Abstract
Objective:
To determine the prevalence, risk factors, and functional impairment associated with peripheral neuropathy in a prospective cohort of adults in rural Uganda.
Methods:
Eight hundred participants (400 HIV− and 400 antiretroviral-naive HIV+) in the Rakai Community Cohort Study underwent detailed neurologic evaluations including assessment of neuropathy symptoms, functional measures (Patient Assessment of Own Functioning Inventory and Karnofsky Performance Status scores), and neurologic evaluation by a trained medical officer. Neuropathy was defined as ≥1 subjective symptom and ≥1 sign of neuropathy on examination. Neuropathy risk factors were assessed using log binomial regression.
Results:
Fifty-three percent of participants were men, with a mean (SD) age of 35 (8) years. Neuropathy was present in 13% of the cohort and was more common in HIV+ vs HIV− participants (19% vs 7%, p < 0.001). Older age (relative risk [RR] 1.04, 95% confidence interval [CI] 1.02–1.06), female sex (RR 1.49, 95% CI 1.04–2.15), HIV infection (RR 2.82, 95% CI 1.86–4.28), tobacco use (RR 1.59, 95% CI 1.02–2.48), and prior neurotoxic medication use (RR 2.08, 95% CI 1.07–4.05) were significant predictors of neuropathy in the overall cohort. Only older age was associated with neuropathy risk in the HIV+ (RR 1.03, 95% CI 1.01–1.05) and HIV− (RR 1.06, 95% CI 1.02–1.10) cohorts. Neuropathy was associated with impaired functional status on multiple measures across all participant groups.
Conclusions:
Peripheral neuropathy is relatively common and associated with impaired functional status among adults in rural Uganda. Older age, female sex, and HIV infection significantly increase the risk of neuropathy. Neuropathy may be an underrecognized but important condition in rural Uganda and warrants further study.
Neurologic complications of HIV infection are common, and peripheral neuropathy is the most common neurologic disorder among HIV+ adults.1,2 Large systematic studies of neuropathy from resource-constrained settings are lacking, and available studies report widely varying neuropathy rates (e.g., 4% in South Africa, 59% in Rwanda).3,4 Importantly, neuropathy rates and severity in resource-constrained settings may differ from those seen in Western contexts because of different antiretroviral (ARV) initiation guidelines, a limited range of available ARV drugs, differences in diet and nutrition, unique environmental exposures, use of other neurotoxic medications such as isoniazid, and differing rates of comorbidities. A better understanding of neuropathy prevalence and risk factors in sub-Saharan African populations are crucial as this is where the majority of the world's HIV+ individuals reside. The WHO estimates that almost 26 million people in sub-Saharan Africa are living with HIV, accounting for 70% of the world's 37 million HIV+ persons.5 Improved knowledge of neuropathy risk factors in this population is imperative to reduce the burden of disease and develop better future treatments for neuropathy.
The goal of this study was to determine the rate of and risk factors for neuropathy among both HIV+ and demographically matched HIV− adults in rural Uganda, a country with a national HIV prevalence of 7.1% and approximately 1.5 million HIV+ persons in 2015.6 This represents the largest study to date of ARV-naive HIV+ adults in an outpatient setting in rural sub-Saharan Africa.
METHODS
Study participants.
Study participants were drawn from the Rakai Community Cohort Study, an open, community-based cohort of adults residing in 50 communities in Rakai District, which is representative of rural Uganda. Eligible participants were ≥20 years old and in 1 of 3 patient groups: (1) HIV+ ARV-naive adults with advanced immunosuppression (CD4 ≤200 cells/μL); (2) HIV+ ARV-naive adults with moderate immunosuppression (CD4 350–500 cells/μL); and (3) HIV− adults who were age-, sex-, and community-matched to the HIV+ participants. (This cohort was also designed to study the effect of HIV subtype on the development of HIV-associated neurocognitive disorders [HAND]. The CD4 strata were used to select participants for assessment of HAND and were not selected specifically for this substudy of neuropathy.) Exclusion criteria included severe systemic illness, inability to provide informed consent, physical disability resulting in an inability to travel to the main Rakai Health Sciences Program clinic, and plans to leave Rakai District within 2 years of enrollment. Leprosy was not a specific exclusion criterion, but no patients with obvious skin lesions of leprosy were included in the study. In addition, while leprosy still occurs in Uganda, it primarily occurs in northern parts of the country and is uncommon in Rakai District, which is located in the south-central region.7
Study procedures.
Participants were enrolled in this observational cross-sectional cohort study between August 2013 and July 2015. Each consenting participant completed a sociodemographic and behavioral interview, depression screen (Center for Epidemiologic Studies–Depression Scale [CES-D]8), functional status assessments (Patient Assessment of Own Functioning Inventory [PAOFI]9 and Karnofsky Performance Status10), and the Timed Gait Test.11 The Modified Total Neuropathy Scale was also administered.12,13 A Ugandan medical officer (i.e., a physician who has completed an internship but no residency training) who was trained specifically for the evaluation of peripheral neuropathy by 2 US neurologists (D.S., N.S.) performed a neuromedical evaluation including assessment of subjective neuropathy symptoms and examination of strength, ankle reflexes, vibratory sensation, and pinprick sensation. Participants also underwent a peripheral blood draw for confirmation of HIV status and, in HIV+ participants, for determination of CD4 cell count. Fasting glucose, comprehensive metabolic panels, and fasting lipids were also obtained for each participant.
Standard protocol approvals, registrations, and patient consents.
Written informed consent for participating in the study was obtained from all study participants. This study was approved by the Johns Hopkins Institutional Review Board, the Uganda Virus Research Institute Research and Ethics Committee, and the Uganda National Council for Science and Technology.
Statistical analysis.
Peripheral neuropathy was defined as ≥1 subjective neuropathy symptom (numbness, paresthesias, or pain in the hands or feet) and ≥1 sign of neuropathy (distal weakness, reduced or absent ankle reflexes, abnormal distal vibratory sensation, or abnormal distal pinprick sensation) on examination. This definition was chosen to minimize false-positives as many participants reported transient tingling but had no objective signs of neuropathy on examination. In addition, because many participants did not regularly wear shoes or regularly engage in manual labor, there was concern that foot calluses would interfere with portions of the sensory examination. As such, the decision was made to require both signs and symptoms in the definition of neuropathy.
Comparative analyses between participant groups and between participants with and without neuropathy were performed using χ2 tests for categorical variables and t tests for continuous variables. Log-binomial regression was performed to determine significant predictors of neuropathy. Analyses were performed using STATA version 10.1 (StataCorp, College Station, TX).
RESULTS
Eight hundred participants were enrolled, of whom 400 were HIV−, 200 were HIV+ with advanced immunosuppression (CD4 count <250 cells/μL), and 200 were HIV+ with moderate immunosuppression (CD4 count 350–500 cells/μL) (table 1). Of all participants, 53% were male with a mean (SD) age of 35 (8) years. Nearly half (48%) of all participants reported alcohol use in the last month, with 13% reporting tobacco use and 2% reporting illicit drug use, which consisted primarily of poppy seeds and marijuana (there is very little IV drug use in the Rakai District). Compared to HIV− participants, HIV+ participants had a lower body mass index (21.8 vs 23.2, p < 0.001), and reported more tobacco (16% vs 10%, p = 0.03) and drug (3% vs 1%, p = 0.02) use. Other significant demographic differences between participant groups are detailed in table 1.
Table 1.
Characteristics by patient group

Neuropathic symptoms and signs were common in the cohort, with each occurring in approximately one-third of patients (table 2). However, both were more common among HIV+ than HIV− participants. Overall, 31% of participants reported neuropathic pain symptoms (HIV+ 40%; HIV− 22%; p < 0.001) and 33% of participants had objective signs of neuropathy on examination (HIV+ 40%; HIV− 26%; p < 0.001). Because the definition of peripheral neuropathy required the presence of both symptoms and signs, 13% of participants overall met this criterion, which was significantly more common among HIV+ (19%) than HIV− participants (7%, p < 0.001). HIV+ participants were more likely to have moderate or severe neuropathy than their HIV− counterparts as assessed by the Modified Total Neuropathy Score.
Table 2.
Neuropathy characteristics by patient group, n (%)

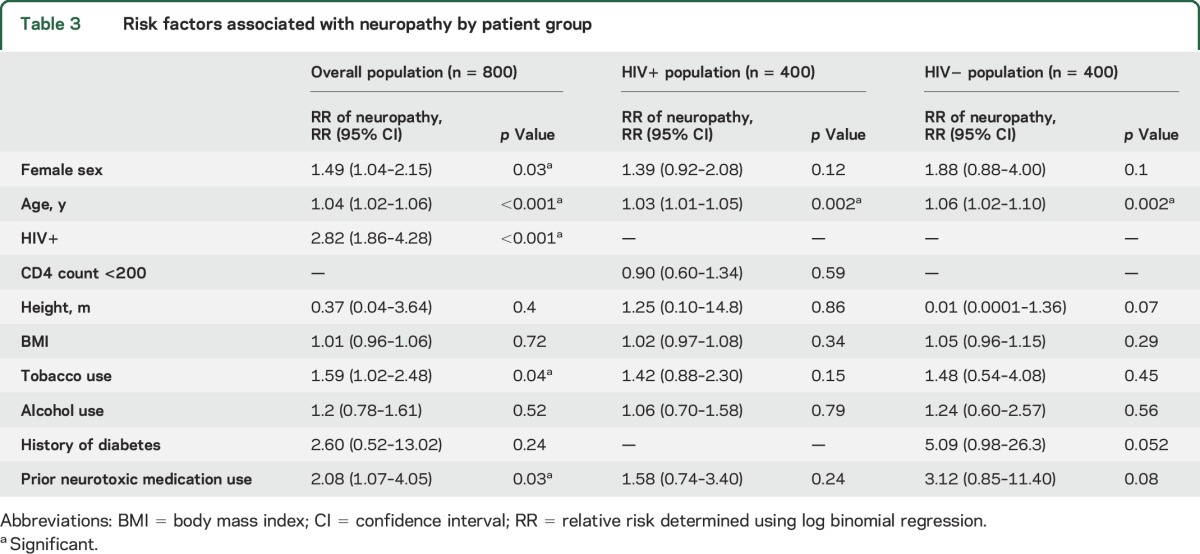
Risk factors for neuropathy are shown in table 3. In the overall cohort, older age, female sex, HIV infection, tobacco use, and prior neurotoxic medication use were significantly associated with neuropathy, with HIV infection conferring the strongest risk (relative risk [RR] 2.82; 95% confidence interval [CI] 1.86–4.28, p < 0.001). Older age was the only significant predictor among HIV+ (RR 1.03, 95% CI 1.01–1.05, p = 0.002) and HIV− (RR 1.06, 95% CI 1.02–1.10, p = 0.002) participants. In addition, advanced immunosuppression was not a significant predictor in the HIV cohort (RR 0.90, 95% CI 0.60–1.34, p = 0.59). A history of diabetes was associated with higher risk of neuropathy among HIV− participants, but this did not reach significance (RR 5.09, 95% CI 0.98–26.3, p = 0.052), although this may have been due to the small number of participants with diabetes. Fasting glucose, liver function tests, creatinine, blood urea nitrogen level, and various other measures of alcohol consumption (e.g., self-reported days of alcohol use per month, number of alcoholic drinks per day, alcohol abuse) did not vary by neuropathy status in any participant group (data not shown).
Table 3.
Risk factors associated with neuropathy by patient group
Participants with peripheral neuropathy showed significant functional impairment by multiple measures when compared to participants without neuropathy (table 4). In the overall cohort, participants with neuropathy were more likely to report fatigue (32% vs 16%, p = 0.04), had slower timed gait (12.8 seconds vs 12.2 seconds, p = 0.04), scored lower on the PAOFI (143 vs 148, p = 0.001), had lower Karnofsky Performance Status scores (89 vs 93, p < 0.001), and had higher depression scores on the CES-D (11.8 vs 7.3, p < 0.001). All of these differences were also significant within the HIV− cohort except for the PAOFI scores. Among HIV+ participants, those with neuropathy had lower PAOFI and Karnofsky Performance Status scores and higher CES-D scores, but there was no significant difference between reported fatigue or timed gait.
Table 4.
Relationship of fatigue, motor, depression, and functional impairment to neuropathy by patient group, mean (SD)

DISCUSSION
This study of adults in rural Uganda revealed high rates of symptomatic neuropathy (19%) among HIV+ participants with an additional 20% of participants having objective signs but no symptoms of neuropathy. This rate is within the range of those found in other studies of HIV-associated peripheral neuropathy from sub-Saharan Africa, which ranged from 4% among HIV+ ARV-naive South African adults to 60% among HIV+ Rwandan outpatients on ARV therapy.3,4 However, most prior studies have found neuropathy rates of 10%–40%, including studies from Ethiopia,14 Kenya,15–17 Nigeria,18,19 Rwanda,20 Uganda,21,22 and Zimbabwe.21,22 Much of the variability likely results from the diversity of patient populations studied as most cohorts included both ARV-naive HIV+ participants and those who had initiated ARV therapy. Study settings varied from exclusively outpatient to inpatient settings, and some included participants from both settings. In addition, the methods used to diagnose neuropathy varied widely from assessment by a neurologist to diagnoses based on peripheral neuropathy screening tools. The most commonly used screening tools were the Brief Peripheral Neuropathy Screen and Subjective Peripheral Neuropathy Screen, which have primarily been validated in the United States but have variable diagnostic utility in resource-limited settings.15,16,20,23,24
A South African study of HIV+ outpatients, which included both ARV-naive and ARV-experienced participants, identified symptomatic neuropathy in 30% of participants with 50% of participants having objective signs of neuropathy on examination.25 These findings are similar to this study, in which 19% of participants had symptomatic neuropathy and 40% had objective signs of neuropathy. The slightly higher rates observed in the South African participants may be attributable to neurotoxic effects of ARV therapy as many first-line ARV medications in sub-Saharan Africa, such as stavudine, confer additional risk of developing neuropathy. Similar differences were found in Kenyan cohorts, in which neuropathy rates rose from 11% in ARV-naive to 36% among HIV+ ARV-experienced outpatients.16,17 These findings suggest that neuropathy rates among HIV+ adults in rural Uganda who have initiated ARV therapy may be even higher than those reported in this study.
Of note, 7% of HIV− participants in our cohort met criteria for neuropathy, with an additional 20% of HIV− participants having objective signs of neuropathy on examination without concurrent symptoms. These rates were much higher than expected given that this cohort included relatively young and healthy adults with low rates of diabetes, renal disease, and liver disease. A prior study of HIV+ participants matched to HIV− controls in Nigeria found no cases of neuropathy among the 100 controls they studied.19 Epidemiologic data of overall peripheral neuropathy rates not linked to one particular etiology are sparse. However, a population-based study in Bombay in which participants who screened positive for neuropathy symptoms on a door-to-door survey were evaluated by a neurologist found an overall neuropathy prevalence of 2.4%, which is much lower than that observed in Rakai.26 Furthermore, many known neuropathy risk factors, including alcohol use, height, and prior neurotoxic medication use, were not associated with neuropathy risk in this study, suggesting that other unstudied risk factors may account for high rates of neuropathy observed in this cohort. Nutritional deficiencies, environmental exposures, and other infectious etiologies may at least partially account for the high neuropathy rate observed in this study but were not assessed in this cohort.
Among HIV+ participants in Rakai, older age was associated with neuropathy. Older age was significantly associated with increased neuropathy risk in other HIV+ cohorts in Uganda, Zimbabwe, Kenya, and Ethiopia.14,16,17,19,21,22 Female sex was also associated with higher risk of neuropathy in all subgroups, although this association was only significant among the overall cohorts. Overall, the effect of sex on neuropathy is controversial. Studies from Kenya, Uganda, and Zimbabwe report an increased neuropathy risk among female participants, but other cohorts from Uganda, Zimbabwe, and Nigeria have found no association.14,21,22 One study of 150 HIV+ Kenyan outpatients initiating ARV therapy found that women were at 10-fold increased risk of developing neuropathy after ARV initiation than their male counterparts, an effect that was partially attenuated by the increased neuropathy risk associated with low hemoglobin levels.17 A study of both HIV+ and HIV− adult women in Rwanda found that approximately half of women in both groups reported neuropathy symptoms.27 These rates were so much higher than expected that the authors posit the rates must be due to overreporting among women, although no objective measures of neuropathy were evaluated to validate this claim. In our study, the diagnosis required both subjective symptoms and objective signs of neuropathy. However, there was no difference in rates of objective signs of neuropathy by sex, which suggests that women may be more likely to report neuropathy symptoms than men. The absence of a sex difference in objective signs of neuropathy observed in this cohort suggests underreporting of symptoms by men rather than overreporting by women.
One unexpected finding in this study was that there was no relationship between CD4 count and neuropathy. In the United States, markers of HIV disease severity, such as low CD4 count and high viral load, have consistently been linked to neuropathy risk.28,29 Studies from sub-Saharan Africa have found no association between CD4 count or duration of HIV infection with peripheral neuropathy.4,19,22,25 Taken together, these findings suggest that factors other than those related to HIV disease severity may be contributing to neuropathy rates in sub-Saharan Africa.
Peripheral neuropathy among both HIV+ and HIV− participants in Rakai was significantly linked to poorer functional status, as has been previously observed in the United States and in other regions of sub-Saharan Africa. For example, in the CNS HIV Antiretroviral Therapy Effects Research Study (CHARTER), neuropathy was associated with reduced quality of life, unemployment, and disability in activities of daily living.29 Another cohort of nearly 1,000 HIV+ adults in the United States found that neuropathy was associated with worsened health status, decreased work productivity, and increased health care utilization.30 While a study from Nigeria found no association between neuropathy and overall mortality in HIV+ adults, a Rwandan study found that neuropathy was associated with reduced quality of life in both physical and psychological domains.19,20
This study has several limitations. First, the diagnosis of peripheral neuropathy was based entirely on clinical findings. Given the resource limitations in rural Uganda, we were unable to obtain skin biopsies, nerve conduction studies, quantitative sensory testing, or other electrophysiologic measures of neuropathy to correlate with the clinical data obtained. In addition, the neurologic evaluation was completed by non-neurologist physicians. However, these physicians received in-person training on assessing neuropathy signs from US-trained neurologists (D.S., N.S.) before the study began and at regular intervals throughout the study. Concomitant diagnoses of conditions predisposing to neuropathy, such as diabetes and thyroid dysfunction, were based only on self-report and are likely underdiagnosed in this rural setting. In addition, our study did not include measures of nutritional status, such as dietary surveys, household income information, or laboratory markers such as mean corpuscular volume. As a result, nutritional deficiencies as risk factors for neuropathy, which may be important contributors in this population, were not fully addressed. Finally, given local resource constraints, a thorough laboratory investigation to assess for causes of peripheral neuropathy was not possible.
This study is important for several reasons. First, it represents one of the largest systematic assessments of peripheral neuropathy in rural sub-Saharan Africa. Inclusion of age-, sex-, and community-matched HIV− participants also offered the opportunity to compare predisposing factors for neuropathy while adequately controlling for the effect of HIV. Furthermore, investigating the prevalence of neuropathy in an ambulatory population is more likely to accurately represent the true population prevalence because neuropathies associated with acute illnesses, which may be common in hospitalized patients, are not included. Finally, the frequency of neuropathy in the HIV+ individuals is not confounded by the use of neurotoxic ARV therapy as all HIV+ individuals were ARV-naive at the time of evaluation.
Given the high rates of neuropathy seen in our study and others from sub-Saharan Africa as well as the significant association of neuropathy with lost productivity, worsened health status, and reduced quality of life, HIV−associated neuropathy is likely a major cause of morbidity in sub-Saharan Africa and likely contributes substantially to lost productivity and health care utilization in this region. Further investigation is needed to determine unique risk factors for neuropathy in both HIV+ and HIV− adults in this region, and the effect of ARV therapy on neuropathy for the HIV+ individuals. In addition, research is urgently needed to improve the treatment of neuropathy in order to reduce the negative effects of neuropathy at an individual, family, community, and societal level in sub-Saharan Africa.
GLOSSARY
- ARV
antiretroviral
- CES-D
Center for Epidemiologic Studies–Depression Scale
- CI
confidence interval
- HAND
HIV-associated neurocognitive disorders
- PAOFI
Patient Assessment of Own Functioning Inventory
- RR
relative risk
AUTHOR CONTRIBUTIONS
Dr. Saylor was involved of the design of the study, analysis and interpretation of data, and drafting and revision of the manuscript. Dr. Nakigozi was involved in the conceptualization of the study and revising of the manuscript. Dr. Nakasujja was involved in the conceptualization of the study and revising of the manuscript. Dr. Robertson was involved in the design and conceptualization of the study and revising of the manuscript. Dr. Gray was involved in the design and conceptualization of the study, the interpretation of the data, and revising of the manuscript. Dr. Wawer was involved in the design and conceptualization of the study, the interpretation of the data, and revising of the manuscript. Dr. Sacktor was involved in the design and conceptualization of the study, the interpretation of the data, and revising of the manuscript.
STUDY FUNDING
This study was sponsored by the NIH (MH099733, MH075673, MH080661-08, L30NS088658, NS065729-05S2) with additional funding from the Johns Hopkins Center for Global Health.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ghosh S, Chandran A, Jansen JP. Epidemiology of HIV-related neuropathy: a systematic literature review. AIDS Res Hum Retroviruses 2012;28:36–48. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia NS, Chow FC. Neurologic complications in treated HIV-1 infection. Curr Neurol Neurosci Rep 2016;16:62. [DOI] [PubMed] [Google Scholar]
- 3.Evans D, Takuva S, Rassool M, Firnhaber C, Maskew M. Prevalence of peripheral neuropathy in antiretroviral therapy naive HIV-positive patients and the impact on treatment outcomes: a retrospective study from a large urban cohort in Johannesburg, South Africa. J Neurovirol 2012;18:162–171. [DOI] [PubMed] [Google Scholar]
- 4.Tumusiime DK, Venter F, Musenge E, Stewart A. Prevalence of peripheral neuropathy and its associated demographic and health status characteristics, among people on antiretroviral therapy in Rwanda. BMC Public Health 2014;14:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Number of People (All Ages) Living With HIV [online]. Available at: who.int/gho/hiv/epidemic_status/cases_all_text/en/. Accessed July 12, 2016. [Google Scholar]
- 6.UNAIDS. Uganda HIV and AIDS Estimates 2015 [online]. Available at: unaids.org/en/regionscountries/countries/uganda. Accessed July 12, 2016. [Google Scholar]
- 7.Uganda Ministry of Health. TB/Leprosy Control Program [online]. Available at: health.go.ug/programs/tb-leprosy-control-program. Accessed March 28, 2016. [Google Scholar]
- 8.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–214. [DOI] [PubMed] [Google Scholar]
- 9.Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and personality correlates of patients' complaints of disability. In: Tarter RE, Goldstein G, editors. Advances in Clinical Neuropsychology. New York: Plenum Press; 1986:95–126. [Google Scholar]
- 10.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macelod CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949:191–205. [Google Scholar]
- 11.Price RW, Sidtis JJ. Evaluation of the AIDS dementia complex in clinical trials. J Acquir Immune Defic Syndr 1993;3:551–560. [PubMed] [Google Scholar]
- 12.Vasquez S, Guidon M, McHugh E, Lennon O, Grogan L, Breathnach OS. Chemotherapy induced peripheral neuropathy: the modified total neuropathy score in clinical practice. Ir J Med Sci 2013;183:53–58. [DOI] [PubMed] [Google Scholar]
- 13.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp KS. The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol 2006;4:W9–W16. [Google Scholar]
- 14.Shurie JS, Deribew A. Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals. Ethiop Med J 2010;48:85–93. [PubMed] [Google Scholar]
- 15.Cettomai D, Kwasa JK, Birbeck GL, et al. Screening for HIV-associated peripheral neuropathy in resource-limited settings. Muscle Nerve 2013;48:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta SA, Ahmed A, Kariuki BW, et al. Implementation of a validated peripheral neuropathy screening tool in patients receiving antiretroviral therapy in Mombasa, Kenya. Am J Trop Med Hyg 2010;83:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta SA, Ahmed A, Laverty M, Holzman RS, Valentine F, Sivapalasingam S. Sex differences in the incidence of peripheral neuropathy among Kenyans initiating antiretroviral therapy. Clin Infect Dis 2011;53:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obiako OR, Ogoina D, Abubakar SA, et al. The frequency and outcome of neuropathies among HIV/AIDS adults treated at a tertiary hospital in Kaduna State, Nigeria. Niger Postgrad Med J 2014;21:319–326. [PubMed] [Google Scholar]
- 19.Ekenze OS, Nwosu CM, Ogunniyi A. Frequency and risk factors for distal sensory polyneuropathy in HIV infection in a developing country. Int J STD AIDS 2014;25:178–183. [DOI] [PubMed] [Google Scholar]
- 20.Biraguma J, Rhoda A. Peripheral neuropathy and quality of life of adults living with HIV/AIDS in the Rulindo district of Rwanda. Sahara J 2012;9:88–94. [DOI] [PubMed] [Google Scholar]
- 21.Arenas-Pinto A, Thompson J, Musoro G, et al. Peripheral neuropathy in HIV patients in sub-Saharan Africa failing first-line therapy and the response to second-line ART in the EARNEST trial. J Neurovirol 2016;22:104–113. [DOI] [PubMed] [Google Scholar]
- 22.Kiwuwa-Muyingo S, Kikaire B, Mambule I, et al. Prevalence, incidence and predictors of peripheral neuropathy in African adults with HIV infection within the DART trial. AIDS 2014;28:2579–2588. [DOI] [PubMed] [Google Scholar]
- 23.Cettomai D, Kwasa J, Kendi C, et al. Utility of quantitative sensory testing and screening tools in identifying HIV-associated peripheral neuropathy in Western Kenya: pilot testing. PLoS One 2010;5:e14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandiah PA, Atadzhanov M, Kvalsund MP, Birbeck GL. Evaluating the diagnostic capacity of a single-question neuropathy screen (SQNS) in HIV positive Zambian adults. J Neurol Neurosurg Psychiatry 2010;81:1380–1381. [DOI] [PubMed] [Google Scholar]
- 25.Maritz J, Benatar M, Dave JA, et al. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve 2010;41:599–606. [DOI] [PubMed] [Google Scholar]
- 26.Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology 1991;41:1315–1317. [DOI] [PubMed] [Google Scholar]
- 27.Tumusiime DK, Musabeyezu E, Mutimurah E, et al. Over-reported peripheral neuropathy symptoms in a cohort of HIV infected and uninfected Rwandan women: the need for validated locally appropriate questionnaires. Afr Health Sci 2014;14:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childs EA, Lyles RH, Selnes OA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 1999;52:607–613. [DOI] [PubMed] [Google Scholar]
- 29.Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 2010;67:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.daCosta DiBonaventura M, Gupta S, Cho M, Mrus J. The association of HIV/AIDS treatment side effects with health status, work productivity, and resource use. AIDS Care 2012;24:744–755. [DOI] [PubMed] [Google Scholar]


