combustion of the tobacco leaf, this further supports a shared mechanism for formation among free radical species.
In the current study, we machine smoked cigarettes using the FTC smoking regimen for comparison purposes. However, differences in human smoking behaviors, such as intensity of smoking, number of puffs per cigarette, and depth of inhalation, are well-known to impact delivery of tobacco smoke and its constituents, such as tar, nicotine and PAHs.61, 62 Thus, it is possible that these factors could similarly impact free radicals leading to greater levels of exposure to free radicals when more intense puffing protocols are used. As such, our findings likely underestimate the overall exposure to smokers and studies are underway to assess these possible differences.
Free radicals have not been included in FDA’s toxicant list perhaps due to the analytical challenges in identifying and quantifying them. Previously, several laboratories have made efforts to measure free radicals; however, most of the studies lack quantitation and have not used standardized smoking protocols, experimental conditions, cigarettes brands, and analytical techniques, probes, or spin traps. Studies that used fluorescent probes to assess free radicals in cigarette smoke lack specificity and can introduce artifacts.63–65 Several groups have used more promising nitroxide-based probes to quantitate and identify tobacco radicals; however, nitroxide-based probes only trap carbon–centered radicals.23, 28, 29, 66 Although it has its own technical limitations, EPR is still the only method that allows for the direct measurement of free radicals. Baum et al. reported on some of the variables that may impact radical quantitation including solvent, spin trap, collection and analysis volume, and EPR tube positioning and thickness.22 We have expanded on their work and developed a robust technique for the measurement of gas-phase as well as particulate-phase radicals in mainstream cigarette smoke with high reproducibility. We found that careful deoxygenation is a critical step to obtain consistent and accurate measurement of gas-phase radicals since any residual oxygen significantly impacts the yields. Results were expressed in nmol (relative to the reference standards used) rather than the number of spins to allow for a more direct comparison with other smoke toxicants as other authors have also suggested.50, 67, 68
It is of interest to compare levels of free radicals in tobacco smoke with other major toxic tobacco smoke constituents currently on the FDA HPHC list including numerous carcinogens. Per cigarette, the observed levels of gas-phase radicals (6.2 nmol) were similar to those repored for other constituents including nitromethane (9.8 nmol), nickel (8.5 nmol), cobalt (1.7 nmol), 2-toluidine (1.3 nmol), hydrazine (1.8 nmol), hetrocyclic A-α-C aromatic amine (1.4 nmol), N′-nitrosonornicotine (NNN, 1.5 nmol), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 1.1 nmol), N-nitrosodiethanolamine (2 nmol), and N-nitrosopiperidine (2.2 nmol).68 Likewise, observed particulate phase radicals per cigarette (65 pmol) were similar to several tar based constituents including benz[a]anthracene (117 pmol), benzo[j]fluoranthene (13 pmol), benzo[a]pyrene (60 pmol), chrsyene (108 pmol), dibenz[a,j]acridine (3.6 pmol), and dibenzo[c,g]carbazole (2.6 pmol).
The Family Smoking Prevention and Tobacco Control Act gives FDA the authority to regulate cigarettes to reduce the impact on public health. FDA’s Center for Tobacco Products can consider regulatory proposals to disclose and set limits on total free radical yields from cigarettes. Much research is warranted to identify all the cigarette radical species and understand the biological implications of individual radicals. We believe our work is a step forward to bringing cigarette free radical emissions under regulatory framework.
Acknowledgments
We would like to thank the Centers For Disease Control and Prevention, Atlanta, USA for analyzing cigarette filter ventilation for us.
Funding Sources: This work was supported in part by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Award Number P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Abbreviations List
- CFP
Cambridge filter pad
- EPR
electron paramagnetic resonance spectroscopy
- EPFRs
Environmentally Persistent Free Radicals
- FTC
Federal Trade Commission smoking regimen
- FDA
Food and Drug Administration
- HPHC
hazardous and potentially hazardous chemical
- PBN
spin-trapping agent phenyl-N-tert-butylnitrone
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
Footnotes
Declaration of Interests: JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience, and has received a research grant and study drug from Pfizer (not relating to cigarette emissions or free radical measurement).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Baker RR. Smoke generation inside a burning cigarette: Modifying combustion to develop cigarettes that may be less hazardous to health. Prog Energ Combust. 2006;32:373–385. [Google Scholar]
- 2.Hertz-Schunemann R, Ehlert S, Streibel T, Liu C, McAdam K, Baker RR, Zimmermann R. High-resolution time and spatial imaging of tobacco and its pyrolysis products during a cigarette puff by microprobe sampling photoionisation mass spectrometry. Anal Bioanal Chem. 2015;407:2293–2299. doi: 10.1007/s00216-014-8447-7. [DOI] [PubMed] [Google Scholar]
- 3.Lyons MJ, Gibson JF, Ingram DJ. Free-radicals produced in cigarette smoke. Nature. 1958;181:1003–1004. doi: 10.1038/1811003a0. [DOI] [PubMed] [Google Scholar]
- 4.Pryor WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of electron spin resonance. Free Radic Biol Med. 1992;13:659–676. doi: 10.1016/0891-5849(92)90040-n. [DOI] [PubMed] [Google Scholar]
- 5.Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pryor WA, Terauchi K, Davis WH., Jr Electron spin resonance (ESR) study of cigarette smoke by use of spin trapping techniques. Environ Health Perspect. 1976;16:161–176. doi: 10.1289/ehp.7616161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellinger B, Khachatryan L, Masko S, Lomnicki S. Free Radicals in Tobacco Smoke. Mini-Rev Org Chem. 2011;8:427–433. [Google Scholar]
- 8.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA. Free radical biology and medicine: it’s a gas, man! Am J Physiol-Reg. 2006;I 291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox bio. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kehrer JP, Klotz LO. Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Crit Rev Toxicol. 2015;45:765–798. doi: 10.3109/10408444.2015.1074159. [DOI] [PubMed] [Google Scholar]
- 11.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int J Env Res Pub Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fearon IM, Phillips G, Carr T, Taylor M, Breheny D, Faux SP. The Role of Oxidative Stress in Smoking-Related Diseases. Mini-Rev Org Chem. 2011;8:360–371. [Google Scholar]
- 13.Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect. 1997;105(Suppl 4):875–882. doi: 10.1289/ehp.97105s4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 15.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci-Landmrk. 2009;14:3128–3144. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen JK, Davies KJ, Forman HJ. Reactive oxygen and nitrogen species in neurodegeneration. Free Radic Biol Med. 2013;62:1–3. doi: 10.1016/j.freeradbiomed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Crotty Alexander LE, Shin S, Hwang JH. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest. 2015;148:1307–1322. doi: 10.1378/chest.15-0409. [DOI] [PubMed] [Google Scholar]
- 19.MacNee W, Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol Med. 2001;7:55–62. doi: 10.1016/s1471-4914(01)01912-8. [DOI] [PubMed] [Google Scholar]
- 20.Pryor WA, Hales BJ, Premovic PI, Church DF. The radicals in cigarette tar: their nature and suggested physiological implications. Science. 1983;220:425–427. doi: 10.1126/science.6301009. [DOI] [PubMed] [Google Scholar]
- 21.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baum SL, Anderson IGM, Baker RR, Murphy DM, Rowlands CC. Electron spin resonance and spin trap investigation of free radicals in cigarette smoke: development of a quantification procedure. Anal Chim Acta. 2003;481:1–13. [Google Scholar]
- 23.Hu N, Green SA. Acetyl Radical Generation in Cigarette Smoke: Quantification and Simulations. Atmos Environ. 2014;95:142–150. doi: 10.1016/j.atmosenv.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh M, Liu CA, Ionita P. Electron spin resonance study of puff-resolved free radical formation in mainstream cigarette smoke. Arkivoc. 2008:318–327. [Google Scholar]
- 25.Yu LX, Dzikovski BG, Freed JH. A Protocol for Detecting and Scavenging Gas-phase Free Radicals in Mainstream Cigarette Smoke. J Vis Exp. 2012 doi: 10.3791/3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson EA, Johnson JD. Methods for Analysis of Free Radicals in Cigarette Smoke. Mini-Rev Org Chem. 2011;8:401–411. [Google Scholar]
- 27.Stevanovic S, Ristovski ZD, Miljevic B, Fairfull-Smith KE, Bottle SE. Application of Profluorescent Nitroxides for Measurements of Oxidative Capacity of Combustion Generated Particles. Chem Ind Chem Eng. 2012;18:653–659. [Google Scholar]
- 28.Bartalis J, Chan WG, Wooten JB. A new look at radicals in cigarette smoke. Anal Chem. 2007;79:5103–5106. doi: 10.1021/ac070561+. [DOI] [PubMed] [Google Scholar]
- 29.Bartalis J, Zhao YL, Flora JW, Paine JB, Wooten JB. Carbon-centered radicals in cigarette smoke: acyl and alkylaminocarbonyl radicals. Anal Chem. 2009;81:631–641. doi: 10.1021/ac801969f. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins CL, Davies MJ. Detection and characterisation of radicals in biological materials using EPR methodology. Bba-Gen Subjects. 2014;1840:708–721. doi: 10.1016/j.bbagen.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Fix BV, Delnevo C, Cummings KM, O’Connor RJ. Trends in market share of leading cigarette brands in the USA: national survey on drug use and health 2002–2013. BMJ. 2016;6:e008813. doi: 10.1136/bmjopen-2015-008813. open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Determination of total and nicotine-free dry particulate matter using a routine analytical cigarette-smoking machine - determination of total particulate matter and preparation for water and nicotine measurements. Coresta Recommended Method N° 23 1991 [Google Scholar]
- 33.Agnew-Heard KA, Lancaster VA, Bravo R, Watson CH, Walters MJ, Holman MR. Multivariate Statistical Analysis of Cigarette Design Features Influence on ISO TNCO Yields. Chem Res Toxicol. 2016;29:1051–1063. doi: 10.1021/acs.chemrestox.6b00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Team, R. C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna; Austria: 2016. [Google Scholar]
- 35.Ghosh M, Ionita P. Investigation of free radicals in cigarette mainstream smoke. Proceedings of the 3rd Biennial Meeting of the Society for Free Radical Research - Asia & 6th Annual Meeting of the Society for Free Radical Research - India. 2007:49–55. [Google Scholar]
- 36.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pankow JF, Watanabe KH, Toccalino PL, Luo W, Austin DF. Calculated cancer risks for conventional and “potentially reduced exposure product” cigarettes. Cancer Epidemiol Biomarkers Prev. 2007;16:584–592. doi: 10.1158/1055-9965.EPI-06-0762. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe KH, Djordjevic MV, Stellman SD, Toccalino PL, Austin DF, Pankow JF. Incremental lifetime cancer risks computed for benzo[a]pyrene and two tobacco-specific N-nitrosamines in mainstream cigarette smoke compared with lung cancer risks derived from epidemiologic data. Regul Toxicol Pharmacol. 2009;55:123–133. doi: 10.1016/j.yrtph.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laugesen M, Fowles J. Scope for regulation of cigarette smoke toxicity according to brand differences in published toxicant emissions. N Z Med J. 2005;118:U1401. [PubMed] [Google Scholar]
- 40.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. Reports of the Surgeon General, US Public Health Service. 2010 [PubMed] [Google Scholar]
- 41.Evans MD, Pryor WA. Damage to human alpha-1-proteinase inhibitor by aqueous cigarette tar extracts and the formation of methionine sulfoxide. Chem Res Toxicol. 1992;5:654–660. doi: 10.1021/tx00029a010. [DOI] [PubMed] [Google Scholar]
- 42.Stone K, Bermudez E, Zang LY, Carter KM, Queenan KE, Pryor WA. The ESR properties, DNA nicking, and DNA association of aged solutions of catechol versus aqueous extracts of tar from cigarette smoke. Arch Biochem Biophys. 1995;319:196–203. doi: 10.1006/abbi.1995.1282. [DOI] [PubMed] [Google Scholar]
- 43.Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441–448. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- 44.Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J Anal Toxicol. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH. Polycyclic Aromatic Hydrocarbons in the Mainstream Smoke of Popular U.S. Cigarettes. Chem Res Toxicol. 2015;28:1616–1626. doi: 10.1021/acs.chemrestox.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson C, Chambers DM. Mainstream Smoke Levels of Volatile Organic Compounds in 50 US Domestic Cigarette Brands Smoked with the ISO and Canadian Intense Protocols. Nicotine Tob Res. 2016;18:1886–1894. doi: 10.1093/ntr/ntw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mons U, Muscat JE, Modesto J, Richie JP, Jr, Brenner H. Effect of smoking reduction and cessation on the plasma levels of the oxidative stress biomarker glutathione–Post-hoc analysis of data from a smoking cessation trial. Free Radic Biol Med. 2016;91:172–177. doi: 10.1016/j.freeradbiomed.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornelius ME, Driezen P, Fong GT, Chaloupka FJ, Hyland A, Bansal-Travers M, Carpenter MJ, Cummings KM. Trends in the use of premium and discount cigarette brands: findings from the ITC US Surveys (2002–2011) Tob Control. 2014;23(Suppl 1):i48–53. doi: 10.1136/tobaccocontrol-2013-051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. CRC Press; 2013. [Google Scholar]
- 50.Wooten JB. Gas-Phase Radicals in Cigarette Smoke: A Re-evaluation of the Steady-State Model and the Cambridge Filter Pad. Mini-Rev Org Chem. 2011;8:412–426. [Google Scholar]
- 51.Kozlowski LT, Mehta NY, Sweeney CT, Schwartz SS, Vogler GP, Jarvis MJ, West RJ. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–375. doi: 10.1136/tc.7.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connor RJ, Hammond D, McNeill A, King B, Kozlowski LT, Giovino GA, Cummings KM. How do different cigarette design features influence the standard tar yields of popular cigarette brands sold in different countries? Tob Control. 2008;17:I1–I5. doi: 10.1136/tc.2006.019166. [DOI] [PubMed] [Google Scholar]
- 53.Wickham RJ. How Menthol Alters Tobacco-Smoking Behavior: A Biological Perspective. Yale J Biol Med. 2015;88:279–287. [PMC free article] [PubMed] [Google Scholar]
- 54.Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cummings KM. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6:S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- 55.Werley MS, Coggins CR, Lee PN. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul Toxicol Pharmacol. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Heck JD. A review and assessment of menthol employed as a cigarette flavoring ingredient. Food Chem Toxicol. 2010;48(Suppl 2):S1–38. doi: 10.1016/j.fct.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Salgado MV, Glantz SA. Direct disease-inducing effects of menthol through the eyes of tobacco companies. Tob Control. 2011;20(Suppl 2):ii44–48. doi: 10.1136/tc.2010.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tobacco induced diseases. 2011;9(Suppl 1):S5. doi: 10.1186/1617-9625-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tobacco induced diseases. 2011;9(Suppl 1):S7. doi: 10.1186/1617-9625-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pryor WA, Church DF, Evans MD, Rice WY, Jr, Hayes JR. A comparison of the free radical chemistry of tobacco-burning cigarettes and cigarettes that only heat tobacco. Free Radic Biol Med. 1990;8:275–279. doi: 10.1016/0891-5849(90)90075-t. [DOI] [PubMed] [Google Scholar]
- 61.Hammond D, Fong GT, Cummings KM, O’Connor RJ, Giovino GA, McNeill A. Cigarette yields and human exposure: a comparison of alternative testing regimens. Cancer Epidemiol Biomarkers Prev. 2006;15:1495–1501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 62.NCI smoking and tobacco control monograph no 13. Bethesda (Maryland): 2001. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. [Google Scholar]
- 63.Forman HJ, Augusto O, Brigelius-Flohe R, Dennery PA, Kalyanaraman B, Ischiropoulos H, Mann GE, Radi R, Roberts LJ, Vina J, Davies KJA. Even free radicals should follow some rules: A Guide to free radical research terminology and methodology. Free Radic Biol Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 64.Zhao JY, Hopke PK. Concentration of Reactive Oxygen Species (ROS) in Mainstream and Sidestream Cigarette Smoke. Aerosol Sci Tech. 2012;46:191–197. [Google Scholar]
- 65.Ou B, Huang D. Fluorescent approach to quantitation of reactive oxygen species in mainstream cigarette smoke. Anal Chem. 2006;78:3097–3103. doi: 10.1021/ac051993s. [DOI] [PubMed] [Google Scholar]
- 66.Miljevic B, Fairfull-Smith KE, Bottle SE, Ristovski ZD. The application of profluorescent nitroxides to detect reactive oxygen species derived from combustion-generated particulate matter: Cigarette smoke - A case study. Atmos Environ. 2010;44:2224–2230. [Google Scholar]
- 67.Hecht SS. More than 500 trillion molecules of strong carcinogens per cigarette: use in product labelling? Tob Control. 2011;20:387–387. doi: 10.1136/tc.2011.042853. [DOI] [PubMed] [Google Scholar]
- 68.Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]


