Abstract
Cardiac arrest is a leading cause of death in the United States, and, currently, therapeutic hypothermia, now called targeted temperature management (TTM), is the only recent treatment modality proven to increase survival rates and reduce morbidity for this condition. Shivering and subsequent metabolic stress, however, limit application and benefit of TTM. Stimulating central nervous system A1 adenosine receptors (A1AR) inhibits shivering and nonshivering thermogenesis in rats and induces a hibernation-like response in hibernating species. In this study, we investigated the pharmacodynamics of two A1AR agonists in development as antishivering agents. To optimize body temperature (Tb) control, we evaluated the influence of every-other-day feeding, dose, drug, and ambient temperature (Ta) on the Tb-lowering effects of N6-cyclohexyladenosine (CHA) and the partial A1AR agonist capadenoson in rats. The highest dose of CHA (1.0 mg/kg, i.p.) caused all ad libitum–fed animals tested to reach our target Tb of 32°C, but responses varied and some rats overcooled to a Tb as low as 21°C at 17.0°C Ta. Dietary restriction normalized the response to CHA. The partial agonist capadenoson (1.0 or 2.0 mg/kg, i.p.) produced a more consistent response, but the highest dose decreased Tb by only 1.6°C. To prevent overcooling after CHA, we studied continuous i.v. administration in combination with dynamic surface temperature control. Results show that after CHA administration control of surface temperature maintains desired target Tb better than dose or ambient temperature.
Introduction
Hypothermia is defined as a body temperature colder than 35°C and is a well-known cause of death in cold climates. Despite this, the American Heart Association (Callaway et al., 2015a) guidelines for cardiopulmonary resuscitation and emergency cardiovascular care strongly recommend induced hypothermia via targeted temperature management (TTM) for treating out-of-hospital cardiac arrest and neonatal resuscitation. Clinical trials for TTM in stroke, the leading cause of adult disability (Mozaffarian et al., 2016), are ongoing (Lyden et al., 2016). Although cooling is neuroprotective, clinical application is complicated by side effects such as shivering.
Paralytics suppress shivering and are used commonly with TTM in comatose patients after cardiac arrest (Bernard et al., 2002). With regard to cooling conscious stroke patients, meperidine (i.v.) in combination with buspirone (oral) is currently the treatment of choice to suppress shivering. Synergy between these two drugs decreases shivering threshold to a core body temperature (Tb) of 33.5°C with minimal risk of respiratory depression (Mokhtarani et al., 2001; Sessler, 2009; Logan et al., 2011); however, a shivering threshold of 33.5°C is not sufficient for optimal control of shivering at colder Tb. The metabolic stress of shivering limits maximum therapeutic benefit of cooling. A recent study (Nielsen et al., 2013) showed no difference in outcome in patients cooled to 33°C versus 36°C and questioned the utility of cooling to 33°C. Importantly, this study reported shivering at 33°C and 36°C, but no differences in adverse effects were seen at these temperatures. Other studies confirm shivering at 36°C (Callaway et al., 2015b).
By examining strategies in species that routinely lower Tb, such as hibernators, we sought a safer, alternative method of inducing TTM without harmful side effects such as shivering. In Arctic ground squirrels (AGS), stimulation of A1 adenosine receptors centrally (intracerebroventricular) or peripherally (i.p.) using N6-cyclohexyladenosine (CHA) decreases oxygen consumption (V̇O2) and leads to a subsequent decrease in Tb in a manner that resembles spontaneous onset of hibernation (Jinka et al., 2011). However, for unknown reasons, the drug is effective only in the hibernation season. Like the hibernation season in AGS, dietary restriction (DR) in rats sensitizes animals to the temperature-lowering effects of CHA when compared with their ad libitum (AL)-fed counterparts (Jinka et al., 2010). Although CHA effectively lowers Tb in DR rats, precise control of target temperature has not been achieved in AL rats; and DR is not a viable option for human emergency medicine. Currently, it is not known how dose and environmental temperature influence final body temperature in AL rats when given CHA, an A1-selective full agonist (van der Wenden et al., 1995), or capadenoson, an A1-selective partial agonist (Albrecht-Kupper et al., 2012). The objective of this study was to characterize how dose of CHA, the partial A1 adenosine receptor (A1AR) agonist capadenoson, and environmental temperature influence Tb in freely fed rats for the purpose of precise control of Tb between 32 and 36°C. We measure the rate of oxygen consumption as an indicator of thermogenesis, define individual variability in response to capadenoson and to CHA at a dose higher than tested previously, and show that ambient temperature alone is not sufficient to control the depth of cooling. We report that dynamic control of surface temperature in rats, designed to mimic conductive cooling used clinically, is the most effective means to regulate Tb after CHA.
Materials and Methods
Animals.
Experiments were done in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council, National Academies Press, 2010), and protocols were approved by University of Alaska Fairbanks Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (approximately 90 days old) were obtained from Simonson Laboratories (Gilroy, CA) (experiment B) or from a University of Alaska Fairbanks colony derived from Simonson Laboratories (experiments A and C). All animals were housed in pairs at 21.5–23.0°C on a 12L:12D photoperiod. A summary of experiments and number of animals used can be seen (Table 1).
TABLE 1.
Summary of experiments
| Experiment | Experimental Test | Drug | Doses | Route | Ambient Temperature | Sample Size |
|---|---|---|---|---|---|---|
| 1 | Diet and oxygen consumption | CHA | 0.5 mg/kg | i.p.-Bolus | 16°C | 15 |
| 2 | ↑ Dose of CHA | CHA | 1.0 mg/kg | i.p.-Bolus | 17°C | 10 |
| Compare partial agonist | Capadenoson | 1.0 and 2.0 mg/kg | i.p.-Bolus | 17°C | 10 | |
| 3 | Surface temperature modulation with i.v. CHA | CHA | 0.25 mg/kg/h | i.v.-Continuous | 16°C–32°C | 2 |
Experiment A: DR and AL, 0.5 mg/kg CHA, i.p.
Prior research had shown that DR increases sensitivity to the Tb-lowering effects of 0.5 mg/kg CHA, but V̇O2 was not measured as an indication of thermogenesis. In this study, we asked whether CHA suppresses V̇O2 prior to the decrease in Tb, consistent with suppression of thermogenesis, and test the influence of 36 days of every-other-day feeding on the thermolytic response to CHA.
Temperature data loggers (iButton; Maxim Integrated, Sunnyvale, CA) were coated with wax and surgically implanted into the abdominal cavity and programmed to record temperature every 10 minutes. After a 10- to 14-day postoperative recovery period, rats were either fed every other day (DR) or AL up to 40 days. Feeding or food removal was done at 10–11 AM every day. Body weights were measured every 4 days. Between 36 and 40 days after starting the DR protocol, animals were moved to a clean cage and housed individually at an ambient temperature of 16.2 ± 0.5°C (mean ± S.D.) for 24 hours prior to treatment. Rats were moved to a metabolic chamber for 3 hours prior to treatment with CHA (0.5 mg/kg, i.p.) or vehicle (1.0 ml/kg, i.p.) and remained in the metabolic chamber for 2 hours postinjection. V̇O2 was measured by open flow respirometry, as detailed below.
Experiment B: AL Feeding, 1.0 mg/kg CHA, 1.0 and 2.0 mg/kg Capadenoson.
We next investigated the effects of 1.0 mg/kg CHA and 1.0 and 2.0 mg/kg partial A1AR agonist capadenoson in AL-fed rats. Rats were instrumented with iButton data loggers, as described for experiment A. All animals, housed in pairs, were placed at an ambient temperature of 17.0 ± 0.5°C (mean ± S.D.) 24 hours before injections and remained at this ambient temperature until 24 hours after injection. Each of the five pairs of animals received a different treatment per week based on a balanced crossover design (Supplemental Table 1). All treatments were given via i.p. injections and consisted of CHA (1.0 mg/kg), CHA vehicle (1.0 mL/kg), capadenoson (1.0 and 2.0 mg/kg), and capadenoson vehicle (1.0 mL/kg). Heart rate was monitored with a digital stethoscope [Littmann Model 4000 electronic stethoscope (3M, St. Paul, MN)].
Experiment C: AL Feeding, i.v. CHA at 0.25 mg/kg/h with Surface Temperature Modulation.
Finally, we applied dynamic control of surface temperature to optimize control over Tb with CHA administered by continuous i.v. infusion. A temperature-controlled cage was built to modulate Tb in animals treated with CHA. Two male rats were implanted with telemetry transmitters (CTA-F40; Data Sciences International, New Brighton, MN) inside the abdominal cavity, and ECG leads were secured to the chest wall. The femoral artery was cannulated using 12 cm 3Fr C30PU-RECA1302 polyurethane catheters (Instech, Plymouth Meeting, PA). The femoral vein was also cannulated using C30PU-RJV1420 catheters; both cannula were passed through an interscapular incision, where they were attached to a two-channel vascular harness (VAD115AB; Instech). For postoperative recovery, animals were housed individually with cotton pads substituted for wood shavings. Sutures were removed 7–10 days after the operation, and catheter maintenance was done by flushing every 5 days using saline, followed by filling with a locking solution of heparin/glycerol (500 IU/mL, 50:50) to prevent clotting. On the day of the experiment, animals were placed on the cage surface with the initial surface temperature set to 17°C. CHA was administered by continuous i.v. infusion (0.25 mg/kg/h). When animals approached a target Tb of 32°C, surface temperature was increased to 32°C to maintain target temperature.
Drugs.
CHA (CAS 36396-99-3) is eliminated with a half-life of approximately 2 hours when given subcutaneously (Tuovinen and Tarhanen, 2004) and is a full A1AR agonist (van der Wenden et al., 1995). CHA (Sigma-Aldrich, St. Louis, MO) was dissolved in 25% (w/v) hydroxypropyl-β-cyclodextrin (TCI America, Portland, OR) and then diluted to 2.5% in physiologic saline. CHA vehicle consisted of 25% (w/v) hydroxypropyl-β-cyclodextrin diluted to 2.5% in physiologic saline. Capadenoson (CAS 544417-40-5) is a partial A1AR agonist relative to 6-chloro-N6-cyclopentyladenosine (CCPA) and shows a half-life of approximately 20 hours (Albrecht-Kupper et al., 2012). Capadenoson (>98% purity; Chemexpress, Monmouth Junction, NJ) was dissolved in 100% polyethylene glycol (PEG400; Med Laboratory Supply, Pompano Beach, FL) and then diluted to 60% polyethylene glycol concentration with sterile water. All substances were USP grade where available. Solutions for injection were sterilized by 0.2 μm filtration (Acrodisc syringe filter; Pall, Port Washington, NY).
Oxygen Consumption (V̇O2).
V̇O2 was measured using open-flow respirometry in conjunction with LabGraph respirometry acquisition and analysis software according to Toien (2013) and Jinka et al. (2011). The accuracy and integrity of the system were calibrated by burning ethanol (100%) following established methodology (Toien, 2013); analyzers were manually calibrated with atmospheric reference air (∼0.03% CO2), zero air (∼0% CO2), and span gas (∼0.51% CO2) before each group of experiments and autocalibrated subsequently every 2 hours. V̇O2 data were synchronized with Tb by subtracting a lag time of 4 minutes calculated as the volume of the chamber and length of the outlet tube.
Statistical Analysis.
Variation in Tb, body mass, and V̇O2 was analyzed using repeated-measures linear mixed-effect models (Domidenko, 2004) to account for within-rat correlations and to model time trajectories after treatment or feeding regimen. These statistical analyses were conducted using the IBM SPSS Statistics 19 Armonk, NY. Post hoc comparisons were performed using t tests with Bonferonni corrections (Excel 2007). The significance criterion was α < 0.05 for all analyses. Data are shown as mean ± S.E.M., unless otherwise indicated.
Results
DR; 0.5 mg/kg i.p. CHA.
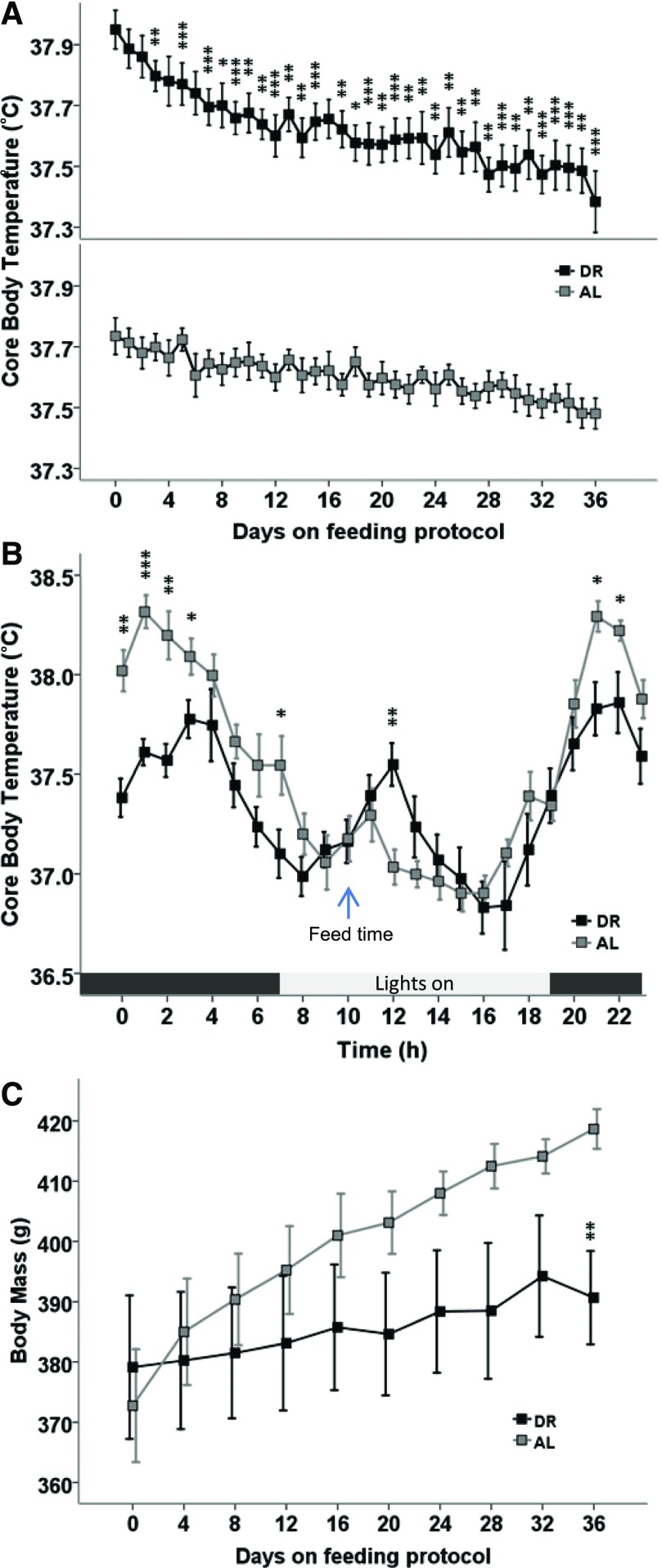
We investigated the influence of every-other-day feeding on whole animal oxygen consumption and on the circadian rhythm in Tb to assess the influence of DR on thermoregulation. DR decreased Tb compared with animals fed AL [diet × time (F[1,13.10] = 7.95, P = 0.014)] and main effect of diet [F(1,12.37) = 8.97, P = 0.011]. Post hoc tests show that the Tb in DR animals in comparison with AL were statistically different on days 4–36, except for days 5, 7, and 17 (P < 0.05), as shown in Fig. 1A. Next, we asked whether DR affected Tb across the circadian rhythm or only during the light or dark phase of the cycle. Analysis of Tb on the day prior to CHA administration (a feeding day; Fig. 1B) shows that DR decreases the amplitude during the dark, active period [main effect of time (F[1,343] = 5.15, P = 0.024) and diet (F[1,43.51] = 7.74, P = 0.008)] with a near-significant interaction between diet and time [F(1,343) = 3.48, P = 0.063]. Assessment of the rhythm in Tb during the lights on (inactive) period was confounded by disturbance associated with feeding and cage cleaning. DR also decreased weight gain relative to AL animals [Fig. 1C; diet × time (F[1,13.50] = 6.28, P = 0.026)].
Fig. 1.
(A) Every-other-day feeding (DR) decreases Tb relative to AL feeding. (B) The decrease in body temperature is greatest during the active period. Light:dark cycle is indicated by colored bar below. (C) The effects of DR did not maintain the same rate of weight gain in comparison with rats fed AL. Ambient temperature (Ta) is 20°C; mean ± S.E.M., n = 7 AL, n = 8 DR; *P < 0.05; **P < 0.01; ***P < 0.001 DR versus AL.
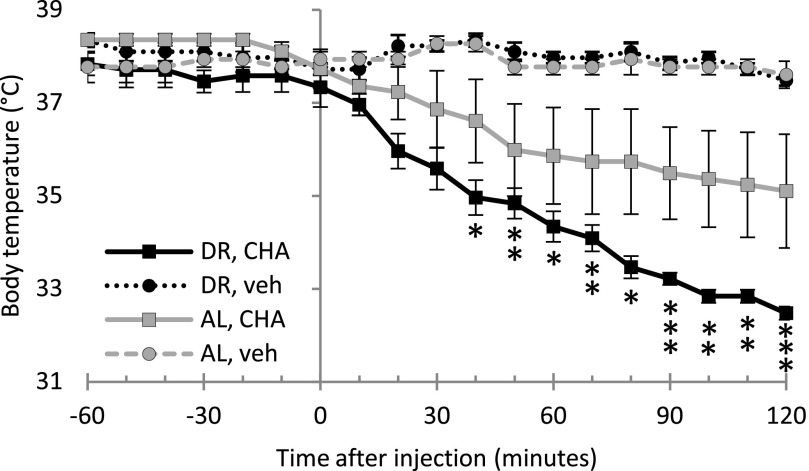
We next assessed the effects of CHA on Tb or V̇O2 in DR- and AL-fed rats. Rats in both DR and AL groups maintained Tb at approximately 37.5°C when given vehicle (Fig. 2). Both groups responded to 0.5 mg/kg i.p. CHA, but the DR group showed a larger, more consistent response than the AL group (n = 4 AL, n = 4 DR). Within 120 minutes of injection, Tb in the AL group reached 35.1 ± 1.2°C, and Tb in the DR group reached 32.5 ± 0.1°C [diet × time × treatment (F[3,140.04] = 18.19, P < 0.001)] with a significant main effect of time [F(1,140.04) = 59.86, P < 0.001]. Post hoc t tests showed that the CHA group was significantly different from vehicle in DR animals (P < 0.05) at 40–120 minutes after injection. The Tb in the AL group after CHA was not different from vehicle (P > 0.05). We observed a bimodal distribution in the four AL-fed rats after giving 0.5 mg/kg CHA; two rats maintained Tb similar to vehicle, whereas the other two showed a decrease in Tb similar to DR rats given CHA (Supplemental Fig. 1).
Fig. 2.
Treating rats with the A1AR agonist CHA at an ambient temperature of 16°C decreases Tb. The decrease in Tb is greater in DR rats than in AL rats. Ambient temperature (Ta) is 16°C, mean ± S.E.M. n = 4 (DR CHA), n = 4 DR VEH, n = 4 (AL CHA), n = 3 (AL VEH). Error bars not shown are smaller than symbols. *P < 0.05; **P < 0.01; ***P < 0.001; DR CHA versus DR vehicle.
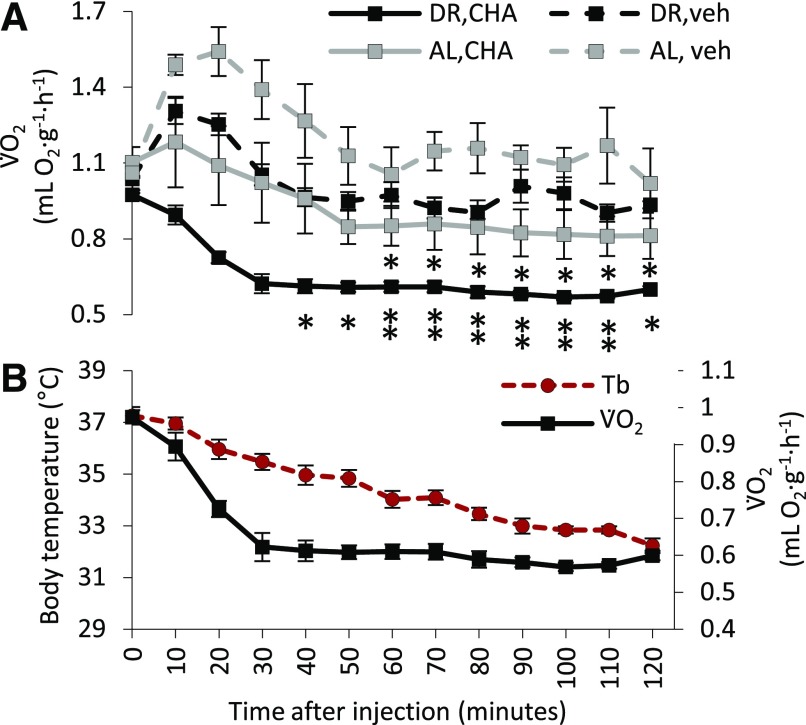
To see whether CHA decreased Tb as a result of an inhibition of thermogenesis, we measured V̇O2 as an indirect measure of both shivering and nonshivering thermogenesis in both DR and AL rats. Compared with rats given vehicle, V̇O2 tended to decrease in both AL and DR rats within 10 minutes after CHA injection (Fig. 3A). V̇O2 stabilized at minimal levels within 30 to 50 minutes after CHA administration and tended to be lowest in the DR group. Pairwise comparisons revealed significant differences between CHA-treated and vehicle-treated DR rats at 40–120 minutes (P < 0.05) and also between AL CHA- and vehicle-treated rats between 70 and 120 minutes (P < 0.05). The i.p. injections with CHA or vehicle tended to produce an immediate increase in V̇O2, except where rats decreased Tb after CHA (Fig. 3B; Supplemental Fig. 2). In these animals, V̇O2 decreased before Tb and is consistent with a decrease in thermogenesis.
Fig. 3.
(A) DR significantly lowered oxygen consumption (V̇O2) for both vehicle- and CHA-treated rats. (B) Simultaneous measurements of Tb and V̇O2 in the DR group show that V̇O2 declines prior to Tb. Ambient temperature (Ta) is 16°C [mean ± S.E.M., n = 4 (DR CHA), n = 4 DR VEH, n = 4 (AL CHA), n = 3 (AL VEH)].
AL Feeding: 1.0 mg/kg i.p. CHA, 1.0 and 2.0 mg/kg Capadenoson, and Vehicles.
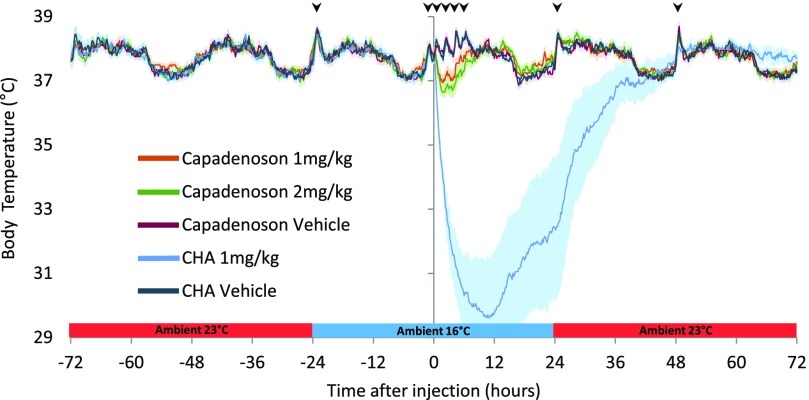
We tested a higher dose of CHA (1.0 mg/kg) and two doses (1.0 and 2.0 mg/kg) of the partial agonist capadenson to test the hypothesis that a maximally effective dose or alternative A1AR agonist would decrease variation in cooling with AL animals. In addition, to assess whether the circadian rhythm of Tb influenced drug response, we graphed Tb for 3 days prior to drug administration. The circadian rhythm of body temperature was noted visually, but not analyzed further (Fig. 4). Both doses of capadenoson cooled Tb to a minimum within 2 hours of injection. Minimum Tb (mean ± S.E.M.) was 37.0 ± 0.2, and 36.6 ± 0.2°C for 1.0 mg/kg, and 2.0 mg/kg, respectively (Fig. 4). Analysis of the minimum core Tb yielded a significant main effect of treatment (1.0 or 2.0 mg/kg capadenoson, or vehicle) [F(2,18) = 17.52, P < 0.001]. Pairwise comparisons of minimum Tb revealed a significant difference between 1.0 mg/kg capadenoson and vehicle (P = 0.008) and 2.0 mg/kg capadenoson and vehicle (P < 0.001) and trended toward significance between 1.0 mg/kg and 2.0 mg/kg injections (P = 0.081). Capadenoson lowered heart rate with a minimum of 77.1% of vehicle baseline for the 1 mg/kg dose and 71% for the 2 mg/kg dose within 2 hours postinjection (Supplemental Fig. 3).
Fig. 4.
CHA (1.0 mg/kg) is more effective than either dose of capadenoson (1.0 and 2.0 mg/kg) at reducing body temperature. Variation in both magnitude and duration of response to CHA was not decreased by the higher dose (indicated by S.E.M.; lighter shaded area). Arrowheads indicate time when rats were picked up for heart rate measurements.
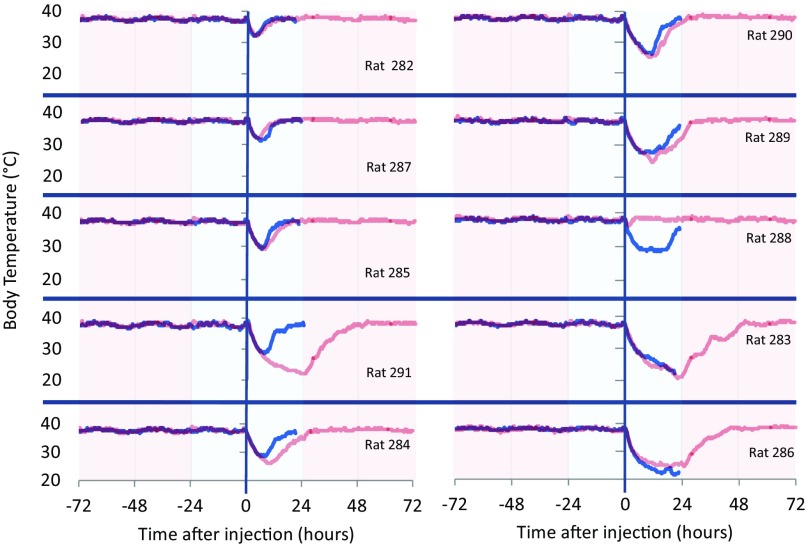
Although a dose of 0.5 mg/kg CHA in AL animals resulted in Tb decreases in two of four animals, the higher dose of CHA (1.0 mg/kg) produced a notable decline in Tb in all animals (10/10). Nonetheless, the magnitude and duration of response still varied between animals (Fig. 4). The lowest minimum Tb recorded was 20.6°C, whereas the highest minimum Tb was 32.5°C. We asked whether this variation was intrinsic to each animal by giving CHA (1.0 mg/kg) to all animals a second time with 1–5 weeks separating the two injections. In 7 of 10 animals, the decline in Tb after the second injection mirrored closely the response to the first injection (Fig. 5); however, in three animals it did not. Statistical analysis on the minimum core Tb within 20.5 hours after injection showed that CHA produced a significant decrease in Tb on both injections [treatment (first injection, second injection, or vehicle): F(2,18) = 36.57, P < 0.001, with significant differences between CHA first injection and vehicle (P < 0.001, t test) and CHA second injection and vehicle (P < 0.001, t test)]. Moreover, there was no significant difference between the first and second injection of CHA (P = 1.000, paired t test). Regression analysis of minimum Tb on the first and second injections yielded an R2 value of just 0.43 (P = 0.043) (Supplemental Fig. 4). Body weight on the day of injection did not predict the magnitude of the cooling response (P = 0.72, first injection; P = 0.25, second injection). Moreover, neither time nor change in body weight between injections predicted response on the second injection (P = 0.82) (Supplemental Table 2). In addition to lowering Tb, CHA caused a 74.5% reduction of heart rate in comparison with vehicle on average within 2 hours of injection (Supplemental Fig. 3). Bradycardia resolved between 24 and 48 hours after injection (Supplemental Fig. 5).
Fig. 5.
Response to CHA (1.0 mg/kg) on the first injection (red lines) did not predict the response on the second injection (blue lines) in 3 of 10 rats. Colored region indicates ambient temperature (red, 23°C; blue, 17°C).
AL Feeding: 0.25 mg/kg/h i.v. CHA at 2 μl/min.
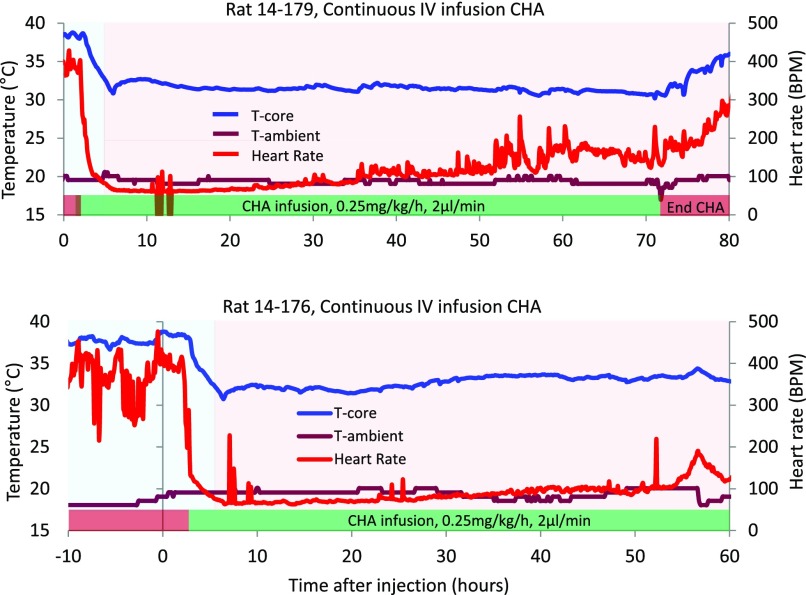
We next asked whether surface cooling would normalize Tb and prevent overcooling. We designed and built a temperature-controlled cage to model surface cooling used clinically and adjusted surface temperature to prevent overcooling. During continuous i.v. infusion of CHA (0.25 mg/kg/h) in the absence of a bolus loading dose, animal Tb approached 32°C within 3 hours on a surface temperature of 17°C. Increasing surface temperature to 32°C maintained target temperature and prevented overcooling (Fig. 6). Heart rate declined rapidly at the start of CHA infusion, and bradycardia persisted throughout the infusion (Fig. 6).
Fig. 6.
To prevent animals from overcooling with CHA on board, cage surface temperature was heated or cooled, as needed. The cage floor was set to 17°C initially (blue region) and brought up to 32°C (red region) as body temperature reached the target temperature of 32°C to prevent overcooling. At this dose of CHA, heart rate drops to about 20% of baseline and is followed by a decrease in body temperature. This temporal relationship between body temperature and heart rate is similar to what is seen at onset of hibernation and is consistent with CHA-induced inhibition of thermogenesis. Ambient temperature within the cage did not vary significantly.
Discussion
Thermolytics include antipyretic drugs such as acetaminophen and certain nonsteroidal anti-inflammatory drugs (Sullivan and Farrar, 2011). In this work, we extend the definition of thermolytic to include drugs that suppress thermogenesis and decrease core Tb. Despite the ability of CHA to suppress thermogenesis, precise control of Tb around a predetermined target temperature had yet to be demonstrated prior to this work. Our objectives were to define how dose of CHA and environmental temperature influence Tb in rats treated with CHA. The i.p. bolus injections using CHA at 0.5 and 1.0 mg/kg failed to produce consistent decreases in Tb; however, use of the higher dose decreased Tb in all animals down to or below our target temperature of 32°C at an ambient temperature of 17°C. From this, we hypothesized that overcooling could be prevented with cage surface temperature modulation and thus facilitate management of target Tb. In this work, we report precise control of Tb using CHA coupled with dynamic control of cage surface temperature and show that modulation of dose alone is not sufficient to precisely manage target Tb.
Our results demonstrate robust thermolytic efficacy of CHA in rats and is a refinement of prior attempts with high doses of purine derivatives. AMP was the first purine reported to induce a torpor-like state in rats (Zhang et al., 2006), and both AMP and ATP were later tested in rats to lower Tb for therapeutic benefit (Zhang et al., 2009, 2013). High doses were necessary to promote sufficient cooling, ultimately producing unwanted effects that discouraged further development. AMP induced a hypothermic response in mice (Swoap et al., 2007) and was later found to act as an A1AR agonist (Muzzi et al., 2013); AMP-induced cooling was blocked using an A1AR antagonist in the CNS (Iliff and Swoap, 2012). Targeting CNS A1AR using CHA to inhibit thermogenesis shows promise as an effective approach to relieve shivering during therapeutic hypothermia (Jinka et al., 2015).
Other nonpurine-based thermolytics currently in development include neurotensin receptor agonists (Choi et al., 2012; Wei et al., 2013), transient receptor potential (TRP) agonists and antagonists (Almeida et al., 2012; Feketa et al., 2014; Feketa and Marrelli, 2015), GABAA agonists (Cerri et al., 2013), and other unique formulations (Katz et al., 2012a,b, 2015). Using a fixed ambient temperature, several studies demonstrate control of target temperature through modulation of dose and dosing regimens alone to maintain Tb or prevent overcooling (Muzzi et al., 2013; Wei et al., 2013; Feketa et al., 2014); however, thermolytic efficacy of other drugs tested to date in rats has not been as great as CHA.
Few preclinical studies combine dynamic temperature control with thermolytics in search for optimal temperature management protocols (Almeida et al., 2012; Katz et al., 2012b; Cerri et al., 2013). In the clinic, induction methods vary, but may include packing ice into axillary and groin areas and infusing ice-cold i.v. saline. Once target temperature is reached, Tb is usually maintained with water-blanket surface cooling (Luscombe and Andrzejowski, 2006) (Blanketrol or Arctic Sun, etc.) or endovascular cooling. Surface temperature control devices are routinely used in clinical settings and are standard protocol at most hospitals (Callaway et al., 2015a).
Although we found in this study that surface temperature modulation prevented overcooling, our data do not explain the large individual variation in Tb response to CHA. This variation was unexpected because prior work suggested more consistent responses between animals (Jinka et al., 2010, 2015). We did not observe significant variation using the same animals with the partial agonist capadenoson, but consistency came at the cost of thermolytic efficacy.
Current knowledge suggests A1AR agonist-induced cooling is due to an inhibition of thermogenesis at a central site of action (Anderson et al., 1994; Tupone et al., 2013). However, peripheral mechanisms such as the inhibition of lipolysis could also impair nonshivering thermogenesis in brown adipose tissue (Asakura, 2004; Viswanadha and Londos, 2006). It is unclear whether these mechanisms are responsible for individual differences in Tb-lowering effects of CHA, but our results reflect what might be expected in a diverse clinical population.
Similar variation in response to CHA is seen in ground squirrels in which sensitivity to CHA depends on the hibernation season. In AGS, stimulation of CNS A1ARs with CHA induces a torpor-like state, but the drug is effective only in the hibernation season (Jinka et al., 2011). Seasonal sensitivity to CHA in AGS precedes a decrease in food intake and is predicted by a gradual decrease in Tb as animals approach the hibernation season (Sheriff et al., 2012; Olson et al., 2013). In rats, prolonged every-other-day feeding increases sensitivity to the temperature-lowering effects of CHA as well as surface expression of A1AR in hypothalamus (Jinka et al., 2010). Although increases in surface expression of A1AR may contribute to increased sensitivity, prolonged restriction of diet is not a viable approach to normalize response to A1AR agonists in emergency medicine.
Shivering is one of the most problematic issues in TTM, which can impede induction of hypothermia by doubling metabolic rate (Badjatia et al., 2008), which leads to a stress-like response. Despite the importance of metabolism reduction, one of the primary desired effects in administering TTM, limited O2 consumption data have been reported for other thermolytics in development. Recently, however, it was revealed that O2 consumption was reduced using TRPv3 agonists to induce hypothermia in mice, but these results could not be replicated in rats (Feketa and Marrelli, 2015). In this work, evidence supporting inhibition of thermogenesis comes from a decrease in the rate of oxygen consumption (V̇O2) that precedes a decrease in Tb. A similar hysteresis of V̇O2 and Tb decline is seen during the onset of hibernation and torpor (Jinka et al., 2011).
Generalization of the current results in rats to other nonhibernating species such as swine and humans is likely because rats do not hibernate naturally. By contrast, many strains of laboratory mice spontaneously enter shallow torpor in response to fasting (Geiser, 2004). Results from studies using mice may not translate to species that do not hibernate, as evident in the study of TRPv3 agonists (Feketa and Marrelli, 2015). For this reason, mice are less preferred in the investigation of thermolytic efficacy in comparison with rats or swine.
One limitation of this study and others using small animals to study whole-body cooling is that surface area to weight ratio is far smaller than in larger animals, including humans. An important next step in evaluating thermolytic efficacy is to use larger animals. In the present study, the rate of cooling was faster following i.p. injection than with continuous i.v. administration because a loading dose was not given prior to i.v. infusion. Another limitation not addressed in this work is the potentially detrimental effects of adenosine receptor-induced bradycardia and hypotension, a side effect of CHA and hypothermia (Nieri et al., 2001). We have found previously that coadministration of the peripherally acting adenosine receptor antagonist, 8-sulfophenyltheophylline, reverses bradycardia and improves survival and neurologic outcome after cardiac arrest in rats (Jinka et al., 2015) without interfering with the thermolytic effect of the drug. Work is in progress to characterize the effects of 8-sulfophenyltheophylline on hypotension during CHA-assisted cooling.
A recent trial (Nielsen et al., 2013) showed no difference in outcome in patients cooled to 33°C versus 36°C and questioned the utility of cooling to 33°C. By contrast, an exhaustive number (over 50) of preclinical studies demonstrate that deeper cooling is better (Lyden et al., 2006; Polderman, 2009). Moreover, Nielsen et al. (2013) noted shivering at 33°C and 36°C, and no differences were found in other adverse effects of 33°C versus 36°C; other studies confirm shivering at 36°C (Callaway et al., 2015b). Importantly, the benefit to risk of colder Tb may increase as severity of brain injury increases (Yenari and Han, 2012). In response to the Nielsen et al. (2013) paper, the original International Liaison Committee on Resuscitation recommendations indicating 32–34°C (Donnino et al., 2015) have been changed to recommend a target Tb between 32°C and 36°C (Donnino et al., 2015). The current study is the first report, to our knowledge, of the effects of capadenoson on body temperature. Capadenoson is a partial agonist that produces 75% of full agonist, CCPA, [35S]GTPγS binding in human cortical membranes. In Langendorff heart preparations, capadenoson reduces heart rate to a maximal of 10% of the bradycardia produced by the full agonist CCPA. At higher doses, CCPA produces complete atrioventricular block (Albrecht-Kupper et al., 2012). The limited bradycardia with capadenoson reported by others is consistent with results reported in this study. Given the absence of cardiovascular risk, the mild hypothermic effect of capadenoson may be useful when a target Tb of 36°C is desired.
In summary, we show pronounced thermolytic efficacy of CHA with unexplained variation that is resolved under DR, but is not resolved with dose in AL-fed animals. Although high thermolytic efficacy produced overcooling in some animals, dynamic control of surface temperature allowed for fine tuning and maintenance of a prescribed target Tb. This approach to reduce and maintain target Tb in rodents is a refinement over fixed ambient temperatures or evaporative cooling protocols in which animals are sprayed with water or alcohol to facilitate heat loss (Klahr et al., 2017), and mimics surface cooling used in the clinic. This new thermolytic class of drugs has potential to facilitate targeted temperature management by inhibiting thermogenesis, providing new avenues for treatment.
Acknowledgments
We thank Carl Murphy, Saurav Bhowmick, and Jeanette Moore for technical assistance.
Abbreviations
- A1AR
A1 adenosine receptor
- AGS
Arctic ground squirrel
- AL
ad libitum
- CCPA
6-chloro-N6-cyclopentyladenosine
- CHA
N6-cyclohexyladenosine
- DR
dietary restriction
- Tb
body temperature
- TRP
transient receptor potential
- TTM
targeted temperature management
Authorship Contributions
Participated in research design: Drew, Bailey, Laughlin, Moore.
Conducted experiments: Drew, Bailey, Laughlin, Bogren, Moore.
Contributed new reagents or analytic tools: Bailey, Laughlin.
Performed data analysis: Drew, Bailey, Laughlin, Barati.
Wrote or contributed to the writing of the manuscript: Drew, Bailey, Laughlin.
Footnotes
This work was supported in part by a graduate student fellowship from Alaska Native Science and Engineering Program (to I.R.B.); American Heart Association postdoctoral fellowship (to Z.B.); National Institutes of Health National Institute of Neurologic Disorders and Stroke [Grant R15NS070779]; Alaska Space Grant Program pilot project; and Alaska INBRE P20GM103395.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Albrecht-Küpper BE, Leineweber K, Nell PG. (2012) Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signal 8 (Suppl 1):91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, et al. (2012) Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32:2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Sheehan MJ, Strong P. (1994) Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol 113:1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H. (2004) Fetal and neonatal thermoregulation. J Nippon Med Sch 71:360–370. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, Buitrago M, Schmidt JM, Ostapkovich ND, Mayer SA. (2008) Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke 39:3242–3247. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, et al. (2015a) Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132 (Suppl 2):S465–S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CW, Elmer J, Guyette FX, Molyneaux BJ, Anderson KB, Empey PE, Gerstel SJ, Holquist K, Repine MJ, Rittenberger JC. (2015b) Dexmedetomidine reduces shivering during mild hypothermia in waking subjects. PLoS One 10:e0129709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, Zamboni G, Amici R. (2013) The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci 33:2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, Yu SP. (2012) A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J 26:2799–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domidenko E. (2004) Mixed Models: Theory and Applications, John Wiley & Sons, Hoboken NJ. [Google Scholar]
- Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, Lang E, Cocchi MN, Xanthos T, Callaway CW, et al. ; ILCOR ALS Task Force (2015) Temperature management after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 132:2448–2456. [DOI] [PubMed] [Google Scholar]
- Feketa VV, Marrelli SP. (2015) Systemic administration of the TRPV3 ion channel agonist carvacrol induces hypothermia in conscious rodents. PLoS One 10:e0141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feketa VV, Zhang Y, Cao Z, Balasubramanian A, Flores CM, Player MR, Marrelli SP. (2014) Transient receptor potential melastatin 8 channel inhibition potentiates the hypothermic response to transient receptor potential vanilloid 1 activation in the conscious mouse. Crit Care Med 42:e355–e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser F. (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274. [DOI] [PubMed] [Google Scholar]
- Iliff BW, Swoap SJ. (2012) Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol 303:R477–R484. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Carlson ZA, Moore JT, Drew KL. (2010) Altered thermoregulation via sensitization of A1 adenosine receptors in dietary-restricted rats. Psychopharmacology (Berl) 209:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Combs VM, Drew KL. (2015) Translating drug-induced hibernation to therapeutic hypothermia. ACS Chem Neurosci 6:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinka TR, Tøien Ø, Drew KL. (2011) Season primes the brain in an Arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci 31:10752–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Frank JE, Glickman LT, McGwin G, Jr, Lambert BH, Gordon CJ. (2015) Effect of a pharmacologically induced decrease in core temperature in rats resuscitated from cardiac arrest. Resuscitation 92:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Frank JE, McGwin G, Jr, Finch A, Gordon CJ. (2012a) Induction of a prolonged hypothermic state by drug-induced reduction in the thermoregulatory set-point. Ther Hypothermia Temp Manag 2:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, McGwin G, Jr, Gordon CJ. (2012b) Drug-induced therapeutic hypothermia after asphyxial cardiac arrest in swine. Ther Hypothermia Temp Manag 2:176–182. [DOI] [PubMed] [Google Scholar]
- Klahr AC, Nadeau CA, Colbourne F. (2017) Temperature control in rodent neuroprotection studies: methods and challenges. Ther Hypothermia Temp Manag 7:42–49. [DOI] [PubMed] [Google Scholar]
- Logan A, Sangkachand P, Funk M. (2011) Optimal management of shivering during therapeutic hypothermia after cardiac arrest. Crit Care Nurse 31:e18–e30. [DOI] [PubMed] [Google Scholar]
- Luscombe M, Andrzejowski JC. (2006) Clinical applications of induced hypothermia. Contin Educ Anaesth Crit Care Pain 6:23–27. [Google Scholar]
- Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, Parker S, Concha M, Hussain S, Agarwal S, et al. Collaborators (2016) Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke 47:2888–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden PD, Krieger D, Yenari M, Dietrich WD. (2006) Therapeutic hypothermia for acute stroke. Int J Stroke 1:9–19. [DOI] [PubMed] [Google Scholar]
- Mokhtarani M, Mahgoub AN, Morioka N, Doufas AG, Dae M, Shaughnessy TE, Bjorksten AR, Sessler DI. (2001) Buspirone and meperidine synergistically reduce the shivering threshold. Anesth Analg 93:1233–1239. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, et al. Writing Group Members. American Heart Association Statistics Committee. Stroke Statistics Subcommittee (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:e38–e360. [DOI] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, Pittelli M, Cavone L, Pugliese AM, Moroni F, et al. (2013) Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab 33:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. TTM Trial Investigators (2013) Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 369:2197–2206. [DOI] [PubMed] [Google Scholar]
- Nieri P, Martinotti E, Calderone V, Breschi MC. (2001) Adenosine-mediated hypotension in in vivo guinea-pig: receptors involved and role of NO. Br J Pharmacol 134:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, Drew KL. (2013) Circannual rhythm in body temperature, torpor, and sensitivity to A1 adenosine receptor agonist in Arctic ground squirrels. J Biol Rhythms 28:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman KH. (2009) Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 37:S186–S202. [DOI] [PubMed] [Google Scholar]
- Sessler DI. (2009) Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke 40:e614–e621. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM. (2012) Thermoregulatory changes anticipate hibernation onset by 45 days: data from free-living Arctic ground squirrels. J Comp Physiol B 182:841–847. [DOI] [PubMed] [Google Scholar]
- Sullivan JEF, Farrar HC, Section on Clinical Pharmacology and Therapeutics. Committee on Drugs (2011) Fever and antipyretic use in children. Pediatrics 127:580–587. [DOI] [PubMed] [Google Scholar]
- Swoap SJ, Rathvon M, Gutilla M. (2007) AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol 293:R468–R473. [DOI] [PubMed] [Google Scholar]
- Toien O (2013) Automated open flow respirometry in continuous and long-term measurements: design and principles. J Appl Physiol (1985) 114:1094-1107. [DOI] [PubMed]
- Tuovinen K, Tarhanen J. (2004) Clearance of cyclopentyladenosine and cyclohexyladenosine in rats following a single subcutaneous dose. Pharmacol Res 50:329–334. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF. (2013) Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 33:14512–14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wenden EM, von Frijtag Drabbe Künzel JK, Mathôt RA, Danhof M, IJzerman AP, Soudijn W. (1995) Ribose-modified adenosine analogues as potential partial agonists for the adenosine receptor. J Med Chem 38:4000–4006. [DOI] [PubMed] [Google Scholar]
- Viswanadha S, Londos C. (2006) Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res 47:1859–1864. [DOI] [PubMed] [Google Scholar]
- Wei S, Sun J, Li J, Wang L, Hall CL, Dix TA, Mohamad O, Wei L, Yu SP. (2013) Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice. Neuroscience 252:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Han HS. (2012) Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13:267–278. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J. (2009) When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metab 29:1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. (2006) Constant darkness is a circadian metabolic signal in mammals. Nature 439:340–343. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li W, Niu G, Leak RK, Chen J, Zhang F. (2013) ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J Cereb Blood Flow Metab 33(1), e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]