Abstract
Objective:
To assess the value of baseline clinical and imaging biomarkers in a cohort of asymptomatic LRRK2 G2019S carriers for predicting conversion to Parkinson disease (PD) at 4 years.
Methods:
Thirty-two asymptomatic carriers of LRRK2 G2019S mutation underwent baseline and 4-year evaluation including clinical examination (Unified Parkinson's Disease Rating Scale, part III, olfaction University of Pennsylvania Smell Identification Test [UPSIT]) and dopamine transporter (DaT) SPECT (123I-ioflupane). Visual and semiquantitative analysis of images was performed. The specific striatal binding ratio was calculated (striatal region of interest [ROI] − occipital ROI/occipital ROI).
Results:
Three carriers, asymptomatic at baseline, had converted to PD at 4-year evaluation. Twenty-three participants were fully evaluated. PD converters had lower striatal DaT binding at baseline than nonconverters (p = 0.002). A baseline scan with a ratio of bilateral striatal uptake below 1 predicted conversion to PD within the 4-year period with high sensitivity and specificity (area under the curve 1; p = 0.006). The slope of DaT binding decline between the 2 scans was similar in PD converters and nonconverters. Age-adjusted UPSIT score at baseline and at 4 years was similar in both groups.
Conclusions:
Semiquantitative DaT-SPECT could be used to predict early conversion to PD in asymptomatic carriers of the LRRK2 G2019S mutation. Rate of conversion to PD at 4 years in this cohort aged ∼64 years was 12%. The slope of DaT binding decline on DaT-SPECT imaging seems to be similar across different stages of the premotor period.
The G2019S mutation of the LRRK2 gene is the commonest known cause of Parkinson disease (PD).1 Clinically and pathologically, this form of the disease is indistinguishable from idiopathic PD, and therefore it constitutes an ideal scenario in which to deepen our knowledge about the disease. The identification and follow-up of carriers of the LRRK2 G2019S mutation who still have not developed motor symptoms of PD represents a unique opportunity for studying the prodromal stage of PD. We lack information on the timing between the beginning of the neurodegenerative process and the appearance of the motor symptoms of PD and the pace of cell loss at the substantia nigra (SN) at the premotor stage. Shedding light into the prodromal stages of the disease is going to be crucial for planning drug trials aiming to modify the disease process early, before most of the damage is already done. The penetrance of the LRRK2 G2019S mutation is age-dependent and only a proportion of LRRK2 G2019S mutation carriers will develop motor symptoms of PD.2 Besides age, no other clinical marker has been proven to be useful to predict conversion to manifest PD in asymptomatic carriers. In a previous study by our group, we characterized a cohort of 32 asymptomatic carriers of the LRRK2 G2019S mutation with brain imaging (123I-ioflupane SPECT and transcranial sonography) and olfaction tests.3 Here we report the findings of a re-evaluation of this cohort at 4 years, including clinical examination, olfaction testing, and 123I-ioflupane SPECT. Our objective was double: first to assess whether any of the biomarkers studied at baseline was helpful to predict conversion to PD within the 4-year period, and second to estimate through sequential 123I-ioflupane imaging the rate of progression of the dopaminergic terminal loss in the premotor stage of LRRK2 G2019S-associated PD.
METHODS
Patients.
This cohort was composed of 32 asymptomatic carriers of LRRK2 G2019S mutation reported in a previous study.3 These were the participants from that study who had completed all the tests (clinical examination, transcranial sonography, 123I-ioflupane SPECT, and olfaction test). All of them were relatives of LRRK2 G2019S PD proband cases identified from a study of 367 consecutive patients with PD attended at a single center.2 Participants belonged to a total of 21 pedigrees.
Clinical assessment.
Approximately 4 years after the baseline evaluation, the 32 participants were invited by telephone to participate in the study. Those who accepted were examined for the presence of signs of PD. We used the Unified Parkinson's Disease Rating Scale, part III (UPDRS-III), and a diagnosis of PD was made according to the Queen Square Brain Bank Criteria.4 All participants were assessed at a single center and by the same research team involved in the baseline evaluation, in which examiners were blind to the genetic status of LRRK2 relatives.3 Clinical examination was done before the rest of the tests were available so examiners were blind to these results. In cases in which the examination led to a diagnosis of PD, we acceded to patient clinical records to check if the patient had already been diagnosed with PD within the 4-year period.
Olfaction testing.
Olfaction was evaluated through the encapsulated odor University of Pennsylvania Smell Identification Test (UPSIT).5 The test was self-administered by using standard 40-odor identification, either at the time of the visit or at the participant's residence, and was returned by mail. Participants were asked to choose a response from the 4 choices listed. Tests including incomplete responses were excluded from the analysis.
123I-ioflupane SPECT protocol.
Acquisition of 123I-ioflupane SPECT images was performed as described elsewere.3 For image processing, filtered backprojection with a Butterworth 0.55 filter and manual Chang attenuation correction with a coefficient of 0.12 cm–1 was applied. The axial slices were reoriented parallel to the frontal-occipital line. Visual and semiquantitative analysis of images was performed by 2 experienced nuclear medicine specialists blinded to clinical data (I.M.-R., M.J.-A.). For the visual analysis, images were categorized as negative or positive (when a decrease in the striatal uptake was observed). For the semiquantitative analysis, 2 regions of interest (ROIs) with the same size for all patients were manually drawn including the left and right striatum, respectively. These ROIs were placed in 3 consecutive axial slices showing the highest striatal uptake. The occipital cortex was selected as background to determine the nonspecific binding, using a rectangular ROI in the same 3 consecutive slices selected for the striatal binding. The specific striatal binding ratio was calculated as follows: striatal binding ratio = striatal ROI − occipital ROI/occipital ROI.
Statistical analysis.
Comparison between quantitative variables was done using Student t test for independent or paired samples or Mann-Whitney test. A χ2 or Fisher exact test was used to analyze categorical variables. Multivariate analysis was performed with multiple lineal and logistic regressions, for quantitative and categorical variables, respectively. In addition to age and sex, variables with significant differences among groups were included in the models in order to adjust for them. The cutoff value of the 123I-ioflupane SPECT distinguishing PD converters from nonconverters was established using a receiver operating characteristic curve. For the evaluation of the olfactory function, mean raw UPSIT scores were compared between groups and afterward the individual scores were categorized (normosmic vs hyposmic) using normative data for age and sex as previously reported by Doty,5 with a dichotomous cut at the 15th percentile. Analyses were performed using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL).
Standard protocol approvals, registrations, and patient consents.
All participants gave their informed consent to participate in the study, which was approved by the ethics board of the University Hospital Marqués de Valdecilla–IDIVAL.
RESULTS
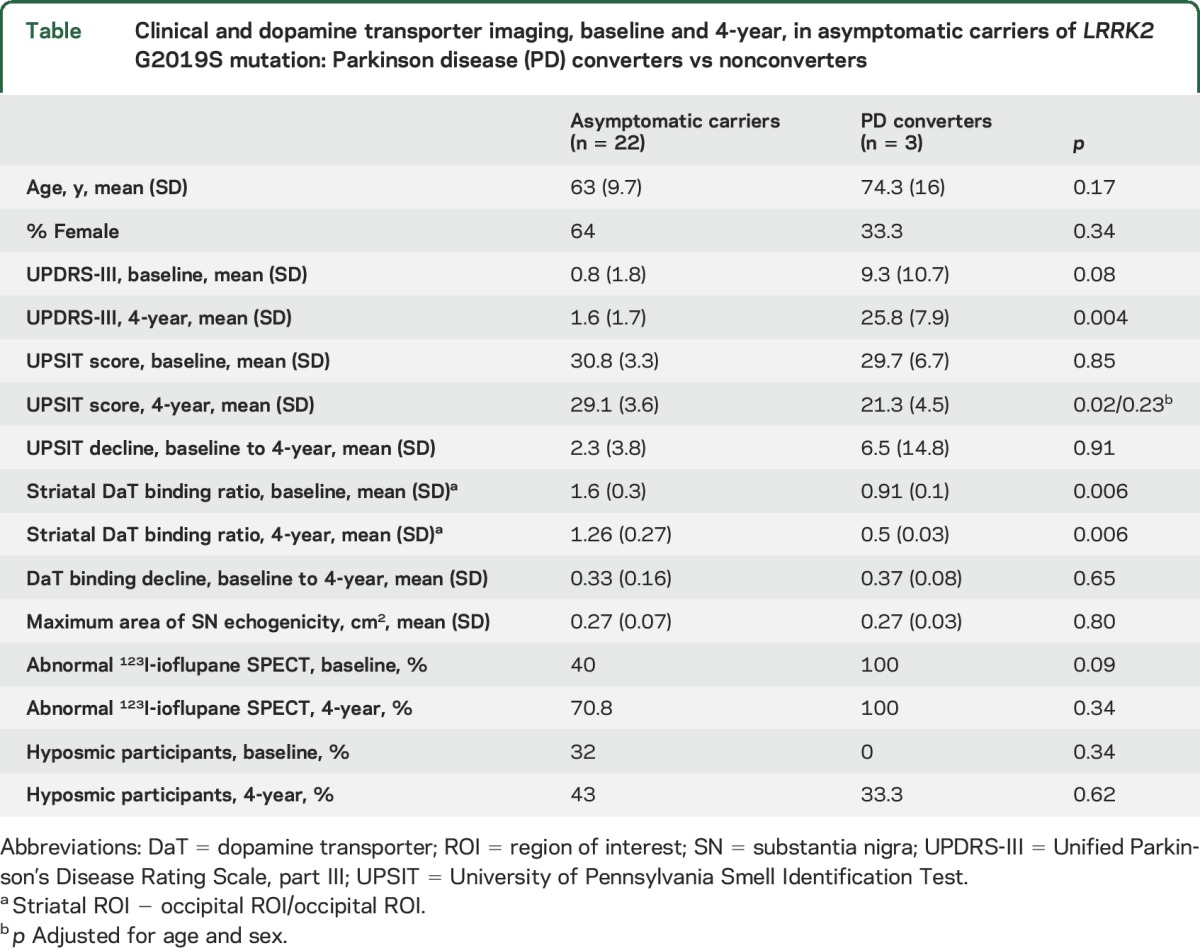
From the initial cohort of 32 participants at baseline, 3 declined to participate in any of the 4-year evaluations and another 4 were available only for the clinical examination but not for olfaction testing and imaging. Mean age of the 29 participants was 64.4 ± 11.9 years, 60.6% female. Mean interval between the baseline and the 4-year clinical and neuroimaging examination was 49.3 ± 4.9 months. Demographic and clinical data of the participants are summarized in the table.
Table.
Clinical and dopamine transporter imaging, baseline and 4-year, in asymptomatic carriers of LRRK2 G2019S mutation: Parkinson disease (PD) converters vs nonconverters
Clinical evaluation.
Three LRRK2 G2019S mutation carriers, asymptomatic at baseline, had converted to PD at the 4-year evaluation. Patients 10 and 17, aged 58 and 75 years, had been diagnosed with PD in 2015 and 2012, respectively, and patient 20, a 91-year-old woman, had been diagnosed with PD in 2013 (in the second year of follow-up). Their UPDRS-III scores at the 4-year evaluation were 21.5, 21, and 35, respectively. Two participants (patients 5 and 24) showed minimal signs of PD with a UPDRS-III score of 6 and 4, respectively, but did not fulfill clinical diagnostic criteria of PD. Compared to the mean UPDRS-III score at baseline, there was a significant increase in the score at 4 years in the entire cohort (1.8 ± 4.4 vs 4.3 ± 8.2; p = 0.03). Mean UPDRS-III in PD converters was higher, although not significantly, than in nonconverters at baseline (9.3 ± 10.7 vs 0.8 ± 1.8; p = 0.3), but was significantly higher at the 4-year evaluation (25.8 ± 7.9 vs 1.6 ± 1.7; p = 0.03). PD converters were older than nonconverters (74.7 ± 16.5 vs 63.3 ± 10.6 years); however, differences were not statistically significant (p = 0.17).
Olfaction testing.
Twenty-five carriers of the mutation completed the UPSIT test at the end of the follow-up. Mean UPSIT score at 4 years was not significantly different from baseline (28.3 ± 4.4 vs 31.1 ± 3.6; p = 0.43). Mean UPSIT decline of the entire cohort during the follow-up was 2.7 points. Mean UPSIT score at baseline was not different between PD converters and nonconverters (29.7 ± 6.6 vs 30.9 ± 3.3; p = 0.8) and that was also the case at 4 years after adjusting for age (21.3 ± 4.5 vs 29.2 ± 3.5; p = 0.02; p-adjusted = 0.23). Among PD converters, patients 10 and 17 had a significant decline in their UPSIT score of 17 and 12 points, respectively; however, patient 20 improved her score by 4 points. The proportion of hyposmic carriers at the 4-year evaluation was 44%, higher compared with 28% at baseline; however, only 1 out of the 3 PD converters was hyposmic at the end of the 4-year follow-up.
Dopamine transporter imaging.
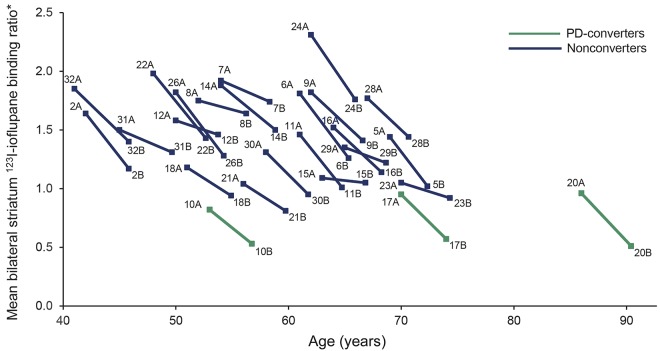
At the 4-year assessment, there was a mean absolute reduction of 123I-ioflupane binding ratio in bilateral striatum of 22.6 ± 10.8% (range 3.7%–47%) compared to baseline measures (1.18 ± 0.35 vs 1.51 ± 0.39; p = 1.56 × 10−10). The absolute rate of decline in PD converters was significantly higher than that observed in nonconverters (40.7 ± 5.8% vs 19.7 ± 8.8%; p = 0.001); however, the slope of the decline was similar between both groups given that PD converters has significantly lower 123I-Ioflupane uptake at baseline than nonconverters (0.91 ± 0.08 vs 1.59 ± 0.33; p = 0.002; figure). At the 4-year scan, mean 123I-ioflupane binding ratio was also significantly lower in PD converters compared to nonconverters (0.54 ± 0.03 vs 1.27 ± 0.27; p = 1.83 × 10−11). A baseline scan with a ratio of bilateral striatal uptake below 1 predicted with 100% sensitivity and specificity conversion to PD within the 4-year period (area under the curve 1; p = 0.006). The mean reduction in the ratio of mean bilateral striatum 123I-ioflupane uptake was 0.33 ± 1.6, with no differences between PD converters and nonconverters (0.37 ± 0.08 vs 0.32 ± 0.16; p = 0.64; p-adjusted [age, sex, time interval between 123I-ioflupane SPECTs] = 0.48). The variable time-adjusted decline in 123I-ioflupane uptake observed between scans was not correlated with any of the variables studied (age, sex, baseline SN echogenicity, or UPSIT score). There was a significant correlation between mean bilateral striatal 123I-ioflupane uptake and UPDRS-III at 4 years (r = −0.65, p = 0.001, p-adjusted = 0.006). Mean bilateral striatal 123I-ioflupane uptake at 4 years was also correlated with age (r = −0.42, p = 0.03). On the contrary, no correlation was observed between the mean maximum area of SN echogenicity and mean bilateral striatal 123I-ioflupane uptake at baseline (r = −0.20, p = 0.33).
Figure. Individual decline of mean bilateral striatum 123I-ioflupane binding ratio between baseline and 4 years.
*Striatum count number − occipital count number/occipital count number. PD = Parkinson disease.
DISCUSSION
In this study, we provide information supported by prospective clinical and imaging data on the premotor stage of LRRK2 G2019S-associated PD. After 4 years of follow-up, 12% of carriers from a cohort aged 59 years at baseline converted to PD. The ratio of mean bilateral striatum dopamine transporter (DaT) binding below 1 on baseline 123I-ioflupane SPECT predicted with a high sensitivity and specificity conversion to motor PD within the 4-year period. The slope of DaT binding decline over the 4-year period was not different between PD converters and nonconverters, implying that most likely the rate of decline is constant along the premotor and earliest clinical stages of the disease.
The neurodegenerative process of patients with PD carrying the LRRK2 G2019S mutation results in a pattern of nigrostriatal dopaminergic dysfunction similar to that observed in idiopathic PD, with identical neurochemical phenotype reported on DaT imaging.6 Although progressive decline in striatal DaT binding in PD is not a direct measure of changes in SN cell counts, results in previous studies are in accordance with postmortem data.7
Previous studies have shown a linear reduction of striatal DaT binding in early PD, with estimated annual rates of decline ranging from 4% to 10%.8–12 In our study, assuming a constant decline, the estimated absolute annual rate of decline of striatal DaT binding was around 5%. It is important to highlight that we could not calculate the relative rate of decline compared to age-expected normal values at the time of the first scan, since we did not have those data. Considering this, the relative annual DaT binding decline in our study would be below 4%, slightly lower than previous studies performed in early idiopathic PD. The slope of the decline was similar between carriers who converted to PD within the 4-year period and those who did not; however, the slope of the decline of striatal DaT binding was widely variable, with some participants showing softer slopes of decline than others (figure). This variability was not correlated with any of the variables studied here. Indeed, participants with a milder slope of decline of DaT binding were no different in terms of age, sex, or DaT binding at baseline compared to those with a more pronounced slope of decline. Our data also indicate that the initiation of dopaminergic terminal loss probably antedates by more than 4 years the onset of motor symptoms, since 75% of carriers with visually abnormal DaTSCAN at baseline did not develop motor symptoms of PD during the 4-year period. A recent study based on serial DaT imaging has estimated the duration of the presymptomatic stage of PD as approximately 10 years.13
Very few studies have reported longitudinal data on DaT imaging in asymptomatic carriers of LRRK2 mutations. One study reported on 2 asymptomatic carriers (aged <55 years) of LRRK2 mutations (different from G2019S) showing a decline of 14% in striatal DaT binding in 2 serial PET scans separated by 4 years.14 In another study, 2 asymptomatic carriers of the R1441C mutation showed no decline on 11C-MP-PET binding in 2 studies separated by 21–36 months.15
Several cross-sectional studies have provided information regarding olfaction as a potential biomarker in LRRK2 G2019S-associated PD. On the one hand, hyposmia has been shown to be approximately 30% less frequent in LRRK2-PD than in idiopathic PD.3,16 On the other hand, asymptomatic carriers of the LRRK2 G2019S mutation are not more hyposmic than their relative noncarriers and healthy controls.3,17 Our data, coming from a longitudinal study, also suggest that olfaction loss is not common at the earliest stages of LRRK2 G2019S-associated PD and therefore is not a good predictor of phenoconversion to PD at the premotor stage. Indeed, 2 out of the 3 PD converters from our study were not hyposmic by the time they developed PD motor symptoms. Interestingly, however, 2 of the PD converters showed a significant decline in their UPSIT score between the baseline and the 4-year evaluation. Interpreted in the context of the established notion that around 50% of LRRK2-PD patients from different cohorts are hyposmic, this suggests that hyposmia in such cases most likely develops shortly before motor symptoms appear or soon after this time.
We had previously shown that an increased area of SN echogenicity was a constant feature (present in 90% of cases) in LRRK2 G2019S asymptomatic carriers.3 As anticipated, this feature was not predictive of phenoconversion to PD within the 4-year period in this study. We also did not find a correlation between the maximum area of SN echogenicity and either the baseline striatal DaT uptake or the slope of its decline over time. This is in accordance with data coming from different studies that indicate that SN echogenicity is not a marker of disease progression but rather a stable feature probably indicating vulnerability of the nigrostriatal system.18
Age is the only variable known to correlate with disease penetrance in carriers of the G2019S mutation of the LRRK2 gene. Penetrance increases with age until 25%–75% at 80 years according to different studies.2,19,20 As expected, PD converters in our study were older (an average of 10 years) than nonconverters, with statistical nonsignificance probably just because of the small number of cases. One of the patients was younger than 60 years when he developed PD motor symptoms.
Our study has several limitations. The main limitation is that the sample is not large and as a consequence only 3 carriers converted to PD despite the long follow-up. Also, we cannot completely exclude that dopaminergic drugs had influenced the results of 123I-ioflupane SPECT in PD converters, since they were already on PD drugs when the second scan was performed. We acknowledge that some studies have suggested that l-dopa might downregulate striatal DaT binding; however, we consider it unlikely that this biased our results, given that 2 out of the 3 PD converters were not taking l-dopa (on low doses of dopamine agonists) and the 3 of them showed similar slopes of decline, also comparable to most of the nonconverters. Also, as acknowledged previously, estimation of the rate of decline of DaT binding over time would have been better interpreted in relation to age-expected normal values, which we did not have. We also acknowledge that a third point of evaluation and imaging acquisition will be necessary to demonstrate whether the rate of decline of DaT binding along time is constant, as we suspect based on our data, or not. For this purpose, we plan to do a further evaluation of this cohort in a 3-year period. This planned follow-up will be also necessary to confirm whether the proposed cutoff value of <1 on the ratio of mean bilateral striatal DaT binding can be used as a reliable predictor of early conversion to PD in this cohort.
The results of this study might be of some help for different aspects related to the design of disease-modifying drug trials and also for genetic counseling. It is widely accepted that the earliest stages of the disease would be the ideal scenario for testing these drugs, before much of the damage is already done and therefore with higher chances of success. Asymptomatic carriers of the LRRK2 G2019S mutation are considered an ideal population for this purpose since patients could be recruited in an early premotor stage of the disease. There are some issues, however; although LRRK2 G2019S carriers are at high risk of developing PD, nearly 50% escape the disease. We therefore need tools to reliably predict which carriers will develop motor symptoms during life and which among them are closest in time to PD conversion. Although findings from this study, due to small sample size, cannot be conclusive and might be nonreproducible, we have shown that in addition to age the ratio of striatum DaT binding on 123I-ioflupane SPECT could be a useful tool for that purpose. According to this notion, asymptomatic carriers with striatal DaT uptake below an established cutoff value might be the best candidates for participating in these clinical trials. Also, if confirmed in further follow-up evaluations of this cohort and others, this information would be of great importance for counseling carriers about their risk of developing PD more accurately. The imaging data from this article highlight the need to further explore DaT imaging with 123I‐ioflupane as a screening tool for those at risk of PD and as a biomarker for monitoring disease progression.
ACKNOWLEDGMENT
The authors thank Coro Sánchez-Quintana for assistance in the genetic analysis of patients, at-risk relatives, and controls; the patients and the families who participated in this study; and HUMV-IDIVAL Biobank for its help in the technical execution of this work.
GLOSSARY
- DaT
dopamine transporter
- PD
Parkinson disease
- ROI
region of interest
- SN
substantia nigra
- UPDRS-III
Unified Parkinson's Disease Rating Scale, part III
- UPSIT
University of Pennsylvania Smell Identification Test
AUTHOR CONTRIBUTIONS
Dr. Sierra: drafting/revising the manuscript, study concept or design, analysis and interpretation of data, acquisition of data. Dr. Sánchez-Juan: revising the manuscript, analysis and interpretation of data, statistical analysis. Dr. Martínez-Rodríguez: revising the manuscript, analysis and interpretation of data, acquisition of data. Dr. González-Aramburu: revising the manuscript, acquisition of data. Dr. Jiménez-Alonso: revising the manuscript, analysis and interpretation of data, acquisition of data, Dr. Sánchez-Rodríguez: revising the manuscript, acquisition of data. Dr. Berciano: revising the manuscript, analysis and interpretation of data Dr. Banzo: drafting/revising the manuscript, analysis and interpretation of data, acquisition of data. Dr. Infante: drafting/revising the manuscript, study concept or design, analysis and interpretation of data, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
Fondo de Investigación Sanitaria-ISCIII (PI1100228) to J.I.
DISCLOSURE
M. Sierra and I. Martínez-Rodríguez report no disclosures relevant to the manuscript. P. Sánchez-Juan was supported by a grant from FIS (PI12/02288), JPND project DEMTEST (PI11/03028), and CIBERNED. I. González-Aramburu, M. Jiménez-Alonso, A. Sánchez-Rodríguez, J. Berciano, and I. Banzo report no disclosures relevant to the manuscript. J. Infante receives research support from the Fondo de Investigación Sanitaria-ISCIII (PI11/00228) and from Centro de Investigación Biomédica en Red de Enfermedades Neurodegenerativas (CIBERNED), has received funding for travel from Abbvie and Zambon, and has received speaker honoraria from Abbvie. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Healy DG, Falchi M, O'Sullivan SS, et al. ; International LRRK2 Consortium. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol 2008;7:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sierra M, González-Aramburu I, Sánchez-Juan P, et al. High frequency and reduced penetrance of LRRK2 G2019S mutation among Parkinson's disease patients in Cantabria (Spain). Mov Disord 2011;26:2343–2346. [DOI] [PubMed] [Google Scholar]
- 3.Sierra M, Sánchez-Juan P, Martínez-Rodríguez MI, et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 2013;80:621–626. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Bhatia KP, Burn DJ, et al. ; Movement Disorders Society Scientific Issues Committee. Movement Disorder Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003;18:467–486. [DOI] [PubMed] [Google Scholar]
- 5.Doty RL. The Smell Identification Test Administration Manual, 3rd ed. Haddon Heights: Sensonics; 1995. [Google Scholar]
- 6.Isaias IU, Benti R, Goldwurm S, et al. Striatal dopamine transporter binding in Parkinson's disease associated with the LRRK2 Gly2019Ser mutation. Mov Disord 2006;21:1144–1147. [DOI] [PubMed] [Google Scholar]
- 7.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114:2283–2301. [DOI] [PubMed] [Google Scholar]
- 8.Pirker W, Holler I, Gerschlager W, Asenbaum S, Zettinig G, Brücke T. Measuring the rate of progression of Parkinson's disease over a 5-year period with beta-CIT SPECT. Mov Disord 2003;18:1266–1272. [DOI] [PubMed] [Google Scholar]
- 9.Hilker R, Schweitzer K, Coburger S, et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol 2005;62:378–382. [DOI] [PubMed] [Google Scholar]
- 10.Marek K, Innis R, van Dyck C, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology 2001;57:2089–2094. [DOI] [PubMed] [Google Scholar]
- 11.Winogrodzka A, Bergmans P, Booij J, van Royen EA, Stoof JC, Wolters EC. [(123)I]beta-CIT SPECT is a useful method for monitoring dopaminergic degeneration in early stage Parkinson's disease. J Neurol Neurosurg Psychiatry 2003;74:294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurmi E, Ruottinen HM, Bergman J, et al. Rate of progression in Parkinson's disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord 2001;16:608–615. [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente-Fernández R, Schulzer M, Kuramoto L, et al. Age-specific progression of nigrostriatal dysfunction in Parkinson's disease. Ann Neurol 2011;69:803–810. [DOI] [PubMed] [Google Scholar]
- 14.Adams JR, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain 2005;128:2777–2785. [DOI] [PubMed] [Google Scholar]
- 15.Nandhagopal R, Mak E, Schulzer M, et al. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology 2008;71:1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaig C, Vilas D, Infante J, et al. Nonmotor symptoms in LRRK2 G2019S associated Parkinson's disease. PLoS One 2014;9:e108982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirelman A, Alcalay RN, Saunders-Pullman R, et al. ; LRRK2 AJ consortium. Nonmotor symptoms in healthy Ashkenazi Jewish carriers of the G2019S mutation in the LRRK2 gene. Mov Disord 2015;30:981–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnke S, Runkel A, Kassar HA, et al. Long-term course of substantia nigra hyperechogenicity in Parkinson's disease. Mov Disord 2013;28:455–459. [DOI] [PubMed] [Google Scholar]
- 19.Marder K, Wang Y, Alcalay RN, et al. ; LRRK2 Ashkenazi Jewish Consortium. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology 2015;85:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latourelle JC, Sun M, Lew MF, et al. The Gly2019Ser mutation in LRRK2 is not fully penetrant in familial Parkinson's disease: the GenePD study. BMC Med 2008;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]