Abstract
Objectives
Neurodynamic exercises aim to improve neural mechanosensitivity in order to promote pain-free movement and function. People with diabetes mellitus (DM) may be candidates for neurodynamic exercises to address common DM-related impairments such as reduced lower extremity range of motion (ROM) and altered neural mechanosensitivity. However, no studies have examined the safety and immediate effects of neurodynamic exercise in people with DM. This study aims to determine the feasibility of applying neurodynamic exercises in adults with DM by evaluating the rate of adverse events and quantifying immediate changes in straight leg raise (SLR) ROM.
Methods
This quasi-experimental study included 20 people with DM who performed a series of neurodynamic exercises on their right leg. Their left leg was used as an internal control. SLR testing was performed before and immediately after these exercises. Adverse events were monitored, including provocation of their neuropathy symptoms or discomfort or pain.
Results
All participants completed the neurodynamic exercises without provocation of their neuropathy symptoms. No pain was reported and only one participant had minor discomfort with one exercise; a <30-s calf cramp. The right SLR ROM increased by an average of 5.2°–5.3° (p < 0.01) with no change on the left.
Discussion
This study demonstrated that lower extremity neurodynamic exercises are safe in adults with DM and may create small immediate improvements in SLR testing. Further research is indicated to investigate the safety and efficacy of neurodynamic exercises performed over multiple sessions.
Level of evidence
3b
Keywords: Nerve, Neurodynamics, Mechanosensitivity, Diabetes mellitus, Straight leg raise, Safety, Feasibility
Introduction
Neurodynamic exercises use specific combinations of spine and limb movements that aim to reduce nerve mechanosensitivity and restore symptom-free limb movement and function.1,2 Current evidence supports using neurodynamic exercises to treat increased nerve mechanosensitivity in patients with neck-arm pain and low back-leg pain.3–6 Since 21 million people in the United States have been diagnosed with diabetes mellitus (DM),7 many patients referred to physical therapy with musculoskeletal diagnoses will have this common comorbidity of DM.8 However, our understanding of responses and tolerance to neurodynamic exercises in adults with DM is limited.
DM has been proposed as a precaution for treatment with neurodynamic exercises.2 Alterations in lower extremity nerve biomechanics and neurodynamic test findings in people with DM provide some support for this proposal. Specifically, adults with DM, both with and without the presence of diabetic peripheral neuropathy (DPN), demonstrated less nerve movement during ankle dorsiflexion in varied hip positions that mimic the straight leg raise (SLR) compared to healthy controls.9,10 Additionally, adults with DM may be less responsive to progressive increases in mechanical loading of neural tissues during the SLR.11 When neural tissues are loaded to a greater degree during the SLR by dorsiflexing the ankle prior to flexing the hip, healthy individuals exhibit less hip flexion range of motion (ROM).12–14 This decrease in hip flexion ROM is thought to be a protective response to the increased neural tissue load.13,14 However, the protective response to ankle dorsiflexion preceding hip flexion during SLR was diminished in people with DM regardless of the presence of DPN and was absent in people with DM with signs of severe DPN.11
Additionally, adults with DM often have reduced lower extremity mobility that may impact their health status.15–17 For example, reduced ankle mobility is commonly associated with DM16–18 and may be a risk factor for developing diabetic foot ulcerations 19,20 that in turn significantly elevate the risk for morbidity (e.g. amputations) and mortality.21,22 Reductions in limb mobility may be related to non-neural structures (i.e. fascia, muscles, and joints) or to neural structures due to increased mechanosensitivity. The latter may be amenable to neurodynamic exercises. However, it is unclear if people with DM can tolerate neurodynamic exercises and if this intervention is safe and appropriate for this population, regardless of the presence of DPN. Recognition of mobility impairments and understanding responses to neurodynamic exercises in adults with DM is important in designing treatments that are not only efficacious but are also safe. However, no study to date has examined the immediate effects and safety of neurodynamic exercises in people with DM.
Therefore, this study aimed to determine the safety of applying neurodynamic exercises in adults with DM by evaluating (1) the rate of adverse events, including symptom provocation and (2) the immediate changes in lower limb ROM as measured by the SLR neurodynamic test after a series of active neurodynamic exercises. This study will provide much needed information regarding the safety of performing neurodynamic exercises in adults with DM.
Materials and methods
Study design
This was a quasi-experimental study investigating the feasibility and safety of providing neurodynamic exercises to people with DM. Study participants attended one session that consisted of a series of five neurodynamic exercises. Occurrence of adverse events was assessed during and after the intervention. Neurodynamic testing was assessed before and immediately after the intervention. All participants performed the interventions on their right lower extremity and received no direct intervention to their left lower extremity, which acted as the within-participant comparison control. Neither the examiner nor the participants were blinded as to which limb received the intervention. However, SLR mobility results were withheld from the participant until the end of the session to minimize biasing the outcome by knowledge of performance.
Participants
A sample of convenience was recruited through local print, electronic, and radio advertisements. Participants were required to be at least 18 years old and have a medical diagnosis of DM (type 1 or type 2). It was required that all participants had <12% on their most recent HbA1c test (within past 3 months) and a blood glucose level between 100 and 250 mg/dL at the time of participation based upon Mayo Clinic guidelines for safe exercise.23 Additional inclusion criteria required bilateral ROM of at least 0–90° hip flexion, 0–90° knee flexion, and ankle motion from 0° dorsiflexion to 30° plantar flexion. The presence of neuropathy was not required for participation in this study as DM was the main risk factor of interest. Potential participants were excluded if they had chemical, drug, or alcohol dependency; medical diagnosis of a herniated disk in the back; trauma to the nerves of the lower extremity; current neck or low back pain; history of lumbar surgery; chemotherapy use within the past year; complex regional pain syndrome; lower extremity amputations proximal to the metatarsophalangeal joint; open wounds; infection in the lower limbs; or were currently pregnant. Informed consent was obtained prior to enrolling participants in the study. The Institutional Review Board at Samuel Merritt University approved this study. The participants in the present study were also evaluated for tibial nerve mobility using ultrasound imaging which has been previously reported elsewhere.10
Clinical examination procedures
After obtaining informed consent, an ACCU-CHEK Aviva glucose meter (Roche Diagnostics, Indianapolis, IN) determined finger capillary blood glucose levels to insure that all participants met this specific eligibility requirement. Participants provided demographic and general health information and completed the self-report Modified Baecke Questionnaire (MBQ), which is a valid and reliable tool to quantify physical activity level with common work, leisure, and sport-related activities.24 Height, weight, and body mass index (BMI) were determined.
Three examinations were performed to characterize the severity of peripheral neuropathy (if present): vibration perception threshold (VPT), the Michigan Neuropathy Screening Instruments (MNSI), and the Michigan Diabetic Neuropathy Score (MDNS). VPT testing was performed bilaterally on the distal hallux using a biothesiometer (60 Hz; Bio-Medical Instruments Company, Newbury, OH, USA). The amplitude of vibration was slowly increased from 0 to 50 V and the participant indicated the moment they first felt the vibration sensation, which was considered their VPT.11 The average score of two repetitions on each limb was recorded (Table 1). Detailed procedures for the MNSI and MDNS are described elsewhere.25–28 The instruments consist of a multimodal clinical examination of nerve function including sensory testing (monofilament, tuning fork, and sharp-dull discrimination), muscle strength testing, deep tendon reflex testing, and visual assessment of the feet. Higher scores on the MNSI (scale of 0–8) indicate signs of neuropathy with a normal range of 0–2.27,28 The MDNS (scale of 0–46) has a scale for grading neuropathy severity, with 0–6 indicating no neuropathy, 7–12 indicating mild neuropathy, 13–29 indicating moderate neuropathy, and 30–46 indicating severe neuropathy.28
Table 1.
Demographic characteristics
| Demographic characteristic | Mean (standard deviation) | Range |
|---|---|---|
| Age (years) | 51.1 (10.8) | 25–66 |
| Height (m) | 1.7 (0.1) | 1.6–1.8 |
| Weight (kg) | 84.9 (28.9) | 52.2–163.3 |
| BMI (kg/m2) | 28.2 (8.4) | 18.3–48.8 |
| Sex (%) | 30% female | N/A |
| 70% male | ||
| Modified Baecke Questionnaire (scale 3–15) | 8.5 (1.1) | 6.3 – 10.9 |
| Years of DM | 12.8 (12.4) | 1 month–52.0 years |
| Type of DM | 40% Type 1 | N/A |
| 60% Type 2 | ||
| Blood glucose at time of participation (mg/dL) | 152.9 (41.3) | 102–249 |
| Hb A1c (%) | 7.5 (1.4) | 5.6–10.9 |
| MDNS (scale 0–46) | 11.7 (8.5) | 0.0–25.0 |
| MNSI (scale 0–8) | 3.2 (1.9) | 0.0–6.0 |
| VPT (scale 0–50 V) | 18.2 (12.5) | 5.0–42.5 |
Note: BMI = body mass index; DM = diabetes mellitus; HB A1c = hemoglobin A1c; MDNS = Michigan diabetes neuropathy score; MNSI = Michigan neuropathy screening instrument; VPT = vibration perception threshold.
Intervention procedures
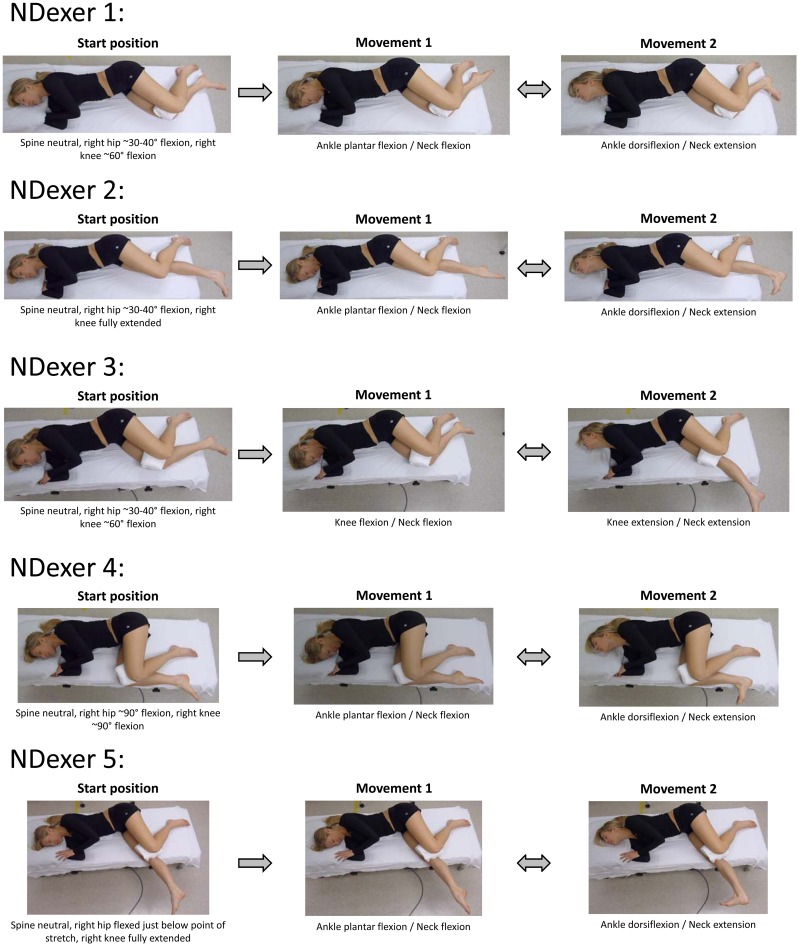
Five separate neurodynamic exercises were performed in a comfortable right side-lying position on a padded mat. Pillows and folded towels supported the participant’s neck and limbs in a comfortable position and assured that the thoracolumbar spine was in neutral alignment. Neurodynamic exercises that simultaneously slacken from one end of the nerve while tensioning from the opposite end are thought to facilitate a sliding movement between the neural tissue and the adjacent non-neural interfacing tissue (sliding technique)1,2,29,30 and are associated with relatively lower levels of nerve strain.1,2,29,31 The exercises included ankle, knee, and/or neck sagittal plane motions performed in various hip and knee positions. Each neurodynamic exercise was performed in a cyclical fashion where each end alternated between tension and slackening (Figure 1 and Supplemental Video). This series of neurodynamic exercises were selected to gradually and progressively preposition the participant into an increasing amount of preloading of the posterior neural structures32,33 by introducing hip and knee positions that are components of the SLR test. The participant performed all movements actively with instructions to create as large a motion as possible without provoking discomfort. If symptoms or discomfort were provoked, the exercise was modified by reducing the ROM to allow movement without symptom provocation.
Figure 1.
Neurodynamic exercises. Participant positioning and movements for each of the five neurodynamic exercises. Start position identifies the pre-positioning prior to beginning the exercises. Movement 1 and movement 2 indicated the end positions involved with the neurodynamic slider exercise. The participant repeatedly moved back and forth between movement 1 position and movement 2 position for 30 s for each exercise. They were given a 60-s rest between each exercise. Courtesy: Authors Own Photographs.
One to two initial trials were performed to assure the participant understood how to perform each movement combination. The participant was then instructed to perform each exercise for 30 s at a slow and comfortable pace of 1 repetition per ~3–4 s. The participants rested for 60 s between each exercise. The total duration of the interventions was approximately 10 min.
Adverse events
In participants with neuropathy, adverse events included any reproduction or exacerbation of the participant’s neuropathy symptoms. For all participants, any reported discomfort or pain from the neurodynamic exercises was also considered an adverse event. Participants described the quality, location, and time to resolve or return to baseline for all neuropathic symptoms or other sensory responses they experienced. Symptoms of stretch or tightness during the SLR testing or during the exercises were not considered to be adverse events as long as the sensation was directly associated with movements into a specific position and resolved immediately once out of that position.
Neurodynamic testing
The SLR test was used to assess pre-intervention to post-intervention changes in ROM. The participant was positioned in supine on a padded plinth with a standardized 2.5 cm foam pad for head support. Two variations of SLR were performed.11,13,34,35 The participant’s ankle was manually placed in full dorsiflexion and then again in full plantar flexion as determined by end range resistance by the examiner. A handheld inclinometer was placed on their anterior leg just distal to the tibial tuberosity. The pelvis was not stabilized during SLR testing to mimic clinical examination procedures. The passive SLR (hip flexion with knee in full extension) was performed up to the point that the participant indicated the first onset of any symptoms or sensory response. The motion was held at that position for 5 s to obtain a measure from the inclinometer and to allow the participant to report any symptoms or sensory responses. Then, the limb was slowly returned to the start position followed by a two-minute rest period between measurements. The handheld inclinometer has excellent reliability and validity when used for SLR testing compared to a highly precise digital inclinometer in healthy individuals.34 To determine the measurement properties of handheld inclinometer in this study, two measurements were performed on each limb before the intervention was applied. The second pre-intervention measurement was used to compare to a single post-intervention measurement of SLR mobility.
Statistical analysis
Power analysis revealed that at least 19 participants were needed to detect an anticipated 10° ROM difference between experimental and control limbs after intervention (15° standard deviation) with 80% power and an alpha of 0.05. Thus, 20 participants were recruited for the present study. Descriptive statistics and frequency distributions were generated for demographic and clinical characteristics. Repeated measurements were used to determine intraclass correlation coefficients (ICC2,1), standard error of the measurement (SEM), and minimal detectible change with a 95% confidence interval (MDC95).36 A general linear model for repeated measures was performed utilizing the following factors: side (two levels: left and right), time (two levels: pre-intervention and post-intervention), and interaction effects (side by time effects). Paired t-tests were utilized for within-limb or between-limb comparisons for ROM changes during SLR testing. Sensory responses (location and quality descriptors) during SLR testing were compared using related samples Wilcoxon signed-rank tests. Statistical analyses were performed using IBM SPSS Statistics, v.22 (IBM Corporation, Somers, NY, USA).
Results
Twenty participants were enrolled and completed all testing. Participant demographic and clinical characteristics are presented in Table 1. This sample included individuals with signs of mild-to-moderate neuropathy, many of whom had significant risk for developing ulcerations due to their diabetic neuropathy. Specifically, MNSI scores were in an abnormal range (>2/8) in 70% of participants (n = 14) and MDNS scores indicated 35% of participants (n = 7) had no signs of neuropathy but that 20% (n = 4) had signs of mild and 45% (n = 9) had signs of moderate neuropathy.27,28 The VPT scores indicated that 50% of the participants (n = 10) had an intermediate risk (16–24 V) to high risk (≥25 V) for developing diabetic ulcerations.37
Measurement properties of handheld inclinometer
Repeated measures of PF/SLR and DF/SLR with the handheld inclinometer demonstrated excellent intra-session, intra-rater, test–retest reliability, and low measurement error (Table 2). The MDC95 was less than 2° for both limbs and both SLR test variations. Thus, differences of 2° or more between pre-intervention and post-intervention measurements were considered beyond measurement error and representative of real change in SLR mobility.
Table 2.
Measurement properties of handheld inclinometer during straight leg raise testing in people with diabetes mellitus
| Reliability | ICC2,1 (95% CI) | SEM | MDC95 |
|---|---|---|---|
| Right PF/SLR | 0.99 (0.98, 1.00) | 0.32° | 0.90° |
| Left PF/SLR | 0.97 (0.93, 0.99) | 0.52° | 1.45° |
| Right DF/SLR | 0.99 (0.98, 1.00) | 0.30° | 0.85° |
| Left DF/SLR | 0.97 (0.97, 1.00) | 0.52° | 1.43° |
Note: DF = dorsiflexion ankle position; PF = plantar flexion ankle position; SLR = straight leg raise; ICC = intraclass correlation coefficient; CI = confidence interval; SEM = standard error of the measurement (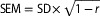 ); MDC95 = minimal detectible change at 95% CI (MDC95 =
); MDC95 = minimal detectible change at 95% CI (MDC95 = 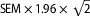 ).
).
Neurodynamic exercise
All participants were able to complete the exercise interventions. The initial 1–2 training trial movements were included in the exercise analysis as they contributed to overall time and ROM utilized during each exercise phase. The average time spent performing exercise #1 was 43.5 s (SD 15.7 s) with 7.3 (SD: 2.9) repetitions. For exercise #2, this was 38.4 s (SD 6.9 s) with 8.2 (SD: 2.5) repetitions. For exercise #3, this was 42.6 s (SD 10.4 s) with 8.6 (SD: 1.8) repetitions. For exercise #4, this was 37.0 s (SD 4.5 s) with 9.5 (SD: 3.3) repetitions. For exercise #5, this was 40.6 s (SD 8.5 s) with 9.5 (SD: 3.0) repetitions. The variability in time and number of repetitions within and between each exercise represents differences between participants in the number of trials needed to learn the movement, the speed chosen to adequately coordinate the movement, and the available ankle (exercises #1, 2, 4, and 5) and knee ROM (exercise #3). On average, total time spent by participants performing all of the neurodynamic exercises was 187.6 s (SD: 36.8).
Adverse events
There was only one minor adverse event during this study. No participants reported reproduction or exacerbation of their neuropathy symptoms with any of the neurodynamic exercises. In addition, no participants reported any pain during any of the exercises. Only one participant reported discomfort during one of the exercises (exercise #4). He experienced a calf cramping sensation after performing the seventh repetition of the movement. The exercise was discontinued, and the cramping sensation completely resolved within 30 s of moving out of that position. The participant was able to continue with the remaining exercises without any return of this or any other symptom.
There were no reports of any sensory responses during the first four exercises (other than the one participant mentioned above). During the fifth exercise, more than half of the participants (55%; n = 11) reported a stretching sensation in the posterior knee (25%; n = 5), calf (30%; n = 6), and plantar aspect of the foot (10%; n = 2) when they moved into ankle dorsiflexion and neck extension. For these participants, this sensation was directly associated with movement into this position and resolved immediately when they moved out of that position and did not stop them from comfortably performing all repetitions of this exercise. No participants reported symptoms or residual sensory responses after completing the exercises and the final rest period.
SLR neurodynamic testing
The general linear model for repeated measures demonstrated significant side by time effects such that the right limb demonstrated significantly greater ROM after the intervention for PF/SLR (p = 0.002) and DF/SLR (p < 0.001).
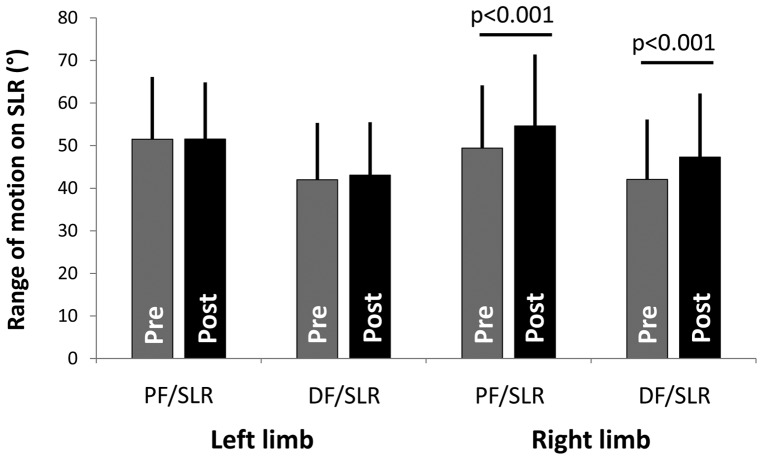
Figure 2 demonstrates the ROM during both versions of the SLR before and after the intervention. Specifically, the PF/SLR ROM was 51.5° (SD: 14.6°) on the left (control) limb and 49.4° (SD: 14.8°) on the right (experimental) limb (paired t-test; p = 0.262) prior to intervention. After the intervention, the PF/SLR on the left was 51.5° (SD: 13.3°) which was not significantly changed from pre-intervention levels (paired t-test; p = 0.828). In contrast, the PF/SLR on the right increased to 54.6° (SD: 16.8°) from pre-intervention levels (paired t-test; p < 0.001). This represents a 5.2° (SD: 5.3°; 95%CI: 2.7°, 7.7°) improvement on the right (experimental) side where 70% (n = 14) of the participants experienced an increase in PF/SLR ROM of at least 2° (threshold based upon the MDC95). In contrast, using this same MDC threshold, 40% (n = 8) of the participants experienced an increase in PF/SLR ROM on the left (control) side.
Figure 2.
ROM during SLR. SLR ROM is presented (in degrees) for the left “control” limb and the right “experimental” limb for both the PF/SLR and the DF/SLR. Pre-intervention ROM is presented in the gray bars, and the post-intervention ROM is presented in the black bars (with SD error bars). There was a significant increase in SLR ROM on the right limb post-intervention compared to pre-intervention for the PF/SLR (p < 0.001) and the DF/SLR (p < 0.001).
The DF/SLR mobility demonstrated a similar side-specific response to these neurodynamic exercises. Before the intervention the DF/SLR ROM to the first onset of symptoms was 42.0° (SD: 13.3°) on the left (control) limb and 42.1° (SD: 14.1°) on the right (experimental) limb (paired t-test; p = 0.967). After the intervention, the DF/SLR on the left was 43.1° (SD: 12.4°) which was not significantly changed from pre-intervention levels (paired t-test; p = 0.574). In contrast, the DF/SLR on the right increased to 47.3° (SD: 15.0°) from pre-intervention levels (paired t-test; p < 0.001). This represents a 5.3° (SD: 3.8°; 95%CI: 3.5°, 7.0°) improvement on the right (experimental) side where 85% (n = 17) of the participants experienced an increase DF/SLR ROM of at least 2° (threshold based upon the MDC95). In contrast, using this same MDC threshold, 20% (n = 4) of the participants experienced an increase in DF/SLR ROM on the left (control) side.
Reductions in SLR ROM after the intervention could be a sign of decreased tolerance to mechanical loading and therefore provide a measure of safety of the neurodynamic exercises. Only one participant (5%) had reduced PF/SLR ROM after the intervention that was greater than the measurement error threshold (MDC95) of 2° (PF/SLR ROM reduced by 3°). This participant had no signs of DPN and reported no sensory responses during the neurodynamic exercises. No participants had reduced DF/SLR ROM after the intervention. These data suggest that the neurodynamic exercises were well tolerated.
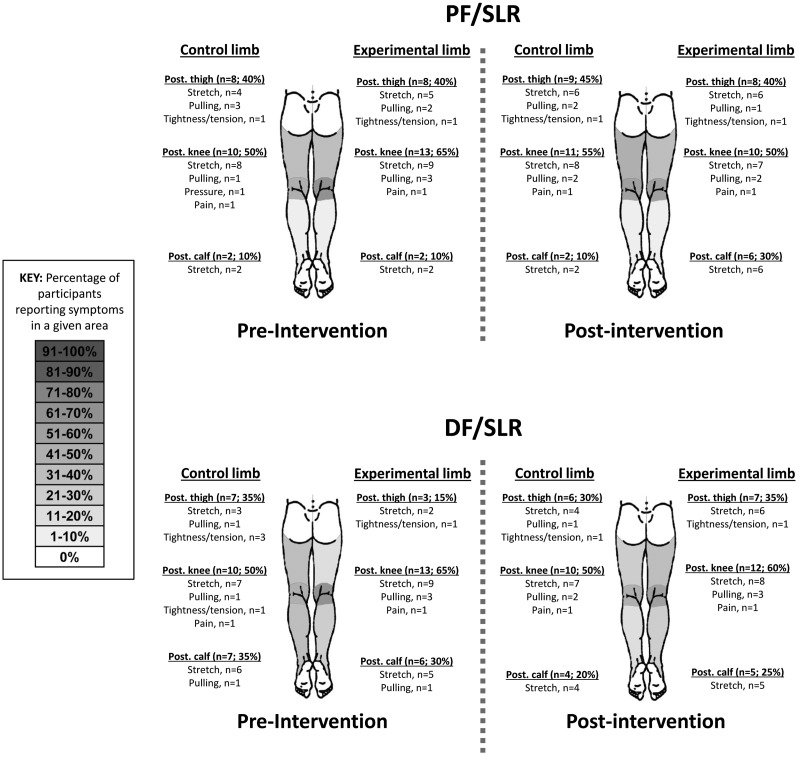
When comparing the sensory provocation during the SLR, there was no significant difference between the sensory responses before and after the intervention procedures (Figure 3). Before the intervention, there were no significant differences between the left and right limbs in the location (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.655; DF/SLR, p = 0.271) or quality descriptors (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.999; DF/SLR, p = 0.180) of sensory responses during SLR testing. After the intervention, there was no significant change in location of sensory responses during SLR testing for the left (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.564; DF/SLR, p = 0.748) nor the right limb (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.366; DF/SLR, p = 0.132). There was also no significant change in quality of sensory responses during SLR testing for the left (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.317; DF/SLR, p = 0.102) nor the right limb (related samples – Wilcoxon signed-rank test; PF/SLR, p = 0.317; DF/SLR, p = 0.317). Only one participant (5%) reported pain (posterior knee) with SLR testing, and they consistently felt this on both sides of the body with both PF/SLR and DF/SLR. This sensory response was not present at rest and immediately ceased upon taking the participant’s limbs out of the SLR testing positions. This response was unchanged after the intervention. No other participants reported pain at any point during the study.
Figure 3.
Sensory responses during SLR. Body charts are presented to represent the frequencies of reported sensory response location and quality descriptors. The left limb was the “control” limb, and the right limb was the “experimental” limb. Pre-intervention and post-intervention body charts are presented for both the PF/SLR and the DF/SLR. The regions are divided into the posterior hip, thigh, knee, leg, and planter surface of the foot. The shaded body chart represents the percentage of participants reporting sensory responses in each of these areas (ex: n = 11, 55%), with darker gray indicating a higher percentage (scale provided). Additionally, the quality descriptors for that area are listed below each region.
Discussion
This is the first study to our knowledge to show that a single session of neurodynamic exercises can be administered safely in adults with DM. There were no significant adverse events associated with a single session of neurodynamic exercises in our sample. None of the participants with documented neuropathy experienced a provocation of their symptoms with these exercises. None of the participants (regardless of neuropathy status) experienced pain during the exercises, and only one reported discomfort in the form of a calf cramp that resolved quickly upon ceasing that specific exercise. That participant completed the remaining exercises without any further discomfort. A sensation of stretching was noted frequently with the last of the exercises (#5), but this sensory response was transient and directly associated with movement into ankle dorsiflexion with neck extension and resolved immediately when participants moved out of that position. None of the participants that felt this stretching sensation required any modifications to the neurodynamic exercises. After completing the exercises, no participants reported symptoms or residual sensory responses provoked by the exercises. In addition, subsequent SLR testing after performing neurodynamic exercises did not alter sensory responses and did not trigger neuropathic symptoms compared to pre-intervention testing. The sensory responses provoked during the SLR tests were similar to previous studies in people with type 2 DM11 and healthy, asymptomatic people without diabetes.13
SLR ROM data after the intervention provide further support for the safety of neurodynamic exercises for people with DM. Reductions in SLR ROM after the intervention could be a sign of decreased tolerance to mechanical loading of lower extremity tissues. Because only one participant experienced a reduction in PF/SLR ROM (decreased by 3°) beyond measurement error and no participants experienced a reduction in DF/SLR ROM after the intervention, it is evident that the neurodynamic exercises were well tolerated by the participants in this study. There is evidence that various forms of exercise can be safe for people with DM and peripheral neuropathy,38–43 but none of these studies investigated neurodynamic exercises. Our findings are the first evidence for the safety of the included neurodynamic exercises for people with DM.
Immediate improvements in SLR ROM suggest that neurodynamic exercise may improve lower extremity ROM and alter mechanosensitivity in lower extremity neural tissues in this population. Admittedly, the ROM increases seen in the PF/SLR test may be additionally due to non-neural structures accommodating greater elongation, such as the hamstring muscles or the hip joint structures. However, it is theorized that when ankle dorsiflexion pre-positioning reduces the amount of hip ROM (during DF/SLR compared to PF/SLR), this is more reflective of the nervous system’s contribution to ROM limitations.11,13,32,33 Thus, DF/SLR can be used to track changes in ROM that can be, at least in part, attributed to changes in the nervous system’s mechanosensitivity. In the present study, the increase in DF/SLR ROM after neurodynamic interventions supports the conclusion that mechanosensitivity improved in these individuals with DM. This suggests that, despite the presence of DM with or without mild-to-moderate neuropathy, the nervous system has the capacity to adapt and allow for more symptom-free movement. In other words, the results of the study demonstrate that this impairment is not fixed.
Although statistically significant, the approximately 5° average improvement in SLR ROM on the experimental limb may not be clinically meaningful in all patient circumstances. However, there is previous evidence that people with DM have mean reductions in ROM during the SLR of 2° to 13° depending on the testing methods.11,13 It is therefore conceivable that a 5° improvement in SLR mobility may be clinically meaningful in some patients as it could at least partially normalize this particular mobility impairment. However, it is also important to recognize that 15–30% of participants did not experience an increase in SLR mobility above the MDC of 2°. Additional research is necessary to determine whether this modest change in SLR ROM has any link to more meaningful functional changes within this population.
Because all five neurodynamic exercises were performed before post-testing of SLR ROM and sensory responses, it is not clear whether the observed changes in the experimental limbs were related to a combination of all five exercises or to the effects of one or more of the exercises. The sequence, number, and order of the exercises were not meant to be used as a protocol, but rather determine the feasibility and safety of each in participants with DM. Since all of the neurodynamic exercises in this study were tolerated well, it is recommended that clinicians choose based on the patient’s ability to perform the exercise without provoking any symptoms, similar to the parameters used in this study. Additionally, biomechanical principles can be considered when choosing neurodynamic exercises. Neurodynamic exercises #2, #3, and #5 are likely to have greater biomechanical effects on the lower extremity neural tissues because pre-loading of the nervous system included both hip flexion and knee extension (exercises #2 and #5) or because a knee extension movement was performed with the hip pre-positioned in flexion (exercise #3).44,45 Therefore, it would seem reasonable clinically to initially consider these (or other similar) neurodynamic exercises when greater loading of the nervous system is indicated and tolerated. Neurodynamic exercises #1 and #4 (or other variations) could be explored when those initial exercises are not tolerated. Further research is needed to determine optimal neurodynamic exercise choice (or combinations of neurodynamic exercises) and appropriate dosage for improving outcomes in patients with DM. Furthermore, it is necessary to determine the safety of performing neurodynamic exercise over multiple treatment sessions and whether that can lead to greater and longer-lasting improvements in lower extremity ROM in this population.
It is important to note that the present study sample included adults with either no neuropathy or mild-to-moderate neuropathy, as rated by the MDNS, without the presence of ulcerations. This sample covers a wide spectrum of patients who may consult a physical therapist with a musculoskeletal disorder that also have DM as a comorbid condition. It is unclear if performing these exercises would be safe in other populations of people with DM such as those with severe neuropathy, ulcerations, or in children. Further research is necessary to elucidate the safety and efficacy of these neurodynamic exercises in different populations.
The limitations present in this study warrant caution when attempting to extrapolate these findings beyond the study sample. Such limitations include not knowing whether functionally relevant improvement is associated with this type of exercise, whether the changes noted would be greater and/or maintained through neurodynamic exercises over multiple sessions, what dosage of such exercises will optimize outcomes, and whether safety and efficacy transfer to other populations of patients who have DM. In addition, we did not control for medications during this single-session trial and the examiner was not blinded to which limb the intervention was applied. Despite these limitations, this is the first study to demonstrate that a single session of neurodynamic exercises can be both safe and effective at creating immediate improvements in lower extremity ROM and improvements in neural tissue mechanosensitivity in people with DM. Results from this study provide a foundation for further investigation into the impacts of neurodynamic exercises in people with DM.
Supplementary material
The supplementary material for this paper is available online at http://dx.doi.10.1080/10669817.2016.1180772.
Conflict of interest statement
The authors declare that they have no conflict or competing interests.
Supplementary Material
Acknowledgements
The authors would like to thank Erin Carlson, Felicia Ferlin, George Haras, Amaya James, and Amanda Welch for their assistance with data collection and analysis. This project was supported in part by a Faculty Research Grant and a Faculty Research Incentives Award from Samuel Merritt University awarded to BSB.
Funding
This work was supported by Samuel Merritt University.
References
- [1].Nee RJ, Butler DS. Management of peripheral neuropathic pain: integrating neurobiology, neurodynamics, and clinical evidence. Phys Ther Sport. 2006;7:36–49. 10.1016/j.ptsp.2005.10.002 [DOI] [Google Scholar]
- [2].Butler DS. The sensitive nervous system. Adelaide: Noigroup Publications; 2000. [Google Scholar]
- [3].Nee RJ, Vicenzino B, Jull GA, Cleland JA, Coppieters MW. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J Physiother. 2012;58(1):23–31. doi: 10.1016/S1836-9553(12)70069-3. [DOI] [PubMed] [Google Scholar]
- [4].Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11(4):279–286. 10.1016/j.math.2005.07.002 [DOI] [PubMed] [Google Scholar]
- [5].Nagrale AV, Patil SP, Gandhi RA, Learman K. Effect of slump stretching versus lumbar mobilization with exercise in subjects with non-radicular low back pain: a randomized clinical trial. J Man Manip Ther. 2012;20(1):35–42. doi: 10.1179/2042618611Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Basson C, Oliver B, Coppieters M, Ellis R, Stewart A, Mudzi W. The effectiveness of neural mobilization in the treatment of neuro-musculoskeletal conditions: a systematic review and meta-analysis. Physiotherapy. 2015;101(Suppl 1):e127–e128. 10.1016/j.physio.2015.03.269 [DOI] [PubMed] [Google Scholar]
- [7].National Diabetes Statistics Report , 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed September 30, 2015. [Google Scholar]
- [8].Kirkness CS, Marcus RL, Lastayo PC, Asche CV, Fritz JM. Diabetes and associated risk factors in patients referred for physical therapy in a national primary care electronic medical record database. Phys Ther. 2008;88(11):1408–1416. doi: 10.2522/ptj.20080129. [DOI] [PubMed] [Google Scholar]
- [9].Boyd BS, Gray AT, Dilley A, Wanek L, Topp KS. The pattern of tibial nerve excursion with active ankle dorsiflexion is different in older people with diabetes mellitus. Clin Biomech. 2012;27(9):967–971. doi: 10.1016/j.clinbiomech.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boyd BS, Dilley A. Altered tibial nerve biomechanics in patients with diabetes mellitus. Muscle Nerve. 2014;50(2):216–223. doi: 10.1002/mus.24155. [DOI] [PubMed] [Google Scholar]
- [11].Boyd BS, Wanek L, Gray AT, Topp KS. Mechanosensitivity during lower extremity neurodynamic testing is diminished in individuals with Type 2 diabetes mellitus and peripheral neuropathy: a cross sectional study. BMC Neurol. 2010;10:75. doi: 10.1186/1471-2377-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boland RA, Adams RD. Effects of ankle dorsiflexion on range and reliability of straight leg raising. Aust J Physiother. 2000;46:191–200. 10.1016/S0004-9514(14)60328-7 [DOI] [PubMed] [Google Scholar]
- [13].Boyd BS, Wanek L, Gray AT, Topp KS. Mechanosensitivity of the lower extremity nervous system during straight-leg raise neurodynamic testing in healthy individuals. J Orthop Sports Phys Ther. 2009;39(11):780–790. doi: 10.2519/jospt.2009.3002. [DOI] [PubMed] [Google Scholar]
- [14].Palmer TB, Akehi K, Thiele RM, Smith DB, Warren AJ, Thompson BJ. Dorsiflexion, plantar-flexion, and neutral ankle positions during passive resistance assessments of the posterior hip and thigh muscles. J Athl Train. 2015;50(5):467–474. doi: 10.4085/1062-6050-49.6.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kwon OY, Minor SD, Maluf KS, Mueller MJ. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture. 2003;18(1):105–113. 10.1016/S0966-6362(02)00166-2 [DOI] [PubMed] [Google Scholar]
- [16].Giacomozzi C, D’Ambrogi E, Cesinaro S, Macellari V, Uccioli L. Muscle performance and ankle joint mobility in long-term patients with diabetes. BMC Musculoskelet Disord. 2008;9:99. 10.1186/1471-2474-9-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanya AO, Obi CS. Range of motion in selected joints of diabetic and non-diabetic subjects. Afr J Health Sci. 1999;6(1):17–21. [PubMed] [Google Scholar]
- [18].Ng TK-W, Lo S-K, Cheing GL-Y. The association between physical characteristics of the ankle joint and the mobility performance in elderly people with type 2 diabetes mellitus. Arch Gerontol Geriatr. 2014;59(2):346–352. doi: 10.1016/j.archger.2014.07.001. [DOI] [PubMed] [Google Scholar]
- [19].Bennett PJ, Stocks AE, Whittam DJ. Analysis of risk factors for neuropathic foot ulceration in diabetes mellitus. J Am Podiatr Med Assoc. 1996;86(3):112–116. doi: 10.7547/87507315-86-3-112. [DOI] [PubMed] [Google Scholar]
- [20].Raspovic A. Gait characteristics of people with diabetes-related peripheral neuropathy, with and without a history of ulceration. Gait Posture. 2013;38(4):723–728. doi: 10.1016/j.gaitpost.2013.03.009. [DOI] [PubMed] [Google Scholar]
- [21].Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Int Med. 1993;233(6):485–491. http://www.ncbi.nlm.nih.gov/pubmed/8501419. Accessed October 22, 2014. 10.1111/joim.1993.233.issue-6 [DOI] [PubMed] [Google Scholar]
- [22].Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–494. http://www.ncbi.nlm.nih.gov/pubmed/12547887. Accessed October 22, 2014. 10.2337/diacare.26.2.491 [DOI] [PubMed] [Google Scholar]
- [23].Diabetes and exercise: when to monitor your blood sugar Mayo Clinic, 2015. http://www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-and-exercise/art-20045697. Accessed October 22, 2014. [Google Scholar]
- [24].Pols MA, Peeters PH, Bueno-De-Mesquita HB, et al. . Validity and repeatability of a modified baecke questionnaire on physical activity. Int J Epidemiol. 1995;24(2):381–388. 10.1093/ije/24.2.381 [DOI] [PubMed] [Google Scholar]
- [25].Fedele D, Comi G, Coscelli C, et al. . A multicenter study on the prevalence of diabetic neuropathy in Italy. Italian Diabetic Neuropathy Committee. Diabetes Care. 1997;20(5):836–843. [DOI] [PubMed] [Google Scholar]
- [26].Feldman EL, Stevens MJ. Clinical testing in diabetic peripheral neuropathy. Can J Neurol Sci. 1994;21(S4):S3–S7. 10.1017/S0317167100040671 [DOI] [PubMed] [Google Scholar]
- [27].Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108(5):477–481. 10.1016/j.clineuro.2005.08.003 [DOI] [PubMed] [Google Scholar]
- [28].Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. 10.2337/diacare.17.11.1281 [DOI] [PubMed] [Google Scholar]
- [29].Coppieters MW, Butler DS. Do “sliders” slide and “tensioners” tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13(3):213–221. 10.1016/j.math.2006.12.008 [DOI] [PubMed] [Google Scholar]
- [30].Coppieters MW, Andersen LS, Johansen R, et al. . Excursion of the sciatic nerve during nerve mobilization exercises: an in vivo cross-sectional study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2015;45(10):1–23. doi: 10.2519/jospt.2015.5743. [DOI] [PubMed] [Google Scholar]
- [31].Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25(7):972–980. 10.1002/(ISSN)1554-527X [DOI] [PubMed] [Google Scholar]
- [32].Coppieters MW, Alshami AM, Babri AS, Souvlis T, Kippers V, Hodges PW. Strain and excursion of the sciatic, tibial, and plantar nerves during a modified straight leg raising test. J Orthop Res. 2006;24(9):1883–1889. 10.1002/(ISSN)1554-527X [DOI] [PubMed] [Google Scholar]
- [33].Boyd BS, Topp KS, Coppieters MW. Impact of movement sequencing on sciatic and tibial nerve strain and excursion during the straight leg raise test in embalmed cadavers. J Orthop Sports Phys Ther. 2013;43(6):398–403. doi: 10.2519/jospt.2013.4413. [DOI] [PubMed] [Google Scholar]
- [34].Boyd BS. Measurement properties of a hand-held inclinometer during straight leg raise neurodynamic testing. Physiotherapy. 2012;98(2):174–179. doi: 10.1016/j.physio.2011.04.352. [DOI] [PubMed] [Google Scholar]
- [35].Boyd BS, Villa PS. Normal inter-limb differences during the straight leg raise neurodynamic test: a cross sectional study. BMC Musculoskelet Disord. 2012;13:245. doi: 10.1186/1471-2474-13-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kovacs FM, Abraira V, Royuela A, et al. . Minimum detectable and minimal clinically important changes for pain in patients with nonspecific neck pain. BMC Musculoskelet Disord. 2008;9:43. 10.1186/1471-2474-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garrow AP, Boulton AJ. Vibration perception threshold–a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev. 2006;22(5):411–419. 10.1002/(ISSN)1520-7560 [DOI] [PubMed] [Google Scholar]
- [38].Kluding PM, Pasnoor M, Singh R, et al. . The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kluding PM, Pasnoor M, Singh R, et al. . Safety of aerobic exercise in people with diabetic peripheral neuropathy: single-group clinical trial. Phys Ther. 2015;95(2):223–234. doi: 10.2522/ptj.20140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568–1579. doi: 10.2522/ptj.20090362. [DOI] [PubMed] [Google Scholar]
- [41].Yang Z, Scott CA, Mao C, Tang J, Farmer AJ. Resistance exercise versus aerobic exercise for type 2 diabetes: a systematic review and meta-analysis. Sports Med. 2014;44(4):487–499. doi: 10.1007/s40279-013-0128-8. [DOI] [PubMed] [Google Scholar]
- [42].Lemaster JW, Reiber GE, Smith DG, Heagerty PJ, Wallace C. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exercise. 2003;35(7):1093–1099. doi: 10.1249/01.MSS.0000074459.41029.75. [DOI] [PubMed] [Google Scholar]
- [43].Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88(11):1385–1398. doi: 10.2522/ptj.20080019. [DOI] [PubMed] [Google Scholar]
- [44].Alshami AM, Babri AS, Souvlis T, Coppieters MW. Strain in the tibial and plantar nerves with foot and ankle movements and the influence of adjacent joint positions. J Appl Biomech. 2008;24(4):368–376. [DOI] [PubMed] [Google Scholar]
- [45].Ridehalgh C, Moore A, Hough A. Normative sciatic nerve excursion during a modified straight leg raise test. Man Ther. 2014;19(1):59–64. doi: 10.1016/j.math.2013.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.