Abstract
Objective:
To identify patterns of noncephalic pain comorbidity in people with episodic migraine (EM; <15 headache-days per month) and chronic migraine (CM; ≥15 headache-days per month) and to examine whether the presence of noncephalic pain is an indicator for the 3-month onset or persistence of CM.
Methods:
Data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, a prospective, web-based study with cross-sectional modules embedded in a longitudinal design, were analyzed at baseline and the 3-month follow-up. Relationships between the number of noncephalic pain sites and 3-month onset of CM or persistent CM were assessed.
Results:
Of 8,908 eligible respondents, 8,139 (91.4%) had EM and 769 (8.6%) had CM at baseline. At 3 months, the incidence of CM among those with baseline EM was 3.4%. When adjusted for demographics and headache-day frequency, the odds of CM onset among those with baseline EM increased by 30% (95% confidence interval [CI] 1.21–1.40, p < 0.001) for each additional noncephalic pain site at baseline. Among those with CM at baseline, 50.1% had persistent CM at the 3-month follow-up. After adjustment for demographics, individuals with CM were 15% (95% CI 1.07–1.25, p < 0.001) more likely to have persistent CM for each additional noncephalic pain site at baseline.
Conclusions:
These results suggest that noncephalic pain may be a marker for headache chronicity that could be used to identify people with EM at risk of the onset of CM and people with CM at risk of persistent CM.
Migraine is a painful neurovascular disorder with an estimated prevalence of ≈15%.1,2 Current diagnostic criteria divide migraine into episodic (EM; <15 headache-days per month) and chronic (CM; ≥15 headache-days per month for >3 months with migraine features on ≥8 days per month) migraine.3 Migraine is comorbid with a large number of pain disorders.4–6 An increase in the number of headache days per month to ≥15 defines clinical progression, with multiple factors contributing.7 Although pain disorders are more common in CM than EM, the influence of comorbid pain on the development of CM has rarely been studied.
Chronic pain typically presents as a dynamic rather than static state8; similarly, headache frequency fluctuates over time.7 Various screening tools to predict long-term pain outcomes have been assessed, recognizing the importance of understanding this in clinical management7,9 and health care use.10 Longitudinal data from the American Migraine Prevalence and Prevention (AMPP) Study and Chronic Migraine Epidemiology and Outcomes (CaMEO) Study demonstrated substantial within-person fluctuations in headache-day frequency using 1-year and 3-month reassessment intervals, respectively.11,12 Reasons for remaining stable or transitioning between EM and CM are unclear, but underlying differences in pain signaling and processing may be involved.13,14 Random variability and regression to the mean may also play a role.11
The presence of noncephalic pain in people with migraine may reflect an underlying biological predisposition to pain chronicity. To help clarify these relationships and patterns, this analysis sought to identify potential factors associated with CM, focusing on the effect of noncephalic pain on progression of EM to CM and persistence of CM over a 3-month period.
METHODS
Study design.
The CaMEO Study is a prospective, web-based study with cross-sectional modules embedded in a longitudinal design in respondents with migraine. CaMEO Study methodology has been described previously.15 Briefly, the study had a screening and recruiting phase and longitudinal assessments approximately every 3 months over 1 year, from September 2012 to November 2013. The present analysis focused on data from the baseline and first 3-month follow-up interviews.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board of the Albert Einstein College of Medicine. Informed consent was not required because participation was voluntary and survey research does not expose participants to known health risks.
Diagnosis.
Respondents were invited to participate if they met American Migraine Study/AMPP Study diagnostic module criteria for migraine,16 which are a modification of International Classification of Headache Disorders (2nd and 3rd editions, beta version) migraine criteria.3,17 We assessed CM on the basis of the Silberstein et al.18 criteria, defined as ≥15 headache-days per month averaged over the past 3 months, with a link to migraine.
The CaMEO Study invited 489,537 people to participate, and 58,418 usable returns were obtained (figure e-1 at Neurology.org). Of these, 16,789 individuals met the inclusion criteria at baseline for EM (n = 15,313 [91.2%]) or CM (n = 1,476 [8.8%]), of whom 12,810 respondents returned completed surveys with valid data.15 Three-month–onset CM was defined as headaches meeting the Silberstein-Lipton18 CM criteria at the 3-month follow-up in those with EM at baseline. Persistent CM was defined as meeting the Silberstein-Lipton CM criteria at both interviews.
Measurements.
The Comorbidity/Endophenotype Module from the CaMEO Study was developed to assess overall physical burden beyond migraine, including common comorbid conditions and other noncephalic pain signs and symptoms. Data were collected on a broad set of indicators (with validated questionnaires when available) encompassing migraine symptoms and features and a variety of comorbidities.
Noncephalic pain data were collected with the Total Pain Index (TPI). The validated TPI assessed pain frequency and intensity in 8 specified body regions (i.e., face, neck or shoulders, back, arms or hands, legs or feet, chest, abdomen or pelvis, other) over the preceding 3 months (table e-1); only head pain was considered cephalic pain for the base analysis. The TPI scored pain frequency (0 = none of the time; 1 = slight bit of the time; 2 = some of the time; 3 = most of the time; 4 = all of the time) for each body region. The number of noncephalic locations with pain most of the time or all of the time was calculated at baseline in people with EM and CM. We also assessed the relation of this variable to progression from EM at baseline to CM at 3 months (i.e., CM onset) and its ability to predict persistent CM at 3 months among those with CM at baseline (i.e., CM persistence).
Data analysis.
Univariate models were used to examine differences in sociodemographic variables by baseline migraine status (EM vs CM). Binary outcomes were modeled with binary logistic regression and reported as odds ratios (ORs) and 95% confidence intervals (CIs). For the continuous covariates (i.e., age, monthly headache-day frequency), mean differences were compared with an independent-samples t test and reported as the mean difference and 95% CI. Annual household income was represented in 4 categories, modeled with ordered logistic regression, and reported as OR and 95% CI.
To evaluate the distribution and pattern of pain locations, TPI data were examined by cross-sectional plotting of the proportion of respondents with noncephalic pain frequency most or all of the time in each of the 8 noncephalic locations by 5-day intervals of the monthly headache days. To assess patterns of pain intensity among respondents who reported pain, the proportion who reported a pain intensity score ≥8 was plotted by monthly headache days. A Mantel-Haenszel test (also known as linear by linear association test), which is a χ2 test used to test for trend across an ordinal variable, was computed for all pain locations for both pain frequency and intensity by headache-day frequency interval. The null hypothesis was that the linear trend parameter was 0; a significant p value (<0.05) for the test is consistent with the presence of a trend.
Multivariable binary logistic regression analysis was used to assess the influence of the number of noncephalic pain locations occurring most or all of the time on CM onset and on persistent CM at 3 months. Models were adjusted for sociodemographics, headache-day frequency, baseline headache-day status (EM or CM), and the interaction of baseline headache-day status by number of pain locations. Regression models were run sequentially, first unadjusted, then adjusted for sociodemographics, and finally adjusted for sociodemographics and baseline headache-day frequency. All models included baseline headache-day status and baseline headache-day status by number of pain locations interaction. Additional exploratory regression models were generated for sensitivity analyses to consider the potential role (confounding vs mediating) of relevant covariates (i.e., generalized anxiety disorder, depression, allodynia, obesity [body mass index >30 kg/m2], headache-day frequency, and use of any acute medication at baseline) on the main effect of number of noncephalic pain locations. Post hoc sensitivity analysis excluding face and neck/shoulder pain as noncephalic pain sites was also undertaken. All analyses were conducted with SPSS Statistics, version 22.0 (IBM, Armonk, NY).
RESULTS
Sample disposition.
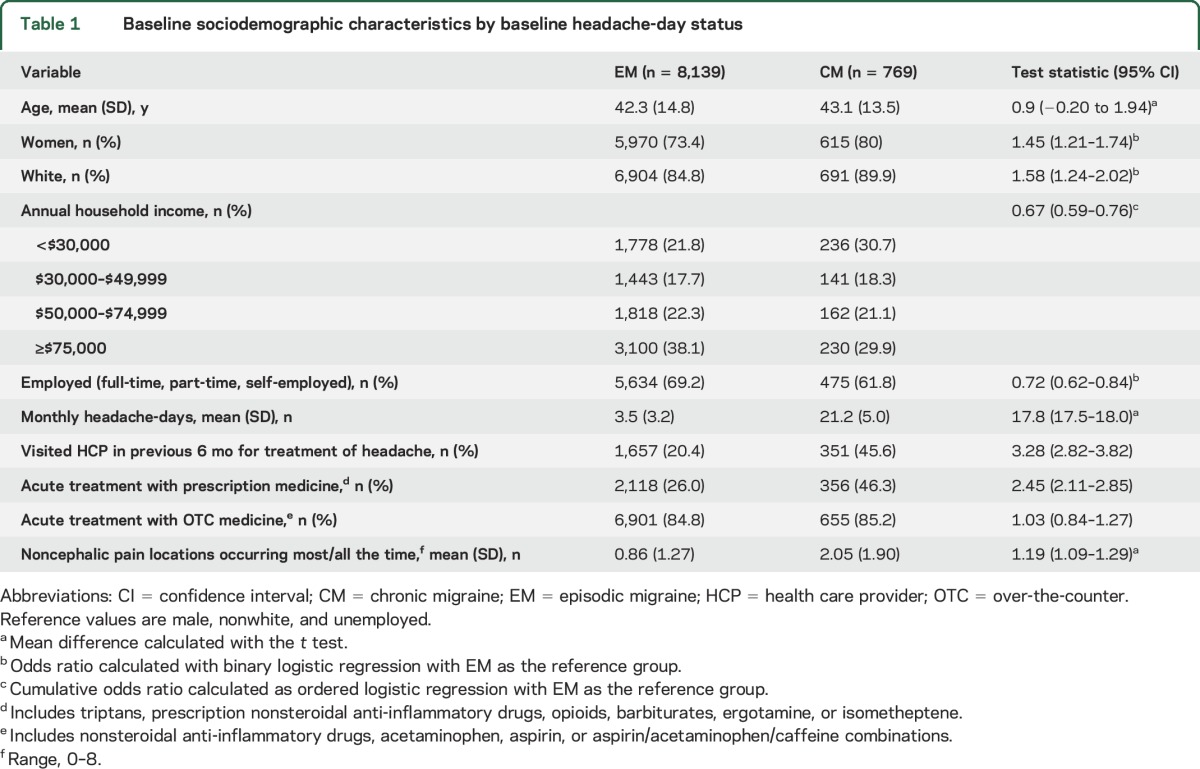
The present analysis included 8,908 CaMEO Study respondents with migraine, including 8,139 with EM (91.4%) and 769 with CM (8.6%), who completed the Comorbidities/Endophenotype Module and the 3-month follow-up assessment (figure e-1). Differences in demographic features and clinical characteristics were noted for respondents with EM or CM at baseline (table 1).
Table 1.
Baseline sociodemographic characteristics by baseline headache-day status
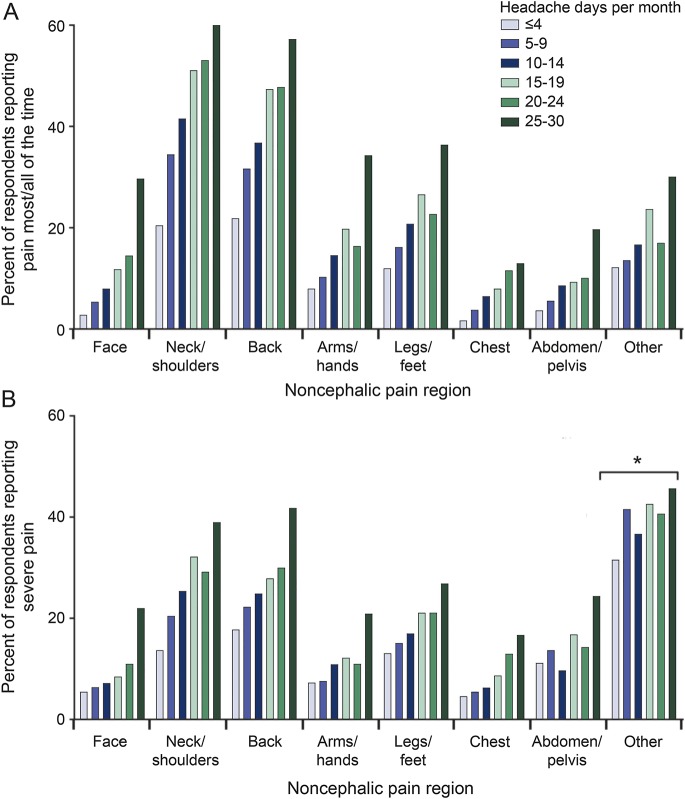
Noncephalic pain by headache-day frequency.
The relative incidence of noncephalic pain described as occurring most or all of the time increased with headache-day frequency for all pain locations (figure 1A). For example, 21.9% of respondents with ≤4 headache-days per month reported back pain most/all of the time vs 57.3% of those with 25 to 30 headache-days per month (figure 1A). Among respondents reporting noncephalic pain, the proportion reporting severe pain (pain intensity scores ≥8) increased with the number of headache days per month (figure 1B). For example, among respondents reporting back pain, the likelihood of reporting severe back pain more than doubled as headache-day frequency increased from ≤4 to 25 to 30 headache-days per month (17.8%–41.8%; figure 1B). All trends were significant when tested with the Mantel-Haenszel test (p < 0.001).
Figure 1. Headache-day frequency by noncephalic pain region.
The frequency of headache days by (A) presence of pain most/all of the time or (B) severe pain intensity. Pain frequency at each location was assessed with the Total Pain Index. Response options included none of the time, a slight bit of time, some of the time, most of the time, and all of the time. Pain severity was assessed by the Total Pain Index. Respondents were asked to rate pain severity on a scale from 0 (no pain) to 10 (worst pain you have had), and severe pain intensity was defined as a score ≥8. *p < 0.05; p < 0.001 for all other comparisons.
Transitions in headache-day status from baseline to 3-month follow-up.
Patterns of transition were apparent at the 3-month observation period (figure 2). Among respondents with EM at baseline, 278 (3.4%) had CM onset within 3 months; in contrast, 384 respondents (49.9%) with CM at baseline remitted to EM. Respondents' sociodemographic and clinical characteristics by baseline and 3-month postbaseline headache-day status are provided in table 2.
Figure 2. Transition in headache-day status.
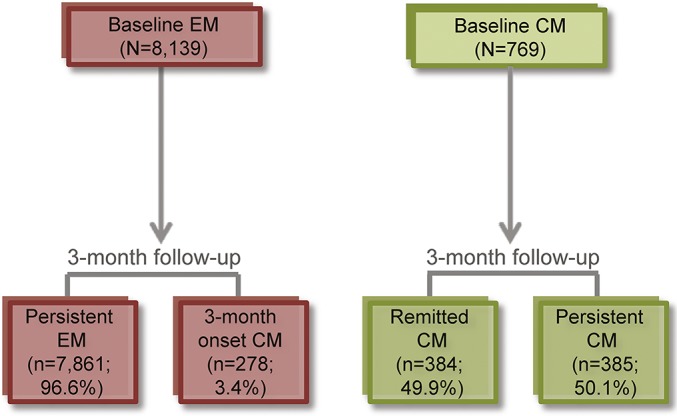
Transition in headache-day status from baseline to 3-month follow-up. CM = chronic migraine (≥15 headache-days per month); EM = episodic migraine (<15 headache-days per month).
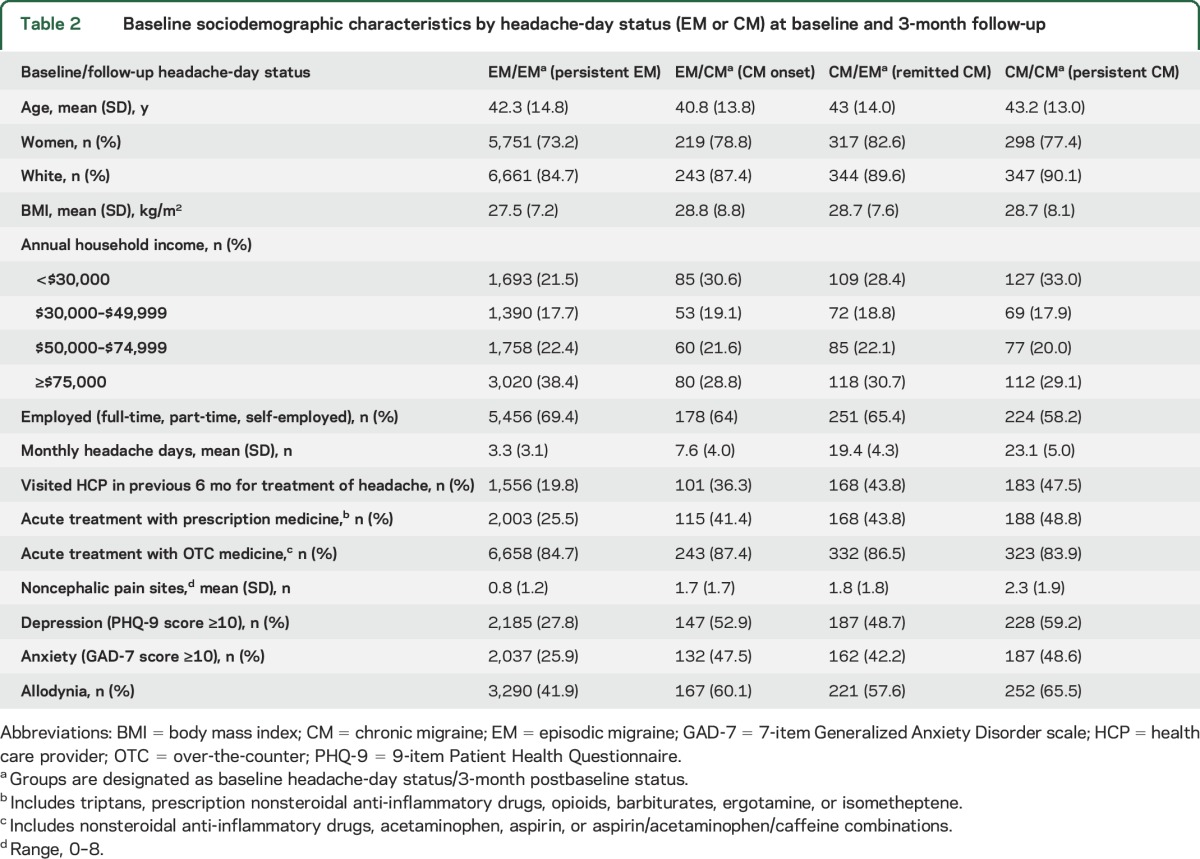
Table 2.
Baseline sociodemographic characteristics by headache-day status (EM or CM) at baseline and 3-month follow-up
Associations of noncephalic pain with CM.
Three-month onset CM.
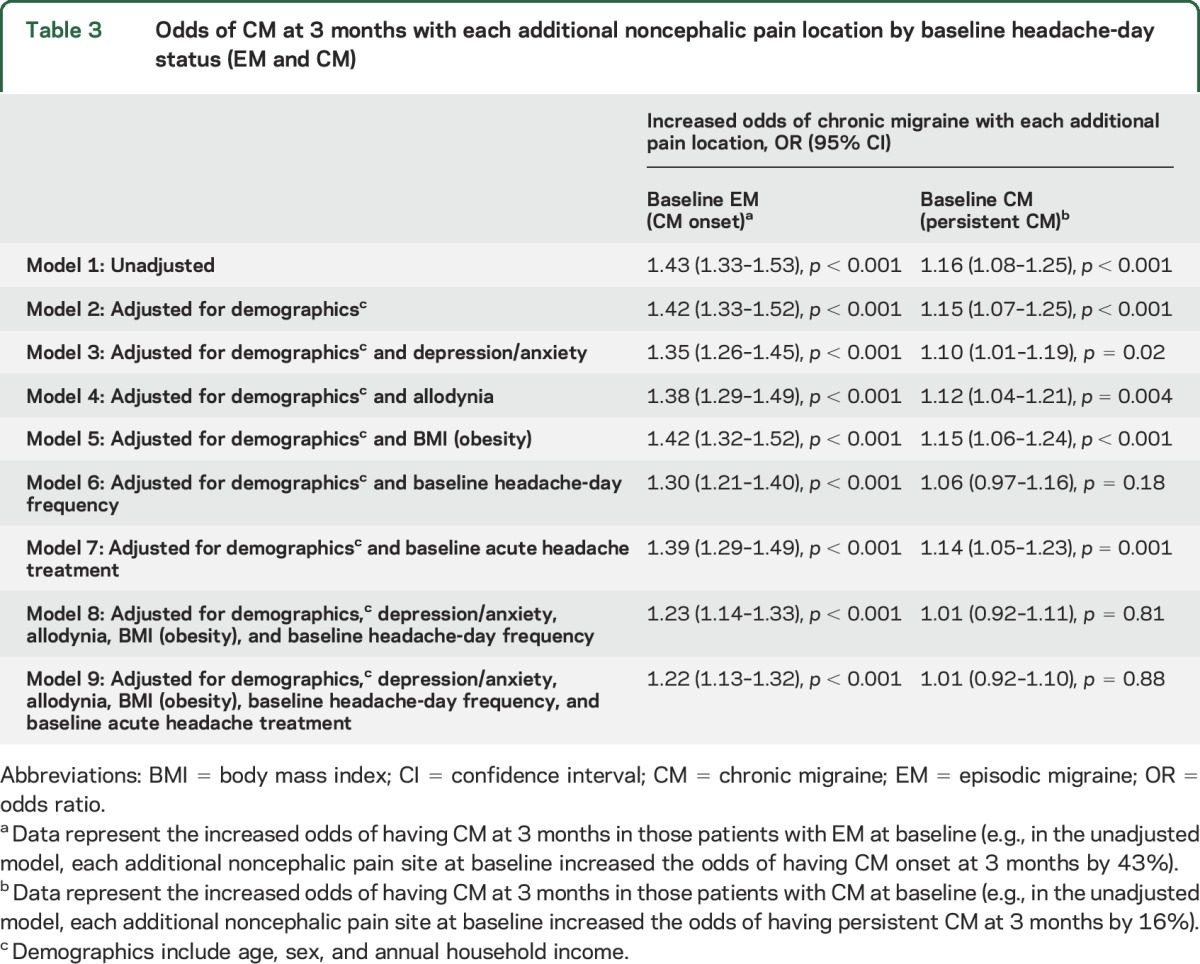
Unadjusted regression analysis demonstrated that the odds of developing CM onset among those with EM at baseline increased by 43% with each additional noncephalic pain site (table 3). After adjustment for demographic features and headache-day frequency, this effect was attenuated but remained significant (OR 1.30, 95% CI, 1.21–1.40, p < 0.001). In sensitivity analyses, the main effect of noncephalic pain locations was diminished but still significant after controlling for the presence of any acute headache medication at baseline and comorbidities (i.e., depression/anxiety, allodynia, and obesity) that may act as mediators or confounders of the relationship between noncephalic pain and CM onset. In the fully adjusted model, accounting for all potentially confounding or mediating factors, the effect of noncephalic pain locations remained significant (OR 1.22, 95% CI, 1.13–1.32, p < 0.001) for each additional noncephalic pain site.
Table 3.
Odds of CM at 3 months with each additional noncephalic pain location by baseline headache-day status (EM and CM)
Persistent CM.
Unadjusted regression analysis demonstrated a 16% increase in the odds of maintaining baseline CM status with each additional noncephalic pain location (OR 1.16, 95% CI 1.08–1.25, p < 0.001; table 3). After adjustment for demographic features, there was a 15% increase in the odds of persistent CM (i.e., people were less likely to remit to EM) for each additional noncephalic pain site (OR 1.15, 95% CI 1.07–1.25, p < 0.001). In nearly all sensitivity analyses, the odds of persistent CM were attenuated but remained significant; however, when adjusted for demographics and headache-day frequency, the odds of persistent CM with each noncephalic pain location were no longer significant (OR 1.06, 95% CI 0.97–1.16, p = 0.18), nor were the odds of persistent CM in the fully adjusted model (OR 1.01, 95% CI 0.92–1.10, p = 0.88).
Sensitivity analyses.
We performed a series of sensitivity analyses to consider whether the mechanism linking comorbid pain with long-term headache outcome may have been mediated by other known prognostic factors. After adjustment for anxiety, depression, allodynia, obesity, headache frequency, and acute medication use at baseline,7 noncephalic pain remained significantly associated with CM onset. The effect of noncephalic pain lost significance for CM persistence after adjustment for baseline headache-day frequency. A post hoc analysis was undertaken with face and neck/shoulder pain excluded from the definition of noncephalic pain because they could be manifestations of migraine; results were not materially altered.
DISCUSSION
This analysis of the CaMEO Study Comorbidity/Endophenotype Module dataset included nearly 9,000 respondents with migraine. We observed that half of the respondents with CM at baseline did not remit to EM after 3 months (i.e., had persistent CM) and that a small but clinically meaningful proportion of respondents (3.4%) with EM at baseline experienced CM onset after 3 months. Previous studies have not examined rates of CM onset over a 3-month period in people with EM. In the AMPP Study, rates of CM onset in those with existing EM ranged from 2.5% to 3.1% over the course of 1 year.19,20 While the incidence rates reported herein may seem high, the natural history of high-frequency headache appears to be volatile.12,21 As reported in AMPP, the relatively constant prevalence of CM in the population may reflect an equilibrium in which rates of CM incidence are counterbalanced by rates of CM remission.12 In the present study, CM persisted in 50.1% of people over 3 months vs 33.9% over 2 years in AMPP (i.e., CM at baseline and both of two 1-year follow-ups).12
We found that noncephalic pain was common in people with migraine, regardless of headache-day frequency. This is consistent with previous work on comorbidity of chronic pain disorders, including migraine, with other pain disorders.4–6 Noncephalic pain appears to be a particularly germane comorbidity in people with headache or migraine.22,23 Chronic pain disorders, including fibromyalgia, back pain, and neck pain, are more common in people with CM than in those with EM.22–25 Furthermore, in our study, the presence of noncephalic pain was a negative predictive factor for both those with EM (i.e., it was associated with CM onset) and those with CM (i.e., it was associated with CM persistence). To the best of our knowledge, no other study has correlated noncephalic pain with the long-term migraine outcome.
After adjustment for anxiety, depression, allodynia, obesity, headache frequency, and any acute medication use at baseline, noncephalic pain was associated with CM onset, suggesting that the effect of noncephalic pain is not accounted for by these factors. Results were somewhat less robust for the effect of noncephalic pain on CM persistence because the effect lost significance after adjustment for baseline headache-day frequency. Post hoc analysis in which the definition of noncephalic pain was modified to exclude face and neck/shoulder pain revealed similar results. We did not capture data on pain medication taken for noncephalic pain and therefore did not adjust for this factor. We note that separating the hypothetical role of acute medication use per se from the underlying indication is problematic in observational studies such as the present one, limiting the inferences that can be made regarding causality.
An exploratory-factor analysis of clusters of multimorbid health conditions found that migraine is clustered with back/neck pain, other chronic pain, and arthritis.26 In other studies, adults with headache were found to be at greater risk of some medical conditions (e.g., rheumatoid arthritis) and psychiatric disorders (e.g., depression, anxiety) than adults without headache.27–29
The association of noncephalic pain with CM vs EM and with CM onset may have a number of possible explanations, including genetic factors30,31 and brain changes occurring in response to chronic pain. For example, greater pain sensitivity is associated with variants of the catechol-O-methyltransferase (COMT) gene.31 COMT variants are also associated with increased-intensity headache pain and more frequent nausea and vomiting.30 In addition, neuroplastic changes may predispose patients to chronic pain.32 For example, compared with those who did not develop chronic pain, patients who developed chronic pain demonstrated greater white matter density in the corticolimbic system but not in pain-related brain regions, as well as smaller amygdala and hippocampal volumes.32 This finding suggests an association between pain chronicity and emotional regulation that is predetermined by underlying neural connections, which may help explain why migraine is also comorbid with psychiatric disorders, especially depression and anxiety.
Depression is a plausible factor common to migraine and other pain disorders. A bidirectional association between depression and migraine has been posited such that the presence of one increases the risk of the other.33 In the AMPP Study, depression was associated with a 2-fold increase in risk of CM onset among those with EM.34 Nevertheless, we observed that the association between noncephalic pain and long-term migraine outcome persisted after adjustment for depression and anxiety.
Strengths of this analysis from the CaMEO Study include the use of cross-sectional data from a large sample of people with migraine. To the best of our knowledge, the influence of comorbid noncephalic pain on migraine status (i.e., EM or CM) across a 3-month period has not been assessed previously. The ability to analyze data on a large pool of potential confounders provides important insight into the time course of factors influencing migraine progression.
One limitation of this study is our reliance on self-reported headache symptoms and not on medical diagnosis. Pain comorbidity is also self-reported by pain location and severity, not a specific diagnosis, and may be related to a “willingness to report” artifact in which people who report one symptom may be more willing to report other symptoms. Data were collected only at baseline and at a 3-month follow-up, not over longer periods of time. Therefore, data reflect a snapshot in time. Those with EM at baseline may have had CM before baseline and vice versa; this could not be determined from the survey results. Furthermore, in the evaluation of the magnitude of ORs for continuous and wide-ranging exposure variables (e.g., age, Migraine Disability Assessment Scores), a small magnitude of effect should not necessarily be construed as being clinically unimportant because the size of the effect is a function of the scaling of the coefficient/exposure variable. The regression coefficient represents the expected change in the outcome for a 1-unit change in the predictor, and the magnitude of that coefficient is determined by the units used. Finally, the causal effect of covariates assessed in this analysis cannot be completely elucidated. Covariates (such as depression) may act as mediators or confounders of the relationship between noncephalic pain and long-term migraine outcome, and we have observed these disorders, which may wax and wane over time, over only a relatively small time period.
Our findings demonstrate the significant comorbidity of noncephalic pain and migraine, each of which is associated with substantial societal and personal burden. Collectively, these conditions seem to be associated with migraine chronicity. There may also be implications for the chronicity of the noncephalic pain conditions. Our findings further underline the importance of assessing the presence of comorbid pain in patients with migraine. Patients with EM and comorbid pain should be monitored more closely, and treatment should be aimed at the prevention of migraine headache.
Analysis of longitudinal headache data in the CaMEO Study has demonstrated that the majority of respondents exhibited substantial within-person fluctuations in headache-day frequency across an ≈1-year period.11 The nature of the variability in self-reported headache-day frequency is complex. It is not clear why some experience an increase in headache-day frequency or CM onset whereas others remain stable or have fewer headache days per month over time, but underlying differences in pain signaling and processing may be involved.13,14 Although further study is required, it seems reasonable that the presence of noncephalic pain in those with migraine could be a marker of an underlying biological predisposition to pain chronicity.
Supplementary Material
GLOSSARY
- AMPP
American Migraine Prevalence and Prevention
- CaMEO
Chronic Migraine Epidemiology and Outcomes
- CI
confidence interval
- CM
chronic migraine
- COMT
catechol-O-methyltransferase
- EM
episodic migraine
- OR
odds ratio
- TPI
Total Pain Index
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Design or conceptualization of the study: A.I.S., D.C.B., K.M.F., A.M.A., R.B.L. Analysis or interpretation of the data: A.I.S., D.C.B., K.M.F., A.M.A., R.B.L. Drafting or revising the manuscript for intellectual content: A.I.S., D.C.B., K.M.F., A.M.K., D.A.F., A.M.A., R.B.L.
STUDY FUNDING
This study and analysis were sponsored by Allergan plc (Dublin, Ireland). The study sponsor was involved in the study design, data collection, data analysis, data interpretation, and writing of the article. Allergan also funded assistance with study design and data collection provided by Vedanta Research (Chapel Hill, NC) and medical writing assistance provided by Complete Healthcare Communications, LLC (Chadds Ford, PA).
DISCLOSURE
A. Scher has received honoraria from and is an advisory board member for Allergan plc, receives grant support from Congressionally Directed Medical Research Programs and Center for Neuroscience and Regenerative Medicine, and is on the editorial boards of Cephalalgia and Pain Medicine. D. Buse has received grant support and honoraria from Allergan, Avanir, Amgen, Dr. Reddy's Laboratories, and Eli Lilly. She is an employee of Montefiore Medical Center, which has received research support from Allergan, Alder, Avanir, CoLucid, Dr. Reddy's, Endo Pharmaceuticals, Labrys, Merck, NuPathe, Novartis, and Zogenix, via grants to the National Headache Foundation or Montefiore Medical Center. She is on the editorial board of Current Pain and Headache Reports, the Journal of Headache and Pain, Pain Medicine News, and Pain Pathways. K. Fanning is an employee of Vedanta Research, which has received research support and honoraria from Allergan, Amgen, CoLucid, Dr. Reddy's Laboratories, Endo Pharmaceuticals, GlaxoSmithKline, Merck, NuPathe, Novartis, and Ortho-McNeil, Zogenix, via grants to the National Headache Foundation. A. Kelly and D. Franznick are medical writers whose activity on this manuscript was funded by Allergan plc. A. Adams is a full-time employee of Allergan plc and owns stock in the company. R. Lipton has received grant support from the NIH, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant to, serves as an advisory board member for, or has received honoraria from Alder, Allergan, Autonomic Technologies, Boston Scientific, Bristol-Myers Squibb, Cognimed, CoLucid, Dr. Reddy's Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache 2015;55:21–34. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. [DOI] [PubMed] [Google Scholar]
- 4.Giamberardino MA, Affaitati G, Martelletti P, et al. Impact of migraine on fibromyalgia symptoms. J Headache Pain 2015;17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speciali JG, Dach F. Temporomandibular dysfunction and headache disorder. Headache 2015;55(suppl 1):72–83. [DOI] [PubMed] [Google Scholar]
- 6.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17:T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol 2016;12:455–464. [DOI] [PubMed] [Google Scholar]
- 8.Von Korff M, Miglioretti DL. A prognostic approach to defining chronic pain. Pain 2005;117:304–313. [DOI] [PubMed] [Google Scholar]
- 9.Von Korff M, Shortreed SM, Saunders KW, et al. Comparison of back pain prognostic risk stratification item sets. J Pain 2014;15:81–89. [DOI] [PubMed] [Google Scholar]
- 10.Von Korff M, Lin EH, Fenton JJ, Saunders K. Frequency and priority of pain patients' health care use. Clin J Pain 2007;23:400–408. [DOI] [PubMed] [Google Scholar]
- 11.Lipton RB, Serrano D, Manack Adams A, Buse DC. Trajectories of headache days in persons with chronic and episodic migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Presented at American Headache Society 2014 Annual Meeting; June 26–29, 2014; Los Angeles.
- 12.Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 2011;76:711–718. [DOI] [PubMed] [Google Scholar]
- 13.Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Curr Opin Neurol 2012;25:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin 2015;7:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manack Adams A, Serrano D, Buse DC, et al. The impact of chronic migraine: the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia 2015;35:563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–657. [DOI] [PubMed] [Google Scholar]
- 17.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 18.Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology 1996;47:871–875. [DOI] [PubMed] [Google Scholar]
- 19.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 2008;48:1157–1168. [DOI] [PubMed] [Google Scholar]
- 20.Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology 2015;84:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–89. [DOI] [PubMed] [Google Scholar]
- 22.Plesh O, Adams SH, Gansky SA. Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache 2012;52:946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buse DC, Manack Adams A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 2010;81:428–432. [DOI] [PubMed] [Google Scholar]
- 24.Kucuksen S, Genc E, Yilmaz H, et al. The prevalence of fibromyalgia and its relation with headache characteristics in episodic migraine. Clin Rheumatol 2013;32:983–990. [DOI] [PubMed] [Google Scholar]
- 25.Yoon MS, Manack A, Schramm S, et al. Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German Headache Consortium study. Pain 2013;154:484–492. [DOI] [PubMed] [Google Scholar]
- 26.Holden L, Scuffham PA, Hilton MF, Muspratt A, Ng SK, Whiteford HA. Patterns of multimorbidity in working Australians. Popul Health Metr 2011;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalaydjian A, Merikangas K. Physical and mental comorbidity of headache in a nationally representative sample of US adults. Psychosom Med 2008;70:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders K, Merikangas K, Low NC, Von Korff M, Kessler RC. Impact of comorbidity on headache-related disability. Neurology 2008;70:538–547. [DOI] [PubMed] [Google Scholar]
- 29.Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JW, Lee KS, Kim JS, Kim YI, Shin HE. Genetic contribution of catechol-O-methyltransferase polymorphism in patients with migraine without aura. J Clin Neurol 2007;3:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005;14:135–143. [DOI] [PubMed] [Google Scholar]
- 32.Vachon-Presseau E, Tetreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain 2016;139:1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breslau N, Davis GC, Schultz LR, Peterson EL. Joint 1994 Wolff Award presentation: migraine and major depression: a longitudinal study. Headache 1994;34:387–393. [DOI] [PubMed] [Google Scholar]
- 34.Ashina S, Serrano D, Lipton RB, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain 2012;13:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.