Abstract
Background/Aims:
The diagnosis of inflammatory bowel disease (IBD) is often delayed due to misdiagnosing patients with irritable bowel syndrome (IBS), mostly because of the under-recognition of high-risk features. The red flag score (RFS) has been recently developed to identify patients with higher risk of IBD rather than IBS. The aim of this study is to estimate the prevalence of high-risk features, according to the RFS, among patients diagnosed with IBS who would consequently be candidates for ileocolonoscopic evaluation.
Patients and Methods:
Adult patients with IBS seen at the general medicine clinic were recruited and surveyed using the RFS. Clinical and demographic data were collected. The prevalence of high-risk features, defined as a RFS >5, was calculated. Logistic regression analysis was used to identify predictors of RFS >5.
Results:
A total of 255 patients with IBS were recruited. The mean age was 30.6 years (±9.9 years); 71.4% of patients were women (182/255), and 90.2% were from Saudi Arabia (230/255). More than half of the patients we surveyed (51.4%) had not visited a gastroenterologist previously. The mean RFS was 6.6 (±3.6) and 54.9% of patients (140/255) scored more than 5 and accordingly were selected for further investigations. Statistical analysis identified no previous visits to a gastroenterologist as the only significant predictor of RFS >5 (OR = 2.2, 95% CI = 1.3–3.7, P = 0.003).
Conclusions:
More than half of the patients known to have IBS are candidates for further investigations to eliminate the possibility of IBD as a diagnosis according to the validated RFS. Patients who did not seek a specialized consultation with a gastroenterologist might be at a higher risk of being misdiagnosed as having IBS.
Keywords: Inflammatory bowel disease, irritable bowel syndrome, red flag score
INTRODUCTION
Inflammatory bowel disease (IBD) is a disease entity comprising ulcerative colitis (UC) and Crohn's disease (CD). Patients with CD typically present with persistent or recurrent symptoms of abdominal pain, diarrhea, and gastrointestinal bleeding because of gastrointestinal tract inflammation. IBD occurs with geographic variations but historically has been considered a disease of the Western world.[1] Its incidence and prevalence has markedly increased since the early 1950s in North America and Europe.[2,3] In other parts of the world, including Asia, South America, and the Pacific region, low prevalence and incidence rates had been reported until recently.[4,5] However, studies have shown increasing trends of IBD diagnosis in countries previously known to have a low incidence. In Asia for instance, although the incidence and prevalence of IBD remain lower than those reported in North America and Europe, recent studies have reported an increase in the prevalence of IBD with a predominance of UC.[6,7] Such a pattern is currently being observed in the Kingdom of Saudi Arabia.[8] This phenomenon has been associated with urbanization but can also be associated with improved medical care and availability of better diagnostic tools.
The symptoms of CD can easily be confused with those of irritable bowel syndrome (IBS), a functional condition associated with stress and anxiety but not with sinister long-term complications, often leading to delayed diagnosis.[9] IBS diagnosis is based on the Rome criteria, and patients are seldom referred for ileocolonoscopy if they exhibit high-risk features. In addition, fecal markers of inflammation such as fecal calprotectin (FC), have recently been developed and currently being used as a screening tool to triage patients with gastrointestinal symptoms toward colonoscopy.[10] This tool, the red flag score (RFS), is a questionnaire designed to discriminate between CD and IBS. In the initial iteration of the RFS, a score of >5 (on a 6-question questionnaire) yielded a sensitivity of 88%; specificity of 96%; positive and negative likelihood ratios of 20.16 and 0.12, respectively; and an area under the curve (AUC) of 0.967, with a diagnostic odds ratio (OR) to discriminate CD from IBS of 169 (95% CI [confidence interval] = 50–572.5, P < 0.0001).[11] Another study used a modified version of the questionnaire that added an eighth question (rectal urgency) and used a cut-off point of RFS >7, which was found to be highly predictive of CD diagnosis, with estimated sensitivity and specificity of 94% (95% CI = 0.88–0.99) and 94% (95% CI = 0.90–0.97), respectively, and positive and negative likelihood ratios of 15.1 (95% CI = 9.3–33.6) and 0.066 (95% CI = 0.013–0.125), respectively, and an AUC of 0.97 (95% CI = 0.94–0.99).[11] However, this questionnaire has so far only been used to screen patients with the first presentation on colonoscopy but not to survey those known to have IBS based on clinical criteria to eliminate the possibility of misdiagnosis. The proportion of patients diagnosed with IBS based on clinical criteria that would have otherwise qualified for ileocolonoscopy based on the RFS is unknown.
The aim of this study is to survey patients who were diagnosed as having IBS based on clinical criteria for high-risk features suggesting CD and to identify predictors of elevated RFS scores.
PATIENTS AND METHODS
After acquiring approval from the Ethics Committee at the King Abdulaziz University (KAU), all patients known to have IBS based on the Rome criteria or on self-reported diagnosis without undergoing previous ileocolonoscopy and being followed at the general medicine outpatient clinics at KAU Hospital were consecutively recruited and asked to provide written informed consent for participation. After recording the patient history and completing physical examination, the patients were asked to complete the original shorter form of the RFS in addition to demographic data. The questions comprising the short RFS questionnaire included any history of the following: 1. Non-healing or complex perianal fistula or abscess or perianal lesions (apart from haemorrhoids), 2. First-degree relative with confirmed IBD, 3. Weight loss (5% of usual body weight) in the last 3 months, 4. Chronic abdominal pain (>3 months), 5. Nocturnal diarrhea, 6. Mild fever in the last 3 months, and 7. Absence of cramps 30–40 minutes after ingesting vegetables.
Statistical analysis and sample size calculation
At baseline, descriptive statistics were calculated including means (±standard deviations [SD]) for all continuous variables and frequencies for categorical variables. The hypothesis was tested using Student t test and Mann–Whitney U test to compare means and medians, respectively, and Chi-squared or Fisher exact test were used for comparisons between frequencies, where appropriate. Simple and multiple linear regression analyses were used to identify predictors of the RFS. Logistic regression analysis was used to identify factors associated with a score of ≥6. STATA 11.2 (StataCorp, Texas, USA) was used in our analysis. P < 0.05 was considered statistically significant.
For sample size calculation, we hypothesized that the prevalence of undiagnosed CD among patients with IBS in the general population is 10%. Assuming a type 1 error of 0.05 and 80% power to detect CD in the patients with IBS by using a test with 93% sensitivity and 94% specificity, we estimated that a sample size of 255 patients would be required.
RESULTS
Baseline characteristics
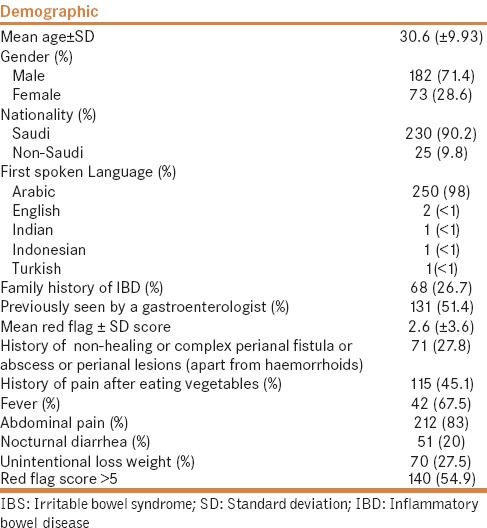
A total of 255 patients were recruited and surveyed. The mean age was 30.6 years (±9.93 years), and most patients were women (71.4%). The majority of patients were natives of Saudi Arabia (90.2%), and Arabic was the most commonly spoken first language (98%). A positive family history of IBD was recorded in 26.7% of the surveyed patients, and 51.4% had not been previously evaluated by a specialized gastroenterologist.
Red flag score
The mean RFS was 2.6 (±3.6) and 140 (54.9%) patients had RFS >5, which places them in the high-risk category. Only 40% of patients with RFS >5 previously visited a specialized gastroenterologist. Furthermore, 27.8% of patients had a history of a perianal fistula, abscess, skin tags, or anal fissure [Table 1].
Table 1.
Baseline demographics for 255 patients with IBS
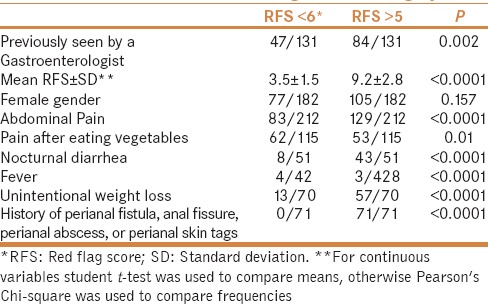
Patients with RFS >5 had higher risks of having abdominal pain; pain after eating vegetables; nocturnal diarrhea; fever; unintentional weight loss; or history of having a perianal fistula, abscess, skin tags, or anal fissure [Table 2].
Table 2.
Clinical differences according to *RFS category
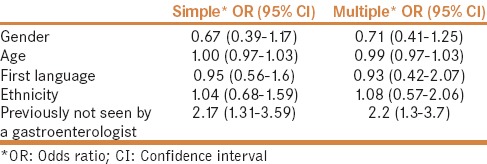
Predictors of red flag score >5
On multiple linear regression analysis, only no previous visits to a gastroenterologist predicted a total RFS (coefficient = 1.53, 95% CI = 0.62–2.43, P = 0.001). Similarly, on multiple logistic regression analysis, no previous visits to a gastroenterologist was the only significant predictor of RFS >5 (OR = 2.2, 95% CI = 1.3–3.7, P = 0.003) [Table 3].
Table 3.
Simple and multiple logistic regression analysis for predictors of a Red flag score >5
DISCUSSION
The recent increase in the incidence of CD in developing countries, such as Saudi Arabia, raises the possibility that patients previously labeled as having IBS might have been misdiagnosed. Moreover, clear evidence presents that delaying the diagnosis of CD increases the risk of complications and the need for bowel resection.[12] Therefore, the ability to detect clinical features of CD early through highly accurate clinical tools and predictive models is of utmost importance. One of the common reasons why patients with CD are diagnosed late is the potential for misdiagnosing them as having IBS.[13] The development of the RFS has provided us with a tool that can be used to “flag” patients with IBS who have high-risk features and therefore triage them toward ileocolonoscopy, which is not a standard investigation when clinically suspecting IBS, particularly in areas where the incidence of CD is increasing, such as Saudi Arabia. In our cohort of patients with IBS, more than half of the patients demonstrated high-risk features, necessitating an endoscopic evaluation and preferably referral to a gastroenterologist for a specialized consultation. This suggests that general practitioners and family physicians require further education about the importance of early diagnosis of CD and clinical features that would prompt early referral.
There is typically a shortage of specialized gastroenterologists worldwide; therefore, patients with symptoms suggestive of IBS tend to be followed by general practitioners. Based on our data, patients with RFS >5 are more likely to have not visited a gastroenterologist, irrespective of the diagnosis being made by a physician or through self-diagnosis. Therefore, it is recommended that patients with symptoms suggestive of IBS be assessed by a physician and be seen by a gastroenterologist at least once to ensure that no high-risk features warranting an ileocolonoscopy exist.
This study has several limitations, including its cross-sectional design, which limits our ability to infer any causal relationship between an elevated RFS and no previous visit to a gastroenterologist. Furthermore, considering that patients were not subsequently tested using objective measures of inflammation, such as FC, and C-reactive protein or undergone diagnostic ileocolonoscopy to eliminate CD, we cannot conclude that the elevated RFS truly reflects misdiagnosis. Accordingly, we can only interpret this observation such that a large proportion of patients previously diagnosed as having IBS who have not undergone an ileocolonoscopy exhibit high-risk features that qualify them for further investigations. Finally, considering that these data were collected from a tertiary care center, referral bias could not be ruled out. Larger, prospective studies that compare RFS scores with diagnostic gold standard testing in similar populations are warranted.
CONCLUSIONS
According to the RFS, in a country with a rising incidence of CD, a large percentage of patients might be misdiagnosed as having IBS. An elevated RFS appears to be associated with not being previously evaluated by gastroenterologists, thus highlighting the need for early specialized referrals.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Loftus EV, Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. doi: 10.1016/s0889-8553(01)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Garland CF, Lilienfeld AM, Mendeloff AI, Markowitz JA, Terrell KB, Garland FC. Incidence rates of ulcerative colitis and Crohn's disease in fifteen areas of the United States. Gastroenterology. 1981;81:1115–24. [PubMed] [Google Scholar]
- 4.Sung JJ, Hsu RK, Chan FK, Liew CT, Lau JW, Li AK. Crohn's disease in the Chinese population. An experience from Hong Kong. Dis Colon Rectum. 1994;37:1307–9. doi: 10.1007/BF02257802. [DOI] [PubMed] [Google Scholar]
- 5.Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, et al. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–7. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian J Gastroenterol. 2007;26:285–9. [PubMed] [Google Scholar]
- 7.Niriella MA, De Silva AP, Dayaratne AH, Ariyasinghe MH, Navarathne MM, Peiris RS, et al. Prevalence of inflammatory bowel disease in two districts of Sri Lanka: A hospital based survey. BMC Gastroenterol. 2010;10:32. doi: 10.1186/1471-230X-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alharbi OR, Azzam NA, Almalki AS, Almadi MA, Alswat KA, Sadaf N, et al. Clinical epidemiology of ulcerative colitis in Arabs based on the Montreal classification. World J Gastroenterol. 2014;20:17525–31. doi: 10.3748/wjg.v20.i46.17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul Rani R, Raja Ali RA, Lee YY. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: Pieces of the puzzle are falling into place. Intest Res. 2016;14:297–304. doi: 10.5217/ir.2016.14.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sood R, Gracie DJ, Law GR, Ford AC. Systematic review with meta-analysis: The accuracy of diagnosing irritable bowel syndrome with symptoms, biomarkers and/or psychological markers. Aliment Pharmacol Ther. 2015;42:491–503. doi: 10.1111/apt.13283. [DOI] [PubMed] [Google Scholar]
- 11.Danese S, Fiorino G, Mary JY, Lakatos PL, D'Haens G, Moja L, et al. Development of red flags for early referral of adults with symptoms and signs suggestive of Crohn's disease: An IOIBD initiative. J Crohns Colitis. 2015;9:601–6. doi: 10.1093/ecco-jcc/jjv067. [DOI] [PubMed] [Google Scholar]
- 12.Schoepfer AM, Dehlavi MA, Fournier N, Safroneeva E, Straumann A, Pittet V, et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108:1744–53. doi: 10.1038/ajg.2013.248. [DOI] [PubMed] [Google Scholar]
- 13.Maconi G, Orlandini L, Asthana AK, Sciurti R, Furfaro F, Bezzio C, et al. The impact of symptoms, irritable bowel syndrome pattern and diagnostic investigations on the diagnostic delay of Crohn's disease: A prospective study. Dig Liver Dis. 2015;47:646–51. doi: 10.1016/j.dld.2015.04.009. [DOI] [PubMed] [Google Scholar]


