Abstract
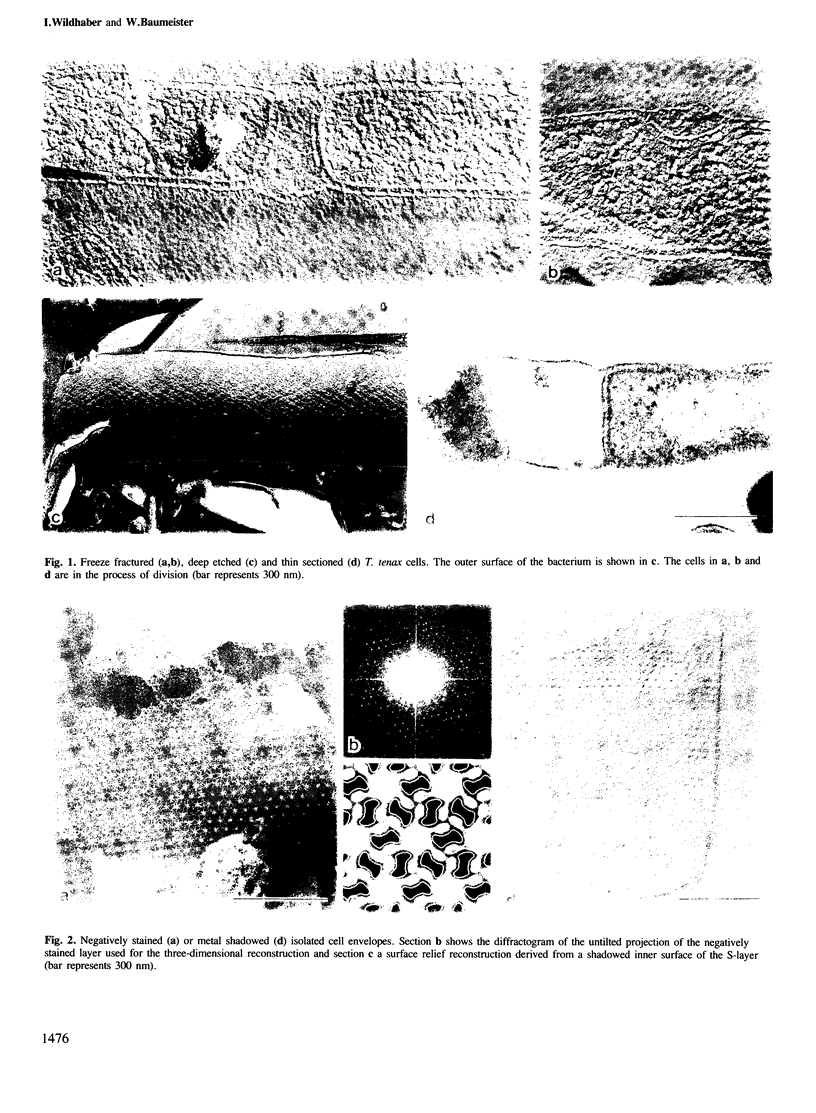
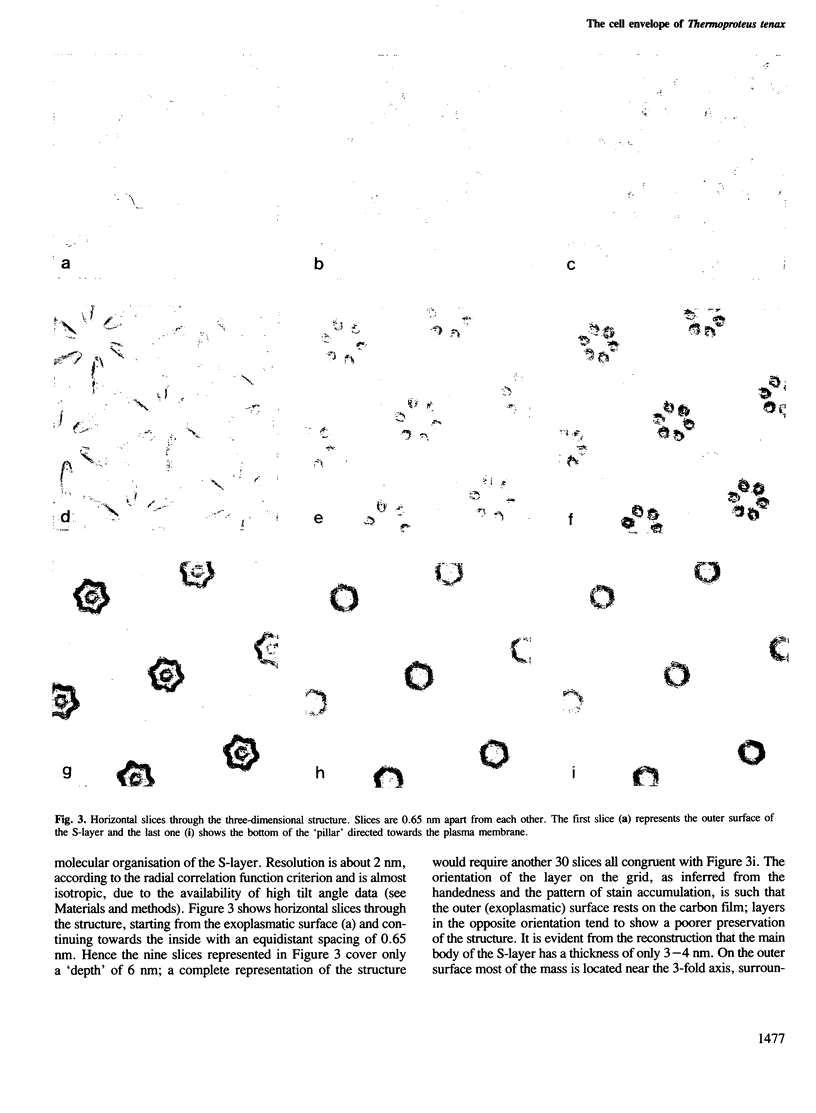
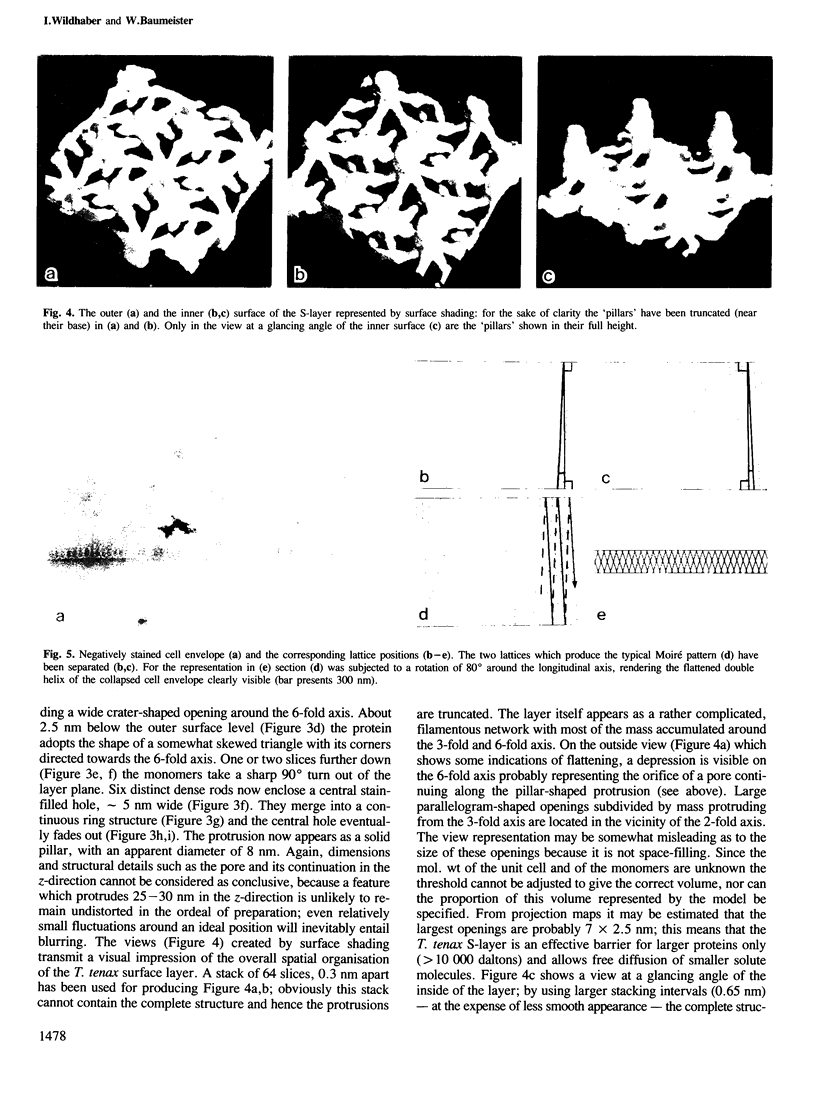
The sulphur-dependent archaebacterium Thermoproteus tenax has a cylindrical cell shape variable in length, but constant in diameter. Its whole surface is covered by a regular protein layer (S-layer). The lattice has p6 symmetry and a lattice constant of 32.8 nm. The three-dimensional reconstruction from a tilt series of isolated and negatively stained S-layer shows a complex mass distribution of the protein: a prominent, pillar-shaped protrusion is located at the 6-fold crystallographic axis with radiating arms connecting neighbouring hexamers in the vicinity of the 3-fold axis. The base vectors of the S-layer lattice have a preferred orientation with respect to the longitudinal axis of the cell. The layer can be seen as a helical structure consisting of a right-handed, two-stranded helix, with the individual chains running parallel. Supposing that new S-layer protein is inserted at lattice faults (wedge disclinations) near the poles, growing of the layer would then proceed by moving a disclination at the end of the helix. The constant shape of the cell, as well as the particular structure of the layer, strongly suggest that this S-layer has a shape-maintaining function.
Keywords: electron microscopy, S-layer, shape determination, Thermoproteus tenax, three-dimensional reconstruction
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Taylor K. A., Amos L. A. Three-dimensional arrangement of the cell wall protein of Sulfolobus acidocaldarius. J Mol Biol. 1983 Jul 15;167(4):823–848. doi: 10.1016/s0022-2836(83)80113-2. [DOI] [PubMed] [Google Scholar]
- Engel A., Baumeister W., Saxton W. O. Mass mapping of a protein complex with the scanning transmission electron microscope. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4050–4054. doi: 10.1073/pnas.79.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. F., Scriven L. E. Function of dislocations in cell walls and membranes. Nature. 1970 Nov 28;228(5274):827–829. doi: 10.1038/228827a0. [DOI] [PubMed] [Google Scholar]
- Hegerl R., Altbauer A. The "EM" program system. Ultramicroscopy. 1982;9(1-2):109–116. doi: 10.1016/0304-3991(82)90233-9. [DOI] [PubMed] [Google Scholar]
- Langmore J. P., Wooley J. C. Chromatin architecture: investigation of a subunit of chromatin by dark field electron microscopy. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2691–2695. doi: 10.1073/pnas.72.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Vigers G. P., Crowther R. A., Pearse B. M. Three-dimensional structure of clathrin cages in ice. EMBO J. 1986 Mar;5(3):529–534. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]