Abstract
Background/Aims:
Celiac disease (CD), a chronic autoimmune condition, is associated with systemic inflammation capable of causing extra intestinal manifestations. Chronic inflammatory process has been implicated in the pathogenesis of accelerated atherosclerosis. Studies examining the burden of coronary artery disease (CAD) in patients with CD are lacking. We evaluated the prevalence of CAD in patients with CD.
Patients and Methods:
Electronic health records from different health care systems were obtained utilizing a Health Insurance Portability and Accountability Act-compliant, patient de-identified web application. Among the 48,642,290 patients, 59,010 were diagnosed with CD. The remaining 48,583,280 patients without CD served as comparison controls.
Results:
The prevalence of CAD was significantly higher in patients with CD than in the controls [5140 (8.7%) vs. 2119060 (4.4%), P < 0.001], with the odds ratio (OR) being 2.09 (95% confidence interval [CI]: 2.03–2.15, P < 0.0001). There was a similarly higher prevalence among younger patients (age, <65 years) with CD compared with those without CD (3.72% vs 1.98% [OR: 1.85, 95% CI: 1.7488–1.9417, P < 0.0001).
Conclusions:
The prevalence of CAD increased nearly two-fold in patients with CD.
Keywords: Atherosclerosis, celiac disease, coronary artery disease, inflammation
INTRODUCTION
Coronary artery disease (CAD) is established as a major cause of mortality and morbidity. CAD alone caused approximately 1 of every 6 deaths in the United States in 2010.[1] The total CAD prevalence in adults older than 20 years in the United States is 6.4%, with the prevalence in men and women being 7.9% and 5.1%, respectively.[2] By 2030, the prevalence of CAD is estimated to increase to approximately 18%.[3] Considerable progress been made with regard to risk stratification, identification, and controlling this risk. A study suggests that inflammation has a role in the etiology of CAD and inflammatory biomarkers, such as C-reactive protein, have a potential role in predicting the risk of CAD and may correlate with the severity of CAD.[4]
Several systemic autoimmune conditions, including rheumatoid arthritis (RA), systemic lupus erythematosus, antiphospholipid syndrome, and primary Sjögren syndrome, are associated with enhanced atherosclerosis, and consequently higher cardiovascular morbidity and mortality rates.[5] Celiac disease (CD), a chronic autoimmune inflammatory disease of the small bowel, is caused by a permanent intolerance of dietary gliadin and related protein, resulting in small bowel mucosal inflammation that leads to villous atrophy and crypt hyperplasia in genetically susceptible individuals. CD is estimated to affect at least 1 in 133 people worldwide.[6] CD can affect multiple organs, including the heart, but few studies have determined the association of CD and CAD. Approximately 2.5 million Americans possibly remain undiagnosed and at a risk of cardiac complications;[7] therefore, we examined the prevalence of CAD in the CD population.
Aims
To estimate the point prevalence of CAD in the CD population
To specify this prevalence in different age groups
To evaluate the incidence of myocardial infarction in the CD population.
PATIENTS AND METHODS
This is a cross-sectional retrospective cohort study conducted using de-identified data, which was obtained using the Explore application on the Explorys Inc. platform. The Explorys technology platform uses a health data gateway (HDG) server behind the firewall of each participating health care organization, all of which are based in the United States. The HDG server collects data from various health information systems: electronic health records, billing systems, laboratory/radiology systems. Next, the de-identified data from each participating health care organization is passed into the Explorys data grid, a cloud-based data store. The data is then standardized and normalized using common ontologies. Each participating health care organization has access to a secure web-based application, which allows for searching and analyzing the data from all participating health care organizations. The data is automatically updated at least once daily. This standardized data is searchable through the Health Insurance Portability and Accountability Act-compliant, patient de-identified web application Explore, Explorys Inc[8] and is totally free to the members of the participating health care organizations.
Study population
All patients aged 18 years or older and who used the participating health care systems from January 1999 to March 2016 were identified from the database. We then identified an aggregated cohort of eligible patients with CD diagnosed code 579.0 from the International Classification of Diseases, Tenth Revision (ICD-10), and the remaining patients without CD in the database formed the control group. CAD included stable angina, unstable angina, non-ST elevation myocardial infarction and ST elevation myocardial infarction[9] were identified using ICD-10 codes 410.0–414.9.
Characteristics, such as age; sex; and history of hypertension, diabetes, cerebrovascular accident, smoking, or peripheral vascular disease (PVD) were abstracted for comparison between the two groups.
This study was approved by the Institutional Review Board (IRB# 14-303) of the Cleveland Clinic Foundation.
Statistical analysis
Categorical variables are reported as counts and percentages; continuous variables are presented as means ± standard deviation. Categorical variables were compared using the Chi-squared test, and continuous data were analyzed using the Student t test or Wilcoxon nonparametric statistics (PASW Statistics version 18, SPSS Inc, Chicago, IL, USA).
RESULTS
Among the 48,642,290 patients in the study (mean age: 52.12 years) 45.7% were men, 54.7% were Caucasians, 2.4% were smokers (current and former), 6.5% had a history of diabetes, 4.5% had hypertension, and 13.5% had hyperlipidemia.
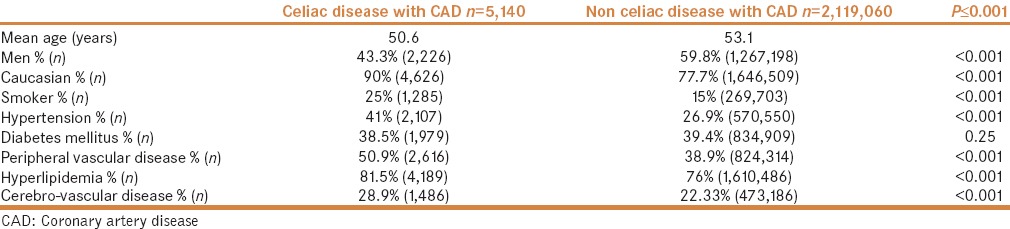
Baseline characteristics of patients with coronary artery disease and without celiac disease
In the CD cohort (n = 59,010), 5140 patients (mean age: 50.6 years) had CAD; 43.3% were men and 90% were Caucasians. In the non-CD cohort (n = 48,583,280), CAD was present in 2,119,060 patients (mean age: 53.1 years); 59.8% were men and 77.7% were Caucasians. The prevalence of hyperlipidemia (81.5% vs 76%), PVD (50.9% vs 38.9%), and hypertension (41% vs. 26.9%) was higher in patients with CD. The prevalence of diabetes (39.3% vs 40.1%) was similar in both groups [Table 1].
Table 1.
Baseline characteristics of patients with coronary artery disease in the celiac and non-celiac disease cohorts
Point prevalence of coronary artery disease in celiac disease
The prevalence of CAD was significantly higher in the CD cohort than the non-CD cohort {5140 (8.7%) vs. 2,119,060 (4.4%), P < 0.001}, with the odds ratio (OR) being 2.09 (95% confidence interval [CI]: 2.03–2.15, P < 0.0001).
In adults ≤65 years of age, the prevalence of CAD was similarly higher in the CD cohort than the non-CD cohort (3.72% vs. 1.98%, P < 0.001), with the OR being 1.84 (95% CI: 1.7488–1.9417, P < 0.001).
Point prevalence of coronary artery disease in celiac disease in different age groups
When comparing by age groups, the prevalence of CAD in CD was 1.1% vs 0.45%, (P < 0.001) in the age group of 18–49 years, 8.65% vs 4.71% (P < 0.001) in the age group of 50–64 years, and 37.67% vs 14.45% (P < 0.001) in the age group of >65 years [Figure 1].
Figure 1.
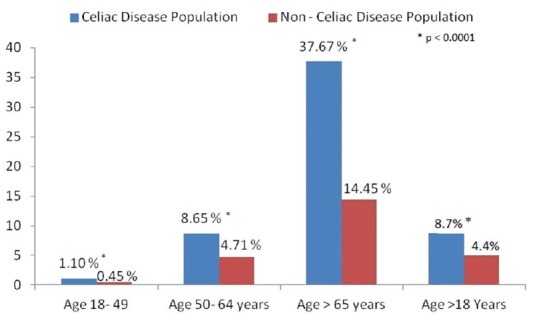
Comparison of the prevalence of cerebral artery disease in patients with and without celiac disease in different age groups
Point prevalence of myocardial infarction in celiac disease
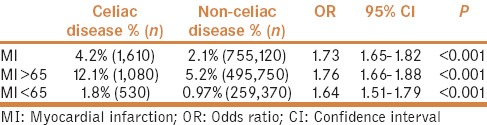
The point prevalence of myocardial infarction (MI) was significantly higher in the CD cohort than in the control cohort (4.2% vs. 2.1%, P < 0.001) with the OR of 1.73 (95% CI: 1.65–1.82, P < 0.001) [Figure 2].
Figure 2.
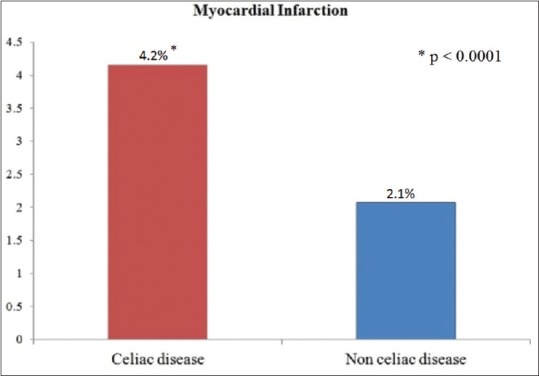
Prevalence of myocardial infarction in patients with and without celiac disease
In younger adults with CD (age, <65 years), the results were similar with a higher point prevalence of MI in the celiac cohort than the non-CD cohort (1.8% vs. 0.97%, P < 0.001), with the OR being 1.64 (95% CI: 1.51–1.79, P < 0.0001). In elderly patients (age: ≥65 years), it was 12.1% in the CD cohort vs. 5.2 in the non-CD cohort (OR: 1.76, 95% CI: 1.66–1.88) [Table 2].
Table 2.
Comparison of the prevalence of myocardial infarction between celiac and non-celiac cohorts
Subgroup comparison
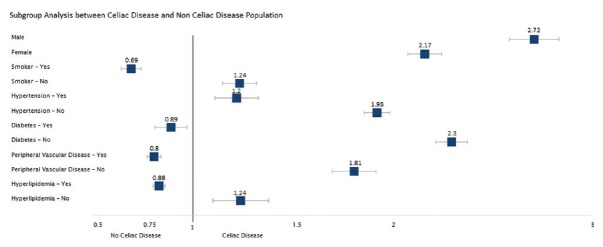
The prevalence of CAD was higher in both men and women in the CD cohort than in the non-CD cohort. Men with CD had a higher prevalence of 14.3% (2,226/15,590) vs 5.7% (1,267,198/22,213,385) in those without CD (OR: 2.72, 95% CI: 2.52–2.92). In women with CD, CAD was present in 6.7% (2,914/43,420) vs 3.2% (851,862/26,369,895; OR: 2.17, 95% CI: 2.08–2.27) in those without CD. In patients with hypertension, CAD was common in patients with CD (32.5%; 2,107/6,480) vs those without CD (25.9%; 570,550/2,204,340), OR: 1.2; 95% CI: 1.11–1.23). The prevalence of CAD seemed lower in patients with CD with diabetes (24.1% vs 26.2%; OR: 0.89, 95% CI: 0.81–0.97), PVD (28.1% vs 32.6%; OR: 0.80, 95% CI: 0.77–0.84), hyperlipidemia (22.4% vs 24.7%; OR: 0.88, 95% CI: 0.84–0.92) and in smokers (16.5% vs 23.3%; OR: 0.69, 95% CI: 0.64–0.74) than in patients without CD. However in normotensive patients (5.8% vs 2.4%; OR: 1.93, 95% CI: 1.86–1.99), non-diabetics (6.2% vs 2.8%; OR: 2.3, 95% CI: 2.22–2.38) and those without PVD (5.1% vs 2.8%; OR: 1.81, 95% CI: 1.74–1.89) or hyperlipidemia (2.4% vs 1.2%; OR: 1.24, 95% CI: 1.16–1.32), the prevalence of CAD was higher in the CD cohort than in the non-CD cohort [Table 3 and Figure 3].
Table 3.
Subgroup analysis and comparison between the celiac and non-celiac disease cohorts
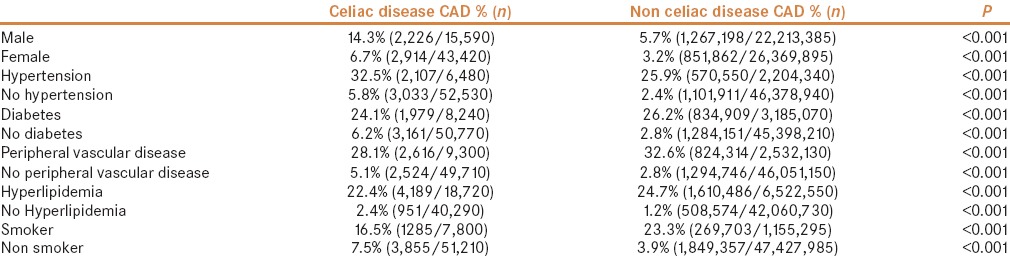
Figure 3.
Subgroup analysis between celiac disease and non-celiac disease cohorts
DISCUSSION
Coronary atherosclerotic heart disease is the most common cause of cardiovascular disability and death in the United States.[10] The total CAD prevalence is 6.4% in the United States in adults older than 20 years, with the prevalence in men and women being 7.9% and 5.1%, respectively. The crude mortality of CAD remains high. CAD alone caused approximately 1 of every 6 deaths in the United States in 2010.[2] By 2030, the prevalence of CAD is estimated to increase to approximately 18%.[3] Over the last decade, numerous studies have indicated that inflammation is associated with accelerated atherosclerosis.[11]
Two hypotheses have been proposed to explain the involvement of inflammation and immune response in the initiation of atherosclerosis. The first is referred to as response to injury, which leads to chronic inflammation in the blood vessel wall, where the macrophage is the primary driver.[11] The second and more recent hypothesis is the autoimmune response, in which the recognition and presentation of atherosclerosis-related autoantigenesis leads to the activation of T cells.[11]
Autoimmune response is defined as immune responses to self-reactive T cells. Self-reactive T cells typically include two subsets of T cells: type 1 helper T cells (Th1) and a recently described subset type 17 helper T cells (Th17). In addition, it is believed that regulatory T cells function to maintain autoimmune responses and that the reduction of regulatory T cells function leads to autoreactivity. Buono et al. showed that the presentation of atherosclerosis-related antigens, including oxidized low-density lipoprotein (LDL), heat shock protein 60, and β2-glycoprotein by antigen-presenting cells (APC), and recognition of these antigens by T cells leads to atherosclerosis.[12] Cheng et al. showed that in patients with acute coronary syndrome, there was a significant increase in peripheral Th17 cells, Th17-related cytokines (e.g., interleukin [IL]-17), and transcription factor (RORγT) levels, as well as a clear decrease in regulatory T cells (Treg), Treg-related cytokines (e.g., IL-10), and transcription factor, suggesting a role of the Th17/Treg imbalance in rupture-prone atherosclerosis.[13] Thus, a strong correlation seems to exist between T cells in blood and plaques or progression of atherosclerosis.
Several systemic autoimmune conditions, including RA, systemic lupus erythematosus, antiphospholipid syndrome, and primary Sjögren syndrome, are associated with enhanced atherosclerosis, and consequently higher cardiovascular morbidity and mortality rates.[5] Pathologically, atherosclerosis and RA share many features, including cellular components (monocytes/macrophages, T cells, and connective tissue cells), extracellular matrix, and pathological mechanisms. With respect to molecular mediators, the two diseases share the involvement of many factors, such as cytokines, matrix metalloproteases (MMP), surface receptors, and transcriptional factors.[5]
CD is an autoimmune inflammatory disorder with characteristics similar to other autoimmune disorders. It is characterized by an intestinal inflammation triggered by gluten, a storage protein found in wheat, rye, and barley. The resulting immune response leads to tissue destruction and production of autoantibodies. HLA-DQA1 and HLA-DQB1 are the two main genes responsible for triggering the immune response in CD.[14]
The deamidation of the gluten peptides by transglutaminase 2 (TG2) creates potent immunostimulatory epitopes that activate CD4 T cells-secreting mainly Th1 cytokines, such as interferon (IFN)-γ, which induces the release and activation of MMP by myofibroblasts.[14] This results in mucosal remodeling and villous atrophy. Additionally, Th2 cytokine production drive the production of auto antibodies to gluten and TG2. Other cytokines, such as IL-18, IFN-α, and IL-21, play a role in polarizing and maintaining the Th 1 response. Gliadin also activates macrophages and dendritic cells via toll-like receptor (TLR) 4, leading to the secretion of inflammatory cytokines, such as IL-1, IL-8, tumor necrosis factor-α, monocyte chemoattractant protein-1, which can potentiate the immune responses of the body.[14,15]
The concern is that this profligacy and persistence of inflammation in patients with CD presents a proinflammatory milieu, favoring the abundance of factors likely to play a contributory role in atherogenesis. The presence of inflammatory mediators and substrates, such as TLRs and cytokines, may probably facilitate and accelerate various stages of atherosclerosis in patients with CD, right from the initiation of atheroma by incorporating LDL, to the propagation, and later rupture of the unstable plaque of the culprit atheromatous lesions by this abundance and possible cross-reaction of the inflammatory process.
Our study shows a higher prevalence of CAD in patients with CD. The incidence of CAD was nearly two-fold higher in patients with CD than in the controls. Similar trends were observed across all age groups, including patients aged <65 years. Based on this trend, our study suggests an association between CD and CAD. This certainly lends to the theory that chronic persisting inflammation contributes to the increased risk of atherogenesis. The same hypothesis can be extended to the extra coronary vascular diseases, whereby atherosclerosis, plaque formation is aided, accelerated and destabilized by the ongoing inflammation. Previous research seems to corroborate this suggestion, particularly in cerebrovascular disease.[16]
Peters et al. report that in patients with CD, 17% (39/238) of the mortality was because of cardiac causes; Viljamaa et al. reported similar higher cardiovascular-related mortality in celiac patients.[17,18] Several reports on cardiac complications, such as atrial fibrillation, myocarditis, and cardiomyopathy, have been associated with CD.[19,20] Approximately 2.5 million Americans with CD may remain undiagnosed, possibly at risk of CAD, thereby increasing the burden of morbidity and mortality associated with cardiac disease if left undiagnosed.
Our study indicates a strong potential association between CD and CAD. Apart from addressing the traditional risk factors for CAD, it may also be important to identify patients with autoimmune disorders, which are associated with accelerated atherosclerosis and treat them appropriately. This may consequently help in reducing cardiovascular morbidity and mortality.
Limitations
Our study has some limitations. The proportion of patients with CAD identified in our study may be smaller than is actually prevalent. This may have resulted from the limitations of using diagnosis codes to retrospectively identify patients, which is dependent on the input by the respective health care provider. Another limitation is that we did not have the data regarding the inflammatory markers and hence could not compare the direct relationship of inflammation with CAD. A larger prospectively designed study would be able to address these limitations and can also further validate our findings.
CONCLUSIONS
Our study highlights the strong association of CAD with CD. Our study has shown a two-fold increase in the prevalence of CAD in patients with CD across all age groups. We suspect the underlying pathogenesis is the autoimmune inflammatory process resulting in accelerated atherogenesis. Early detection and aggressive treatment of CD may result in favorable outcomes of cardiovascular complications associated with this chronic disorder.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). Prevalence of coronary heart disease - United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011;60:1377–81. [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: A report from the American heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the American heart association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–33. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Pedrera C, Perez-Sanchez C, Ramos-Casals M, Santos-Gonzalez M, Rodriguez-Ariza A, Cuadrado MJ. Cardiovascular risk in systemic autoimmune diseases: Epigenetic mechanisms of immune regulatory functions. Clin Dev Immunol. 2012;2012:974648. doi: 10.1155/2012/974648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–44. doi: 10.1038/ajg.2012.219. [DOI] [PubMed] [Google Scholar]
- 8.Kaelber DC, Foster W, Gilder J, Love TE, Jain AK. Patient characteristics associated with venous thromboembolic events: A cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012;19:965–72. doi: 10.1136/amiajnl-2011-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan SL, Behrend L, Vu MB, Meier A, Griffith JM, Pignone MP. Individuals' responses to global CHD risk: A focus group study. Patient Educ Couns. 2009;76:233–9. doi: 10.1016/j.pec.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q. Inflammation, autoimmunity, and atherosclerosis. Discov Med. 2009;8:7–12. [PubMed] [Google Scholar]
- 12.Buono C, Lichtman AH. Co-stimulation and plaque-antigen-specific T-cell responses in atherosclerosis. Trends Cardiovasc Med. 2004;14:166–72. doi: 10.1016/j.tcm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, et al. The Th17/treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Schuppan D, Junker Y, Barisani D. Celiac disease: From pathogenesis to novel therapies. Gastroenterology. 2009;137:1912–33. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Kupfer SS, Jabri B. Pathophysiology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:639–60. doi: 10.1016/j.giec.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkind MS. Inflammatory mechanisms of stroke. Stroke. 2010;41(10 Suppl):S3–8. doi: 10.1161/STROKEAHA.110.594945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566–72. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 18.Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38:374–80. doi: 10.1016/j.dld.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Goel NK, McBane RD, Kamath PS. Cardiomyopathy associated with celiac disease. Mayo Clin Proc. 2005;80:674–6. doi: 10.4065/80.5.674. [DOI] [PubMed] [Google Scholar]
- 20.Frustaci A, Cuoco L, Chimenti C, Pieroni M, Fioravanti G, Gentiloni N, et al. Celiac disease associated with autoimmune myocarditis. Circulation. 2002;105:2611–8. doi: 10.1161/01.cir.0000017880.86166.87. [DOI] [PubMed] [Google Scholar]