Abstract
Background/Aims:
Hepatitis C virus (HCV) infection is one of the most common infections worldwide. Several epidemiologic studies have suggested that patients with HCV infection might be at an increased risk of osteoporosis. However, the data on this relationship remains inconclusive. This meta-analysis was conducted with the aim to summarize all available evidence.
Materials and Methods:
A literature search was performed using MEDLINE and EMBASE databases from inception to June 2016. Studies that reported relative risks, odd ratios (OR), or hazard ratios comparing the risk of osteoporosis among HCV-infected patients versus those without HCV infection were included. Pooled OR and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method.
Results:
Four studies met our eligibility criteria and were included in the analysis. We found a higher risk of osteoporosis among patients with chronic HCV with OR of 1.65 (95% CI: 0.98–2.77). Sensitivity analysis including only studies with higher quality yielded a higher OR, and the result was statistically significant (OR: 2.47; 95% CI: 1.03–5.93).
Conclusions:
Our study demonstrated a higher risk of osteoporosis among HCV-infected patients. Further studies are required to clarify how this risk should be addressed in clinical practice.
Keywords: Bone mineral density, hepatitis C virus, meta-analysis, osteopenia, osteoporosis
INTRODUCTION
Osteoporosis is a common medical condition in older adults characterized by reduced bone mass and increased risk of fragility fracture.[1] Well-established risk factors of osteoporosis include immobility, aging, menopause, diabetes, chronic use of glucocorticoid, vitamin D deficiency, and inadequate calcium intake.[2] Osteoporosis affects more than 200 million patients globally.[3] It has been estimated that 9 million osteoporotic fractures occur every year worldwide.[4]
Hepatitis C virus (HCV) infection is one of the leading causes of cirrhosis affecting approximately 180 million patients worldwide.[5] Interestingly, a recent meta-analysis has demonstrated a lower bone mineral density (BMD) and increased risk of fracture among patients with human immunodeficiency virus (HIV) and HCV coinfection compared with either HIV monoinfection or healthy controls.[6,7] However, data on the association of HCV monoinfection and osteoporosis remains inconclusive.[8,9,10,11,12,13] This systematic review and meta-analysis was conducted with the aim to summarize all available evidence to assess the risk of osteoporosis among HCV-infected patients.
MATERIALS AND METHODS
Search strategy
Two investigators (K.W. and P.U.) independently searched published studies indexed in MEDLINE and EMBASE databases from inception to June 2016 using the search strategy that included the terms for “hepatitis C virus” and “osteoporosis,” as described in online supplementary data 1. No language limitation was applied. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (1) case-control, cross-sectional, or cohort studies published as original articles to evaluate the risk of osteoporosis among HCV-infected patients compared with individuals without HCV infection, (2) odds ratios (OR), relative risks (RR), hazard ratios (HR) or standardized incidence ratios (SIR) with 95% confidence intervals (CI), or sufficient raw data to calculate these ratios were provided.
Study eligibility was independently determined by three investigators (K.W., C.T., and P.U.). Differences in the determination of study eligibility were resolved by mutual consensus. The quality of each study was also independently evaluated by each investigator using the validated Newcastle–Ottawa quality assessment scale.[14] This scale evaluated each study in three domains including the selection of the participants; the comparability between the groups, as well as ascertainment of the exposure of interest for case-control study; and the outcome of interest for cohort study. The modified Newcastle–Ottawa scale, as described by Herzog et al., was used for the cross-sectional study.[15]
Data extraction
A standard data collection form was used to extract the following data from each study – title of the study, name of the first author, year of study, year of publication, country where the study was conducted, number of participants, demographic data, method used to identify and verify HCV infection, as well as the event of interest (osteoporosis), adjusted effect estimates with 95% CI, and covariates that were adjusted in the multivariate analysis.
To ensure the accuracy of data extraction, this process was independently conducted by three investigators (K.W., C.T., and P.U.). Any data discrepancy was also resolved by referring to the original articles.
Statistical analysis
Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates and standard errors from individual study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study based on its variance.[16] Because the outcome of interest was relatively uncommon, we used RR of cohort study as an estimate for OR to combine with OR from cross-sectional and case-control study. In light of the possible high between-study variance due to different study designs and populations, we used a random-effect model rather than a fixed-effect model. Cochran's Q test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0–25% represents insignificant heterogeneity, greater than 25% but less than or equal to 50% represents low heterogeneity, greater than 50% but less than or equal to 75% represents moderate heterogeneity, and greater than 75% represents high heterogeneity.[17]
RESULTS
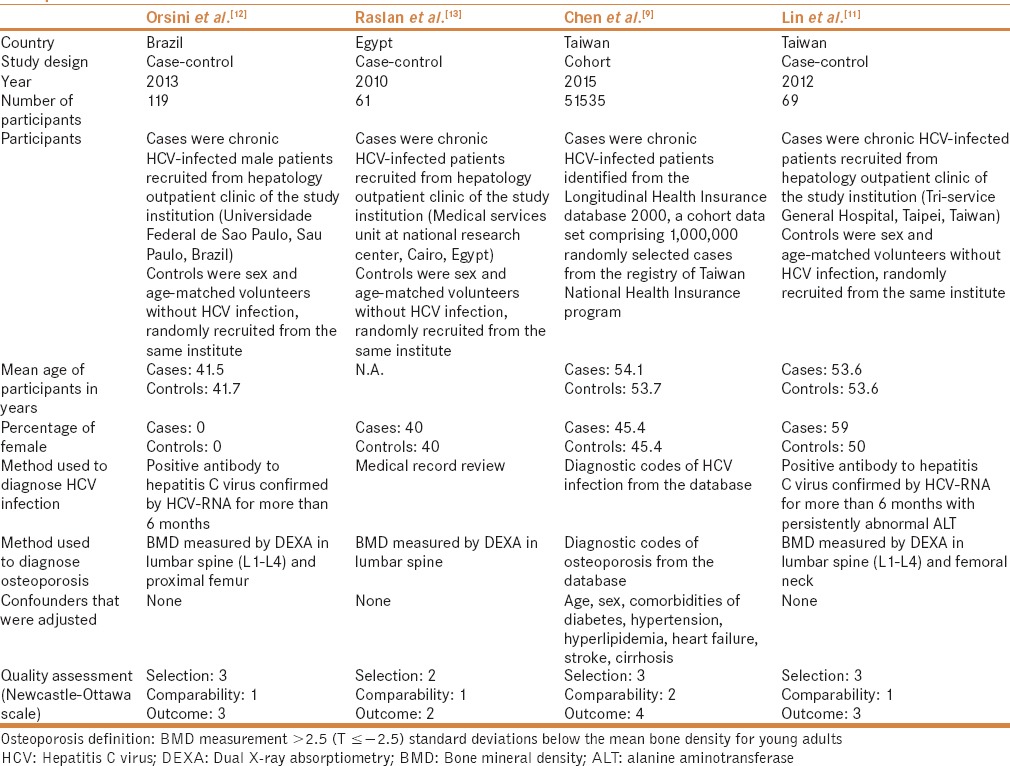
Our search strategy yielded 1115 potentially relevant articles (438 articles from Medline and 677 articles from EMBASE). After the exclusion of 422 duplicated articles, 693 underwent title and abstract review. Six hundred and fifty-nine articles were excluded at this stage because they were case reports, letters to editor, review articles, basic science studies, animal studies or interventional studies, leaving 34 articles for a full-length article review. Twenty-five of them were excluded because they did not report the outcome of interest whereas 5 articles were excluded because they were descriptive studies without comparators. Therefore, 4 studies (3 case-control studies and 1 cohort study) were included in the data analysis.[9,11,12,13] Figure 1 outlines the literature review and study selection process. The clinical characteristics and the quality assessment of the included studies are described in Table 1. PRISMA (Preferred reporting Items for Systematic Reviews and Meta-Analysis) is provided as online supplementary data 2. It should be noted that the inter-rater agreement for quality assessment using the Newcastle-Ottawa scale was high with kappa statistic of 0.77.
Figure 1.
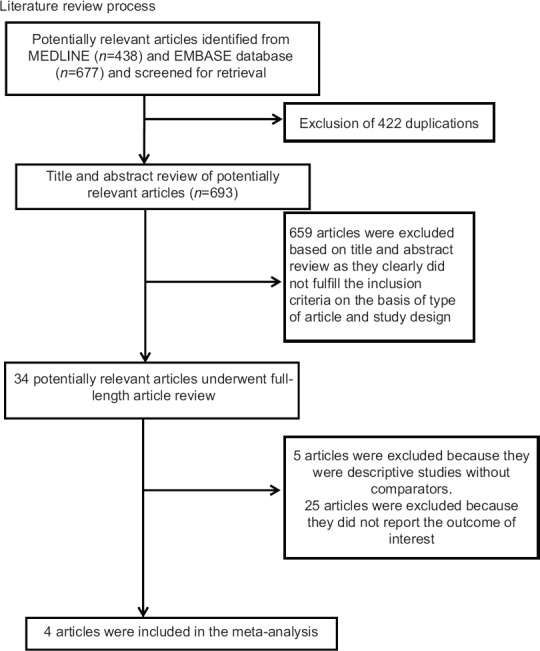
Literature review process
Table 1.
Main characteristics of the studies included in this meta-analysis of the association between HCV infection and risk of osteoporosis
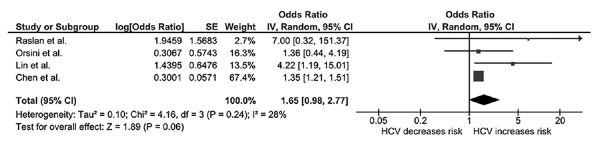
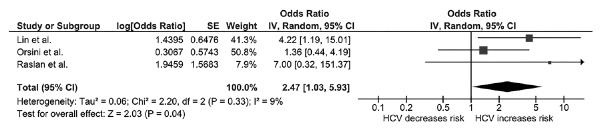
In the overall analysis, we found a higher risk of osteoporosis among patients with chronic HCV compared with individuals without HCV infection with an OR of 1.65 (95% CI: 0.98–2.77; I2: 28%), as shown in Figure 2. Sensitivity analysis excluding the only study that used diagnostic codes without case/event verification[9] yielded a higher OR (OR: 2.47; 95% CI: 1.03 -5.93; I2: 9%). The pooled effect estimate from the sensitivity analysis did reach statistical significance [Figure 3].
Figure 2.
Forest plot of the complete analysis
Figure 3.
Forest plot of the sensitivity analysis excluding the study without case verification
Evaluation for publication bias
Funnel plot to evaluate publication bias is shown in Figure 4. The graph is asymmetric, suggesting the presence of publication bias in favor of positive studies.
Figure 4.
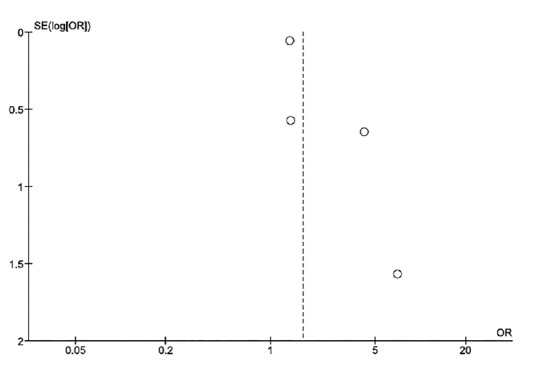
Funnel plot
DISCUSSION
This study is the first systematic review and meta-analysis to assess the risk of osteoporosis among HCV-infected patients. We found that the risk of osteoporosis was higher among HCV-infected patients compared with those without HCV infection, with approximately 65% excess risk. Sensitivity analysis including only studies with more reliable case/event verification yielded a higher OR and reached statistical significance.
The exact mechanisms behind the association of osteoporosis and HCV infection are not known. It is possible that either chronic liver disease associated with HCV infection or the infection itself is responsible for the reduced BMD.
Abnormal bone metabolism resulting in low BMD and osteoporosis is a known complication of chronic liver disease. It has been demonstrated that patients with cirrhosis have an increase in receptor-activator ratio of nuclear factor kappa ligand (RANKL) and osteoprotegerin (OPG), resulting in increased bone resorption, and, ultimately, bone loss.[18,19] Bilirubin is known to inhibit osteoblast proliferation.[20] Therefore, hyperbilirubinemia associated with chronic liver disease might interfere with bone production.[21] Moreover, patients with cirrhosis from chronic HCV infection had significantly lower level of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP-3), compared with patients with chronic HCV infection but without cirrhosis and healthy controls.[9,20] Decreased levels of these two growth factors may contribute to the development of osteoporosis because both IGF-1 and IGFBP-3 play an essential role in osteoblast differentiation and proliferation.[13,22] Furthermore, patients with chronic liver disease tend to have impaired hepatic hydroxylation of cholecalciferol, the first step in the synthesis of the active form of vitamin D, which could result in vitamin D deficiency and, ultimately, osteopenia/osteoporosis.[23,24]
The implication of HCV infection itself on bone metabolism is less known. It has been reported that patients with chronic HCV infection, even without cirrhosis, have an increased serum level of C-terminal cross-linking telopeptide of type I collagen (CTX), which suggests an increased bone resorption activity.[11] The mechanism of this increased resorptive activity is not known and requires further investigations.
Chronic inflammation associated with chronic HCV infection might also have deleterious effect on bone metabolism.[25] Inflammation modulates the bone-remodeling pathway through pro-inflammatory cytokines, such as tumor necrotic factor (TNF)- α, interleukin (IL)-1, IL-6, and IL-17, that can promote the differentiation of osteoclast from their precursor cells.[26,27] These cytokines are also capable of downregulating bone anabolic pathway, resulting in loss of balance between bone resorption and production.[28]
Although most of the included studies were of high quality, as reflected by the high quality assessment scores, we acknowledged that this meta-analysis had some limitations. Therefore, the results should be read with caution.
First, most of the primary studies included in this meta-analysis did not adjust their effect estimates for several known risk factors for osteoporosis such as diabetes, use of glucocorticoids, and immobility. Thus, it is possible that the observed association is a result of confounding effect, rather than HCV infection itself. Second, as previously mentioned, it is very likely that publication bias in favor of positive studies was present. Third, the primary studies included in this meta-analysis were conducted in three regions (Asia, Middle East and South America). Therefore, the results might not be generalizable to other populations including Caucasians and Africans.
In summary, this meta-analysis demonstrated an increased risk of osteoporosis among HCV-infected patients. Further studies are required to clarify how this risk should be addressed in clinical practice.
Disclosure
The authors have no commercial associations that might be a conflict of interest in relation to this article.
Authors' contributions
All authors had access to the data and a role in writing the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Raisz LG. Clinical practice. Screening for osteoporosis. N Engl J Med. 2005;353:164–71. doi: 10.1056/NEJMcp042092. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Minor Res. 1994;9:1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 3.Reginster JY, Burlet N. Osteoporosis: A still increasing prevalence. Bone. 2006;38:S4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 5.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill TJ, Rivera L, Struchkov V, Zaheen A, Thein HH. The effect of HIV-hepatitis C co-infection on bone mineral density and fracture: A meta-analysis. PloS One. 2014;9:e101493. doi: 10.1371/journal.pone.0101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong HV, Cortes YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: A systematic review and meta-analysis. Aids. 2014;28:2119–31. doi: 10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 9.Chen CH, Lin CL, Kao CH. Relation Between Hepatitis C Virus Exposure and Risk of Osteoporosis: A Nationwide Population-Based Study. Medicine. 2015;94:e2086. doi: 10.1097/MD.0000000000002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AB, Omland LH, Krarup H. Obel N Study Dc. Fracture risk in hepatitis C virus infected persons: Results from the DANVIR cohort study. J Hepatol. 2014;61:15–21. doi: 10.1016/j.jhep.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Lin JC, Hsieh TY, Wu CC, Chen PJ, Chueh TH, Chang WK, et al. Association between chronic hepatitis C virus infection and bone mineral density. Calcif Tissue Int. 2012;91:423–9. doi: 10.1007/s00223-012-9653-y. [DOI] [PubMed] [Google Scholar]
- 12.Orsini LG, Pinheiro MM, Castro CH, Silva AE, Szejnfeld VL. Bone mineral density measurements, bone markers and serum vitamin D concentrations in men with chronic non-cirrhotic untreated hepatitis C. PloS One. 2013;8:e81652. doi: 10.1371/journal.pone.0081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raslan HM, Elhosary Y, Ezzat WM, Rasheed EA, Rasheed MA. The potential role of insulin-like growth factor 1, insulin-like growth factor binding protein 3 and bone mineral density in patients with chronic hepatitis C virus in Cairo, Egypt. Trans R Soc Trop Med Hyg. 2010;104:429–32. doi: 10.1016/j.trstmh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Minor Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 19.Moschen AR, Kaser A, Stadlmann S, Millonig G, Kaser S, Muhllechner P, et al. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005;43:973–83. doi: 10.1016/j.jhep.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581–6. doi: 10.1172/JCI117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guanabens N, Cerda D, Monegal A, Pons F, Caballeria L, Peris P, et al. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348–56. doi: 10.1053/j.gastro.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Kawai M, Rosen CJ. The insulin-like growth factor system in bone: Basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41:323–33. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han YP, Kong M, Zheng S, Ren Y, Zhu L, Shi H, et al. Vitamin D in liver diseases: From mechanisms to clinical trials. J Gastroenterol Hepatol. 2013;28:49–55. doi: 10.1111/jgh.12016. [DOI] [PubMed] [Google Scholar]
- 24.Stokes CS, Volmer DA, Grunhage F, Lammert F. Vitamin D in chronic liver disease. Liver Int. 2013;33:338–52. doi: 10.1111/liv.12106. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Okanoue T, Ohnishi N, Sakamoto M, Nishioji K, Nakagawa Y, et al. Serum levels of soluble tumor necrosis factor receptors and effects of interferon therapy in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1999;94:1332–40. doi: 10.1111/j.1572-0241.1999.01083.x. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9:103. doi: 10.1186/ar2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakchbandi IA. Osteoporosis and fractures in liver disease: Relevance, pathogenesis and therapeutic implications. World J Gastroenterol. 2014;20:9427–38. doi: 10.3748/wjg.v20.i28.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR, et al. Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFalpha induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Minor Res. 2009;24:1434–49. doi: 10.1359/jbmr.090305. [DOI] [PubMed] [Google Scholar]