Abstract
The process of aging is considered to be tightly related to mitochondrial dysfunction. One of the causes of aging is an increased sensitivity to the induction of mitochondrial permeability transition pore (mPTP) opening in the inner membrane of mitochondria. Melatonin, a natural antioxidant, is a hormone produced by the pineal gland. The role of melatonin whose level decreases with aging is well understood. In the present study, we demonstrated that long-term treatment of aged rats with melatonin improved the functional state of mitochondria; thus, the Ca2+ capacity was enhanced and mitochondrial swelling was deaccelerated in mitochondria. Melatonin prevented mPTP and impaired the release of cytochrome c and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) from mitochondria of both young and aged rats. Our data suggest that melatonin retains CNPase inside mitochondria, thereby providing the protection of the protein against deleterious effects of 2′,3′-cAMP in aging.
Keywords: Melatonin; Rat liver mitochondria; Permeability transition pore; Aging; 2′,3′-Cyclic nucleotide 3′-phosphodiesterase
1. Introduction
Mitochondria are dynamic, plastic organelles linked to the cellular biochemical powerhouse, since they produce the greatest part of ATP through oxidative phosphorylation and carry out several other crucial functions in cells [1]. The damage to the mitochondrial function is considered as the main factor in aging, ischemia/reperfusion, septic shock, and neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, and Huntington disease [2]. Mitochondria are the major intracellular source of ROS, which cause oxidative stress [3,4]. The factors responsible for mitochondrial function damage enhance the generation of reactive oxygen species (ROS) and activation of inducible NO-synthesis as well as the down-regulation of respiratory chain enzyme activities and induction of the mitochondrial permeability transition pore (mPTP). In response to oxidative stress, or when the mitochondrial matrix is overloaded by calcium, an increase of inner membrane permeability (permeability transition) occurs, and the nonspecific pore (mPTP) forms. The composition of mPTP and the regulation of its functioning are not yet completely understood [5]. The mPTP is a multicomponent protein complex formed in the inner membrane of mitochondria, which allows the passage of solutes with a molecular weight of up to 1.5 kDa [6]. The Ca2+-induced pore opening leads to the depolarization of the inner membrane, the uncoupling of respiration, the swelling of mitochondria, and the release of proapoptotic factors. In mitochondria isolated from the liver, heart, and brain of aged animals, an increased sensitivity to mPTP opening was found [7–10]. The functioning of mPTP is considered to be an important factor related to aging, though the exact mechanism underlying the age-dependent mitochondrial changes has not yet been established [4]. Therefore, a lot of experiments are performed to study the protective properties of antioxidants with the aim of suppressing oxidative stress and diminish the age-related oxidative cellular damage and mitochondrial dysfunction [11]. In this connection, melatonin (MEL) is considered as an effective antioxidant, a natural compound, and a neuroendocrine hormone produced by the pineal gland (epiphysis) [12], where the enzyme tryptophan hydroxylase converts tryptophan to 5-hydroxytryptophan and further to MEL. Recently it has been established that MEL is produced not only by the pineal gland but also in many other tissues [13–16]. Being a highly lipophilic molecule, MEL penetrates cell membranes, easily reaching the subcellular structures [17]. MEL is found in vertebrates, invertebrates, plants, unicellular eukaryotes, algae, and even in bacteria [14,18,19]. The content of MEL decreases sharply in aging [20,21].
MEL accomplishes its action via a number of pathways. Many cells are provided with membrane receptors, which allow them to respond to the circadian MEL message [22,23]. In addition to being a broad spectrum antioxidant [24], MEL is a ligand of several G protein-coupled receptors [25]. There are two mammalian isoforms of the MEL receptor in the brain and peripheral tissues, MT1 and MT2 [25–27].
Chronic treatment with MEL in a pharmacological dose affects the mitochondrial function and prevents mitochondrial dysfunction under experimental diabetes and intoxication, demonstrating the mitochondrion-specific activity of MEL [28–31]. It was shown that, in addition to scavenging mitochondrial ROS, MEL targets mitochondrial Ca2+-induced mPTP in astrocytes for the protection during Ca2+-mediated apoptosis. It maintained the mitochondrial membrane potential (ΔΨm) and not only prevented mPTP induction but also retained ΔΨm-dependent ATP formation [32].
Recently, a number of neuroscientists have demonstrated that MEL influences the morphological features of the nerve tissue. In particular, it was shown using various experimental injury models that MEL has positive effects on the number of axons and myelin sheath [33] and promotes myelination in the white matter [34].
Recently a neuroprotective protein has been identified as 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in rat brain mitochondria (RBM), and it was shown that CNPase protects mitochondria from mPTP opening [35]. The enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP, EC 3.1.4.37) catalyzes the hydrolysis of 2′,3′-cyclic nucleotides to form the corresponding 2′-monophosphates [36]. The functions of CNPase in mitochondria are unclear nowadays. CNPase substrates (2′, 3′-cAMP and 2′,3′-cNADP) were found to be able to enhance Ca2+-activated and CsA-sensitive pore opening in RBM as well as in liver mitochondria [35] which indicates potential interaction of CNPase with modulators of mPTP in mitochondria. CNPase interacts with RNA, calmodulin, and the cytoskeleton. CNPase was also shown to inhibit translation, to modulate mitochondrial membrane permeability, and to have putative ATP/GTPase activity. The addition of anti-CNP antibody to a mitochondrial suspension was found to protect mitochondria from mPTP opening [37, 38]. Interestingly, MEL interacts with calmodulin, G-proteins, protein kinase-alpha, and adenylyl cyclase, which are involved in calcium signaling and the cAMP-signaling cascade [39]. Moreover, recently we have shown that, under mPTP stimulation, CNPase itself can be released from mitochondria in parallel with cytochrome c, AIF, and Endo G [40]. These data allowed us to raise a question about the synergistic effect of CNPase and MEL on the parameters of mPTP opening in rat liver mitochondria (RLM). Therefore, the goal of the present study was to examine the effect of 2′,3′-cAMP (CNPase substrate) on mPTP opening in RLM isolated from young and aged rats treated and untreated with MEL.
2. Material and methods
2.1. Animals and treatment
In experiments, twenty-four young and aged male were used, six animals in a group. For each separate experiment, one rat was used; thus, six replicates were done for each experimental group. Animals were maintained in a temperature controlled room (22 °C) (two individuals in a cage) and kept on a standard diet with full access to water and food. A group of young and aged animals was given orally melatonin for 2 months starting at 1 month for young and 16 months for aged animals after which rats were sacrificed. Animals were sacrificed at the same time, at 10.00 a.m. All experiments were performed according to the European Community Guide for animal care. Melatonin was dissolved in sterile water diluted to 100 μg/mL, which was made available to animals ad libitum as drinking water. The volume of daily water intake was 33 ± 3 mL per rat, which made up approximately 7 mg of melatonin per kg body weight per day [41]. After two months, RLM were isolated from the liver of rats in each group.
2.2. Isolation of rat liver mitochondria
Mitochondria were isolated from rats by a standard method [42] using a homogenization medium containing 210 mM mannitol, 70 mM sucrose, 1 mM EGTA, 0.05% bovine serum albumin fraction V, and 10 mM Tris (pH 7.3). The homogenate was centrifuged at 800g for 10 min to pellet nuclei and damaged cells. The supernatant containing mitochondria was centrifuged for 10 min at 9000g. Sedimented mitochondria were washed twice in medium containing EGTA and BSA for 10 min at 9000g and resuspended in the same medium. The protein concentration was determined using the Bradford assay.
2.3. Evaluation of mitochondrial functions
The Ca2+ capacity of RLM was determined with a Ca2+-sensitive electrode (Nico, Russia), and the oxygen consumption rate was measured with a Clark-type O2 electrode in a 1-mL measuring chamber [43].
Mitochondria (1 mg protein/mL) were incubated in a medium containing 125 mM KCl, 10 mM Tris, 2 mM K2HPO4, pH 7.4, at 25 °C. In experiments, succinate (5 mM) was used as a substrate, and rotenone (5 μM) was added to the measuring medium in order to block Complex I dehydrogenases. The respiratory control index (RCI) was measured in a closed chamber after the addition of 200 μM ADP. The RCI of mitochondria from young rats (1st group) was used as a control. The mPTP opening in RLM was induced by a threshold Ca2+ load. A threshold Ca2+ load is that load of calcium added to a mitochondrial suspension at which calcium ions (when accumulating in mitochondria) induce the mPTP opening. For Ca2+-loading of RLM, each addition was 50 nmol of Ca2+ per mg of protein with 1.0 mg protein/mL in the chamber. The Ca2+ capacity was calculated as the amount of calcium loaded before mPTP opened and Ca2+ was released from RLM.
The swelling of RLM was measured as a change in light scattering in a mitochondrial suspension at 540 nm (A540) using a Tecan I-Control infinite 200 spectrophotometer at 25 °C. The standard incubation medium for the swelling assay contained 125 mM KCl, 10 mM Tris, 2 mM KH2PO4, 5 mM succinate, and 5 μM rotenone. The concentration of the mitochondrial protein in a well was 0.5 mg protein/mL. Swelling was initiated by the addition of 300 nmol of Ca2+ per mg of protein. The swelling process was characterized by the time needed to reach the half-maximal light scattering signal (T1/2).
2.4. Sample preparation
Aliquots (100 μL) were taken from the chamber, placed in an Eppendorf tube, and centrifuged for 5 min at 15,000 × g. Then, 60 μL of the supernatant was placed in another Eppendorf tube, and 20 μL of 4× Laemmli buffer was added. Samples were heated to 95 °C for 5 min and applied to the gel. 40 μL of a sample with the supernatant was applied and subjected to electrophoresis followed by Western blot analysis.
2.5. Electrophoresis and immunoblotting of mitochondrial proteins
Supernatant samples were separated under denaturing conditions in 12.5% SDS-PAGE gels and transferred to a nitrocellulose membrane. Precision Plus Pre-stained Standards from Bio-Rad Laboratories (Hercules, CA, USA) were used as markers. After overnight blocking, the membrane was incubated with the appropriate primary antibody. Anti-melatonin related receptor antibody (rabbit polyclonal) was used at the 1:1000 dilution (abcam, USA). The monoclonal anti-CNP antibody (anti-CNP Ab) was obtained as described [44] and used at the 1:10,000 dilution, the monoclonal anti-cytochrome c antibody (# DLN 06724 from Dianova; Hamburg, Germany) was used at the 1:2000 dilution, and the Tom20 antibody (Cell Signaling, USA) was used to rule out the contamination of the supernatant with mitochondria and as a loading control (1:1000 dilution). The immunoreactivity was detected using the appropriate secondary antibody conjugated to horseradish peroxidase (Jackson Immuno Research, West Grove, PA, USA). The peroxidase activity was detected using ECL chemiluminescence reagents (Pierce, Rockford, IL, USA).
2.6. Statistical analysis
For statistical analysis, the relative levels of protein density were expressed as mean ± SD from at least five to six independent experiments. The statistical significance of the difference between the mean values was evaluated using the Student's t-test. The difference was considered significant at p < 0.05.
3. Results
The RCI indicates the effectiveness of mitochondria in promoting the oxidative phosphorylation. Table 1 presents the results showing the RCI of RLM isolated from rats of all groups. According to the experimental data, no substantial changes in RCI and ADP/O were observed as a function of aging, indicating that aging, as well as the treatment of rats with MEL, does not bring about substantial alterations to the mitochondrial membrane.
Table 1.
Effect of MEL on the respiratory control index (RCI) in rat liver mitochondria (RLM) from rats of each group.
| RCI | ADP/O | |
|---|---|---|
| Young | 7.09 ± 0.49 | 2.23 ± 0.21 |
| Young + melatonin | 7.2 ± 0.32 | 2.4 ± 0.26 |
| Aged | 7.25 ± 0.45 | 2.4 ± 0.28 |
| Aged + melatonin | 7.85 ± 0.19* | 2.3 ± 0.24 |
RLM were incubated in standard medium as described in Section 2. Oxidative phosphorylation was initiated by adding 200 μM ADP. RCI values with ±SD of six independent experiments are presented.
p < 0.05 in comparison with the control (RLM from young rats, the first group).
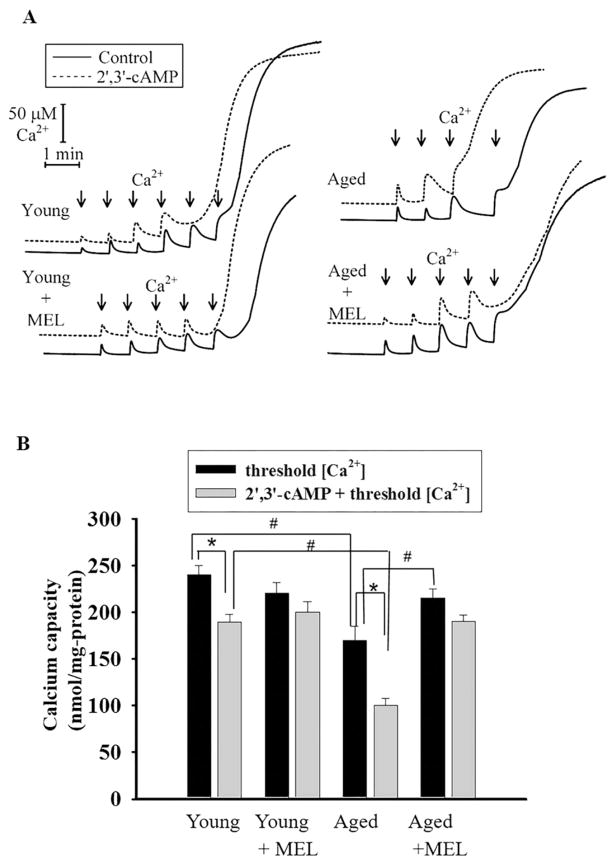
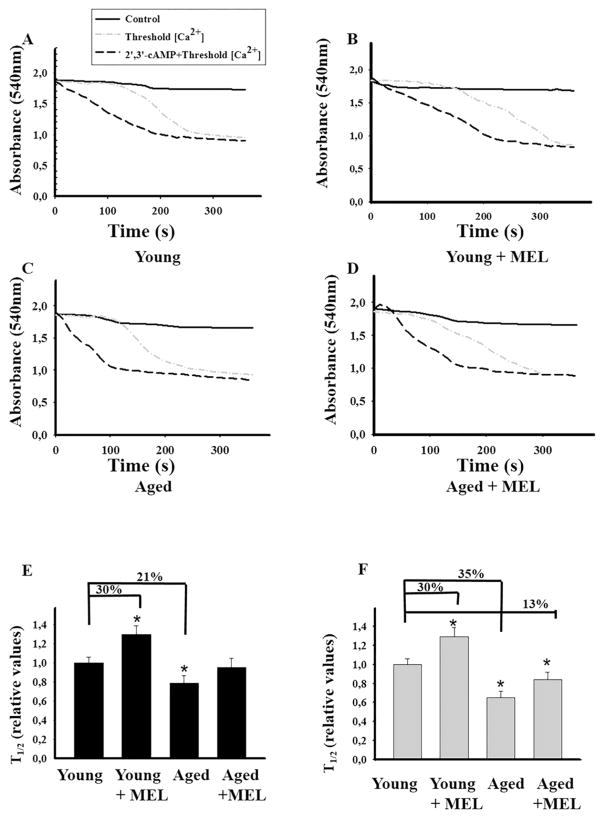
Taking into consideration that 2′,3′-cAMP in the concentration range of 1–5 μM facilitated the Ca2+-induced mPTP opening in nonsynaptic brain mitochondria [35] and 2′,3′-cAMP (5 μM) caused a comparable acceleration of mPTP opening in mitochondria isolated from 18-month-aged rats [45], we decided to examine the influence of MEL on the Ca2+ capacity of RLM from all groups of rats upon mPTP opening (Fig. 1). In RLM from each experimental group, the first addition of Ca2+ leads to an active accumulation of Ca2+ in mitochondria with subsequent restoration (Fig. 1A). However, the sixth addition of Ca2+ to RLM from young rats and the fifth addition of Ca2+ to RLM from young MEL-treated rats led to the mPTP opening. Even after the forth addition of Ca2+ to mitochondria from aged rats and after the fifth addition of Ca2+ to RLM from aged MEL-treated rats, an irreversible release of Ca2+ occurred. Here, we compared the influence of 2′,3′–cAMP on the Ca2+ capacity of RLM from each experimental group. The threshold Ca2+ concentration was reached after the forth addition of Ca2+ to both RLM from young animals and young MEL-treated animals. In RLM from aged rats, the second addition of Ca2+ led to Ca2+ release, while in RLM from aged MEL-treated rats, the forth addition of Ca2+ resulted in Ca2+ irreversible release. Fig. 1(B) demonstrates quantitative changes in the Ca2+ capacity of Ca2+-loaded mitochondria in the absence and presence of 2′,3′-cAMP. The threshold Ca2+ concentration declined by 28% in mitochondria from aged rats. No difference in Ca2+ capacity was found between RLM isolated from MEL-treated aged, MEL-treated young and young rats not treated with MEL; however, the Ca2+ capacity of RLM from MEL-treated aged rats increased by 26% as compared to aged rats not treated with MEL. Thus, it is clear that the Ca2+-capacity changes with age, which is consistent with our previous data [9,45]. In particular, the Ca2+-capacity of mitochondria decreased in aging, and MEL treatment prevented this decline. The effect of 2′,3′-cAMP on Ca2+ capacity was observed in mitochondria isolated from the liver of aged rats. 2′,3′-cAMP reduced the Ca2+ capacity of RLM from young rats by 25% (1st group); however, in RLM of the 2nd group no difference in the Ca2+capacity from the corresponding control was revealed. In RLM of the 3rd experimental group, we observed a significant acceleration of mPTP opening in the presence of 2′,3′-cAMP, and the threshold Ca2+ concentration decreased by 41% as compared with the value obtained without 2′,3′-cAMP, while the difference between Ca2+ capacity with/without 2′,3′-cAMP in RLM of the 4th experimental group was negligible. MEL protected mitochondria against the age-dependent activation of mPTP in both young and aged rats. Calcium-induced mitochondrial swelling is one of the characteristics of the mPTP opening. The addition of Ca2+ at the threshold concentration to the mitochondrial suspension incubated in the standard medium caused a decrease in light scattering, which is indicative of swelling, and mitochondria became permeable to low-molecular-weight substances. We compared the swelling of RLM from young and aged, MEL-treated and untreated rats. Fig. 2(A–D) shows the curves of Ca2+-activated swelling of RLM isolated from rats of four groups examined. Fig. 2(E) demonstrates the average half time (T1/2) of mitochondrial Ca2+-activated swelling. The half time of mitochondrial swelling of aged rats decreased by 21%. Thus, the rate of swelling of RLM from aged rats became faster as compared to the mitochondrial swelling of young ones, while the time needed for the swelling of RLM from young MEL-treated rats increased by 30%. The swelling of RLM from MEL-treated aged rats tended to decelerate compared with the swelling in untreated animals; moreover, its T1/2 value approached T1/2 of mitochondrial swelling in young control rats, pointing to the preventive effect of MEL in the age-dependent activation of RLM swelling. Fig. 2(F) shows the average half time (T1/2) of mitochondrial swelling activated by Ca2+ and 5 μM 2′,3′-cAMP. 2′,3′-cAMP slows down the swelling of RLM from the second group by 30% and accelerates the swelling of RLM of aged rats from the third group by 35%, while the mitochondrial swelling of RLM in the fourth group was enhanced inessentially, by 13%. MEL prevented the RLM swelling in aged rats caused by 2′,3′-cAMP–dependent stimulation, but to a lesser degree than in the experiment without 2′,3′-cAMP.
Fig. 1.
Comparative analysis of Ca2+-capacity in the presence/absence of 2′,3′-cAMP in rat liver mitochondria isolated from young and aged rats treated and untreated with MEL. Isolated RLM (1.0 mg protein/ml) were incubated in the electrode chamber under conditions described in Section 2. A - Recordings are shown for the mitochondria isolated from each experimental group for Ca2+ fluxes. Arrows show the times at which CaCl2 (50 nmol of Ca2+per mg of protein) was applied. B - Quantitative analysis of Ca2+-capacity in the presence/absence of 2′,3′-cAMP in RLM. The values shown are the means ± SD from six independent experiments, *p < 0.05 vs the Ca2+-capacity without 2′,3′-cAMP for each group of rats, #p < 0.05 in comparison with the corresponding values (with/without 2′,3′-cAMP) of Ca2+-capacity of RLM from young untreated rats (the first group).
Fig. 2.
Influence of 2′,3′–cAMP on the swelling of RLM from rats of each group under mPTP opening. RLM incubated in standard medium as described in Section 2. A - Swelling of RLM from young rats, B - Swelling of RLM from young rats treated with MEL, C - Swelling of RLM from aged rats, D - Swelling of RLM from aged rats treated with MEL, E - Quantitative analysis of the half time (T1/2) of mitochondrial swelling initiated by Ca2+. The average T1/2 value in RLM from the first group was taken as a control. (F) - Quantitative analysis of T1/2 of mitochondrial swelling initiated by Ca2+ in the presence of 2′,3′–cAMP (5 μM); the average T1/2 value in RLM from the first group was taken as control. The values shown are the means ± SD from six independent experiments, *p < 0.05 vs control (RLM from young rats, the first group).
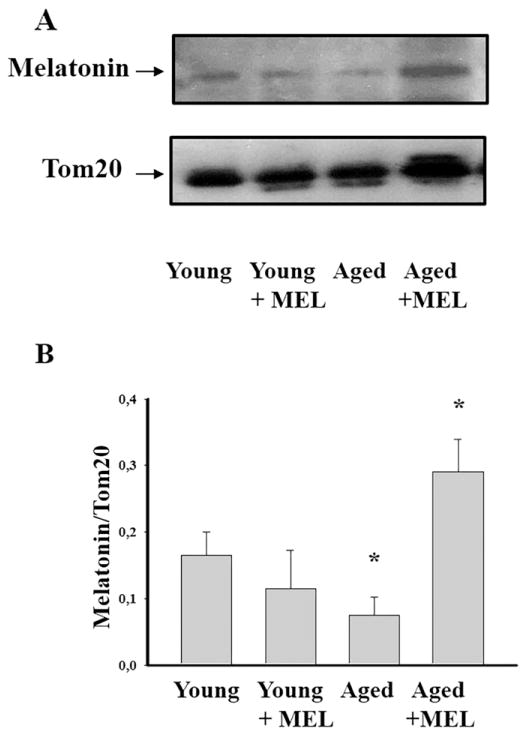
Earlier we have noted that the content of MEL decreased sharply in aging [20,21]. Here we performed a comparative analysis of the MEL content in RLM isolated from each experimental group. Fig. 3A shows Western blots of the MEL level in RLM from rats of different age, treated and not treated with MEL. The quantitative analysis of MEL levels is shown in Fig. 3B. Protein bands were quantified after normalization with respect to Tom20. The MEL levels in RLM from aged rats were by 55% lower than in RLM from young rats (Fig. 3B). The treatment with MEL did not change the MEL level in RLM from young rats but increased the MEL level in RLM from aged rats by 75% (Fig. 3B).
Fig. 3.
Level of MEL in RLM isolated from each experimental groups. A - Western blot of mitochondrial suspension obtained after isolated RLM as ascribed Section 2. Membranes were stained for the presence of MEL by anti-MEL related receptor antibody. Protein concentration was 30 μg per lane. B - Quantitation of immunostaining using computer-assisted densitometry. Bar graphs representing MEL immunoreactivity in RLM isolated from each experimental rat groups. Protein bands were quantified after normalization with respect to Tom20. The bar graphs in B show mean ± SD from three independent experiments. *P < 0.05, significant difference for samples isolated from young MEL-treated, aged, aged MEL-treated rats in comparison with those from young rats.
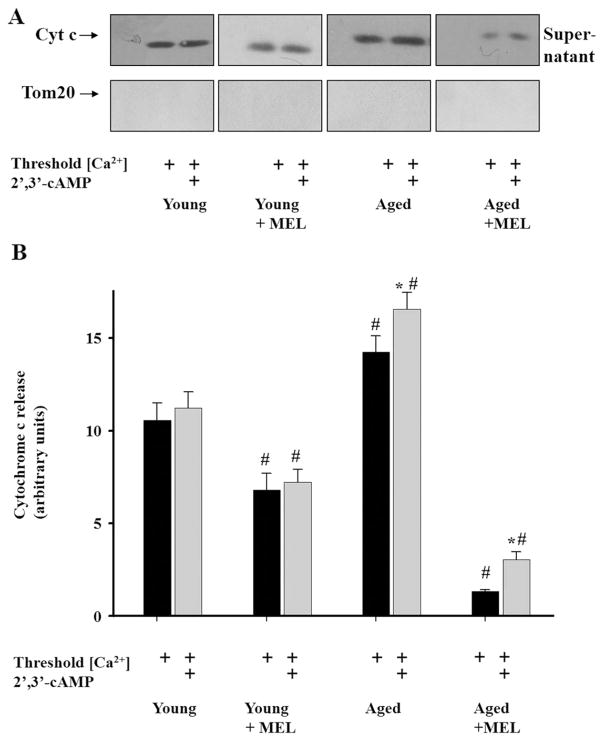
Then, we investigated the effect of MEL on the release of cytochrome c (as an apoptotic factor) from all types of RLM after the mPTP opening in the absence and presence of 2′,3′-cAMP. As shown in Fig. 4(A), no cytochrome c release was detected under control conditions (without added Ca2+) in the supernatant of RLM isolated from the liver of rats of all four groups. However, in the presence of Ca2+ at the threshold concentration, the cytochrome c release was observed, being significantly decreased (by 36%) in mitochondria from MEL-treated young rats. In mitochondria isolated from the liver of aged untreated rats, the level of cytochrome c release was higher (by 36%) in comparison with mitochondria isolated from young rats in the presence of the threshold calcium concentration. The treatment of aged rats with MEL led to a strong decline in the cytochrome c release from RLM, demonstrating the ability of MEL to inhibit cytochrome c release and prevent mitochondrial dysfunction. Further, we examined the effect of 2′,3′-cAMP on cytochrome c release in the presence of the threshold calcium concentration. We observed a significant enhancement of cytochrome c release from mitochondria of aged MEL-treated and untreated rats in comparison with the corresponding values recorded at threshold [Ca2+] in the groups examined. We did not find the effect of 2′,3′-cAMP on cytochrome c release from mitochondria of young MEL-treated and untreated rats. The main result of this set of experiments is that the treatment of rats with MEL prevented the release of cytochrome c from mitochondria, which was especially pronounced in aged rats.
Fig. 4.
Cytochrome c release from RLM from rats of each group. Cytochrome c releases from RLM in control conditions without added Ca2+ and from Ca2+-overloaded mitochondria in the absence and presence of 2′,3′-cAMP. A - Western blot of supernatants obtained as described in Section 2. The anti-cytochrome c antibody was used in the 1:2000 dilution. Supernatants were tested for the presence of Tom20 to rule out the contamination of the supernatant with mitochondria. B - Quantitation of immunostaining by computer-assisted densitometry. The values represent the means ± SD from three independent experiments. *p < 0.05 vs the Ca2+-capacity without 2′,3′-cAMP for each group of rats, #p < 0.05 in comparison with the corresponding values (with/without 2′,3′-cAMP) of Ca2+-capacity of RLM from young untreated rats (the first group).
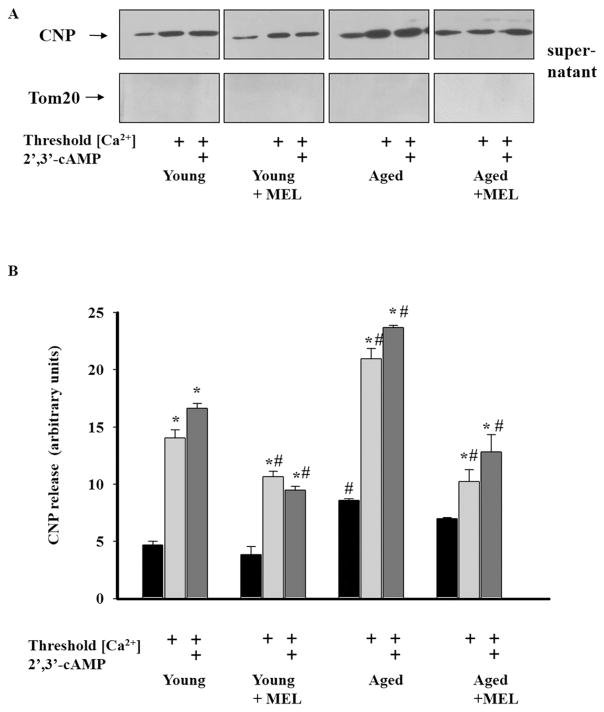
Since the effect of 2′,3′-cAMP was related to CNPase (2′,3′-cAMP was a CNPase substrate) and, along with the cytochrome c release from Ca2+-overloaded rat nonsynaptic mitochondria, the release of CNPase occurred [40], we next studied whether MEL affected the CNPase release from RLM in rats of each experimental group. Anti-CNP antibodies were used for the visualization of CNPase on the same membrane as was applied before to detect the cytochrome c release. As shown in Fig. 5(A), the amount of CNPase released under control conditions (without Ca2+) from mitochondria isolated from rats of each group was small. The CNPase release in the third group of rats was about twice as much and in the fourth group 1.5 times as much as that in the control (young rats). The induction of mPTP increased the CNPase release from RLM of young untreated rats about threefold as compared with control (without Ca2+). The CNPase release from calcium-overloaded mitochondria increased by about 44% in the third group and decreased by 24% in the second group and by 27% in the fourth group of rats in comparison with control conditions (Ca2+-overloading of mitochondria in the first group). We observed a more pronounced decrease in the CNPase release from Ca2+-overloaded mitochondria isolated from aged MEL-treated but not young MEL-treated rats as compared to the corresponding controls. The addition to mitochondria of 2′,3′-cAMP inducing the mPTP opening led to negligible changes in the CNPase release from mitochondria of each group relative to the Ca2+-induced CNPase release in the corresponding controls. Thus, these data suggest that MEL retains CNPase inside Ca2+-overloaded mitochondria in the presence/absence of 2′,3′-cAMP in RLM from both young and aged rats.
Fig. 5.
CNPase release from rat liver mitochondria from rats of each group. CNPase is released from RLM in control conditions without added Ca2+ and from Ca2+-overloaded mitochondria in the absence and presence of 2′,3′-cAMP. A - Western blot of supernatants obtained as described in Section 2. The anti-CNP antibody was used in the 1:10,000 dilution. Supernatants were tested for the presence of Tom20 to rule out the contamination of the supernatant with mitochondria; B - Quantitation of immunostaining by computer-assisted densitometry. The values represent the means ± SD from three independent experiments. *p < 0.05 vs the Ca2+-capacity without 2′,3′-cAMP for each rat group (of rats), #p < 0.05 vs the corresponding values (with/without 2′,3′-cAMP) of Ca2+-capacity of RLM from young untreated rats (the first group).
4. Discussion
Mitochondrial functions are impaired with aging. Oxidative stress and the decreased activity of the calcium-transporting mitochondrial system are considered as important factors in age-related mitochondrial dysfunction [3,4]. The cell death associated with mitochondrial dysfunction can be caused by an increase in the nonselective permeability of the inner membrane, which occurs due to an elevated (exceeding the threshold) concentration of calcium ions in the matrix and mPTP opening [5,46]. MEL is able to prevent mPTP opening and cytochrome c release in heart rat mitochondria [47]. We have reported earlier that MEL treatment prevents the cumene hydroperoxide-induced swelling of brain mitochondria isolated from young and aged rats. MEL abolished the pro-oxidant effect of cumene hydroperoxide [48].
Mitochondrial coupling is an important feature of the functional state of mitochondria. According to our results, mitochondria isolated from every group were coupled (the better biochemical function, Table 1). Recently we showed that mitochondria isolated from aged animals displayed an enhanced activation of the mPTP opening because of a decrease in the threshold calcium concentration [9,45]. Chronic MEL treatment in a pharmacological dose was found to affect the mitochondrial function [30]. Recently we showed that the opening of the Ca2+-induced nonselective pore in brain mitochondria isolated from aged animals after long-term MEL treatment led to an increase in the threshold Ca2+ concentration and prevented the pore opening [49]. Interestingly, chronic MEL treatment improved the mitochondrial function in aged animals and made them more resistant to the Ca2+-induced increase in mPTP (Fig. 1). These data allow us to conclude that long-term administration of MEL facilitates the functioning of liver mitochondria and prevents the mPTP opening in the inner membrane and, therefore, may contribute to the energy transduction of cell.
Furthermore, we revealed that mitochondrial swelling, another important characteristic of mitochondrial function, occurred more rapidly in liver mitochondria from aged rats than in young animals and was retarded in RLM from both young and aged rats exposed to chronic MEL treatment in comparison with the corresponding controls (Fig. 2).
Earlier, we observed that 2′,3′-cAMP induced the mPTP opening in brain and liver mitochondria [35] and accelerated the mPTP opening in rat brain mitochondria in aging [45]. Here, we detected that MEL enhanced Ca2+ capacity in RLM of both young and aged rats (Fig. 1). Moreover, mitochondrial swelling was accelerated in mitochondria isolated from aged rats and slowed down in mitochondria from MEL-treated aged and young rats as compared with corresponding control (Fig. 2F). MEL retarded the mitochondrial swelling in the presence of 2′,3′-cAMP in aging. The difference in the decrease of the half time of swelling in mitochondria from aged versus young animals confirms the age-dependent effect of accumulated MEL during mPTP opening.
The level of MEL decreases in aging [20,21]. The decrease in MEL levels is considered to be one of the major factors of elevated oxidative stress, which in turn is responsible for various disorders [50]. According to our experiments, the level MEL reduced in RLM from aged rats and greatly increased in RLM from aged MEL-treated rats. Enhanced Ca2+ capacity and delayed mitochondrial swelling probably occur due to the accumulation of MEL in RLM from aged rats after chronic MEL treatment. We did not detect the accumulation of MEL in RLM from young MEL-treated rats, although in mitochondria isolated from the brain of young MEL-treated rats the accumulation of MEL was observed (unpublished data). Presumably, the distribution of MEL in different tissues proceeds differently.
Mitochondria are a reservoir for apoptogenic factors and play a central role in Ca2+ signaling, oxidative stress, and regulation of the life span of cells with both necrotic and apoptotic outcome. The cytochrome c release from mitochondria triggers a set of events leading to the activation of programmed cell death [51,52]. It was demonstrated in some investigations that aging increases apoptosis in various cells (neuron, cardiomyocytes, hepatocytes, and lymphocytes) [53–55]. It was found that the cytosolic content of cytochrome c in cardiocytes of aged rats was significantly higher than in young animals [56]. In this work, we observed an increase in cytochrome c release from isolated RLM of aged rats under mPTP opening in comparison with young rats (Fig. 4). The decreased Ca2+ capacity and accelerated swelling were in accord with enhanced cytochrome c release in aging. MEL protects mitochondria against oxidative stress and hence has the antioxidant property [57]. The data obtained in our experiments showed that chronic MEL administration to young and aged rats produces a decrease in cytochrome c release. The enhancement in Ca2+ capacity, the deceleration of mitochondrial swelling, and a decrease in cytochrome c release support a protective effect of MEL in aging. It is possible that MEL interacts with the mitochondrial lipid bilayer, in which MEL is able to prevent peroxidation of cardiolipin [58], stabilizing the mitochondrial inner membrane [59]. Recently, we identified CNPase in rat brain mitochondria; the protein was localized in both mitoplasts and the outer mitochondrial membrane. We supposed that CNPase participates in the regulation of mPTP, and CNPase substrates such as 2′,3′-cAMP and 2′,3′-cNADP activate mPTP in RBM and RLM [35]. One of the properties of CNPase is that it hydrolyzes 2′,3′-cAMP to 2′-AMP in the mouse brain in vivo, and this event provides an important source of adenosine production [60]. The participation of the mitochondrial CNPase pool in the aging process was supported by our results indicating the acceleration of mPTP opening in the presence of the CNPase substrate 2′,3′cAMP in mitochondria from both young and aged animals [45]. These results suggest that the age-dependent facilitation of the mPTP opening might generally contribute to the enhanced susceptibility of several tissues to cell damage in the aging process. Moreover, recently we observed that, when mPTP was opened, CNPase was released from RBM to cytosol. Under Ca2+-overloading conditions, 2′,3′-cAMP enhanced the CNPase release, without affecting the cytochrome c release in RBM [40]. Verrier et al. hypothesize that the 2′,3′-cAMP-adenosine pathway is a mechanism to rid the cell of a toxic product (2′,3′-cAMP) and in turn transform it to a protective metabolite (adenosine); CNPase is a critically important enzyme in the 2′,3′-cAMP-adenosine pathway and participates in the production of adenosine [60]. MEL acts via high-affinity G-protein-coupled receptors [61], but in the late 80s of the last century, the computer analysis of the amino acid sequence of CNPase revealed the presence of a consensus sequence of G-proteins [62]. We suppose that MEL may mediate its effect on mitochondrial function through CNPase.
Earlier, we examined whether changes in the CNPase level in mitochondria correlate with the activation of mPTP opening during aging. Our previous results [45] confirmed that RBM from aged rats, which contain a lower, compared with young animals, amount of CNPase, are more susceptible to Ca2+-induced mPTP activation. Here we observed that the lowering of the threshold Ca2+ concentration and an acceleration of mPTP opening in RLM isolated from aged rats resulted in an enhanced CNPase release in mitochondria from aged rats in comparison with young animals. During chronic MEL treatment, we observed CNPase release under conditions when the mPTP was closed (without Ca2+ and 2′,3′-cAMP) (Fig. 5) in liver mitochondria isolated from rats of every group. Under conditions of mPTP opening, massive CNPase release occurred in RLM from both young and especially aged rats. MEL prevented CNPase release in RLM from both young and aged rats.
In summary (Fig. 6), reduced Ca2+ capacity, acceleration of mitochondrial swelling, and an increase in cytochrome c and CNPase release were observed in RLM during aging. These effects are abolished by chronic MEL treatment, which supports the protective effect of MEL, and suggest the involvement of MEL in the process improving the mitochondrial function in aging. Added 2′,3′-cAMP might probably be a second messenger leading to mPTP opening; on the other hand, accumulated intramitochondrial 2′,3′-cAMP could cause cell injury. CNPase is an important enzyme, which participates in the production of adenosine from 2′,3′-cAMP by the 2′,3′-cAMP–adenosine pathway. In aging, the level of CNPase decreases, and cells become vulnerable to damage. MEL retains CNPase inside mitochondria to protect cells against the deleterious effects of 2′,3′-cAMP in aging.
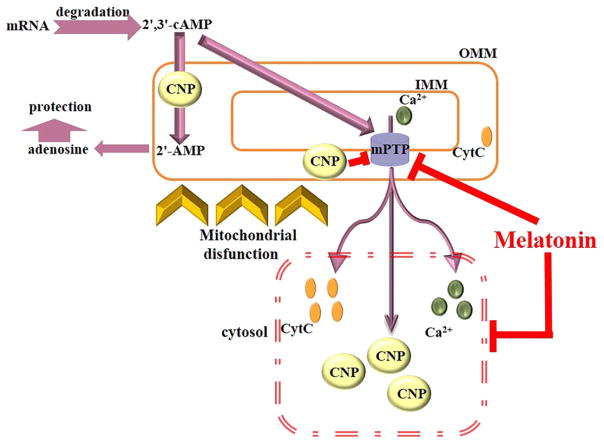
Fig. 6.
The interaction of CNPase and 2′,3′-cAMP with mPTP. Influence of MEL on the mPTP. CNPase is a myelin protein, which was also detected in nonmyelin tissues. It was found to decrease the Ca2+-sensitivity of mPTP, and 2′,3′-cAMP, a substrate for CNPase, increases the Ca2+ sensitivity of mPTP by yet unknown mechanisms [35,45]. 2′,3′-cAMP is formed in cells as a result of RNA degradation, and its formation is stimulated by cell injury/stress/mitochondrial dysfunction signals [63,64]. It is metabolized via extracellular, intracellular, and transcellular routes (2′,3′ cAMP - adenosine pathway(s)) to adenosine [65]. Intracellular/mitochondrial accumulation of 2′,3′-cAMP reduces the mitochondrial Ca2+ retention capacity and facilitates the activation of mPTP [35]. Cell injury and stress signals also lead to dysregulation of cellular Ca2+, which promotes mPTP opening. In our model, mitochondrial CNPase protects these organelles against the deleterious effects of elevated levels of 2′,3′-cAMP by degrading it to 2′-AMP. MEL is a highly lipophilic molecule that penetrates cell membranes, easily reaching the subcellular structures, and accumulates in mitochondria in high concentrations [17]. The level of CNPase during mitochondrial dysfunction decreases, and cells become vulnerable to damage. We showed that MEL prevents mPTP opening and diminishes cytochrome c and CNPase release from mitochondria; in addition, it retains of CNPase inside the mitochondria, providing the protection of the cell against deleterious effects of 2′,3′-cAMP in aging.
Acknowledgments
The work was supported by grants from the Russian Federation Government (N14.Z50.0028 to J.L.) and RFBR (14-04-00625, 16-04-00927).
Footnotes
Author contributions
Conceived and designed the study and the experiments: O Krestinina, Yu Baburina; I Odinokova;
Performed the experiments: Yu Baburina, I Odinokova, O Krestinina;
Analyzed the data: Yu Baburina, I Odinokova, O Krestinina, T Azarashvili;
Contributed reagents/materials/analysis tools: V Akatov, O Krestinina, J Lemasters;
Wrote the manuscript: O Krestinina, Yu Baburina, T Azarashvili, I Odinokova, V Akatov.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011;11:221–240. doi: 10.2174/156802611794863517. [DOI] [PubMed] [Google Scholar]
- 3.Crompton M. Mitochondria and aging: a role for the permeability transition? Aging Cell. 2004;3:3–6. doi: 10.1046/j.1474-9728.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Lisa F, Bernardi P. Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res. 2005;66:222–232. doi: 10.1016/j.cardiores.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front Physiol. 2013;4:95. doi: 10.3389/fphys.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Goodell S, Cortopassi G. Analysis of oxygen consumption and mitochondrial permeability with age in mice. Mech Ageing Dev. 1998;101:245–256. doi: 10.1016/s0047-6374(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 8.Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krestinina OV, Kruglov AG, Grachev DE, Baburina YL, Evtodienko YV, Moshkov DA, Santalova IM, Azarashvili TS. Age-related changes of mitochondrial functions under the conditions of Ca2+-induced opening of permeability transition pore. Biol Membr. 2010;27:177–183. [Google Scholar]
- 10.Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–608. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- 11.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 12.Poeggeler B. Introduction. Melatonin and the light-dark zeitgeber in vertebrates, invertebrates and unicellular organisms. Experientia. 1993;49:611–613. doi: 10.1007/BF01923940. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Arto M, Hamilton TR, Gallego M, Gaspar-Torrubia E, Aguilar D, Serrano-Blesa E, Abecia JA, Perez-Pe R, Muino-Blanco T, Cebrian-Perez JA, Casao A. Evidence of melatonin synthesis in the ram reproductive tract. Andrology. 2016;4:163–171. doi: 10.1111/andr.12117. [DOI] [PubMed] [Google Scholar]
- 14.Hardeland R. Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci. 2008;65:2001–2018. doi: 10.1007/s00018-008-8001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, Garcia-Corzo L, Lopez LC, Reiter RJ, Acuna-Castroviejo D. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado MD, Mora-Santos M, Naji L, Carrascosa-Salmoral MP, Naranjo MC, Calvo JR. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol Res. 2010;62:282–287. doi: 10.1016/j.phrs.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 1993;15:59–69. doi: 10.1111/j.1600-079x.1993.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 18.Hardeland R, Poeggeler B. Non-vertebrate melatonin. J Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 19.Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. Phytomelatonin: a review. J Exp Bot. 2009;60:57–69. doi: 10.1093/jxb/ern284. [DOI] [PubMed] [Google Scholar]
- 20.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Reiter RJ. Chronobiological aspects of the mammalian pineal gland. Prog Clin Biol Res. 1981;59C:223–233. [PubMed] [Google Scholar]
- 22.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 23.Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006;41:297–305. doi: 10.1111/j.1600-079X.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 24.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 25.Drew JE, Barrett P, Mercer JG, Moar KM, Canet E, Delagrange P, Morgan PJ. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J Neuroendocrinol. 2001;13:453–458. doi: 10.1046/j.1365-2826.2001.00651.x. [DOI] [PubMed] [Google Scholar]
- 26.Pozo D, Garcia-Maurino S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res. 2004;37:48–54. doi: 10.1111/j.1600-079X.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 27.Coto-Montes A, Tomas-Zapico C, Escames G, Leon J, Rodriguez-Colunga MJ, Tolivia D, Acuna-Castroviejo D. Specific binding of melatonin to purified cell nuclei from mammary gland of swiss mice: day-night variations and effect of continuous light. J Pineal Res. 2003;34:297–301. doi: 10.1034/j.1600-079x.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheshchevik VT, Lapshina EA, Dremza IK, Zabrodskaya SV, Reiter RJ, Prokopchik NI, Zavodnik IB. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol. 2012;261:271–279. doi: 10.1016/j.taap.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Zavodnik IB, Lapshina EA, Cheshchevik VT, Dremza IK, Kujawa J, Zabrodskaya SV, Reiter RJ. Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J Physiol Pharmacol. 2011;62:421–427. [PubMed] [Google Scholar]
- 30.Cheshchevik VT, Dremza IK, Lapshina EA, Zabrodskaya SV, Kujawa J, Zavodnik IB. Corrections by melatonin of liver mitochondrial disorders under diabetes and acute intoxication in rats. Cell Biochem Funct. 2011;29:481–488. doi: 10.1002/cbf.1775. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Aranda A, Fernandez-Vazquez G, Mohammad ASM, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zucker diabetic fatty rats. J Pineal Res. 2014;57:103–109. doi: 10.1111/jpi.12147. [DOI] [PubMed] [Google Scholar]
- 32.Jou MJ. Melatonin preserves the transient mitochondrial permeability transition for protection during mitochondrial Ca(2+) stress in astrocyte. J Pineal Res. 2011;50:427–435. doi: 10.1111/j.1600-079X.2011.00861.x. [DOI] [PubMed] [Google Scholar]
- 33.Turgut M, Kaplan S. Effects of melatonin on peripheral nerve regeneration. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:100–108. doi: 10.2174/187221411799015336. [DOI] [PubMed] [Google Scholar]
- 34.Villapol S, Fau S, Renolleau S, Biran V, Charriaut-Marlangue C, Baud O. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr Res. 2011;69:51–55. doi: 10.1203/PDR.0b013e3181fcb40b. [DOI] [PubMed] [Google Scholar]
- 35.Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol. 2009;296:C1428–C1439. doi: 10.1152/ajpcell.00006.2009. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, O'Neill RC, Park MW, Gravel M, Braun PE. Mitochondrial localization of CNP2 is regulated by phosphorylation of the N-terminal targeting signal by PKC: implications of a mitochondrial function for CNP2 in glial and non-glial cells. Mol Cell Neurosci. 2006;31:446–462. doi: 10.1016/j.mcn.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Myllykoski M, Seidel L, Muruganandam G, Raasakka A, Torda AE, Kursula P. Structural and functional evolution of 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Baburina YL, Gordeeva AE, Moshkov DA, Krestinina OV, Azarashvili AA, Odinokova IV, Azarashvili TS. Interaction of myelin basic protein and 2′,3′-cyclic nucleotide phosphodiesterase with mitochondria. Biochemistry (Mosc) 2014;79:555–565. doi: 10.1134/S0006297914060091. [DOI] [PubMed] [Google Scholar]
- 39.de Faria Poloni J, Feltes BC, Bonatto D. Melatonin as a central molecule connecting neural development and calcium signaling. Funct Integr Genomics. 2011;11:383–388. doi: 10.1007/s10142-011-0221-8. [DOI] [PubMed] [Google Scholar]
- 40.Baburina Y, Azarashvili T, Grachev D, Krestinina O, Galvita A, Stricker R, Reiser G. Mitochondrial 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNP) interacts with mPTP modulators and functional complexes (I–V) coupled with release of apoptotic factors. Neurochem Int. 2015;90:46–55. doi: 10.1016/j.neuint.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G. Increased susceptibility to Ca(2+)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res. 2010;48:340–346. doi: 10.1111/j.1600-079X.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 42.Allshire A, Bernardi P, Saris NE. Manganese stimulates calcium flux through the mitochondrial uniporter. Biochim Biophys Acta. 1985;807:202–209. doi: 10.1016/0005-2728(85)90123-9. [DOI] [PubMed] [Google Scholar]
- 43.Azarashvili T, Grachev D, Krestinina O, Evtodienko Y, Yurkov I, Papadopoulos V, Reiser G. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium. 2007;42:27–39. doi: 10.1016/j.ceca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Stricker R, Lottspeich F, Reiser G. The myelin protein CNP ′,3′-cyclic nucleotide (2 3′-phosphodiesterase): immunoaffinity purification of CNP from pig and rat brain using a monoclonal antibody and phosphorylation of CNP by cyclic nucleotide-dependent protein kinases. Biol Chem Hoppe Seyler. 1994;375:205–209. [PubMed] [Google Scholar]
- 45.Krestinina O, Azarashvili T, Baburina Y, Galvita A, Grachev D, Stricker R, Reiser G. In aging, the vulnerability of rat brain mitochondria is enhanced due to reduced level of 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNP) and subsequently increased permeability transition in brain mitochondria in old animals. Neurochem Int. 2015;80:41–50. doi: 10.1016/j.neuint.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, Fiore T, Paradies G. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009;297:H1487–H1493. doi: 10.1152/ajpheart.00163.2009. [DOI] [PubMed] [Google Scholar]
- 48.Krestinina OV, Odinokova IV, Baburina YL, Azarashvili TS. Age-related effect of melatonin on permeability transition pore opening in rat brain mitochondria. Biol Membr. 2013;30:304–312. [Google Scholar]
- 49.Krestinina OV, Baburina YL, Azarashvili TS. Effect of melatonin on stress-induced opening of non-selective pore in mitochondria from brain of young and old rats. Biochem Mosc Suppl Ser. 2015;9:116–123. [Google Scholar]
- 50.Reiter RJ, Tan DX, Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29:325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 51.Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 52.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 53.Higami Y, Shimokawa I. Apoptosis in the aging process. Cell Tissue Res. 2000;301:125–132. doi: 10.1007/s004419900156. [DOI] [PubMed] [Google Scholar]
- 54.Morrison JH, Hof PR, Bloom FE. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 55.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 56.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282:R423–R430. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 57.Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antiox-idant vitamin. J Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 58.Costa EJ, Shida CS, Biaggi MH, Ito AS, Lamy-Freund MT. How melatonin interacts with lipid bilayers: a study by fluo rescence and ESR spectroscopies. FEBS Lett. 1997;416:103–106. doi: 10.1016/s0014-5793(97)01178-2. [DOI] [PubMed] [Google Scholar]
- 59.Garcia JJ, Reiter RJ, Pie J, Ortiz GG, Cabrera J, Sainz RM, Acuna-Castroviejo D. Role of pinoline and melatonin in stabilizing hepatic microsomal membranes against oxidative stress. J Bioenerg Biomembr. 1999;31:609–616. doi: 10.1023/a:1005425213253. [DOI] [PubMed] [Google Scholar]
- 60.Verrier JD, Jackson TC, Bansal R, Kochanek PM, Puccio AM, Okonkwo DO, Jackson EK. The brain in vivo expresses the 2′,3′-cAMP-adenosine pathway. J Neurochem. 2012;122:115–125. doi: 10.1111/j.1471-4159.2012.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams LM, Drew JE, Bunnett NW, Grady E, Barrett P, Abramovich DR, Morris A, Slater D. Characterization of an antibody to the human melatonin mt1 receptor. J Neuroendocrinol. 2001;13:94–101. doi: 10.1046/j.1365-2826.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- 62.Sprinkle TJ. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- 63.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry. 1994;33:7408–7414. doi: 10.1021/bi00189a047. [DOI] [PubMed] [Google Scholar]
- 65.Jackson EK. The 2′,3′-cAMP-adenosine pathway. Am J Physiol Ren Physiol. 2011;301:F1160–F1167. doi: 10.1152/ajprenal.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]