Abstract
Facile cleavage C-terminal to ornithine residues in gas phase peptides has been observed and termed the ornithine effect. Peptides containing internal or C-terminal ornithine residues, which are formed from deguanidination of arginine in solution, were fragmented to produce either a y-ion or water loss, respectively, and the complementary b-ion. The fragmentation patterns of several peptides containing arginine were compared to those of the ornithine analogues. Conversion of arginine to ornithine results in a decrease of the gas phase proton affinity of the residue, thereby increasing the mobility of the ionizing proton. This alteration allows the nucleophilic amine to facilitate a neighboring group reaction to induce a cleavage of the adjacent amide bond. The selective cleavage at the ornithine residue is proposed to result from the highly favorable generation of a six-membered lactam ring. The ornithine effect was compared with the well-known proline and aspartic acid effects in peptide fragmentation using angiotensin II, DRVYIHPF and the ornithine analogue, DOVYIHPF. Under conditions favorable to either the aspartic acid (i.e. singly protonated peptide) or proline effect (i.e. doubly protonated peptide), the ornithine effect was consistently observed to be the more favorable fragmentation pathway. The highly selective nature of the ornithine effect opens up the possibility for conversion of arginine to ornithine residues to induce selective cleavages in polypeptide ions. Such an approach may complement strategies that seek to generate non-selective cleavages of the related peptides.
Keywords: selective cleavage, ornithine, lactam formation, deguanidination
Introduction
Mass spectrometry has emerged as the leading tool for identifying both proteins and peptides. With the advent of soft ionization methods such as matrix-assisted laser desorption ionization (MALDI)[1] and electrospray ionization (ESI),[2,3] large molecules, particularly biomolecules, can be readily ionized without inducing fragmentation thereby facilitating mass determination. Primary structure determination is usually approached by fragmenting ions along the peptide backbone. For protonated proteins and peptides, activation of the ions via collisions or absorption of IR photons results most commonly in backbone fragmentation at the amide bonds. Such behavior is common to all dissociation methods that give rise to vibrationally excited even-electron peptide and protein cations, such as, for example, collision-induced dissociation (CID),[4–6] surface-induced dissociation[7–9] and infrared multiphoton dissociation.[10–13]
The mobile proton model[14–20] is widely accepted as rationalizing the fragmentation behavior of protonated peptides in the gas phase. Basic sites, such as the N-terminal amine, and the side chains of arginine, lysine and histidine are generally accepted to be the locations for excess protons in ions of low internal energy. Activation of the peptide ion increases the mobility of the proton,[16,19] allowing it to populate different sites along the peptide backbone. When a backbone amide bond is protonated, cleavage is facilitated to produce b- and y-type ions.[21] Although cleavage at other bonds in the peptide backbone may occur, this is the most common cleavage observed for CID experiments.
In several cases, some of these backbone cleavages are found to be much more favorable than others, depending on the amino acid composition of the peptide (i.e. there can be strong side-chain effects). There are currently many examples of residues that exhibit selective cleavages under specific conditions.[22–33] Cleavages observed at aspartic acid[23,26–28] and proline[22,23,25,31] residues are among the most commonly observed of these facile cleavages. These cleavages are related to the mobile proton effect, yet the individual processes are quite different. For the aspartic acid effect, if a proton is sequestered away from the aspartic acid residue, and therefore not mobile, cleavage C-terminal to the aspartic acid is observed by cyclization of the side chain to produce a terminal succinic anhydride and a new primary amine. This type of cleavage that does not directly involve the ionizing proton is sometimes described as a charge-remote fragmentation pathway and is readily observed with a nearby arginine residue, which serves to sequester the proton away from aspartic acid. Alternatively, if there is a mobile proton, the amide bond of proline has a higher proton affinity than neighboring amide bonds, resulting in a highly favorable cleavage N-terminal to the proline due to the higher basicity of the tertiary nitrogen. In this scenario, the ionizing proton is directly involved in the cleavage and is therefore said to be a charge-directed fragmentation pathway. Both of these cleavage phenomena have been found to dominate spectra under conditions favorable to the particular pathways.
Ornithine is an amino acid that is not coded by DNA but is produced in nature via deguanidination of arginine and plays an important role in the urea cycle. Chemical deguanidination of arginine in a polypeptide results in the conversion of a guanidine containing side-chain to a δ-amine-containing side chain. The proton affinity of ornithine, the side chain of which is one methylene group shorter than lysine, is therefore expected to be very similar to that of lysine and much less than that of arginine.[34,35] The conversion of arginine to provide enhanced sequence coverage has been explored previously.[36–38] Conversion of arginine to an ornithine residue in a polypeptide, therefore, might be expected to alter the fragmentation pattern of an ion derived from the peptide simply due to more facile proton mobility in the ornithine case, much like the difference observed between the same peptide with a lysine exchanged for an arginine. However, the conversion of arginine to ornithine has a much greater effect than does the substitution of a lysine for an arginine. Specifically, the introduction of ornithine exhibits a classic case of a neighboring group effect,[39] which leads to a highly selective cleavage C-terminal to the ornithine residue. The conversion of arginine to ornithine residues within the context of the tandem mass spectrometry (MALDI-post-source decay) of N-terminal tris(2,4,6-trimethoxyphenyl)phosphonium derivatization has been reported,[38] but the effect described here, although observed as a minor process, was not discussed. In this report, the ornithine effect is described and compared with the aspartic acid and proline effects using a peptide in which all three processes can compete. We have found that the ornithine effect appears to dominate. The conversion of arginine to ornithine in peptide and protein ions may therefore be useful when it is desirable to introduce a highly selective decomposition route.
Experimental section
Materials
The peptide AAAAARA was custom synthesized from NeoBioSci (Cambridge, MA). Angiotensin II human (DRVYIHPF) and hydrazine hydrate were purchased from Sigma Aldrich (St. Louis, MO). The peptides YGGFLR and YGGFLK were purchased from CPC Scientific (Sunnyvale, CA). The peptides GAILPGAILR and GAILDGAILR were purchased from SynPep (Dublin, CA).
Arginine hydrazinolysis
Aqueous peptide solutions were prepared to concentrations up to approximately 100 mM. The procedure used has been modified slightly from previous reactions.[38,40] 50 μl of hydrazine hydrate was added to 50 μl of the peptide solution in a 1.5 ml vessel. The reaction mixture was then mixed briefly and allowed to react in a 60 °C water bath for periods between 2 and 16 h. Complete hydrazinolysis was observed for the longer time, while a mixture of modified and unmodified peptides was observed after 2 h of reaction. The deguanidination reaction is illustrated in Scheme 1. For peptides containing additional arginine residues, it has been found to be beneficial, but not necessary, to use a slightly greater amount of hydrazine.
Scheme 1.

Conversion of arginine to ornithine.
Mass spectrometry
Mass spectrometry experiments were performed on QqQ tandem mass spectrometers (QTRAP 2000 and QTRAP 4000, AB Sciex, Concord, ON, Canada), modified with a home-built, alternately pulsed nanoelectrospray ionization (nESI) source.[41] Following positive mode nESI, cations were transferred to Q3 where they were mass selected. Following isolation, the ions were activated and subsequently fragmented via ion trap CID. Ions were mass analyzed via mass-selective axial ejection.[42]
Results and discussion
The ornithine effect
A comparison of the fragmentation patterns of arginine- versus ornithine-containing peptides is shown in Fig. 1, with the peptide AAAAAXA, where X is either R or O. Figure 1(a) shows CID of [AAAAARA + H]+, which produces several sequence ions as well as many neutral losses. In the case of Fig. 1(b), [AAAAAOA + H]+ is cleaved C-terminal to ornithine, forming two fragments, b6 and b5. As the N-terminal amine of the b-ion is the only basic site remaining, the proton is assumed to be bound there. However, if there was a basic residue C-terminal to ornithine, one would expect to see a large abundance of the corresponding y-ion. It should be noted that although it is commonly accepted that b-ions are terminated in cycles,[21] as either a diketopiperazine or an oxazalone, the b-ions formed from the ornithine effect are terminated with a lactam.
Figure 1.

Comparison of the fragmentation patterns between arginine-and ornithine-containing peptides. (a) CID of [AAAAARA+H]+ produces several sequence fragments, neutral losses, and unidentifiable peaks. (b) CID of [AAAAAOA + H]+ produces a major cleavage C-terminal to ornithine. Ammonia loss is labeled with an asterisk (*), water loss is labeled with a degree sign (°). Precursor ion fragmented (M) is denoted with a lightning bolt (
 ).
).
This dramatic difference between the spectra of Fig. 1 is proposed to arise from a facile new fragmentation channel open to ornithine-containing peptides. An initial nucleophilic attack of the carbonyl carbon by the γ-amine of ornithine, resulting in cyclization and the formation of a six-membered lactam, gives rise to the abundant b6 ion. This mechanism gives rise to what is being termed the ‘ornithine effect’, as illustrated in Scheme 2. For N-terminal or internal ornithine residues, the R in Scheme 2 is an amide nitrogen, such that HR results in the formation of a primary amine (H2N-peptide). For C-terminal ornithine residues, the R in Scheme 2 is an OH, such that HR is water (H2O). It should be noted that the ionizing proton is not shown in the mechanism of Scheme 2 as this process involves the nucleophilic attack from an unprotonated amine. This has been tested by acylating the N-terminal amine of AAAAAOA with a fixed charge, trimethyammoniumbutyrate (TMAB), to form the cationic but unprotonated peptide [TMAB-AAAAAOA]+. Activation of this peptide resulted in a highly selective cleavage C-terminal to the ornithine, producing a b6 ion (Fig. S-1).
Scheme 2.

(Left) Nucleophilic attack of the carbonyl carbon by the amine results in the formation of (Right) a six-membered lactam and cleavage at the C-terminal carbonyl carbon bond of ornithine. If ornithine is N-terminal or internal, HR is an amine (HNH-R); if ornithine is the C-terminal residue, HR is water loss (H2O).
Subsequent fragmentation of the b6 ion in Fig. 1(b) via an MS3 experiment produced almost exclusively the b5 ion (Fig. S-2(d)), which suggests that the b5 ion in Fig. 1(b) could arise largely or exclusively via sequential fragmentation. Collisional activation of the [AAAAAOA+ H]+ precursor at a higher amplitude than was used to generate Fig. 1(b) gave rise to an abundant b5 ion (Fig. S-2(c)), which is consistent with sequential fragmentation via the b6 ion. Similar fragmentation behavior of the lactam-terminated bn ion to produce the bn−1 has been observed in lysine-containing peptides by Kish and Wesdemiotis.[29] For lactam-terminated bn ions, fragmentation produces both the bn−1 ion and 3-amino-2-piperidinone(Fig. S-3).
Peptides containing a c-terminal ornithine
A simple example of a case in which ornithine is the C-terminal residue is provided in Fig. 2, which compares the CID behavior of YGGFLX, where X is either R (Fig. 2(a)), K (Fig. 2(b)) or O (Fig. 2(c)). Lysine- and arginine-terminated peptides are generally produced from tryptic digestion of proteins, while the ornithine-terminated peptide would be produced from deguanidination of the arginine-terminated peptide. The behavior of a C-terminal ornithine in a peptide is therefore relevant for a strategy that would involve the conversion of arginine to ornithine in tryptic peptides. Figures 2(a) and 2(b) both show extensive backbone cleavage with a somewhat higher degree of spectral complexity, as reflected in the appearance of more low abundance fragments, observed in Fig. 2(a) from protonated YGGFLR. The latter observation is likely due to the somewhat higher activation amplitude required to generate a similar extent of precursor ion depletion with protonated YGGFLR than for protonated YGGFLK. The greater kinetic stability of arginine protonated peptides is fully consistent with the mobile proton model.[16] Figure 2(c) shows almost exclusive loss of water from the peptide, which is presumed to result from the formation of a lactam. The enhanced loss of water from ornithine has been observed previously[43–45] and has been ascribed to ring size effects. CID of the water loss product in Fig. 2(c) produces almost entirely the intact b5 ion (see Fig. S-4(d)). This result is fully consistent with the hypothesis that the loss of H2O is from the C-terminal lactam formation and that subsequent fragmentation leads to loss of 3-amino-2-piperidinone to generate ab5 ion. When the protonated YGGFLO ion was subjected to a higher activation amplitude, the same products observed in the CID of the water loss ion (Fig. S-4(d)) were observed (see Fig. S-4(c)), and no additional products were seen, which suggests that the greater degree of fragmentation at higher activation amplitudes arises largely from sequential decomposition following water loss. This hypothesis was further tested by esterifying the carboxy group of YGGFLO, such that activation of YGGFLO-OMe should produce a methanol loss while any water loss observed would be from an alternate fragmentation pathway.[46,47] Activation of [YGGFLO-OMe + H]+ was found to produce almost exclusively loss of methanol from the precursor, with a much smaller loss of water observed as well (Fig. S-5). We note that protonated YGGFLK should also be able to undergo an analogous process with the C-terminal lysine generating a seven-membered lactam ring.[29] This may very well occur, as there is significant water loss, and many of the products could result from sequential fragmentation from the water loss product. However, there are also several relatively abundant fragments in the data for protonated YGGFLK, such as the y4 and y5 ions, which cannot arise from the water loss process. Analogous ions are notably absent in the protonated YGGFLO data (see Fig. S-4(c)). Apparently, the ability to form the six-membered lactam makes the reaction of Scheme 2 significantly more competitive than the analogous reaction with protonated YGGFLK to form the seven-membered lactam.
Figure 2.

Comparisons of CID behavior of small peptides with basic C-terminal residues. (a) CID of [YGGFLR + H]+; (b) CID of [YGGFLK + H]+; (c) CID of [YGGFLO + H]+. Ammonia loss is labeled with an asterisk (*), water loss is labeled with a degree sign (°). Precursor ion fragmented (M) is denoted with a lightning bolt (
 ).
).
It is of interest to examine the C-terminal ornithine effect summarized above in peptides where the proline or aspartic acid effects can also play roles. The model tryptic peptides GAILPGAILX and GAILDGAILX, where X = R or O, were therefore investigated. The proline effect is considered for singly protonated peptides [GAILPGAILR + H]+ and [GAILPGAILO + H]+ in Fig. 3. A mobile proton is regarded to be a prerequisite for the observation of a dominant proline effect (i.e. cleavage at the N-terminal side of proline).[22,23,25,31] In the case of [GAILPGAILR + H]+, the proton is effectively sequestered on the arginine residue, thereby significantly reducing the preferential N-terminal cleavage pathway. The product ion spectrum from CID of this peptide ion is shown in Fig. 3(a). A variety of products are observed that includes sequence ions and internal cleavages. Products expected from a proline effect (i.e. y6 and b4 ions) are observed but are not particularly dominant. Conversion of arginine to ornithine is expected to enhance proton mobility, and, as expected, this modification substantially changes the fragmentation spectrum, as observed in Fig. 3(b) for CID of [GAILPGAILO + H]+. Evidence for a more pronounced proline effect is reflected in the abundant y6-H2O product ion, which is indicated in the figure as . Interestingly, only a very small y6 ion is observed. Although the loss of water after formation of the y6 ion cannot be precluded, CID of the H2O loss of [GAILPGAILO + H]+ (Fig. 3(c)) produces a nearly identical spectrum to that of CID of intact [GAILPGAILO + H]+ (Fig. 3(b)). The only significant difference is that there are no complete y-ions, only dehydrated y-ions, in the case of dehydrated [GAILPGAILO + H]+. The similarity between the two spectra is consistent with the interpretation that most of the product ions in Fig. 3(b) arise from sequential fragmentation following water loss from the precursor ion with concomitant lactam formation (Scheme 2). At lower activation amplitude, water loss dominates the product ion spectrum of [GAILPGAILO + H]+ (Fig. S-6(b)), which further supports the proposed sequence of events. In this scenario, the prominent b9 ion arises from the loss of 3-amino-2-piperidinone from the initial water loss ion, which competes with the proline effect to generate the ion. These results therefore suggest that the ornithine effect in C-terminal ornithine containing peptides is stronger than the proline effect when both are potentially active.
Figure 3.

Comparisons of the proline effect between arginine- and ornithine-containing peptides. (a) CID of [GAILPGAILR +H]+; (b) CID of [GAILPGAILO + H]+; (c) CID of [GAILPGAILO+ H-H2O]+. Ammonia loss is labeled with an asterisk (*), water loss is labeled with a degree sign (°). Precursor ion fragmented (M) is denoted with a lightning bolt (
 ).
).
The aspartic acid effect tends to be prominent when there is relatively little proton mobility in peptides that contain an aspartic acid residue. In this case, cleavage is favored at the C-terminal side of aspartic acid. This effect is clearly evident in the CID of [GAILDGAILR + H]+, shown in Fig. 4(a). The y5 ion, produced by fragmentation C-terminal to aspartic acid, is essentially the only fragment ion observed. Figure 4(b) provides a 25× magnification of this CID spectrum, revealing that other sequence ions can be generated, but in much lower abundance. Conversion of arginine to ornithine makes a profound difference in the fragmentation of this and similar peptide sequences, as shown in Fig. 4(c). The initial impact of the presence of ornithine rather than arginine is that there is less proton sequestration, thereby allowing a more uniform distribution of cleavages to occur. Nearly full sequence coverage is obtained with significant abundances of most of the possible b an y sequence ions. The major water loss apparent in the product ion spectrum of [GAILDGAILO + H]+ and the prominent b9 that may arise from the loss of 3-amino-2-piperidinone from the water loss ion, along with other plausible sequential decomposition products following water loss, indicate that the ornithine effect is also prominent in this case.
Figure 4.

Comparisons of the aspartic acid effect with arginine- and ornithine-containing peptides. (a) CID of [GAILDGAILR+ H]+;(b) 25× zoom in of CID of [GAILDGAILR + H]+ from top; (c) CID of [GAILDGAILO+ H]+. Ammonia loss is labeled with an asterisk (*), water loss is labeled with a degree sign (°). Precursor ion fragmented (M) is denoted with a lightning bolt (
 ).
).
Peptides containing an internal ornithine
Proteins, non-tryptic peptides and tryptic peptides with missed cleavages frequently contain arginine residues at intermediate locations in the amino acid sequence. The data of Fig. 1 indicate that the ornithine effect is operative when arginine residues at intermediate locations in the sequence are deguanidinated. Human Angiotensin II, DRVYIHPF, was examined to explore the contribution of the ornithine effect at an intermediate location in the sequence when the aspartic acid and proline effects are also possible.
As illustrated in Fig. 4(a), the aspartic acid effect can be preponderant in the absence of a mobile proton, such as is the case when an ionizing proton is sequestered at an arginine residue. CID of [DRVYIHPF + H]+ (Fig. 5(a)) results largely in the formation of the y7 product ion, corresponding to cleavage C-terminal to aspartic acid. The loss of water from the precursor may arise from a number of sites, such as, for example, the carboxylic acids of the C-terminus or aspartic acid side chain or the hydroxyl group of tyrosine. Conversion of the arginine to ornithine significantly alters the fragmentation behavior. CID of [DOVYIHPF +H]+ (Fig. 5(b)) shows selective cleavage C-terminal to ornithine, as reflected by the highly abundant y6 ion. Other y ions were also observed at much lower abundance, and a relatively small b6 ion, which is consistent with the proline effect, appeared in the spectrum. The comparison of Figs. 5(a) and 5(b) shows that deguanidination of angiotensin II diminishes the aspartic acid effect and opens up the ornithine effect. This is interpreted to result from the replacement of the highly basic arginine residue with ornithine, which opens up the dissociation pathway for formation of the favored six-membered lactam ring.
Figure 5.
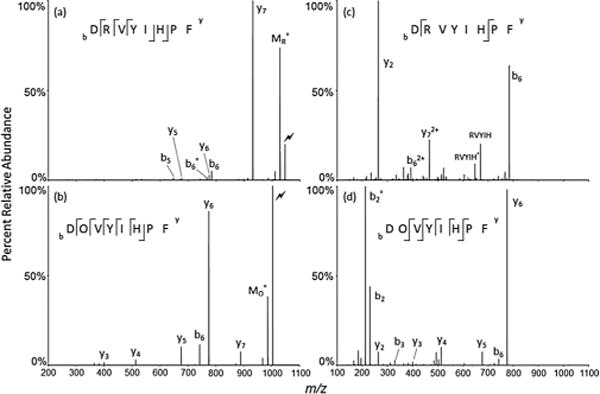
Comparison of CID of Angiotensin II (human), DRVYIHPF and ornithinated DOVYIHPF. (a) CID of [DRVYIHPF+H]+; (b) CID of [DOVYIHPF + H]+; (c) CID of [DRVYIHPF + 2H]+; (d) CID of [DOVYIHPF + 2 H]+. Water loss is labeled with a degree sign (°). Precursor ion fragmented (M) is denoted with a lightning bolt (
 ).
).
When angiotensin II is doubly protonated, the proline effect gives rise to the predominant fragmentation channel, as illustrated in Fig. 5(c). CID of [DRVYIHPF + 2H]2+ leads to b6 and y2 as the most abundant fragments, which arise from cleavage between the histidine and proline residues. The aspartic acid effect also appears to contribute as reflected by the appearance of the ion. An internal fragment, singly protonated RVYIH, and its water loss product are also observed and appear to arise from sequential cleavages arising from both the proline and aspartic acid effects (i.e. cleavage N-terminal to proline and C-terminal to aspartic acid). The proline effect becomes important for the doubly protonated peptide while it is not important for the singly protonated peptide presumably due to the second proton being sufficiently mobile to transfer to the tertiary nitrogen of the proline amide nitrogen. Following conversion of arginine to ornithine, CID of the doubly protonated peptide, [DOVYIHPF+ 2H]2+, results in a dramatic change in fragmentation behavior, as shown in Fig. 5(d). The product ion spectrum is dominated by fragments generated from cleavage C-terminal to ornithine (i.e. the y6,b2 and ions) with relatively minor products from cleavages at other amide linkages. From the standpoint of proton mobility, there is no obvious reason why the proline effect would be diminished by replacing arginine with ornithine. It appears that the ornithine effect is clearly favored over the proline effect when both are potentially active.
Conclusions
The presence of an ornithine residue in a polypeptide cation introduces a facile peptide backbone cleavage channel C-terminal to ornithine via the formation of a stable six-membered lactam ring terminated b-ion. The complementary product is a water molecule when the ornithine is present at the C-terminus or a y-ion in all other cases. The mechanism for this ‘ornithine effect’ is also open to lysine residues. However, the formation of a seven-membered lactam ring is less favored, which presumably underlies the much weaker effect with lysine residues. The lactam terminated b-ion can undergo a facile sequential cleavage to generate a smaller b-ion and loss of 3-amino-2-piperidinone. The ornithine-related cleavage appears to be even more favored than other selective cleavages known for CID of peptide cations, such as those N-terminal to proline and C-terminal to aspartic acid. The presence of an ornithine residue in a polypeptide cation introduces a particularly ‘weak spot’ for CID.
Selective cleavages of peptide ions can be a complication when the objective is to generate full peptide sequence. However, there may be instances in which selective cleavages can be useful because they introduce a degree of specificity that is analogous to that associated with selective enzymes in solution. The common gas phase selective cleavages, however, lack generality. That is, certain conditions must be met before they are particularly dominant (e.g. mobile protons for the proline effect, lack of mobile protons for the aspartic acid effect). For this reason, a practical approach to top-down protein identification that takes advantage of selective cleavages has not been developed. The ornithine effect may prove to be more general. At least, this appears to be the case based on the peptides we have examined to date. The conversion of arginine to ornithine via a simple solution-phase reaction is a relatively simple way to introduce ornithine into a polypeptide, which both replaces a highly basic site with a less basic site and opens up the facile cleavage channel associated with ornithine. We are currently exploring alternate means for converting arginine residues to ornithine residues as well as analytical strategies that take advantage of the ornithine effect.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under Grant GM 45372.
Footnotes
Supporting information
Supporting information may be found in the online version of this article.
References
- 1.Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10, 000 daltons. Anal Chem. 1987;60:2299. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- 2.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science. 1989;246:64. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 3.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray Ionization-Principles and Practice. Mass Spectrom Rev. 1990;9:37. [Google Scholar]
- 4.de Hoffmann E. Tandem Mass Spectrometry: A Primer. J Mass Spectrom. 1996;31:129. [Google Scholar]
- 5.Shukla AK, Futrell JH. Tandem Mass Spectrometry: Dissociation of Ions by Collisional Activation. J Mass Spectrom. 2000;35:1069. doi: 10.1002/1096-9888(200009)35:9<1069::AID-JMS54>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Jennings KR. The Changing Impact of the Collision-Induced Decomposition of Ions in Mass Spectrometry. Int J Mass Spectrom. 2000;200:479. [Google Scholar]
- 7.Mabud MA, DeKrey MJ, Cooks RG. Surface-Induced Dissociation of Molecular Ions. Int J Mass Spectrom Ion Processes. 1985;67:285. [Google Scholar]
- 8.Williams ER, Henry KD, McLafferty FW, Shabanowitz J, Hunt DF. Surface-Induced Dissociation of Peptide Ions in Fourier-Transform Mass Spectrometry. J Am Soc Mass Spectrom. 1990;1:413. doi: 10.1016/1044-0305(90)85022-E. [DOI] [PubMed] [Google Scholar]
- 9.Laskin J, Denisov EV, Shukla AK, Barlow SE, Futrell JH. Surface-Induced Dissociation in a Fourier Transform Ion Cyclotron Resonance Mass Spectrometer: Instrument Design and Evaluation. Anal Chem. 2002;74:3255. doi: 10.1021/ac025514q. [DOI] [PubMed] [Google Scholar]
- 10.Basov NG, Markin EP, Oraevski AN, Pankratov AV, Shachkov AN. Stimulation of Chemical Processes by Infrared Laser Radiation. JETP Letters. 1971;14:251. [Google Scholar]
- 11.Little DP, Speir JP, O’Connor PB, McLafferty FW. Infrared Multiphoton Dissociation of Large Multiply Charged Ions for Biomolecule Sequencing. Anal Chem. 1994;66:2809. doi: 10.1021/ac00090a004. [DOI] [PubMed] [Google Scholar]
- 12.Laskin J, Futrell JH. Activation of Large Ions in FT-ICR Mass Spectrometry. Mass Spectrom Rev. 2005;24:135. doi: 10.1002/mas.20012. [DOI] [PubMed] [Google Scholar]
- 13.Polfer NC, Oomens J. Reaction Products in Mass Spectrometry Elucidated with Infrared Spectroscopy. Phys Chem Chem Phys. 2007;9:3804. doi: 10.1039/b702993b. [DOI] [PubMed] [Google Scholar]
- 14.McCormack AL, Somogyi Á, Dongré AR, Wysocki VH. Fragmentation of Protonated Peptides: Surface-Induced Dissociation in Conjunction with a Quantum Mechanical Approach. Anal Chem. 1993;65:2859. doi: 10.1021/ac00068a024. [DOI] [PubMed] [Google Scholar]
- 15.Somogyi Á, Wysocki VH, Mayer I. The Effect of Protonation Site on Bond Strengths in Simple Protonated Peptides: Application of Ab Initio and MNDO Bond Orders and MNDO Energy Partitioning. J Am Soc Mass Spectrom. 1994;5:704. doi: 10.1016/1044-0305(94)80002-2. [DOI] [PubMed] [Google Scholar]
- 16.Dongré AR, Jones JL, Somogyi Á, Wysocki VH. Influence of Peptide Composition, Gas-Phase Basicity, and Chemical Modification on Fragmentation Efficiency: Evidence for the Mobile Proton Model. J Am Chem Soc. 1996;118:8365. [Google Scholar]
- 17.Gu C, Somogyi Á, Wysocki VH, Medzihradszky KF. Fragmentation of Protonated Oligopeptides XLDVLQ (X-L, H, K or R) by Surface Induced Dissociation: Additional Evidence for the ‘Mobile Proton’ Model. Anal Chim Acta. 1999;397:247. [Google Scholar]
- 18.Tsaprailis G, Nair H, Somogyi Á, Wysocki VH. Influence of Secondary Structure on the Fragmentation of Protonated Peptides. J Am Soc Mass Spectrom. 1999;121:5142. [Google Scholar]
- 19.Wysocki VH, Tsaprailis G, Smith LL, Breci LA. Mobile and Localized Protons: A Framework for Understanding Peptide Dissociation. J Mass Spectrom. 2000;35:1399. doi: 10.1002/1096-9888(200012)35:12<1399::AID-JMS86>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Paizs B, Suhai S. Fragmentation Pathways of Protonated Peptides. Mass Spectrom Rev. 2005;24:508. doi: 10.1002/mas.20024. [DOI] [PubMed] [Google Scholar]
- 21.Paizs B, Suhai S. Towards Understanding the Tandem Mass Spectra of Protonated Oligopeptides. 1: Mechanism of Amide Bond Cleavage. J Am Soc Mass Spectrom. 2004;15:103. doi: 10.1016/j.jasms.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz BL, Bursey MM. Some Proline Substituent Effects in the Tandem Mass Spectrum of Protonated Pentaalanine. Biol Mass Spectrom. 1992;21:92. doi: 10.1002/bms.1200210206. [DOI] [PubMed] [Google Scholar]
- 23.Yu W, Vath JE, Huberty MC, Martin SA. Identification of the Facile Gas-Phase Cleavage of the Asp-Pro and Asp-Xxx Peptide Bonds in Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry. Anal Chem. 1993;65:3015. doi: 10.1021/ac00069a014. [DOI] [PubMed] [Google Scholar]
- 24.Hu P, Loo JA. Gas-Phase Coordination Properties of Zn2+,Cu2+,Ni2+, and Co2+ with Histidine-Containing Peptides. J Am Chem Soc. 1995;117:11314. [Google Scholar]
- 25.Vaisar T, Urban J. Probing the Proline Effect in CID of Protonated Peptides. J Mass Spectrom. 1996;31:1185. doi: 10.1002/(SICI)1096-9888(199610)31:10<1185::AID-JMS396>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Tsaprailis G, Somogyi Á, Nikolaev EN, Wysocki VH. Refining the Model for Selective Cleavage at Acidic Residues in Arginine-Containing Protonated Peptides. Int J Mass Spectrom. 2000;195/196:467. [Google Scholar]
- 27.Sullivan AG, Brancia FL, Tyldesley R, Bateman R, Sidhu K, Hubbard SJ, Oliver SG, Gaskell SJ. The Exploitation of Selective Cleavage of Singly Protonated Peptide Ions Adjacent to Aspartic Acid Residues Using a Quadrupole Orthogonal Time-of-Flight Mass Spectrometer Equipped with a Matrix-Assisted Laser Desorption/Ionization Source. Int J Mass Spectrom. 2001;210/211:665. [Google Scholar]
- 28.Huang Y, Wysocki VH, Tabb DL, Yates JR., III The Influence of Histidine on Cleavage C-terminal to Acidic Residues in Doubly Protonated Tryptic Peptides. Int J Mass Spectrom. 2002;219:233. [Google Scholar]
- 29.Kish MM, Wesdemiotis C. Selective Cleavage at Internal Lysine Residues in Protonated vs. Metalated Peptides. Int J Mass Spectrom. 2003;227:191. [Google Scholar]
- 30.Zhang Q, Perkins B, Tan G, Wysocki VH. The Role of Proton Bridges in Selective Cleavage of Ser-, Thr-, Cys-, Met-, Asp-, and Asn-Containing Peptides. Int J Mass Spectrom. 2011;300:108. [Google Scholar]
- 31.Bleiholder C, Suhai S, Harrison AG, Paizs B. Towards Understanding the Tandem Mass Spectra of Protonated Oligopeptides. 2: The Proline Effect in Collision-Induced Dissociation of Protonated Ala-Ala-Xxx-Pro-Ala (Xxx = Ala, Ser, Leu, Val, Phe, and Trp) J Am Soc Mass Spectrom. 2011;22:1032. doi: 10.1007/s13361-011-0092-1. [DOI] [PubMed] [Google Scholar]
- 32.Shaw JB, Ledvina AR, Zhang X, Julian RR, Brodbelt JS. Tyrosine Deprotonation Yields Abundant and Selective Backbone Cleavage in Peptide Anions upon Negative Electron Transfer Dissociation and Ultraviolet Photodissociation. J Am Chem Soc. 2012;134:15624. doi: 10.1021/ja3032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu L, Chen T, Xue G, Zu L, Fang W. Selective Cleavage Enhanced by Acetylating the Side Chain of Lysine. J Mass Spectrom. 2013;48:128. doi: 10.1002/jms.3136. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder OE, Andriole EJ, Hessler K, Carver KL, Coyler KE, Poutsma JC. Proton Affinity of Lysine Homologues from the Extended Kinetic Method. J Phys Chem A. 2004;108:326. [Google Scholar]
- 35.Wu Z, Fenselau C. Proton Affinity of Arginine Measured by the Kinetic Approach. Rapid Commun Mass Spectrom. 1992;6:403. [Google Scholar]
- 36.Dikler S, Kelly JW, Russell DH. Improving Mass Spectrometric Sequencing of Arginine-containing Peptides by Derivatization with Acetylacetone. J Mass Spectrom. 1997;32:1337–1349. doi: 10.1002/(SICI)1096-9888(199712)32:12<1337::AID-JMS599>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Kuyama H, Nakajima C, Nakazawa T, Nishimura O. Conversion of Arginine to Ornithine for Improving the Fragmentation Pattern of Peptides Labeled with the N-Terminal Tris(2,4,6-Trimethoxyphenyl)-Phosphonium Group in Tandem Mass Spectrometry. Anal Methods. 2010;2:1792. [Google Scholar]
- 38.Kuyama H, Nakajima C, Nakazawa T, Nishimuram O. Enzymatic Conversion of Arginine to Citrulline for Improving Fragmentation of Nα-tris(2,4,6-trimethoxyphenyl)phosphonium-acetylated Peptides by Tandem Mass Spectrometry. Anal Methods. 2011;3:2829–2835. [Google Scholar]
- 39.O’Hair RAJ. The Role of Nucleophilie-Electrophile Interactions in the Unimolecular and Bimolecular Gas-Phase Ion Chemistry of Peptides and Related Systems. J Mass Spectrom. 2000;35:1377–1381. doi: 10.1002/1096-9888(200012)35:12<1377::AID-JMS83>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 40.Shemyakin MM, Vinogadova EI, Alakhof YuB, Lipkin VM, Rosinov BV, Fonina LA. Mass Spectrometric Amino Acid Sequence Determination in Arginine Containing Peptides. Tetrahedron. 1969;25:5785. doi: 10.1016/s0040-4020(01)83087-8. [DOI] [PubMed] [Google Scholar]
- 41.Liang X, Xia Y, McLuckey SA. Alternately Pulsed Nano-electrospray Ionization/Atmospheric Pressure Chemical Ionization for Ion/Ion Reactions in an Electrodynamic Ion Trap. Anal Chem. 2006;78:3208. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Londry FA, Hager JW. Mass Selective Axial Ion Ejection from a Linear Quadrupole Ion Trap. J Am Soc Mass Spectrom. 2003;14:1130. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 43.Leclercq PA, Desiderio DM. Chemical Ionization Mass Spectra of Amino Acids and Derivatives. Occurrence and Fragmentation of Ion-Molecule Reaction Products. Org Mass Spectrom. 1973;7:515–533. [Google Scholar]
- 44.Weinkam RJ. Reactions of Protonated Diamino Acids in the Gas Phase. J Org Chem. 1978;43:2581–2586. [Google Scholar]
- 45.Kulik W, Heerma W. A Study of the Positive and Negative Ion Fast Atom Bombardment Mass Spectra of α-Amino Acids. Biomed Environ Mass Spectrom. 1988;15:419–427. doi: 10.1002/bms.1200150803. [DOI] [PubMed] [Google Scholar]
- 46.Ballards KD, Gaskell SJ. Dehydration of Peptide [M + H]+ Ions in the Gas Phase. J Am Soc Mass Spectrom. 1993;4:447–481. doi: 10.1016/1044-0305(93)80005-J. [DOI] [PubMed] [Google Scholar]
- 47.Reid GE, Simpson RJ, O’Hair RAJ. A Mass Spectrometric and Ab Initio Study of the pathways for dehydration of Simple Glycine and Cysteine-Containing Peptide [M + H]+ Ions. J Am Soc Mass Spectrom. 1998;9:945–956. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


