Abstract
Purpose
Neovascular age-related macular degeneration (AMD) is a major cause of legal blindness in the elderly. Diets with omega3-long-chain-polyunsaturated-fatty-acid (ω3-LCPUFA) correlate with a decreased risk of AMD. Dietary ω3-LCPUFA versus ω6-LCPUFA inhibits mouse ocular neovascularization, but the underlying mechanism needs further exploration. The aim of this study was to investigate if adiponectin (APN) mediated ω3-LCPUFA suppression of neovessels in AMD.
Methods
The mouse laser-induced choroidal neovascularization (CNV) model was used to mimic some of the inflammatory aspect of AMD. CNV was compared between wild-type (WT) and Apn−/− mice fed either otherwise matched diets with 2% ω3 or 2% ω6-LCPUFAs. Vldlr−/− mice were used to mimic some of the metabolic aspects of AMD. Choroid assay ex vivo and human retinal microvascular endothelial cell (HRMEC) proliferation assay in vitro was used to investigate the APN pathway in angiogenesis. Western blot for p-AMPKα/AMPKα and qPCR for Apn, Mmps, and IL-10 were used to define mechanism.
Results
ω3-LCPUFA intake suppressed laser-induced CNV in WT mice; suppression was abolished with APN deficiency. ω3-LCPUFA, mediated by APN, decreased mouse Mmps expression. APN deficiency decreased AMPKα phosphorylation in vivo and exacerbated choroid-sprouting ex vivo. APN pathway activation inhibited HRMEC proliferation and decreased Mmps. In Vldlr−/− mice, ω3-LCPUFA increased retinal AdipoR1 and inhibited NV. ω3-LCPUFA decreased IL-10 but did not affect Mmps in Vldlr−/− retinas.
Conclusions
APN in part mediated ω3-LCPUFA inhibition of neovascularization in two mouse models of AMD. Modulating the APN pathway in conjunction with a ω3-LCPUFA-enriched-diet may augment the beneficial effects of ω3-LCPUFA in AMD patients.
Keywords: age-related macular degeneration, omega-3 long-chain polyunsaturated fatty acids, adiponectin, neovascularization
Choroidal neovascularization (CNV) and retinal angiomatous proliferation (RAP) in age-related macular degeneration (AMD) is a major cause of vision loss in elderly people. CNV leads to exudation, hemorrhage, neural retinal dysfunction, and eventually loss of central vision. Therefore, prevention and suppression of CNV is of great interest to improve quality of life. The causes of CNV still remain incompletely defined, but genetic deficiencies, inflammation, extracellular matrix (ECM) integrity and remodeling, and dietary lipid and metabolites could all contribute to the development of CNV.1–3 Dietary lipids and their metabolites have been implicated to alter many of these processes.1,2 In this work we focus on the joint actions of fatty acids and adiponectin (APN) on neovascular AMD and their downstream mediators, inflammation and ECM integrity.
In clinical investigations, dietary intake of fish containing omega 3-long-chain-polyunsaturated-fatty-acid (ω-3 LCPUFA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) is associated with a decreased risk of AMD.4–7 The Age-Related Eye Disease Study (AREDS) reported 30% less central geographic atrophy and neovascular AMD in participants with a diet containing high ω-3 LCPUFA, DHA, and EPA (0.106% of total energy intake) than those with low ω-3 LCPUFA intake (0.013% of total energy intake).8 In a large cohort of female health professionals in the United States, there was a 42% reduced incidence of AMD with higher (two servings of fish per week) versus lower fish (and ω-3 LCPUFA) intake.9 Some studies show that there is no improvement in AMD progression associated with DHA and EPA capsule supplementation, although these patients had a high baseline serum ω-3 LCPUFA level or were otherwise well-nourished.10–12 The effects of ω-3 LCPUFA on modulating neovessel formation needs further exploration, particularly for patients on a Western diet with low ω-3 LCPUFA intake.13,14 Previous studies show that a ω-3 LCPUFA-enriched diet reduces laser-induced CNV in mice15–17; however, the complete underlying mechanisms are still unknown.16–19
APN plays anti-angiogenic and anti-inflammatory roles in vascular diseases.20,21 In the mouse model of oxygen-induced proliferative retinopathy, APN is involved in ω-3 LCPUFA's inhibition of retinal neovascularization.22 Therefore, the APN pathway may be involved in ω-3 LCPUFA's protection in neovascular AMD. We hypothesized that the APN signaling pathway mediates ω-3 LCPUFA protection against choroidal/retinal NV. We explored this question in two animal models: the laser-induced CNV model and very low density lipoprotein receptor deficient mice (Vldlr−/−) with retinal angiomatous proliferation (RAP) and CNV.
Methods
Ethics Statement
All animal studies adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at Boston Children's Hospital (ARCH protocol number 16-06-3155R). C57BL/6J and Apn−/− (backcrossed with C57BL/6J for 11 generations) from the Jackson Laboratory were used.
Laser-Induced CNV in Mice
Four laser burns were induced by a green Argon laser pulse (Micron IV, Phoenix Research Laboratories, Pleasanton, CA, USA) with duration of 70 ms and power of 240 mW in 6- to 8-week-old C57BL/6J and Apn−/− mice. Mice were fed defined rodent diets with either 2% ω-3 (1% DHA and 1% EPA) or 2% ω-6 LCPUFA (AA)23 (Supplementary Table S1) 7 days before and after laser photocoagulation. ARASCO (42% AA), DHASCO (43% DHA), and MEG-3 (45% EPA and 24% DHA), respectively, were obtained from DSM Nutritional Products (TE Heerlen, Netherlands) and were integrated into the rodent feed at Research Diets (New Brunswick, NJ, USA). The eyes were enucleated and fixed in 4% paraformaldehyde (PFA) for 1 hour at room temperature. The choroid was penetrated with 1% Triton X-100 PBS for 1 hour at room temperature and stained overnight with fluorescent Griffonia Bandeiraea Simplicifolia Isolectin B4 (Alexa Fluor 594, I21413, Molecular Probes, Grand Island, NY, USA; 10 μg/mL) in 1 mM CaCl2 in 1% Triton X-100 PBS. The choroid was washed with PBS and whole mounted, and images were taken at 100× or 200× magnification on a Zeiss AxioObserver.Z1 microscope. Lesion area was quantified and exclusion criteria were followed per previous publications.15,24
Very Low Density Lipoprotein Receptor Deficient (Vldlr−/−) Mice
Vldlr−/− mice develop pathological RAP similar to AMD.25 Neovessels extend from the deep retinal vascular layer of the outer plexiform layer (OPL) toward the retinal pigment epithelium (RPE). Mice were fed defined rodent diets with either 2% ω-3 (DHA and EPA) or 2% ω-6 LCPUFA (AA)23 from postnatal day (P) 1. At P16, the eyes were enucleated and fixed in 4% PFA for 1 hour at room temperature. The retinas were dissected and stained overnight with fluorescent Griffonia Bandeiraea Simplicifolia Isolectin B4 (Alexa Fluor 594, I21413, Molecular Probes, 10 μg/mL) in 1 mM CaCl2 in PBS, then whole mounted with photoreceptors facing up. Images were taken at 50× magnification on a Zeiss AxioObserver.Z1 microscope and merged to form one image with AxioVision 4.6.3.0 software. Vascular lesions were analyzed using the SWIFT_MACTEL method, a plugin in ImageJ.25
Choroid Assay Ex Vivo
Three-week-old C57BL/6J and Apn−/− mice were killed. RPE/choroid/sclera complex (“choroid explants”) from the peripheral area was dissected and cut into approximately 1 × 1 mm pieces.26 The choroid explants were immediately embedded in 30 μL growth factor–reduced Matrigel (BD Biosciences, San Jose, CA, USA; Cat. 354230) in 24-well tissue culture plates (day 0, D0). The explants were grown in CSC complete medium (Cell Systems, Kirkland, WA, USA; Cat. 420-500) supplemented with growth factor Boost and 1% Penicillin/Streptomycin (GIBCO, Grand Island, NY, USA; Cat. 15142) at 37°C with 5% CO2. At D6, images were taken under 25× magnification. The sprouting area was quantified using ImageJ.
Human Retinal Microvascular Endothelial Cell (HRMEC) Proliferation Assay
HRMECs (passage 7 or 8) were grown in EGM2 and 1% antibiotic-antimycotic (GIBCO, #15240) on 0.1% gelatin-coated 96-well cell culture plate. HRMECs were treated with APN receptor agonist adipoRon (2.5, 5.0, 10, and 25 μM) and vehicle (0.1% DMSO) for 24 hours. Ten microliters MTT reagent (ATCC, #30-1010K) was added to 100 μL EGM2 in each well. After 6-hour incubation at 37°C, cells were washed with PBS and 100 μL DMSO was added per well. The absorbance was recorded at 570 nm.
HRMECs (passage 7 or 8) were grown on 0.1% gelatin-coated six-well cell culture plate and treated with APN receptor agonist adipoRon (25 μM) or vehicle for 2 hours. The cells were collected for RNA extraction.
Real-Time PCR
Freshly isolated sclera/choroid/RPE/retina complex or retinas or HRMECs were lysed with QIAzol lysis reagent and incubated on ice for 15 minutes, and 20% chloroform was added and incubated for 5 minutes at room temperature. The mixture was centrifuged at 12,000g for 15 minutes, and the supernatant was collected for RNA extraction according to the manufacturer's instructions using a PureLink RNA Mini Kit (#12183018A; Ambion, Grand Island, NY, USA). RNA was then reverse transcribed using iScript cDNA synthesis kit (#1708891; Bio-Rad, Hercules, CA, USA). The sequences of primers were Apn (F: 5′-GAA GCC GCT TAT GTG TAT CGC-3′, R: 5′-GAA TGG GTA CAT TGG GAA CAG T-3′); AdipoR1 (F: 5′-TCT TCG GGA TGT TCT TCC TGG-3′, R: 5′-TTT GGA AAA AGT CCG AGA GAC C-3′); Vegfa (F: 5′-GGA GAT CCT TCG AGG AGC ACT T-3′, R: 5′-GCG ATT TAG CAG CAG ATA TAA GAA-3′); Mmp2 (F: 5′-TGG GAG CAT GGA GAT GGA TAC-3′, R: 5′-AAG TGA GAA TCT CCC CCA ACA C-3′); Mmp9 (F: 5′-ACT GCG GGC TCT TCT GAG G-3′, R: 5′-CCC TGG ATC TCA GCA ATA GCA-3′); hMmp2 (F: 5′-TAC AGG ATC ATT GGC TAC ACA CC-3′, R: 5′-GGT CAC ATC GCT CCA GAC T-3′); hMmp9 (F: 5′-TGT ACC GCT ATG GTT ACA CTC G-3′, R: 5′-GCA GGG ACA GTT GCT TCT-3′). Quantitative analysis of gene expression was generated using an Applied Biosystems (Grand Island, NY, USA) 7300 Sequence Detection System with the SYBR Green Master mix kit, and gene expression was calculated relative to internal control Cyclophilin A (F: 5′-CAG ACG CCA CTG TCG CTT T-3′; R: 5′-TGT CTT TGG AAC TTT GTC TGC AA-3′) for mouse retinas and 18S ribosomal RNA (F: 5′-ACGGAAGGGCACCACCAGGA-3′, R: 5′-CACCACCACCCACGGAATCG-3′) for HRMECs using the ΔΔCt method. The relative mRNA levels were presented as the ratio of change versus internal control.
Western Blot
Choroidal-retinal explants were homogenized and protein was extracted in radioimmunoprecipitation assay buffer (RIPA) (#89900; Pierce, Grand Island, NY, USA) supplemented with phosphatase inhibitor (1:100, P0044, Sigma-Aldrich Corp., St. Louis, MO, USA) and protease inhibitor (1:1000, Sigma, P8340). Forty micrograms protein lysate were used to detect the levels of phosphor-AMPKα27 (p-AMPKα, 1:500, #2535; Cell Signaling, Beverly, MA, USA) and AMPKα28 (1:500, #2532, Cell Signaling) overnight at 4°C. Signals were detected using 1:5000 corresponding horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL, Pierce). GAPDH (1:3000, sc-32233; Santa Cruz, Dallas, TX, USA) was used as internal control.
Statistical Analysis
Animal data are presented as mean ± SEM. All data were used except for low quality images that were not sufficient for analysis. Both male and female mice were used. For drug treatments, the mice were randomly assigned to treatment and vehicle control in the same litter. For in vitro study, the experiment was repeated two or three independent times. Two-tailed unpaired t-test, ANOVA with Bonferroni's multiple comparison test was used for comparison of results as specified in the figure legends (Prism v5.0; GraphPad Software, Inc., San Diego, CA, USA). Statistically significant difference was set at P ≤ 0.05.
Results
Dietary Intervention of ω-3 Versus ω-6 LCPUFA Decreased Laser-Induced CNV in Mice
In the mouse model of laser-induced CNV, there was a 20% decreased lesion area (P = 0.0435. Fig. 1; Supplementary Table S2) in mice with ω-3 versus ω-6 LCPUFA-enriched diets, as shown in our previous reports.15–17
Figure 1.
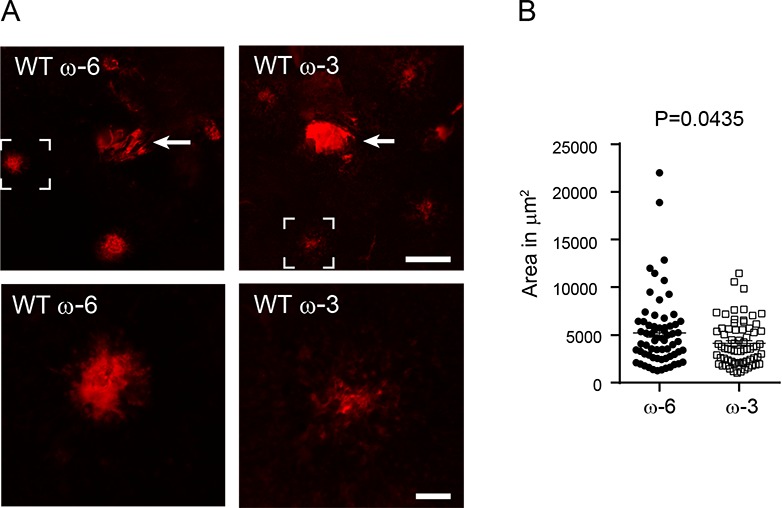
Dietary ω-3 LCPUFA versus ω-6 LCPUFA suppressed laser-induced CNV. Eight-week-old C57BL/6J (WT) mice were fed ω-3 or ω-6 LCPUFA-enriched diets starting 1 week before and continuing until 1 week after laser-induced CNV (day 0). At day 7, choroidal neovessels were examined. (A) Representative neovessel lectin-stained (red) choroidal whole-mounts. The brackets in the top panel are shown at the bottom. (B) Significantly reduced lesion areas were found in ω-3 versus ω-6 LCPUFA-fed retinas. n = 12 to 14 mice per group. Unpaired t-test. Arrow (top): optic nerve head. Scale bar: 200 μm (top); 50 μm (bottom).
APN Pathway Mediated ω-3 LCPUFA Protection Against Laser-Induced CNV
In laser-induced CNV with dietary ω-3 LCPUFA supplementation, the lesion area was increased 60% in Apn−/− versus WT mice (P = 0.0033, Figs. 2A–B). However, APN deficiency did not affect lesion area in mice fed a ω-6 LCPUFA-enriched diet (Figs. 2C–D), indicating that APN played a major role in ω-3 LCPUFA dependent inhibition in CNV. We also examined the lesion severity in mice fed animal chow (with undefined composition) provided by our animal facility. APN deficiency worsened CNV lesion formation (P = 0.0438; Supplementary Fig. S2A; Supplementary Table S3) and activation of the APN pathway with APN receptor agonist AdipoRon administration significantly reduced the lesion size (P = 0.0233; Supplementary Fig. S2B; Supplementary Table S3).
Figure 2.
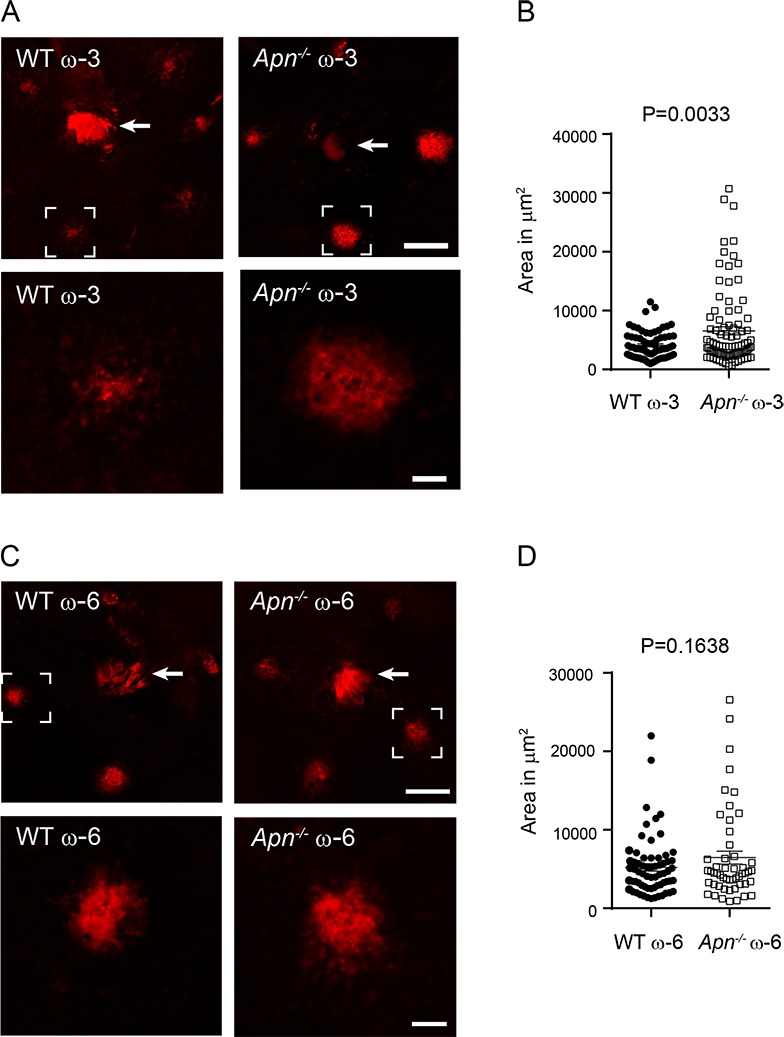
APN deficiency abolished dietary ω-3 LCPUFA protection against laser-induced CNV. Eight-week-old Apn−/− and WT mice were fed ω-3 or ω-6 LCPUFA-enriched diets from 1 week before until 1 week after laser-induced CNV (day 0). At day 7, choroidal neovessels were examined. Representative lectin-stained (red) vessels in choroidal whole-mounts (A, C) and quantification of choroidal lesion area (B, D). The brackets in the top panel are shown at the bottom (A, C). Significantly larger lesions were observed in ω-3 LCPUFA-fed Apn−/− versus WT mice but not in ω-6 LCPUFA-fed Apn−/− versus WT mice (B, D). n = 9 to 14 mice per group (ω-3). n = 8 to 12 mice per group (ω-6). Unpaired t-test. Arrow (top): optic nerve head. Scale bar: 200 μm (top, panel A, C); 50 μm (bottom, panel A, C).
Activation of the APN Pathway by Dietary ω-3 LCPUFA Supplementation Inhibited Matrix Metalloproteinase (MMP) Expression in Laser-Induced CNV
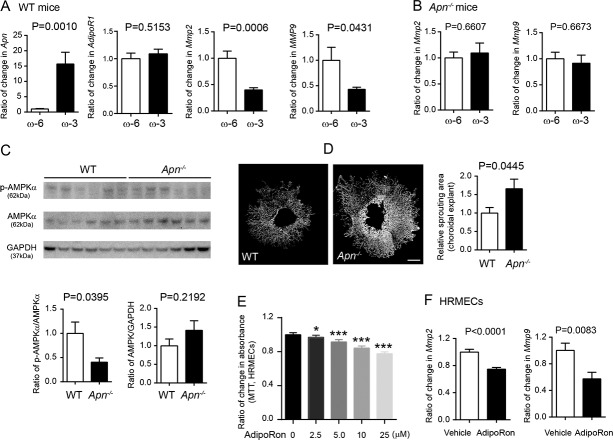
To explore the underlying mechanism of APN pathway activation causing CNV suppression, we examined changes in potential APN downstream targets Mmp2 and Mmp9 in WT and Apn−/− mice with either ω-3 or ω-6 LCPUFA-enriched diets. ω-3 LCPUFA supplementation increased Apn mRNA in WT choroid-retina complex (P = 0.0010; Fig. 3A). Dietary ω-3 versus ω-6 LCPUFA decreased Mmp2 and Mmp9 in WT choroid-retina complex (Mmp2: ω-3 to ω-6 = 0.4, P = 0.0006; Mmp9: ω-3 to ω-6 = 0.43, P = 0.0431; Fig. 3A). The decreases were abolished with APN deficiency (Mmp2: ω-3 to ω-6 = 1.1, P = 0.6607; Mmp9: ω-3 to ω-6 = 0.91, P = 0.6673; Fig. 3B). We then investigated the downstream target of the APN pathway AMPKα,29,30 which transcriptionally regulates the expression of MMPs.31,32 A decreased choroidal-retinal p-AMPKα/AMPKα ratio (Apn−/− to WT = 0.4) was found in Apn−/− versus WT CNV mice fed with ω-3 LCPUFA (Fig. 3C). Our findings suggested that dietary ω-3 LCPUFA supplementation activated the APN pathway to phosphorylate AMPKα and reduce MMP2 and MMP9 levels, which in turn inhibited neovessel formation.
Figure 3.
The APN pathway inhibited neovessel formation through the suppression of MMP expression in laser-induced CNV. (A) WT mice fed ω-3 versus ω-6 LCPUFA-enriched diets had increased Apn, and decreased Mmp2 and Mmp9, as well as unchanged AdipoR1 expression in choroidal-retinal explants. n = 3 mice per group. Unpaired t-test. (B) APN deficiency abolished ω-3 LCPUFA inhibition of choroidal-retinal Mmp2 and Mmp9 levels. n = 3 to 4 mice per group. Unpaired t-test. (C) APN deficiency decreased the levels of phosphorylated AMPKα in choroidal-retinal CNV explants. n = 6 mice per group. Unpaired t-test. (D) In the choroidal sprouting assay ex vivo, choroidal explants from Apn−/− mice showed larger sprouting areas than WT mice. n = 4 to 6 explants per group. Unpaired t-test. Scale bar: 500 μm. (E) In HRMEC MTT assay in vitro, activation of the APN pathway with APN receptor agonist adipoRon inhibited endothelial cell proliferation. n = 10 replicates per group. Unpaired t-test. (F) AdipoRon treatment (25 μM) decreased Mmp2 and Mmp9 expression in HRMECs. Results were repeated in two independent experiments. Unpaired t-test.
Activation of the APN Pathway Inhibited Endothelial Cell Activity Ex Vivo and In Vitro
The inhibitory effects of the APN pathway on ocular vessel growth were further confirmed in the choroidal sprouting assay ex vivo26 and HRMECs proliferation assay in vitro. APN deficiency resulted in a larger choroid sprouting area (Apn−/− to WT = 1.67; P = 0.0445; Fig. 3D). Activation of the APN pathway with adipoRon (a dual agonist for APN receptor ADIPOR1 and ADIPOR2)33 dose-dependently inhibited HRMEC proliferation (Fig. 3E). AdipoRon treatment significantly decreased Mmp2 (AdipoRon to vehicle = 0.57; P = 0.0083; Fig. 3F) and Mmp9 expression in HRMECs in vitro (AdipoRon to vehicle = 0.75; P < 0.0001; Fig. 3F). The results were consistent with activation of the APN pathway mediating ω-3 LCPUFA inhibition of laser-induced CNV through activation of AMPKα and reduction in Mmp2 and Mmp9 mRNA expression (Fig. 4).
Figure 4.
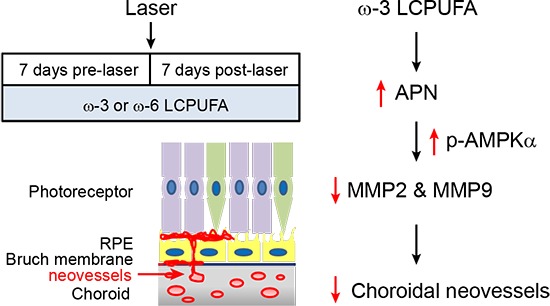
Schematic of the APN pathway in mediating ω-3 LCPUFA inhibition on laser-induced CNV.
Dietary ω-3 LCPUFA Supplementation Suppressed Lesion Formation in Vldlr−/− Mice
In addition to the inflammatory contribution to CNV formation,34 metabolic alterations may also lead to disease progression of AMD.35,36 In Vldlr−/− mice, the absence of VLDLR is associated with RAP,25 similar to that seen with neovascular AMD.37 To assess if dietary intervention of ω-3 LCPUFA also attenuated vascular lesion formation caused by dysregulated metabolism, Vldlr−/− mice were fed either ω-3 or ω-6 LCPUFA-enriched diets from P1. The mother's milk reflects the lipid content of their diet.23 In Vldlr−/− mice with ω-3 versus ω-6 LCPUFA diets, there was no difference in retinal Apn but an increase in AdipoR1 mRNA levels (ω-3 to ω-6 = 3.9; P = 0.008; Fig. 5A). Retinal AdipoR1 was increased during development from P3 to P17, and induced in Vldlr−/− versus Vldlr-+/+ mice (Fig. 5B). At P16, dietary ω-3 versus ω-6 LCPUFA decreased the number of neovascular lesions by 18% (P = 0.0099) and total lesion area by 29% (P = 0.0095) (Figs. 5C–E). Interestingly, ω-3 versus ω-6 LCPUFA diets did not impact retinal Mmp2 and Mmp9 expression in Vldlr−/− mice (Fig. 5F). Instead, there were increased anti-inflammatory marker IL-10 mRNA levels in ω-3 versus ω-6 LCPUFA-fed Vldlr−/− mice (ω-3 to ω-6 = 13.3; P = 0.0009; Fig. 5F). Activation of the APN pathway with adipoRon (50 mg/kg, oral gavage daily from P3–16) also increased IL-10 expression (Fig. 5G) and decreased Mmp2 and Mmp9 (Supplementary Fig. S3) in Vldlr−/− mouse retinas. However, in the laser-induced CNV model, ω-3 versus ω-6 LCPUFA did not change IL-10 expression in WT choroid-retina complex (ω-3 to ω-6 = 0.67; P = 0.1554; Fig. 5H). In mice fed with animal chow provided by animal facility with undefined composition, APN deficiency increased gene expression of Mmp2 and Mmp9 but not IL-10 in laser-induced CNV (Supplementary Fig. S4). These observations suggested that activation of the APN pathway might target different downstream pathways to modulate neovascularization in different models, such as laser-induced CNV and Vldlr−/− mice. APN is one but not the only way mediating omega-3 protective effects in Vldlr−/− mice.
Figure 5.
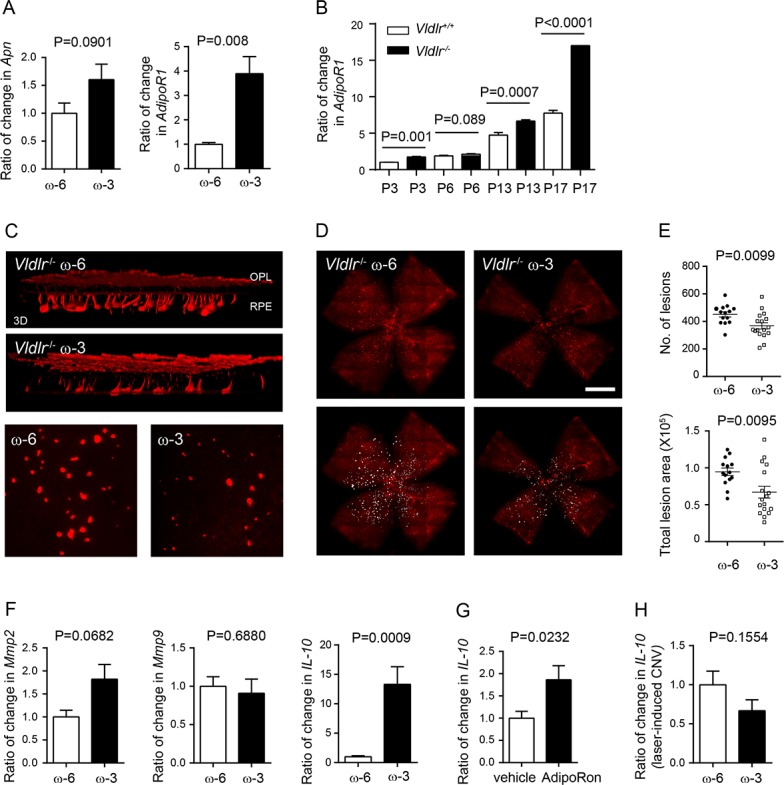
Dietary ω-3 LCPUFA decreased retinal neovascular lesions in Vldlr−/− mice. Vldlr−/− mice were fed either ω-3 or ω-6 LCPUFA-enriched diets from postnatal day (P) P1. (A) In Vldlr−/− retinas, ω-3 LCPUFA induced gene expression of APN receptor adipoR1 but not Apn (n = 3 to 4 mice per group). Unpaired t-test. (B) Increased AdipoR1 mRNA levels in Vldlr−/− versus Vldlr+/+ retinas from postnatal day 3 to 17. Unpaired t-test. (C) At P16, retinal neovascularization was examined. 3D reconstruction demonstrated blood vessels invading from the OPL toward RPE; representative images of neovascular lesion (200× magnification). (D) Representative images of isolectin-stained vessels (red) in retinal whole mounts. (E) Dietary ω-3 versus ω-6 LCPUFA supplementation decreased the number and total lesion area of neovascular lesions (n = 7 to 10 mice per group). Unpaired t-test. (F) ω-3 versus ω-6 LCPUFA did not affect retinal Mmp2 and Mmp9 expression in Vldlr−/− mice. Retinal IL-10 was increased in ω-3 LCPUFA-fed Vldlr−/− mice. n = 3 to 4 mice per group. Unpaired t-test. (G) Change in IL-10 in Vldlr−/− mice treated with either 50 mg/kg adipoRon or vehicle from P3 to 15. n = 3 to 4 retinas per group. (H) In the model of laser-induced CNV, there was no change in IL-10 expression in choroidal-retinal complex from WT mice fed ω-3 versus ω-6 LCPUFA-enriched diets. n = 3 mice per group. Unpaired t-test.
Discussion
Many signaling pathways including lipids are associated with the development of CNV.38–40 Because many clinical studies suggest a strong protective effect of dietary ω-3 LCPUFA on neovascular AMD, it is particularly important to understand the underlying mechanisms. Laser-induced CNV is a widely used model of neovascularization driven by inflammation.34,35 Vldlr−/− mice as a model of metabolically driven neovascular AMD have abnormal lipid metabolism and photoreceptor energy deficits that drive RAP (and CNV).25 Our data showed that ω-3 LCPUFA protects against AMD-like neovascularization in these two animal models through the activation of the APN pathway.
There are indications in other systems that ω-3 LCPUFA and APN are linked. APN is an important metabolic modulator mainly derived from white adipocytes.41 Dietary intake of ω-3 LCPUFA increases circulating APN levels in premature infants, diabetic patients, and the elderly.42–45 ω-3 LCPUFA activates peroxisome proliferator-activated receptor γ, an upstream transcriptional regulator of APN expression.18,46 Dietary supplementation of ω-3 LCPUFA reduces white-adipose endoplasmic reticulum stress and increases the production of APN to inhibit hypoxia-induced retinal neovascularization in vivo and white adipocytes in vitro.22 Interestingly, mutations in APN or ADIPOR1 are associated with severe AMD.47,48
Exploring the role of APN and neovascularization, we examined retinal vascular development. AdipoR1 gradually increased during retinal vascular formation in both Vldlr−/− and WT mice from P3 to P17, demonstrating a key role of the APN pathway during the period of normal retinal neurovascular development.49 Vldlr−/− had consistently higher expression of AdipoR1 during this period, suggesting that the APN/AdipoR1 pathway might also be involved in the pathologic NV in Vldlr−/− retinas. ω-3 LCPUFA versus ω-6 LCPUFA increased APN receptor mRNA AdipoR1 in WT mice, suggesting that ω-3 LCPUFA could act through APN. In laser-induced CNV, APN deficiency abolished dietary ω-3 LCPUFA-induced inhibition of CNV formation but did not affect CNV in mice fed a ω-6 LCPUFA-enriched diet, confirming that APN mediated ω-3 and not ω-6 LCPUFA prevention of CNV.
APN receptor activation is known to promote anti-angiogenic, and anti-inflammatory functions.20,21,50,51 However, the neovascular inhibitory effects of APN activation were independent of vascular endothelial growth factor A (VEGFA) in both models (Supplementary Fig. S1). Interestingly, although APN mediated the protective effect of dietary ω-3 LCPUFA on neovascularization in both the laser-induced CNV model and in Vldlr−/− mice, the downstream mechanisms were different. ω-3 LCPUFA inhibited laser-induced CNV through the suppression of MMP2 and MMP9 but inhibited NV in Vldlr−/− mice through the induction of IL-10. In laser-induced CNV mice, APN deficiency reduced AMPKα phosphorylation and completely reversed ω-3 LCPUFA-induced effects on MMP2 and MMP9, proteases which degrade ECM and basement membrane, to promote tumor growth and angiogenesis.52–55 MMP2 and MMP9 are expressed in vitreous56 and retina57,58 in human eyes with neovascular AMD and are genetically associated with AMD.59,60 Plasma MMP9 levels are higher in subjects with CNV versus control groups.61 Inhibition of AMPK activity partially abolished metformin inhibition of Mmp2 and Mmp9 mRNA levels.31 Phosphorylation of AMPKα leads to the reduction in MMP9 levels in mouse embryonic fibroblasts.32 AMPKα phosphorylation is increased with activation of the APN pathway.62,63 Therefore, ω-3 LCPUFA, mediated by APN, inhibits MMPs production. As previously reported, ω-3 LCPUFA is associated with decreased MMP2 and MMP9 activity.64–67
Surprisingly, unlike in laser-induced CNV, MMP2 and MMP9 were not decreased in Vldlr−/− mice on a ω-3 LCPUFA diet. However, the anti-inflammatory factor IL-10 was increased 13-fold in ω-3 LCPUFA versus ω-6 LCPUFA-fed Vldlr−/− mice. A dual agonist of AdipoR1/R2, AdipoRon, increased IL-10 in Vldlr−/− mice, suggesting that the inhibitory regulation of ω-3 LCPUFA on CNV was through APN/AdipoR1 and IL-10. There was no measurable change in IL-10 expression in ω-3 LCPUFA versus ω-6 LCPUFA-fed laser-induced CNV mice, suggesting that ω-3 LCPUFA may exert protective effects through APN but with different downstream mechanisms in the two neovascular AMD models with respect to inflammation and metabolic alterations.
Further work is needed to explore how to manipulate the APN pathway to facilitate ω-3 LCPUFA's effect in CNV prevention. Our observations suggest that APN could potentially be a biomarker for the effects of dietary ω-3 LCPUFA in AMD progression, and indicate a plausible target to treat AMD and other choroidal/retinal neovascular diseases. The current treatment for CNV, anti-VEGF therapy, does not treat all patients effectively and requires repeated intravitreous injections, associated with complications.68–70 The inhibitory effects of ω-3 LCPUFA in AMD seen in the present study were independent of VEGFA. The potential use of ω-3 LCPUFA and pharmaceutical modifiers of its protective effects on neovascular AMD is of great interest. Increased dietary ω-3 LCPUFA consumption in conjunction with the modulation of the APN pathway may prevent disease progression in neovascular AMD through inflammation and metabolic alterations.
Supplementary Material
Acknowledgments
Supported by Grants NIH EY024864, EY017017, EY022275, P01 HD18655, BCH IDDRC, 1U54HD090255; Lowy Medical Research Institute, European Commission FP7 project 305485 PREVENT-ROP (LEHS); The Swedish Research Council (DNR# 2016-01131), and Gothenburg County Council (ALFGBG-507741) long-term support by De Blindas Vänner and Kronprinsessan Margaretas Arbetsnämnd för synskadade, European Commission FP7 project 305485 PREVENT-ROP (AH); European Commission FP7 Project 305485 PREVENT-ROP (CL); Knights Templar Eye Foundation and Blind Children's Center (ZF); Knights Templar Eye Foundation (CHL); Boston Children's Hospital OFD/BTREC/CTREC Faculty Career Development Grant (YS); The German Research Foundation (DFG), and Li2650/1-1 (RL).
Disclosure: Z. Fu, None; R. Liegl, None; Z. Wang, None; Y. Gong, None; C.-H. Liu, None; Y. Sun, None; B. Cakir, None; S.B. Burnim, None; S.S. Meng, None; C. Löfqvist, None; J.P. SanGiovanni, None; A. Hellström, None; L.E.H. Smith, None
References
- 1. Ding X, Patel M, Chan CC. . Molecular pathology of age-related macular degeneration. Progress Retinal Eye Res. 2009; 28: 1– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stahl A, Krohne TU, Sapieha P,et al. . Lipid metabolites in the pathogenesis and treatment of neovascular eye disease. British J Ophthalmol. 2011; 95: 1496– 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw PX, Stiles T, Douglas C,et al. . Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016; 3: 196– 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merle BM, Delyfer MN, Korobelnik JF,et al. . High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr. 2013; 143: 505– 511. [DOI] [PubMed] [Google Scholar]
- 5. Tan JS, Wang JJ, Flood V, Mitchell P. . Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009; 127: 656– 665. [DOI] [PubMed] [Google Scholar]
- 6. Ho L, van Leeuwen R, Witteman JC,et al. . Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol. 2011; 129: 758– 766. [DOI] [PubMed] [Google Scholar]
- 7. Seddon JM, George S, Rosner B. . Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006; 124: 995– 1001. [DOI] [PubMed] [Google Scholar]
- 8. Sangiovanni JP, Agron E, Meleth AD,et al. . ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009; 90: 1601– 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christen WG, Schaumberg DA, Glynn RJ, Buring JE. . Dietary omega-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol. 2011; 129: 921– 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel CK, Fung TH, Muqit MM,et al. . Non-contact ultra-widefield imaging of retinopathy of prematurity using the Optos dual wavelength scanning laser ophthalmoscope. Eye (Lond). 2013; 27: 589– 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kabasawa S, Mori K, Horie-Inoue K,et al. . Associations of cigarette smoking but not serum fatty acids with age-related macular degeneration in a Japanese population. Ophthalmology. 2011; 118: 1082– 1088. [DOI] [PubMed] [Google Scholar]
- 12. Souied EH, Aslam T, Garcia-Layana A,et al. . Omega-3 fatty acids and age-related macular degeneration. Ophthalmic Res. 2015; 55: 62– 69. [DOI] [PubMed] [Google Scholar]
- 13. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. . Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011; 93: 950– 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simopoulos AP. . Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006; 60: 502– 507. [DOI] [PubMed] [Google Scholar]
- 15. Gong Y, Li J, Sun Y,et al. . Optimization of an image-guided laser-induced choroidal neovascularization model in mice. PLoS One. 2015; 10: e0132643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gong Y, Fu Z, Edin ML,et al. . Cytochrome P450 oxidase 2C inhibition adds to omega-3 long-chain polyunsaturated fatty acids protection against retinal and choroidal neovascularization. Arterioscl Thromb Vascular Biol. 2016; 36: 1919– 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gong Y, Shao Z, Fu Z,et al. . Fenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesis. EBioMedicine. 2016; 13: 201– 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stahl A, Sapieha P, Connor KM,et al. . Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circul Res. 2010; 107: 495– 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sapieha P, Stahl A, Chen J,et al. . 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011; 3: 69ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higuchi A, Ohashi K, Kihara S, Walsh K, Ouchi N. . Adiponectin suppresses pathological microvessel formation in retina through modulation of tumor necrosis factor-alpha expression. Circul Res. 2009; 104: 1058– 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohashi K, Ouchi N, Matsuzawa Y. . Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012; 94: 2137– 2142. [DOI] [PubMed] [Google Scholar]
- 22. Fu Z, Lofqvist CA, Shao Z,et al. . Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr. 2015; 101: 879– 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Connor KM, SanGiovanni JP, Lofqvist C,et al. . Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature Med. 2007; 13: 868– 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lambert V, Lecomte J, Hansen S,et al. . Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nature Protocols. 2013; 8: 2197– 2211. [DOI] [PubMed] [Google Scholar]
- 25. Joyal JS, Sun Y, Gantner ML,et al. . Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nature Med. 2016; 22: 439– 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shao Z, Friedlander M, Hurst CG,et al. . Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One. 2013; 8: e69552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma ZG, Dai J, Wei WY,et al. . Asiatic acid protects against cardiac hypertrophy through activating AMPKalpha signalling pathway. Int J Biol Sci. 2016; 12: 861– 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almudena GH, Nuria B, Oscar E,et al. . Severe brown fat lipoatrophy aggravates atherosclerotic process in male mice. Endocrinology. 2016; 157: 3517– 3528. [DOI] [PubMed] [Google Scholar]
- 29. Iwabu M, Yamauchi T, Okada-Iwabu M,et al. . Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010; 464: 1313– 1319. [DOI] [PubMed] [Google Scholar]
- 30. Kadowaki T, Yamauchi T. . Adiponectin and adiponectin receptors. Endocrine Rev. 2005; 26: 439– 451. [DOI] [PubMed] [Google Scholar]
- 31. Esfahanian N, Shakiba Y, Nikbin B,et al. . Effect of metformin on the proliferation, migration, and MMP-2 and -9 expression of human umbilical vein endothelial cells. Molecular Med Rep. 2012; 5: 1068– 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morizane Y, Thanos A, Takeuchi K,et al. . AMP-activated protein kinase suppresses matrix metalloproteinase-9 expression in mouse embryonic fibroblasts. J Biol Chem. 2011; 286: 16030– 16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okada-Iwabu M, Yamauchi T, Iwabu M,et al. . A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013; 503: 493– 499. [DOI] [PubMed] [Google Scholar]
- 34. Parmeggiani F, Romano MR, Costagliola C,et al. . Mechanism of inflammation in age-related macular degeneration. Mediators Inflamm. 2012; 2012: 546786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barron MJ, Johnson MA, Andrews RM,et al. . Mitochondrial abnormalities in ageing macular photoreceptors. Invest Ophthalmol Vis Sci. 2001; 42: 3016– 3022. [PubMed] [Google Scholar]
- 36. Ghaem Maralani H, Tai BC, Wong TY,et al. . Metabolic syndrome and risk of age-related macular degeneration. Retina. 2015; 35: 459– 466. [DOI] [PubMed] [Google Scholar]
- 37. Yannuzzi LA, Negrao S, Iida T,et al. . Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001; 21: 416– 434. [DOI] [PubMed] [Google Scholar]
- 38. Wang Z, Cheng R, Lee K,et al. . Nanoparticle-mediated expression of a Wnt pathway inhibitor ameliorates ocular neovascularization. Arteriosclerosis Thromb Vascular Biol. 2015; 35: 855– 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Z, Liu CH, Sun Y,et al. . Pharmacologic activation of Wnt signaling by lithium normalizes retinal vasculature in a murine model of familial exudative vitreoretinopathy. Am J Pathol. 2016; 186: 2588– 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gong Y, Shao Z, Fu Z,et al. . Fenofibrate inhibits cytochrome P450 epoxygenase 2C activity to suppress pathological ocular angiogenesis. EBioMedicine 2016; 13: 201– 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karbowska J, Kochan Z. . Role of adiponectin in the regulation of carbohydrate and lipid metabolism. J Physiol Pharmacol. 2006; 57 suppl 6: 103– 113. [PubMed] [Google Scholar]
- 42. Nakano Y, Itabashi K, Sakurai M, Aizawa M, Dobashi K, Mizuno K. . Accumulation of subcutaneous fat, but not visceral fat, is a predictor of adiponectin levels in preterm infants at term-equivalent age. Early Hum Develop. 2014; 90: 213– 217. [DOI] [PubMed] [Google Scholar]
- 43. Ito R, Satoh-Asahara N, Yamakage H,et al. . An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. J Atherosclerosis Thromb. 2014; 21: 248– 260. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Wang C, Li L,et al. . Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br J Nutr. 2012; 108: 1455– 1465. [DOI] [PubMed] [Google Scholar]
- 45. Olza J, Mesa MD, Aguilera CM,et al. . Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin Nutr. 2010; 29: 31– 37. [DOI] [PubMed] [Google Scholar]
- 46. Liu M, Liu F. . Transcriptional and post-translational regulation of adiponectin. Biochem J. 2009; 425: 41– 52. [DOI] [PubMed] [Google Scholar]
- 47. Cao G, Chen Y, Zhang J,et al. . Effects of adiponectin polymorphisms on the risk of advanced age-related macular degeneration. Biomarkers. 2015; 20: 266– 270. [DOI] [PubMed] [Google Scholar]
- 48. Kaarniranta K, Paananen J, Nevalainen T,et al. . Adiponectin receptor 1 gene (ADIPOR1) variant is associated with advanced age-related macular degeneration in Finnish population. Neurosci Lett. 2012; 513: 233– 237. [DOI] [PubMed] [Google Scholar]
- 49. Dorrell MI, Friedlander M. . Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Progress Retinal Eye Res. 2006; 25: 277– 295. [DOI] [PubMed] [Google Scholar]
- 50. Higuchi A, Ohashi K, Shibata R, Sono-Romanelli S, Walsh K, Ouchi N. . Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arteriosclerosis Thromb Vascular Biol. 2010; 30: 46– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fu Z, Gong Y, Lofqvist C, Hellstrom A, Smith LE. . Review: adiponectin in retinopathy. Biochimica Biophys Acta. 2016; 1862: 1392– 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Egeblad M, Werb Z. . New functions for the matrix metalloproteinases in cancer progression. Nature Rev Cancer. 2002; 2: 161– 174. [DOI] [PubMed] [Google Scholar]
- 53. Mira E, Lacalle RA, Buesa JM,et al. . Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004; 117: 1847– 1857. [DOI] [PubMed] [Google Scholar]
- 54. Kvanta A, Shen WY, Sarman S, Seregard S, Steen B, Rakoczy E. . Matrix metalloproteinase (MMP) expression in experimental choroidal neovascularization. Curr Eye Res. 2000; 21: 684– 690. [PubMed] [Google Scholar]
- 55. Lambert V, Wielockx B, Munaut C,et al. . MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003; 17: 2290– 2292. [DOI] [PubMed] [Google Scholar]
- 56. De La Paz MA, Itoh Y, Toth CA, Nagase H. . Matrix metalloproteinases and their inhibitors in human vitreous. Invest Ophthalmol Vis Sci. 1998; 39: 1256– 1260. [PubMed] [Google Scholar]
- 57. Steen B, Sejersen S, Berglin L, Seregard S, Kvanta A. . Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1998; 39: 2194– 2200. [PubMed] [Google Scholar]
- 58. Zeng J, Jiang D, Liu X, Zhu X, Tang L. . Matrix metalloproteinases expression in choroidal neovascular membranes. Yan Ke Xue Bao. 2004; 20: 191– 193. [PubMed] [Google Scholar]
- 59. Fiotti N, Pedio M, Battaglia Parodi M,et al. . MMP-9 microsatellite polymorphism and susceptibility to exudative form of age-related macular degeneration. Genetics Med. 2005; 7: 272– 277. [DOI] [PubMed] [Google Scholar]
- 60. Fritsche LG, Igl W, Bailey JN,et al. . A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature Genetics. 2016; 48: 134– 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. . Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond). 2007; 21: 1511– 1515. [DOI] [PubMed] [Google Scholar]
- 62. Yamauchi T, Kadowaki T. . Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metabol. 2013; 17: 185– 196. [DOI] [PubMed] [Google Scholar]
- 63. Yamauchi T, Nio Y, Maki T,et al. . Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nature Med. 2007; 13: 332– 339. [DOI] [PubMed] [Google Scholar]
- 64. SanGiovanni JP, Chew EY. . The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Progress Retinal Eye Res. 2005; 24: 87– 138. [DOI] [PubMed] [Google Scholar]
- 65. Harris MA, Hansen RA, Vidsudhiphan P,et al. . Effects of conjugated linoleic acids and docosahexaenoic acid on rat liver and reproductive tissue fatty acids, prostaglandins and matrix metalloproteinase production. Prostaglandins Leukot Essent Fatty Acids. 2001; 65: 23– 29. [DOI] [PubMed] [Google Scholar]
- 66. Suzuki I, Iigo M, Ishikawa C,et al. . Inhibitory effects of oleic and docosahexaenoic acids on lung metastasis by colon-carcinoma-26 cells are associated with reduced matrix metalloproteinase-2 and -9 activities. Int J Cancer. 1997; 73: 607– 612. [DOI] [PubMed] [Google Scholar]
- 67. Liu XH, Rose DP. . Suppression of type IV collagenase in MDA-MB-435 human breast cancer cells by eicosapentaenoic acid in vitro and in vivo. Cancer Lett. 1995; 92: 21– 26. [DOI] [PubMed] [Google Scholar]
- 68. Dedania VS, Bakri SJ. . Sustained elevation of intraocular pressure after intravitreal anti-VEGF agents: what is the evidence? Retina. 2015; 35: 841– 858. [DOI] [PubMed] [Google Scholar]
- 69. Falavarjani KG, Nguyen QD. . Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond). 2013; 27: 787– 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cheung N, Lam DS, Wong TY. . Anti-vascular endothelial growth factor treatment for eye diseases. BMJ. 2012; 344: e2970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.