Abstract
Marek's disease (MD) is a lymphoproliferative disease of chickens induced by a herpesvirus, the MD virus (MDV). Because MD is a significant economic problem to the poultry industry, there is great interest in enhancing genetic resistance, which is controlled by multiple genes. The influence of the MHC has been clearly demonstrated, and several relevant quantitative trait loci have been mapped; however, no single gene influencing MD resistance has been identified. Transcription of SORF2 is perturbed in the MDV recombinant clone RM1 due to a solo insertion of the reticuloendotheliosis virus long terminal repeat, which may explain the loss of oncogenicity for this strain. Hypothesizing that SORF2-interacting host proteins are involved in MD resistance, we screened a chicken splenic cDNA library by the yeast two-hybrid assay using SORF2 as bait. The chicken growth hormone (GH) structural peptide was identified, and the specific interaction was verified by coimmunoprecipitation. Immunohistochemical staining and indirect immunofluorescence assay indicated that GH and SORF2 can be coexpressed in MDV-infected cells both in vitro and in vivo. Furthermore, polymorphism in the GH gene (GH1) is associated with the number of tissues with tumors in commercial White Leghorn chickens with the MHC B*2/B*15 genotype. We conclude that GH1 may well be a MD resistance gene.
Poultry is an important food source and agricultural commodity worldwide. From 1988 to 1998, world consumption of poultry meat and eggs rose 77% far outpacing the 34% and 5% rises, respectively, in pork and beef consumption (1). This achievement to meet consumer demands has been attained in part by breeding to generate chicken lines with superior growth and production traits and by more concentrated chicken rearing. Although both reasons allow for more economical meat and egg production, the latter one has the unfortunate consequence that disease outbreaks occur more frequently.
Marek's disease (MD) is the most serious chronic concern to the poultry industry. MD is a lymphoproliferative disease caused by the MD virus (MDV), an oncogenic avian herpesvirus (2). More than 30 years of research have led to the following summary of events, which has been reviewed (3). Because of the ubiquitous distribution of MDV, all chickens are exposed at an early age in poultry-rearing facilities to cell-free MDV through inhalation of contaminated dust (4). Macrophages phagocytize the particles and carry them to the lymphoid organs. The B cells are the initial targets of viral replication, resulting in a productive, cytolytic infection between 3 and 6 days, causing cytopathy of lymphoid organs, especially the thymus and bursa (5). Around 7 days, the infection switches to activated T cells and MDV becomes latent, a hallmark of herpesvirus infections. The immune response (6, 7), especially cell-mediated immunity (8), is necessary to initiate this switch to latency. Transient immunosuppression also is observed at this period, which may be attributable to macrophage function (9). MDV-infected lymphocytes in the peripheral blood distribute the virus to other tissues. In susceptible chickens, a second round of cytolytic infection occurs around 14 days. At 21 days and later, chronic inflammation of the peripheral nerves is often seen and changes in lymphoid cells may progress to form frank lymphomas. Only in the feather follicle epithelium is cell-free MDV produced (10), which is the source of infectious material for bird-to-bird spread.
The main control strategy for MD is vaccination. The first U.S. vaccine was HVT, an antigenically related nonpathogenic herpesvirus of turkey, introduced in 1970 (11, 12). Since then, additional vaccines with better efficacies have been introduced. This work has been necessary because of the appearance of MDV strains with increasing virulence in the field (13). Based on pathogenicity shifts, it has been suggested that a new vaccine is useful for about 10 years (14). The continuing evolution of MDV strains with higher virulence indicates that alternative strategies to augment existing vaccinal control are needed (15).
Genetic resistance to MD is an attractive solution because it is reliable, long lasting, and environmentally sound. Also, chicken lines selected for MD resistance have been shown to have greater vaccinal immunity and higher egg production than susceptible lines (16–18). If genes conferring genetic resistance to MD could be identified or located, poultry breeders would be able to directly select for enhanced MD resistance through the use of genetic markers, eliminating the need for progeny or sibling testing and the use of pathogenic agents. As resistance to MD is complex and controlled by multiple genes (quantitative trait loci or QTL), we are taking several approaches to identify the causative genes; namely, (i) performing genomewide scans to identify the QTL (19, 20), (ii) developing recombinant congenic strains (21, 22) to simplify the genetic complexity and provide functional information on each QTL, (iii) generating comparative maps to the “information-rich” human genome for the QTL regions to identify potential positional candidate genes (23), and (iv) integrating DNA microarrays and genetic mapping to screen for genes with expression variation between MD-resistant and susceptible lines that lie within a QTL region.
In addition, virus-host protein interactions may be useful for identifying QTL relevant to MD resistance. Because MDV uses the host cell machinery to replicate, viral-host protein interactions are likely to be of significance to influence the disease outcome. The identification of these interactions and the mapping of their corresponding host genes should provide positional candidates for QTL and potentially identify pathways for disease resistance or progression.
The unique biological properties of the MDV recombinant clone RM1 stimulated our interest to characterize SORF2, especially with regard to its potential involvement in the immune response. RM1 was derived from the oncogenic MDV strain JM/102W after retrovirus insertional mutagenesis (24) resulting in a reticuloendotheliosis virus long terminal repeat being inserted immediately upstream of SORF2 (25). Interestingly, RM1 is attenuated for oncogenicity but retains other in vivo properties of virulent viruses including efficient replication, thymic and bursal atrophy, early immunosuppression, and contact spread (26). Normally, attenuated MDV strains lack one or more of these characteristics. In addition, chickens infected with RM1 were protected against challenge with virulent MDV, and the level of protection by RM1 exceeded those of other MDV vaccine strains (26). Molecular analysis indicated that RM1 overexpressed a 3.2-kb transcript initiated from the long terminal repeat promoter, which extended across the coding sequences of SORF2 and two more downstream genes (US1 and US10) (25).
The SORF2 gene, encoding a 179-aa protein, is found only in serotype 1 (virulent) MDV strains (27, 28), although it is not essential for either viral replication or tumor formation (27, 29). Furthermore, SORF2 shows homology to fowlpox ORF4 and fowl adenovirus ORF4, suggesting gene transfer between these unrelated avian viruses as well as a role in defining host range (25, 30, 31). Finally, SORF2 also shows homology to human cytomegalovirus US22 and UL36, and the human herpesvirus 6 EPLF3, members of a family of proteins with transactivation ability (32–35).
In this article, we report that (i) chicken growth hormone (GH) was identified in a screen of chicken proteins that interact with MDV SORF2, (ii) SORF2 and GH can be coexpressed in MD tumors and nerve lesions, and (iii) the GH gene (GH1) is associated with MD resistance in commercial chickens having the MHC B*2/B*15 genotype.
Materials and Methods
Yeast Two-Hybrid Screen.
SORF2 was inserted in-frame into the pLexA vector (CLONTECH) downstream of the binding domain between the BamHI and NotI sites. This construct was used to screen yeast strain EGY48 containing a reporter plasmid and a chicken cDNA splenic T cell library fused into the pB42AD vector (CLONTECH). Approximately 1.5 × 106 yeast transformants were selected on synthetic medium lacking His/Trp/Ura and His/Leu/Trp/Ura and screened for β-galactosidase activity. The DNA sequence of the positive clones was determined by dye-terminator fluorescence sequencing on an ABI 377 automatic DNA sequencer (Perkin–Elmer).
In Vitro Binding Assays.
A glutathione S-transferase (GST)-GH fusion protein and the GST protein alone were purified on glutathione Sepharose 4B beads according to the manufacturer's instructions (Amersham Pharmacia). For the binding assay, SORF2 was expressed in vitro using the Single Tube Protein System 3 (Novagen) in the presence of [35S]methionine and incubated with the GST-containing proteins. After several washes with PBS, bound proteins were released with elution buffer (10 mM reduced glutathione in 50 mM Tris⋅HCl, pH 8.0) and subjected to 8% SDS/PAGE analysis followed by autoradiography.
Coimmunoprecipitation.
GH cDNA was cloned into the eukaryotic expression vector pcDNA3 (Invitrogen) by using the EcoRI and XhoI sites. Cell lysates from MDV-infected or mock-infected chicken embryo fibroblasts were prepared by using a mild lysis buffer (145 mM KCl/5 mM MgCl2/10 mM Hepes, pH 7.5/1 mM EGTA/0.2% Nonidet P-40/protease inhibitors), followed by centrifugation to remove insoluble materials and incubation with normal rabbit serum and protein A-Sepharose for 1 h. Cell lysates were incubated with in vitro-translated structural GH peptide (unlabeled or 35S-labeled) and 10% (vol/vol) protein A Sepharose. After addition of either rabbit anti-SORF2 polyclonal antibodies or mouse anti-GH mAb (provided by Luc Berghman, Texas A & M, College Station, TX), immunocomplexes were precipitated with protein A-Sepharose. Precipitates were washed extensively and analyzed by SDS/PAGE and autoradiography.
Immunohistochemical Staining.
Uninfected and MDV-infected (JM/102W strain) chickens were obtained from the U.S. Department of Agriculture, Agricultural Research Service, Avian Disease and Oncology Laboratory. The appropriate tissues were harvested, fixed in 10% buffered formalin, embedded into paraffin, and sectioned into 3-μm thick sections. Immunostaining of tissues for SORF2 or GH was done by using the biotinylated horseradish peroxidase complex (ABC) system (Vector Laboratories). Sections were incubated sequentially with blocking serum, antibodies, and washing buffers according to the manufacturer's instructions. Nova red was used as the chromogen for color development.
Indirect Immunofluorescence Assay.
Various organs from uninfected and MDV-infected (JM strain inoculated at 1 week of age) Avian Disease and Oncology Laboratory line 7 chickens were harvested at 6 weeks of age, frozen in OCT Embedding Medium (Sakura Finetek, Torrance, CA), and sectioned to a thickness of 8 μm on a cryostat. Samples were incubated with rabbit anti-SORF2 antiserum or mouse anti-GH antiserum diluted 1:1,000 in PBS for 30 min at room temperature. Sections then were washed several times with PBS at 5-min intervals. The secondary antibodies, either goat anti-rabbit FITC-conjugated IgG or goat anti-mouse Texas red-conjugated IgG (Kirkegaard & Perry Laboratories), were diluted 1:1,000 in PBS and incubated with samples for an additional 30 min. The slides then were rinsed extensively and sealed with 50% glycerol in PBS. All samples were viewed with a laser scanning confocal microscope (Zeiss).
MD Resource Families and Association Analysis.
Genetic mapping of GH1 used the East Lansing reference panel (36), standard restriction fragment length polymorphism analysis of a MspI site, and linkage analysis with map manager (37). For the association study, two commercial White Leghorn pure lines were intermated to produce backcross families. In each family, a single line 1 grandsire was mated to a single line 2 grandam to produce seven F1 sires. Each F1 sire was mated to ≈15 random line 1 dams to produce ≈20 daughters in a single hatch. All progeny were vaccinated with bivalent vaccine (HVT and SB-1) at 1 day of age, inoculated intraabdominally at 1 week of age with MDV strain 648 (500 plaque-forming units), reared in an environmentally controlled house, and observed until moribund or 20 weeks of age. The MHC genotype was determined by serology for the B blood group. All of the animals were necropsied and scored for MD lesions in various nerves (vagus, sciatic, and brachial) and visceral tissues (thymus, proventriculus, spleen, heart, liver, lung, gonad). To determine GH1 genotype, the primers ACC TGG AAG AAG GGA TCC AAG and GGC CGT CGT GGA GCT GTG AGC were used to amplify the fourth intron, followed by digestion with SacI and 1% agarose gel electrophoresis. Statistical analysis between GH1 genotype and MD traits were analyzed by ANOVA for continuous traits (survival, number of tissues with tumors) and χ2 for nonparametric traits (e.g., vagus, gonad) using jmp (38); the Wilcoxon test gave the same results for the continuous traits (data not shown).
Results
MDV SORF2 Interacts with Chicken GH.
A splenic cDNA library was chosen for our yeast two-hybrid assay as it was enriched for genes present in B and T lymphocytes, the cell types that MDV preferentially infects. Seven β-galactosidase-positive clones were identified when SORF2 was used as bait in the system. DNA sequencing and database searches for two of these clones revealed that both were identical to the 216-aa chicken GH (39).
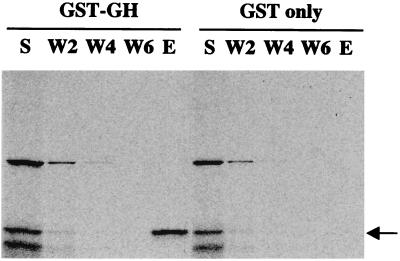
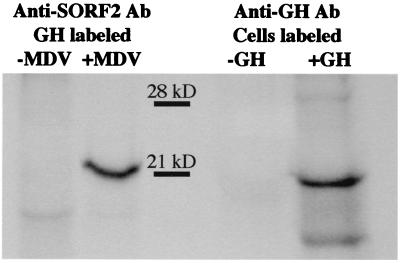
To confirm the interaction between SORF2 and GH, in vitro-translated SORF2 was incubated with GST fusion proteins. After extensive washing, SORF2 was retained by the GST-GH protein but not by GST alone (Fig. 1). Additional support for this specific interaction came from coimmunoprecipitation assays (Fig. 2). Chicken embryo fibroblasts, which support MDV replication, were used; however, because chicken embryo fibroblasts do not express GH, in vitro-translated GH was added to the cell lysates. Only in MDV-infected cells in the presence of added GH could SORF2 or GH be bound by anti-GH or anti-SORF2 antibodies, respectively, demonstrating that the interaction between SORF2 and GH is a direct and specific interaction that does not require other intermediary factors (e.g., yeast proteins).
Figure 1.
GH and SORF2 interact in vitro. S (supernatant) shows the input of 35S-labeled SORF2 (indicated by the arrow) to the affinity columns. After two, four, and six washes (W2, W4, and W6, respectively), SORF2 is retained and eluted (E) with the GST-GH fusion protein but not with GST alone.
Figure 2.
Coimmunoprecipitation analysis of SORF2 and GH. Lysates from uninfected and MDV-infected cells were precipitated with antibody to SORF2 or GH and separated on a SDS/PAGE. Alternative labeling of GH or SORF2 was used because both proteins are of similar molecular weight. Anti-SORF2 antibody coimmunoprecipitates 35S-labeled GH only in MDV-infected cells where SORF2 is expressed. Likewise, the ability for anti-GH antibody to coimmunoprecipitate 35S-labeled SORF2 depends on the addition of GH (unlabeled) to lysates from MDV-infected cells.
Expression of SORF2 and GH in MDV-Infected Chickens.
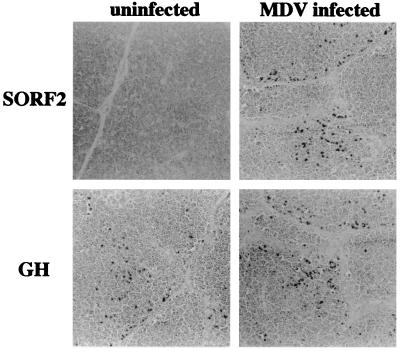
To determine whether GH and SORF2 are expressed in vivo, various tissues with or without gross lesions were examined. Moderate to strong signals for SORF2 were found in organs from 10-week-old line 7 birds challenged with JM/102W (passage 13) at 1 day of age as judged by immunohistochemical staining (Fig. 3). Positive staining for SORF2 was not observed in tissues from unchallenged birds or when the primary antibody was omitted. As judged by the distribution of SORF2 staining, vagus and feather follicles exhibited fewer SORF2-expressing cells compared with MDV-infected spleen, thymus, and bursa (data not shown).
Figure 3.
SORF2 and GH are expressed in MDV-infected tissues. Organs from uninfected and MDV infected birds were sectioned and stained with anti-SORF2 antibody or anti-GH antibody to detect SORF2 or GH expression, respectively. All of the examples shown are from the thymus. (Magnification: 20 × 1.25.)
GH is known widely to be secreted from the pituitary gland. However, it has been reported that GH also is expressed in immune-responding tissues (40). To confirm this observation, histochemical staining of tissues from uninfected and MDV-infected birds was performed. Positive staining for GH in unchallenged birds was observed (Fig. 3) as previously reported (40), indicating that GH is not expressed solely from the pituitary gland. Furthermore, similar positive GH staining also was clearly detected in tissues (e.g., spleen, thymus, ovary, kidney, and liver) from MDV-infected birds, demonstrating that MDV infection does not inhibit GH expression.
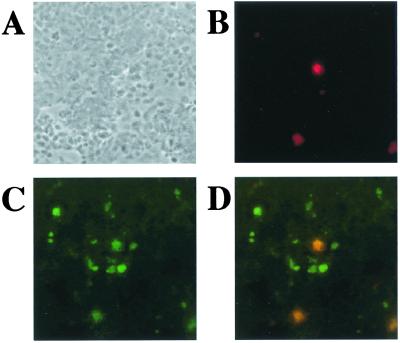
To examine potential colocalization in vivo, double staining for GH and SORF2 using indirect immunofluorescence assay in tissues from MDV-infected chickens was performed. Secondary antibodies to GH and SORF2 were conjugated with Texas red and FITC fluorescent labels, respectively. The result was examined by confocal microscopy. As shown (Fig. 4), some cells stained positive for both GH and SORF2. This result indicates that both proteins can be coexpressed in the same cell and supports the potential interaction of the two proteins in vivo.
Figure 4.
GH and SORF2 can be colocalized in MD tumors. A MDV-induced tumor of the spleen was frozen, sectioned, and incubated with primary and secondary antibodies (SORF2, FITC; GH, Texas red), and visualized by confocal microscopy. (A) Phase contrast of the section. (B) The same section as in A except showing GH expression. (C) The same section as in A except showing SORF2 expression. (D) Images in B and C superimposed. (Magnification: ×100.)
GH1 Is Associated with MD Resistance.
There is growing evidence that GH modulates the immune system in many species (41, 42), and it has been reported that the frequency of GH1 alleles change in chicken strains in response to selection for MD resistance (43). To rule out the possibility that the alteration in GH1 allele frequency in chicken strains was caused by random genetic drift, genetic mapping was performed to determine whether GH1 was linked to a previously identified MD QTL (19, 20, 44). Linkage analysis of a MspI polymorphism in the East Lansing reference panel (ref. 36 and http://poultry.mph.msu.edu) placed GH1 on linkage group E59 as predicted by conserved linkage to SLC4A1 (45). This region shows conserved synteny to human chromosome 17. Unfortunately, this region was not surveyed in the MD QTL scans.
An association study was conducted in a commercial White Leghorn resource population. This population was derived from matings between two pure lines that have relatively high levels of inbreeding (60–80%) and exhibit differences in resistance to MD. Each one of the 274 progeny (BC1) females in the two families was challenged with the highly virulent MDV strain 648A and measured for MHC genotype and traits indicative or associated with MD (e.g., vagus enlargement). The disease incidence was very high (97%) and the mean length of survival was 63 days. The phenotypic correlations for the MD traits were significant only between number of tissues with tumors and all other traits except length of survival (data not shown). A PCR-restriction fragment length polymorphism assay that detects a SacI polymorphism in the fourth intron was used to determine the GH1 genotype (Fig. 5). The incidence of MD associated traits ranged from 13% (gonad tumors) to 66% (vagus lesions). Although the MHC is known to influence genetic resistance to MD, even with B*2 (MD resistant) and B*15 (MD susceptible) haplotypes segregating, the MHC had no influence on any MD-associated trait in this population (Table 1) demonstrating the complexity of MD resistance. No significant associations were identified when all animals were analyzed together. However, when subdivided by MHC genotype, the B*2/B*15 chicks showed a significant association (P ≤ 0.01; R2 = 6%) for the number of tissues with tumors, and near significant association for enlargement of the vagus nerve (P ≤ 0.06; R2 = 3%) and length of survival (P ≤ 0.07; R2 = 4%) (Table 1).
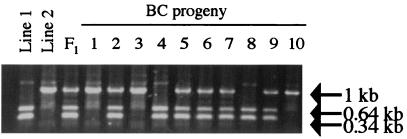
Figure 5.
PCR-restriction fragment length polymorphism analysis of GH1 in the MD resource family. Polymorphisms were obtained by digestion of PCR products with SacI restriction enzyme followed by resolution with agarose gel electrophoresis. The BC progeny display all three possible GH genotypes as other parents (not shown) used to generate the resource population were often heterozygous.
Table 1.
Effect of MHC genotype or GH genotype within MHC genotype on MD-associated traits in MD resource population
| Trait | MHC genotype
|
GH genotype in B2/B2 chicks
|
GH genotype in B2/B15 chicks
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B2/B2 | B2/B15 | P value | A/A | A/B | B/B | P value | A/A | A/B | B/B | P value | |
| Number of chicks | 121 | 141 | NA | 6 | 57 | 58 | NA | 12 | 82 | 47 | NA |
| Mean survival (days) | 67 | 67 | 0.84 | 61 | 70 | 65 | 0.46 | 81 | 63 | 68 | 0.07 |
| % with vagus lesions | 71 | 62 | 0.17 | 83 | 72 | 68 | 0.67 | 58 | 70 | 49 | 0.06 |
| % with sciatic lesions | 21 | 20 | 0.66 | 16 | 19 | 24 | 0.80 | 8 | 25 | 13 | 0.11 |
| % with heart tumors | 33 | 38 | 0.35 | 33 | 30 | 35 | 0.80 | 25 | 42 | 34 | 0.39 |
| % with gonad tumors | 11 | 14 | 0.51 | 16 | 9 | 14 | 0.66 | 17 | 16 | 11 | 0.69 |
| % with proventriculus tumors | 19 | 18 | 0.81 | 33 | 14 | 22 | 0.36 | 8 | 23 | 11 | 0.12 |
| Mean number of tissues with tumors per chick | 1.7 | 1.7 | 0.86 | 2.0 | 1.6 | 1.8 | 0.48 | 1.4 | 1.9 | 1.3 | 0.01 |
ANOVA for continuous traits and χ2 for nonparametric traits. NA, not applicable.
Discussion
Genetic resistance to MD and the accompanying immune response to MDV infection is complex as it controlled by many genes, each of small effect, that may be influenced by environmental conditions and other genes. The requirement of animal experiments, variation in viral replication and other disease-related traits even between individuals from inbred lines, and the lack of reagents especially to avian cytokines make it even more challenging to identify the molecular determinants and mechanisms. Consequently, although there has been considerable increase in knowledge of MDV virulence, and genetic differences in MD resistance are consistently demonstrated, functional pathways still remain largely unknown and no gene has been identified to confer MD resistance.
The best understood factor for MD genetic resistance is the MHC. Identified initially as the polymorphic B blood group (46), this gene cluster contains numerous genes encoding antigen processing and presenting molecules as well as other immunologically related genes. Congenic chicken lines varying only in B haplotype have clearly demonstrated the influence the amount of the MHC on the incidence of MD after challenge with MDV (47). B alleles do not influence MDV replication early in infection; thus, the MHC is likely to play a key role in the timing of the induction of the immune response and the efficiency of the response. Certain B haplotypes express lower levels of MHC class I, and this reduced expression has been associated with increased resistance to MD (48). Vaccinal immunity is strongly influenced by B haplotype (49, 50) although cell-mediated immunity as bursectomy has no effect (51).
Genes outside the MHC also are known to influence MD resistance. As a group, these non-MHC genes contribute more to MD genetic resistance than the MHC itself (52). Chickens with the same MHC B*2 haplotype [e.g., lines 6 (MD resistant) and 7 (MD susceptible)] have been shown to exhibit great differences in MDV viremia levels, confirming the influence of non-MHC genes on viral replication or spread (53, 54). This non-MHC genetic resistance may be because of differences in innate immunity or the efficiency of the immune response as B and T lymphocytes are more abundant and adjacent to cells with productive MDV infections in line 7 chickens compared with similarly treated line 6 chickens (55). After MDV infection, lymphocytes from line 6 were more able to reduce the number of viral plaques and exhibited cell-mediated cytotoxicity against MD tumor cells than did lymphocytes from line 7 (53). This observation suggests that non-MHC genes have both cellular antiviral and antitumor activities. Previous experiments have identified non-MHC QTL that account for variation in the number or distribution of tumors whereas other QTL influence MDV viremia levels (19, 20).
GH1 is proposed as being one of these non-MHC genes that can account for part of the genetic resistance to MDV infection. The direct involvement of GH with regard to MD is supported by the interaction of GH with MDV SORF2. This finding also argues against the idea that a gene genetically linked to GH1 is responsible for the MD resistance effect.
The ability to observe an effect of GH1 only in one MHC genotype might be caused by conditional neutrality, a situation where the gene effect is expressed only under certain conditions. The MHC is an appropriate example because, even though there is a clear B haplotype effect on MD resistance, several studies have shown that this effect depends on the genetic background. For example, our results demonstrate a lack of MHC effect on MD incidence even though the B*2 and B*15 haplotypes, described previously as “moderately resistant” and “susceptible” alleles to MD, respectively (56), are segregating. When line M (B*2, B*13, B*14, and B*21) was mated to line G (B*2), B haplotype effects were observed as progeny inheriting the B*2, B*13, B*14, and B*21 alleles had 27%, 42%, 43%, and 9% incidences of MD, respectively (57). However, when line M was mated to another strain, line R (B*15), there was no significant B haplotype effect as the progeny displayed similar MD incidences of 21%, 29%, 17%, and 30% for the B*2, B*13, B*14, and B*21 alleles, respectively (57). In fact, it could be suggested that the B*21 haplotype exhibits antagonistic pleiotrophy because the MD incidence rankings are reversed in the two matings. Similarly, the MD-resistant Cornell strain C is another example of context dependency as it contains the B*13 haplotype, which is supposedly “MD susceptible” (58). These studies and others have led to the conclusion that the existence and possible direction of effects for specific B haplotypes on MD resistance needs to be evaluated in each breeding line (59). Epistatic effects also could be a contributing factor, which has been observed with our MD QTL (19). Also, although similar to the phenotypic variation accounted for by the 14 QTL conferring resistance to MD previously identified (20), the effect of GH1 (6%) is small, and thus difficult to detect especially if the size of effect diminishes in other genetic backgrounds. In short, QTL conferring resistance to MD appear to act like QTL in other species in that they can be of small effect, and the size and direction of the effect may depend on the genetic background or interaction with other genes. These context-specific (e.g., sex, environment, or genotype) and epistatic effects probably will be observed more frequently in poultry as our power to identify and resolve gene effects increases, as has been the case for model organisms such as Drosophila (60).
SORF2 and GH were shown to be coexpressed in MDV-infected cells. However, as yet there is no information on the molecular basis of how these proteins interact and how this interaction influences MD resistance. GH is known to modulate the immune response and a variety of direct and indirect influences have been shown (41, 42). Higher GH levels activate and stimulate the proliferation of more B and T lymphoctyes both in vitro and in vivo in humans and mammals (61–64). GH also increases the ability of T cells to traffic to the peripheral lymphoid organs and has been suggested to produce a microenvironment conducive for virally induced lymphocyte transformation (63).
Chickens selected for MD resistance are smaller in body and proportional lymphoid organ weight (65), which implies an association between GH levels and MDV replication and/or tumor formation. This association is further supported by the observation that sex-linked dwarf chickens, which have a defective GH receptor, have increased MD resistance (66). The primary cells infected and transformed by MDV are chicken lymphocytes, and these cells both produce and secrete GH (40). GH production appears to have an autocrine effect because GH antisense oligonucleotides block GH synthesis and lymphocyte proliferation, which can be overcome by the addition of either GH sense oligonucleotides or exogenous GH (67). Therefore, local levels of GH may be critical in immunoregulation. It is possible that GH levels may increase after MDV infections, similar to the stimulation of T cell mitogens on mammalian lymphocytes where the number of GH-secreting cells and the amount of GH released increases (68). Human monocytes treated with GH are more susceptible to herpes simplex virus type 1 in vitro (69), suggesting that elevated GH levels increase the susceptibility of lymphocytes to MDV infection. This prediction is supported by our DNA microarrays studies where GH1 is one of 55 genes differentially expressed between line 6 and 7 lymphocytes after MDV infection with line 7 (MD susceptible) having higher GH expression (unpublished results).
Chicken GH can be found in monomers, dimers, and trimers as well as other size and charge variants (70). These multimeric forms may represent a mixture of GH monomers with different posttranslational modifications (71). The distribution of the GH size variants changes with the age of the chicken. This finding is highly relevant as GH variants display different biological activity (72). GH levels might influence GH activity by altering the ratio of structural variants. Therefore, it is possible that GH variants may explain why GH1 heterozygous individuals have a different phenotype than either GH1 homozygous class, assuming that GH1 alleles varying in GH levels or temporal expression.
Administration of GH antisera in mice causes thymic atrophy (73), and GH promotes thymic growth, the proper architecture and repopulation after damage (74). In chickens infected with the RM1 mutant (which expresses elevated levels of SORF2), one could expect that unbound GH would be lower than in birds infected with other MDV strains. Thus, it is possible that the modulation of normal GH movement or activity by SORF2 binding is related to the T cell depletion and severe thymic atrophy observed as phenotypes for the RM1 strain.
In conclusion, we have presented three independent observations that suggest GH is involved in resistance to MD. First, the yeast two-hybrid system revealed an interaction between GH and MDV SORF2. Second, immunohistochemical staining and indirect immunofluorescence assay indicate that GH and SORF2 can be coexpressed in MDV-infected cells in vivo. And third, an independent and extensive mapping project using outbred commercial chicken lines identified an association between GH1 and various MD-associated traits. Previous publications from several laboratories also have implied an association between GH and MD resistance, and a large amount of literature demonstrates a role of GH in the immune response. Although none of these studies by themselves conclusively confirm the association between GH and MD resistance, each study points to the same conclusion, that GH is a factor in the resistance to MD in the chicken. Despite the fact that GH accounts for only a small percentage of the phenotypic variation for MD resistance, it provides a point of reference on a biological pathway specifying genetic resistance to MD on which to base further investigations that dissect the complex immune response to MD.
Acknowledgments
We thank Laurie Molitor and Valencia Rilington for excellent technical support, Luc Berghman for the anti-GH mAb, and Jerry Dodgson and Lyman Crittenden for helpful advice and comments on the manuscript. This work was supported in part by funding from the United States Department of Agriculture National Research Initiative Competitive Grants Program (Grant 99-35205-8259 to H.H.C.).
Abbreviations
- GH
growth hormone
- GH1
GH gene
- GST
glutathione S-transferase
- MD
Marek's disease
- MDV
MD virus
- QTL
quantitative trait loci
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Roenigk W P. Poultry Sci. 1999;78:722–728. doi: 10.1093/ps/78.5.722. [DOI] [PubMed] [Google Scholar]
- 2.Churchill A E, Biggs P M. Nature (London) 1967;215:528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- 3.Calnek B W, Witter R L. In: Diseases of Poultry. Calnek B W, Barnes H J, Beard C W, McDougald L R, Saif Y M, editors. Ames: Iowa State Univ. Press; 1997. pp. 369–413. [Google Scholar]
- 4.Purchase H G. Cancer Res. 1970;30:1898–1908. [PubMed] [Google Scholar]
- 5.Payne L N. In: Marek's Disease. Payne L N, editor. Boston: Martinus Nijhoff; 1985. pp. 43–75. [Google Scholar]
- 6.Buscaglia C, Calnek B W. J Gen Virol. 1988;69:2809–2818. doi: 10.1099/0022-1317-69-11-2809. [DOI] [PubMed] [Google Scholar]
- 7.Volpini L M, Calnek B W, Sekellick M J, Marcus P I. Vet Microbiol. 1995;47:99–109. doi: 10.1016/0378-1135(95)00056-g. [DOI] [PubMed] [Google Scholar]
- 8.Schat K A, Calnek B W, Fabricant J. Infect Immun. 1981;31:199–207. doi: 10.1128/iai.31.1.199-207.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee L F, Sharma J M, Nazerian K, Witter R L. J Immunol. 1978;120:1554–1559. [PubMed] [Google Scholar]
- 10.Calnek B W, Adldinger H K, Kahn D E. Avian Dis. 1970;14:219–233. [PubMed] [Google Scholar]
- 11.Okazaki W, Purchase H G, Burmester B R. Avian Dis. 1970;14:413–429. [PubMed] [Google Scholar]
- 12.Witter R L, Nazerian K, Purchase H G, Burgoyne G H. Am J Vet Res. 1970;31:525–538. [PubMed] [Google Scholar]
- 13.Witter R L. Avian Dis. 1997;41:149–163. [PubMed] [Google Scholar]
- 14.Kreager K. In: Diagnosis and Control of Neoplastic Disease of Poultry. Fadly A M, Schat K A, Spencer J L, editors. Kennett Square, PA: American Association of Avian Pathologists; 1997. pp. 23–26. [Google Scholar]
- 15.Witter R L. Poultry Sci. 1998;77:1197–1203. doi: 10.1093/ps/77.8.1197. [DOI] [PubMed] [Google Scholar]
- 16.Von Krosigk C M, McClary C F, Vielitz E, Zander D V. Avian Dis. 1972;16:11–19. [PubMed] [Google Scholar]
- 17.Spencer J L, Gavora J S, Grunder A A, Robertson A, Speckmann G W. Avian Dis. 1974;18:33–44. [PubMed] [Google Scholar]
- 18.Gavora J S, Spencer J L. Comp Immun Microbiol Infect Dis. 1979;2:359–371. doi: 10.1016/0147-9571(79)90022-5. [DOI] [PubMed] [Google Scholar]
- 19.Vallejo R L, Bacon L D, Liu H-C, Witter R L, Groenen M A M, Hillel J, Cheng H H. Genetics. 1998;148:349–360. doi: 10.1093/genetics/148.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonash N, Bacon L D, Witter R L, Cheng H H. Anim Genet. 1999;30:126–135. doi: 10.1046/j.1365-2052.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 21.Demant P, Hart A A M. Immunogenetics. 1986;24:416–422. doi: 10.1007/BF00377961. [DOI] [PubMed] [Google Scholar]
- 22.Bacon L D, Hunt H D, Cheng H H. Poultry Sci. 2000;79:1082–1093. doi: 10.1093/ps/79.8.1082. [DOI] [PubMed] [Google Scholar]
- 23.Suchyta S P, Cheng H H, Burnside J, Dodgson J B. Anim Genet. 2001;32:12–18. doi: 10.1046/j.1365-2052.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- 24.Isfort R J, Jones D, Kost R G, Witter R L, Kung H. Proc Natl Acad Sci USA. 1992;89:991–995. doi: 10.1073/pnas.89.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones D, Brunovskis P, Witter R, Kung H-J. J Virol. 1996;70:2460–2467. doi: 10.1128/jvi.70.4.2460-2467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witter R L, Li D, Jones D, Lee L F, Kung H-J. Avian Dis. 1997;41:407–421. [PubMed] [Google Scholar]
- 27.Parcells M S, Anderson A S, Cantello J L, Morgan R W. J Virol. 1994;68:8239–8253. doi: 10.1128/jvi.68.12.8239-8253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang H-K, Ono M, Kim T-J, Kzumiya Y, Damiani A M, Matsumura T, Niikura M, Kai C, Mikami T. Virus Res. 1998;58:137–147. doi: 10.1016/s0168-1702(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 29.Parcells M S, Anderson A S, Morgan R W. J Virol. 1995;69:7888–7898. doi: 10.1128/jvi.69.12.7888-7898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunovskis P, Velicer L F. Virology. 1995;206:324–338. doi: 10.1016/s0042-6822(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 31.Tulman E R, Afonso C L, Lu Z, Zsak L, Rock D L, Kutish G F. J Virol. 2000;74:7980–7988. doi: 10.1128/jvi.74.17.7980-7988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomley F, Binns M, Campbell J, Boursnell M. J Gen Virol. 1988;69:1025–1040. doi: 10.1099/0022-1317-69-5-1025. [DOI] [PubMed] [Google Scholar]
- 33.Stasiak P C, Mocarski E S. J Virol. 1992;66:1050–1058. doi: 10.1128/jvi.66.2.1050-1058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanshanchi R, Thompson J, Sadaie M R, Doniger J, Duvall J, Brady J N, Rosenthal L J. Virology. 1994;201:95–106. doi: 10.1006/viro.1994.1269. [DOI] [PubMed] [Google Scholar]
- 35.Nicholas J, Martin M E E. J Virol. 1994;68:597–610. doi: 10.1128/jvi.68.2.597-610.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crittenden L B, Provencher L, Santangelo L, Levin I, Abplanalp H, Briles R W, Briles W E, Dodgson J B. Poultry Sci. 1993;72:334–348. [Google Scholar]
- 37.Manly K F. Mamm Genome. 1993;4:303–313. doi: 10.1007/BF00357089. [DOI] [PubMed] [Google Scholar]
- 38.SAS Institute. JMP Statistics and Graphics Guide. Cary, NC: SAS Institute; 1995. , Version 3.1. [Google Scholar]
- 39.Tanaka M, Hosokawa Y, Watahiki M, Nakashima K. Gene. 1992;112:235–239. doi: 10.1016/0378-1119(92)90382-y. [DOI] [PubMed] [Google Scholar]
- 40.Render C L, Hull K L, Harvey S. Endocrine. 1995;3:729–735. doi: 10.1007/BF03000205. [DOI] [PubMed] [Google Scholar]
- 41.Gala R R. Proc Soc Exp Biol Med. 1991;198:513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- 42.Auernhammer C J, Strasburger C J. Eur J Endocrinol. 1995;133:635–645. doi: 10.1530/eje.0.1330635. [DOI] [PubMed] [Google Scholar]
- 43.Kuhnlein U, Ni L, Weigend S, Gavora J S, Fairfull W, Zadworny D. Anim Genet. 1997;28:116–123. doi: 10.1111/j.1365-2052.1997.00076.x. [DOI] [PubMed] [Google Scholar]
- 44.Bumstead N. Avian Pathol. 1998;27:S78–S81. [Google Scholar]
- 45.Toye A A, Van Hest B J, Sheldon B L, Moran C. Anim Genet. 1997;28:457–458. doi: 10.1111/j.1365-2052.1997.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 46.Briles W E, McGibbon W H, Irwin M R. Genetics. 1950;35:633–652. doi: 10.1093/genetics/35.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacon L D. Poultry Sci. 1987;66:802–811. doi: 10.3382/ps.0660802. [DOI] [PubMed] [Google Scholar]
- 48.Kaufman J, Volk H, Wallny H-J. Immunol Rev. 1995;143:63–68. doi: 10.1111/j.1600-065x.1995.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 49.Bacon L D, Witter R. Avian Dis. 1994;38:65–71. [PubMed] [Google Scholar]
- 50.Bacon L D, Witter R. Poultry Sci. 1994;73:481–487. doi: 10.3382/ps.0730481. [DOI] [PubMed] [Google Scholar]
- 51.Else R W. Vet Res. 1974;95:182–187. doi: 10.1136/vr.95.9.182. [DOI] [PubMed] [Google Scholar]
- 52.Groot A J C, Albers G A A. Proc World's Poultry Congress. 1992;1:185–188. [Google Scholar]
- 53.Lee L F, Powell P C, Rennie M, Ross L J N, Payne L N. J Natl Cancer Inst. 1981;66:789–796. [PubMed] [Google Scholar]
- 54.Bumstead N, Sillibourne J, Rennie M, Ross N, Davison F. J Virol Methods. 1997;65:75–81. doi: 10.1016/s0166-0934(96)02172-6. [DOI] [PubMed] [Google Scholar]
- 55.Baigent S J, Davison T F. Avian Pathol. 1999;28:287–300. doi: 10.1080/03079459994786. [DOI] [PubMed] [Google Scholar]
- 56.Longenecker B M, Tosmann T R. Immunogenetics. 1981;13:1–23. doi: 10.1007/BF00524601. [DOI] [PubMed] [Google Scholar]
- 57.Hartmann W. Prog Clin Biol Res. 1989;307:221–231. [PubMed] [Google Scholar]
- 58.Bacon L D, Polley C R, Cole R K, Rose N R. Immunogenetics. 1981;12:339–349. doi: 10.1007/BF01561675. [DOI] [PubMed] [Google Scholar]
- 59.Blankert J J, Albers G A A, Briles W E, Vrielink-van Ginkel M, Groot A J C, te Winkel GP, Tilanus M G J, van der Zijpp A J. Avian Dis. 1990;54:818–823. [PubMed] [Google Scholar]
- 60.Mackay T F C. Nat Rev Genet. 2001;2:11–20. doi: 10.1038/35047544. [DOI] [PubMed] [Google Scholar]
- 61.Villanua M A, Szary A, Bartke A, Esquilino A L. J Endocrinol Invest. 1992;15:587–595. doi: 10.1007/BF03344930. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida A, Ishioka C, Kimata H, Mikawa H. Acta Endocrinol. 1992;126:524–529. doi: 10.1530/acta.0.1260524. [DOI] [PubMed] [Google Scholar]
- 63.Murphy W J, Durum S K, Anver M, Frazier M, Longon D L. Brain Behav Immun. 1992;6:355–364. doi: 10.1016/0889-1591(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 64.Kimata H, Yoshida A. J Clin Endocrinol Metab. 1994;78:635–641. doi: 10.1210/jcem.78.3.8126135. [DOI] [PubMed] [Google Scholar]
- 65.Gavora J S, Spencer J L. Anim Blood Groups Biochem Genet. 1983;14:159–180. doi: 10.1111/j.1365-2052.1983.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 66.Merat P. World's Poultry J. 1984;40:10–18. [Google Scholar]
- 67.Weigent D A, Blalock J E, LeBoeuf R D. Endocrinology. 1991;128:2053–2057. doi: 10.1210/endo-128-4-2053. [DOI] [PubMed] [Google Scholar]
- 68.Baxter J B, Blalock J E, Weigent D A. J Neuroimmunol. 1991;33:43–54. doi: 10.1016/0165-5728(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 69.Cipollaro de L'Ero G, Marcatili A, Folgore A, Donnarumma G, Petrillo G. Microbiologica. 1998;21:213–220. [PubMed] [Google Scholar]
- 70.Houston B, Goddard C. J Endocrinol. 1988;116:35–41. doi: 10.1677/joe.0.1160035. [DOI] [PubMed] [Google Scholar]
- 71.Aramburo C, Luna M, Carranza M, Reyes M, Martinez-Coria H, Scanes C G. Proc Soc Exp Biol Med. 2000;223:67–74. doi: 10.1046/j.1525-1373.2000.22309.x. [DOI] [PubMed] [Google Scholar]
- 72.Aramburo C, Campbell R M, Scanes C G. Life Sci. 1989;45:2201–2207. doi: 10.1016/0024-3205(89)90060-x. [DOI] [PubMed] [Google Scholar]
- 73.Pierpaoli W, Sorkin E. J Immunol. 1968;101:1036–1043. [PubMed] [Google Scholar]
- 74.Beschorner W E, Divic J, Pulido H, Yao X, Kenworthy P, Bruce G. Transplantation. 1991;52:879–884. doi: 10.1097/00007890-199111000-00024. [DOI] [PubMed] [Google Scholar]