ABSTRACT
As a formin protein, Daam1 (Dishevelled-associated activator of morphogenesis 1) is reported to regulate series of cell processes like endocytosis, cell morphology and migration via its effects on actin assembly in mitosis. However, whether Daam1 plays roles in female meiosis remains uncertain. In this study, we investigated the expression and functions of Daam1 during mouse oocyte meiosis. Our results indicated that Daam1 localized at the cortex of oocytes, which was similar with actin filaments. After Daam1 morpholino (MO) microinjection, the expression of Daam1 significantly decreased, which resulted in the failure of oocyte polar body extrusion. These results might be due to the defects of actin assembly, since the decreased fluorescence intensity of actin filaments in oocyte cortex and cytoplasm were observed. However, Daam1 knockdown seemed not to affect the meiotic spindle movement. In addition, we found that fascin might be the down effector of Daam1, since the protein expression of fascin decreased after Daam1 knockdown. Thus, our data suggested that Daam1 affected actin assembly during oocyte meiotic division via the regulation of fascin expression.
KEYWORDS: actin, Daam1, fascin, meiosis, oocyte
Introduction
During mouse oocyte meiosis, the primary oocyte generates a functional haploid gamete and 2 small polar bodies with minimal cytoplasmic content, while the larger ovum preserves a haploid genome and most cytoplasmic content, which is essential for fertilization and early embryo development. When maturing in vitro, the oocyte initiates meiosis after germinal vesicle breakdown (GVBD), then the meiotic spindle forms near the center and moves to the cortex in metaphase I (MI), after polar body extrusion, the oocyte is arrested in metaphase II (MII) until fertilized. In mammalian oocyte meiotic progress, many of the events like spindle position, cortical polarization and cytokinesis depend on actin dynamics.1 For spindle position, F-actin networks are the key players in spindle migration,2,3 and cortical actin filaments reorganize and form an F-actin enriched domain termed the actin cap above the spindle. At anaphase, actin constitutes contractile ring, a pivotal organelle to the cytokinesis. When inhibiting microfilament, cleavage can be blocked, leading to the failure of polar body extrusion.4
Formins are the most important actin nucleators, which are classified into 7 subfamilies including Diaphanous (Dia), Disheveled-associated activator of morphogenesis (DAAM), formin related gene in leukocytes (FRL), formin homology domain containing protein (FHOD), formin-like protein (FMN), inverted formin (INF) and Delphilin. All of the formins have formin-homology-1 (FH1) and FH2 domians. The FH2 domain directly nucleates actin assembly, binds to actin filament barded ends and protects these ends from elongation inhibitory.5 The FH1 domain binds to profilin and enhances the actin filament elongation rate.6,7 Daam1 is a formin protein, which has been showed to regulate actin assembly through FH1 and FH2 domain.8,9 Daam1 can nucleate, elongate 8 and bundle actin,10 then regulates series of cell processes from cell shapes change to migration.11 In recent years Daam1 is also shown to be related with the pronephric tubulogenesis,12 congential heart defect,13 and required for myocardial maturation and sarcomere assembly.14 There are several proteins that communicate with Daam1 including fascin,10 Rho GTPases RhoA, Cdc42, CIP4, Src,15 Profilin,16 Piccolo,17 EphB 18 and Dvl.19 Generally, Daam1 is in an auto-inhibited conformation, resulting from the interaction between GTPase-binding domain (GBD) and Diaphanous auto-regulatory domain (DAD). Rho binding to the GBD breaks the interaction between GBD and DAD, leading to Daam1 activation, however, it is reported that Daam1 is not significantly activated by Rho binding but rather by its interaction with Dishevelled (Dvl).20 Although Daam1 is shown to be involved into multiple critical biological processes during mitosis, its physiological functions and the signaling cascade involved during oocyte meiosis have not been investigated.
Based on the previous findings, we proposed that Daam1 might act as the critical regulator in oocyte meiosis. In the present study, by using knockdown analysis, we found that Daam1 predominately expressed in mouse oocyte, and specific reduction of Daam1 expression prevented polar body emission and disrupted actin assembly through affecting fascin expression.
Results
Localization of Daam1 during mouse oocyte meiotic maturation
To investigate the expression pattern of Daam1, oocytes were cultured for 0, 4, 8, or 12 h, corresponding to the time points when most oocytes reached the GV, GVBD, MI, MII stages, respectively. Daam1 antibody was used to observe the Daam1 localization in oocytes. As Daam1 was demonstrated to be an actin related protein, we also performed double staining of Daam1 and actin. As shown in Fig. 1, Daam1 localized at the cortex of oocytes, which co-localized with actin through the whole oocyte meiosis. The distribution pattern of Daam1 indicated that Daam1 might function in meiosis through its effect on actin.
Figure 1.
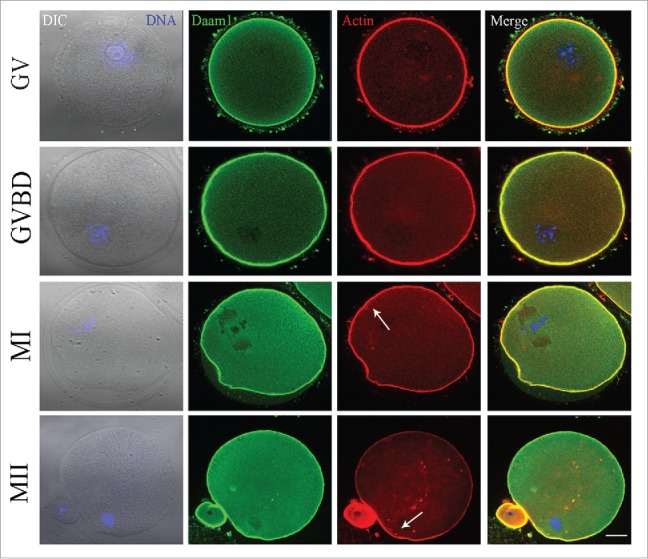
Localization of Daam1 during mouse oocyte meiotic maturation. Confocal imaging analysis of Daam1 and actin localization during mouse oocyte meiotic maturation based on staining with an anti-Daam1 antibody. Daam1 co-localized with actin at the cortex of oocyte from the GV stage to the MII stage. Arrow indicates the location of actin cap. Blue: chromatin; Green: Daam1; Red: actin. Bar = 20 μm.
Daam1 knockdown (KD) leads to the failure of first polar body extrusion in mouse oocyte
To explore the function of Daam1 during oocyte meiosis, Daam1 MO was used to decrease the expression of Daam1 protein. As shown in Fig. 2A, Daam1 expression was significantly reduced after Daam1 MO injection (1 vs 0.26 ± 0.15, n = 150, p < 0.01, western blot analysis). The oocytes injected with Daam1 MO were cultured for 12 h when the normal oocytes reached to MII stage. Fig. 2B showed that polar body extrusion was disturbed greatly compared with control groups (67.79 ± 6.34% vs 31.93 ± 18.69%, n = 50, p < 0.01). These results suggested the reduction of Daam1 caused the failure of polar body extrusion, indicating a vital role of Daam1 during mouse oocyte meiotic maturation.
Figure 2.
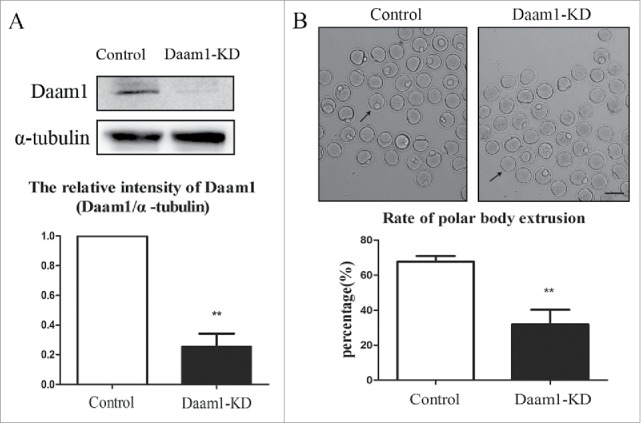
Daam1 knockdown (KD) leads to the failure of mouse oocyte first polar body extrusion. (A) After Daam1 MO injection, Daam1 protein expression was siginficantly reduced by Western blot examination. Band intensity analysis by Image J showed that the intensity of Daam1 significantly decreased after Daam1 knock down. (B) DIC picture showed that oocytes failed to extrude polar body after Daam1 MO injection. Arrows showed that the control oocytes with the polar body while the treated oocytes failed to extrude the polar body. Bar = 100 μm. Analysis of the rate of polar body extrusion showed a significantly decrease after Daam1 knock down. **:significant difference (p < 0 .01).
Daam1 regulates actin assembly but not spindle positioning
As actin dynamics and spindle positioning were essential for oocyte meiosis, we examined the actin assembly and spindle positioning in control and treated oocytes after 8 h of culture using immunofluorescent staining. As shown in Fig. 3A, Daam1 knockdown caused an arresting alteration in the arrangement of the actin cytoskeleton. Immunofluorescence analysis showed that the accumulation of actin signals in the cortex or cytoplasm were weaker in Daam1-knock down oocytes as compared with control oocytes. Furthermore, the decreased actin expression in Daam1 KD oocytes was investigated by a statistical analysis of fluorescence intensity levels. Fig. 3B showed that actin intensity in cortex of treated groups was significantly weaker than that of control groups (1 vs 0.61 ± 0.14, n = 30, p < 0.001). Similarly, actin intensity in cytoplasm of treated groups was decreased than control groups (1 vs 0.67 ± 0.17, n = 30, p < 0.01). Thus, our results suggested that Daam1 played a vital role in actin assembly during mouse oocyte meiosis.
Figure 3.
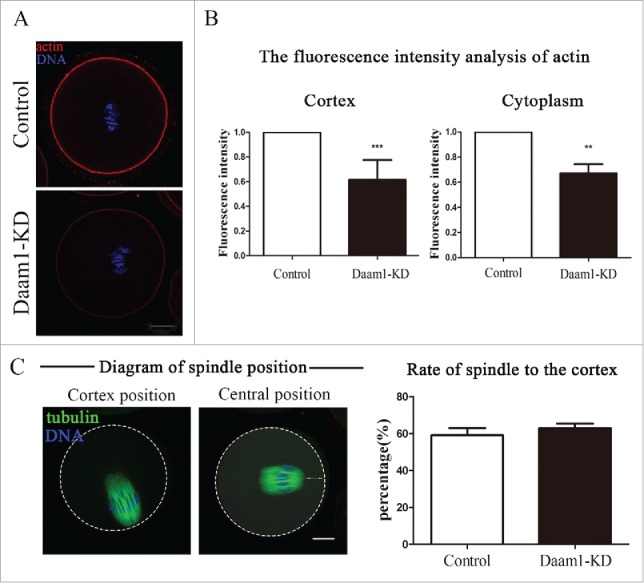
Daam1 affects actin assembly but not spindle position. (A) Actin distribution in oocyte cortex and cytoplasm at MI stage. After Daam1 knock down, the actin distribution was disrupted at both cortex and cytoplasm. Red: actin; Blue: chromatin. Bar = 20 μm. (B) The fluorescence intensity of actin decreased in cortex and cytoplasm after Daam1 knock down. ***: significant difference (p < 0.001). **: significant difference (p < 0.01). (C) For spindle location in the ooctyes, analysis of the rate of spindle to the cortex showed that there was no difference between control groups and treatment groups (p > 0.05). Green, α-tubulin; Blue: chromatin, Bar = 20 μm.
We then next examined the spindle location in the oocytes after Daam1 knock down. Fig. 3C showed the typical spindle position (cortex position and central position) in mouse oocyte during MI stage. After statistically analyzing of the spindle position, no difference was found between the control and treated groups (59.12 ± 7.68% vs 62.89 ± 5.13%, n = 35, p > 0.05). Therefore, it suggested that Daam1 did not affect spindle position.
Daam1 knockdown affects the expression of fascin in mouse oocytes
To investigate the possible mechanism of Daam1 for actin assembly, we explored the expression of fascin in oocytes after Daam1 knock down. As shown in Fig. 4, the protein expression of fascin was significantly decreased after Daam1 knock down. Western blot and quantitative analysis results showed the relative intensity (fascin/α-tubulin) was reduced, compared with the control groups (1 vs 0.44 ± 0.10, n = 100, p < 0.05). Taken together, these results suggested that Daam1 was likely essential for fascin expression during mouse oocyte meiotic maturation.
Figure 4.
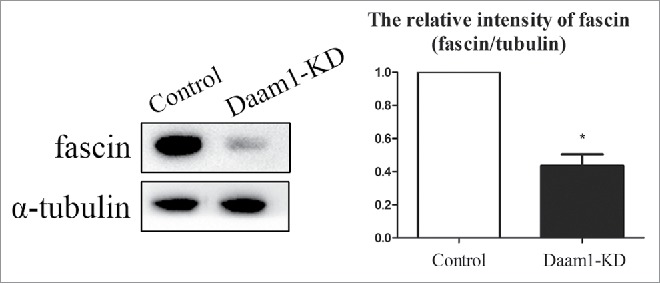
Daam1 knockdown affects the expression of Fascin in mouse oocytes. Fascin protein expression was decreased after Daam1 knock down showing with Western blot analysis. The band intensity of fascin measured by Image J also confirmed this. *: significant difference (p < 0.05).
Discussion
In this study we explored the localization and functions of Daam1 during mouse oocyte meiotic maturation. Our results showed that Daam1 localized specifically at cortex of oocyte. Daam1 MO injection affected polar body extrusion, actin assembly, and fascin expression. Therefore, the results indicated that Daam1 played a vital role in mouse oocyte maturation and Daam1 promoted meiotic division via the regulation of fascin expression.
As a formin protein which functioned in actin nucleation and elongation, Daam1 is involved in a series of cell progress associated with actin. However, its function and mechanisms during mouse oocyte meiosis have remained uncertain. In this study, we first determined whether Daam1 expressed in mouse oocytes. Our results revealed that Daam1 co-localized with actin at the cortex of oocyte, which was similar to previous results that Daam1 co-localized with actin stress fibers.21 Moreover, in primary human macrophages, endogenous Daam1 localized to filopodia and F-actin-rich uptake structures.22 Based on the localization pattern of Daam1, we speculated that Daam1 might be involved in actin-related cellular processes during mouse oocyte maturation. Then, we used Daam1 MO to explore the roles of Daam1 in mouse oocyte meiosis. And we found that compared with control oocytes, the majority of oocytes injected with Daam1 MO failed to undergo cytokinesis and extrude the polar body. Thus, these findings suggested that Daam1 was an important regulator for cytokinesis during oocyte meiosis.
As reported, the extrusion of polar body depended on cortical polarization and spindle position,4 and both of them relied on actin dynamics.2,23 In addition, previous studies demonstrated that chromosomes signal the establishment of cortical polarity.24 To figure out how Daam1 was involved in cytokinesis in mouse oocyte meiosis, we examined actin filament distribution and spindle position. Our results showed actin filament distribution in oocyte cytoplasm and cortex was significantly disrupted. Several previous reports had demonstrated that Daam1 was linked to actin dynamics.8,15,25-27 Depletion of Daam1 expression impaired presynaptic-F-actin assembly in neurons.17 Additionally, a similar phenomenon showed that the subapical actin web was disrupted when Daam1 knockdown in Xenopus epidermis.28 Active Daam1 could induce actin polymerization in endothelial cells.29 In addition, abrogation of Daam1 resulted in disorganized filamentous actin and α-tubulin in habenula.30 Thus, our results suggested that Daam1 might play conserved roles between difference species or cell types, and positively affect actin assembly, followed by disrupted polar body extrusion in mouse oocytes.
In mammalian oocytes, actin filaments drive spindle to the cortex, resulting in asymmetric division.31 Recently, a vesicle-driven mechanism for the regulation of the F-actin cytoplasmic meshwork during oocyte asymmetric division had been proposed.32 And several molecules like LIMK1/2,33 ROCK,34 WASH,35 Dynamin 2 36 were reported to regulate actin for spindle migration in oocytes. Whereas we found that Daam1 knock down did not affect spindle positioning in mouse oocytes, despite the reduction of actin intensity in cytoplasm. This result raised the question about the relationship between actin and spindle movement. Recently some actin nucleators in oocyte like Cdc42,37 N-WASP 38 were also reported to influence actin assembly but not spindle migration. One possibility is that the cytokinesis needs more actin filaments power while spindle movement needs less actin filaments power, since we could observe strong actin signal at the oocyte cortex but weak signal in the oocyte cytoplasm. While knock down of Daam1 reduced the actin filaments, however, the actin filaments are still enough for the movement of the spindle. Further work needs to be performed to study how exactly actin pushes the spindle to the cortex and how actin mediates cytokinesis in mammalian oocytes.
We also explored the possible signaling pathway for Daam1 functions in oocytes, and we found that fascin expression was decreased after Daam1 KD. Fascin was a actin-binding protein, it was shown to cross-link actin filaments into unipolar and tightly packed bundles.39,40 Besides, fascin was demonstrated to have a vital role in cell adhesion and motility.41 A recent study also reported that Daam1 and fascin collaborated to promote filopodia formation.10 One possibility is that Daam1 is necessary for the binding of fascin to the actin filaments: once there is no Daam1, the assembly and transport of facsin protein is disturbed since the lack of signal. Further study is needed to explore the accurate interaction and mechanism between Daam1 and fascin. Therefore, we suggested that Daam1 cooperated with fascin to affect oocyte maturation.
In conclusion, our results suggested that Daam1 affected actin assembly for polar body extrusion during oocyte maturation via the regulation of fascin.
Materials and methods
Antibodies and chemicals
Goat polyclonal anti-Daam1 antibody for immunofluorescence staining was purchased from Santa Cruz (Santa Cruz, CA). Phalloidin-TRITC and mouse monoclonal anti-α-tubulin-FITC antibodies were obtained from Sigma (St. Louis, MO, USA). FITC-conjugated rabbit anti-goat IgG was from Zhongshan Golden Bridge Biotechnology (Beijing). Mouse monoclonal anti-Daam1 antibody (for Western blot) and rabbit monoclonal anti-fascin antibody were purchased from Abcam (Cambridge, UK). α-tubulin (11H10) Rabbit mAb and HRP-conjugated anti-mouse IgG were from Cell Signaling Technology (Beverly, MA, USA). HRP-conjugated goat anti-rabbit IgG was bought from Vazyme (Nanjing, China). All other chemicals and reagents were from Sigma-Aldrich Corp., unless otherwise stated.
Oocyte collection and culture
All the operations with mice were conducted followed the guidelines of Animal Research Institute Committee of Nanjing Agricultural University. Germinal vesicle-intact oocytes were obtained from ovaries of 3- to 5-week old ICR mice, cultured with M16 medium (Sigma Chemical Co., St. Louis, MO) under the paraffin oil and placed at 37°C with an atmosphere of 5% CO2. The oocytes cultured to different time points were used for immunostaining, microinjection and western blot.
Daam1 morpholino microinjection
Morpholino (MO) for mouse Daam1 (5′-TGA TAT TTC TAT ACT CTA CTG GCC C-3′) was purchased from Gene Tools Corp. and diluted with reagent grade water (Sigma) to give a stock concentration of 2 mM. Each GV oocyte was microinjected with 5–10 pl MO solution by Eppendorf FemtoJet (Eppendorf AG, Hamburg, Germany) under an inverted microscope (Olympus IX53, Japan). After injection, oocytes were cultured with M16 medium containing 2.5 μM milrinone for 20–24 h, then washed 5 times (2 min each wash) and cultured in milrinone-free M16 medium under paraffin oil at 37°C in an atmosphere of 5% CO2 in air. For the control group, oocytes were microinjected with 5–10 pl water.
Confocal microscopy
For double staining of Daam1 and actin, oocytes were fixed in 4% paraformaldehyde (in PBS) for 30 min and permeabilized with 0.5% Triton X-100 in PBS for 20min, then blocked in blocking buffer (1% BSA-supplemented PBS) for 1 h at room temperature. For Daam1 staining, oocytes were incubated with goat polyclonal anti-Daam1 antibody (1:100) overnight at 4°C. After washing for 3 times (2 min each time) with wash buffer (0.1% Tween 20 and 0.01% Triton X-100 in PBS), oocytes were incubated with FITC-anti-rabbit IgG (1:100) for 1 h at room temperature. For co-staining with actin, oocytes were incubated with Phalloidin-TRITC (5 μg/ml in PBS) at room temperature for 1 h. After washing oocytes were co-staining with Hoechst 33342 for 10 min at room temperature, and then mounted on glass slides. A confocal laser-scanning microscope (Zeiss LSM 700 META, Germany) were adopted to examine the oocytes, at least 30 oocytes were tested for each group.
Western blot analysis
The oocytes for western blot were first lysed in 10 μl Laemmli sample buffer (SDS sample buffer and 2-Mercaptoethanol), then heated at 100°C for 10 min and frozen at -20°C. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 180 v for 90 min, and electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) at 20 V for 60 min. After transferring, we used TBST (TBS containing 0.1% Tween 20) containing 5% non-fat milk to block the membranes at room temperature for 1 h, then mouse monoclonal anti-Daam1 (1:1000), a rabbit monoclonal anti-fascin (1:10000) or a rabbit monoclonal anti-α-tubulin antibody (1:2000) were used to incubate the corresponding membrane overnight at 4°C. After washing in TBST for 3 times (10 min each time), the membranes were incubated with HRP-conjugated anti-mouse IgG (1:1000) and HRP-conjugated goat anti-rabbit IgG (1:5000) for 1 h at room temperature. The membranes were tested by chemiluminescence reagent (Millipore, Billerica, MA). Different samples were used to repeat experiment for at least 3 times.
Statistical analysis
For fluorescence intensity analysis, the control and treated oocytes were mounted on the same glass slide and used the same parameters to normalize across the replicates. Using Image J, the average fluorescence intensity per unit area within the region of interest (ROI) of immunofluorescence images was determined. The independent measures which used sized ROIs were used for the cell cortex. We omitted the abnormal oocytes with strong or weak fluorescence intensity. Average intensities of control and treated oocytes were made after testing all of the measurements.
For western blot results, the band intensity was measured by Image J and control band intensity was set to 1. Three replicates were used for the analysis.
Each analysis used at least 3 replicates and each replicate was finished by an independent experiment. Results were endowed as means ± SEM. Statistical evaluation of the data was performed with a 2-tailed Student's t test in prism. A p-value of < 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are particularly grateful to Sijing Song, Ruxia Jia and Yue Zhang for their technical assistance.
Funding
This work was supported by the National Natural Science Foundation of China (31622055, 31571547, 31601204); the Natural Science Foundation of Jiangsu Province, China (BK20140030; BK20150674); the Fundamental Research Funds for the Central Universities (KJJQ201501, KJTZ201602, KJYQ201701), China; the Postdoctoral Science Foundation of China (2015M580441); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2015R1D1A1A01057629) and the Biogreen 21 Program (PJ011126), RDA, Republic of Korea.
References
- [1].Azoury J, Verlhac MH, Dumont J. Actin filaments: key players in the control of asymmetric divisions in mouse oocytes. Biol Cell 2009; 101:69-76; PMID:19076067; https://doi.org/ 10.1042/BC20080003 [DOI] [PubMed] [Google Scholar]
- [2].Almonacid M, Terret ME, Verlhac MH. Actin-based spindle positioning: new insights from female gametes. J Cell Sci 2014; 127:477-83; PMID:24413163; https://doi.org/ 10.1242/jcs.142711 [DOI] [PubMed] [Google Scholar]
- [3].Woolner S, O'Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol 2008; 182:77-88; PMID:18606852; https://doi.org/ 10.1083/jcb.200804062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Maddox AS, Azoury J, Dumont J. Polar body cytokinesis. Cytoskeleton 2012; 69:855-68; PMID:22927361; https://doi.org/ 10.1002/cm.21064 [DOI] [PubMed] [Google Scholar]
- [5].Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell 2005; 16:1-13; PMID:15509653; https://doi.org/ 10.1091/mbc.E04-07-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004; 119:419-29; PMID:15507212; https://doi.org/ 10.1016/j.cell.2004.09.039 [DOI] [PubMed] [Google Scholar]
- [7].Higgs HN. Formin proteins: a domain-based approach. Trends Biochem Sci 2005; 30:342-53; PMID:15950879; https://doi.org/ 10.1016/j.tibs.2005.04.014 [DOI] [PubMed] [Google Scholar]
- [8].Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. <Structure of the FH2 domin of Daam1_implications for formin regulation of actin assembly.pdf>. J Mol Biol 2007; 369:1258-69; PMID:17482208; https://doi.org/ 10.1016/j.jmb.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vidali L, van Gisbergen PA, Guerin C, Franco P, Li M, Burkart GM, Augustine RC, Blanchoin L, Bezanilla M. Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc Natl Acad Sci U S A 2009; 106:13341-6; PMID:19633191; https://doi.org/ 10.1073/pnas.0901170106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jaiswal R, Breitsprecher D, Collins A, Correa IR Jr, Xu MQ, Goode BL. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Curr Biol 2013; 23:1373-9; PMID:23850281; https://doi.org/ 10.1016/j.cub.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kida Y, Shiraishi T, Ogura T. Identification of chick and mouse Daam1 and Daam2 genes and their expression patterns in the central nervous system. Brain Res Dev Brain Res 2004; 153:143-50; PMID:15464228; https://doi.org/ 10.1016/j.devbrainres.2004.07.014 [DOI] [PubMed] [Google Scholar]
- [12].Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol 2011; 22:1654-64; PMID:21804089; https://doi.org/ 10.1681/ASN.2010101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bao B, Zhang L, Hu H, Yin S, Liang Z <Deletion of a single-copy Daam1 gene in xongenital heart defect_ a case report.pdf>. BMC Med Genet 2012; 13:63; PMID:22857009; https://doi.org/ 10.1186/1471-2350-13-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ajima R, Bisson JA, Helt JC, Nakaya MA, Habas R, Tessarollo L, He X, Morrisey EE, Yamaguchi TP, Cohen ED. DAAM1 and DAAM2 are co-required for myocardial maturation and sarcomere assembly. Dev Biol 2015; 408:126-39; PMID:26526197; https://doi.org/ 10.1016/j.ydbio.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res 2006; 312:2180-94; PMID:16630611; https://doi.org/ 10.1016/j.yexcr.2006.03.013 [DOI] [PubMed] [Google Scholar]
- [16].Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development 2006; 133:4219-31; PMID:17021034; https://doi.org/ 10.1242/dev.02590 [DOI] [PubMed] [Google Scholar]
- [17].Wagh D, Terry-Lorenzo R, Waites CL, Leal-Ortiz SA, Maas C, Reimer RJ, Garner CC. Piccolo directs activity dependent F-actin assembly from presynaptic active zones via Daam1. PloS One 2015; 10:e0120093; PMID:25897839; https://doi.org/ 10.1371/journal.pone.0120093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci U S A 2007; 104:6708-13; PMID:17412835; https://doi.org/ 10.1073/pnas.0608946104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol 2006; 174:701-13; PMID:16943183; https://doi.org/ 10.1083/jcb.200605006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A 2008; 105:210-5; PMID:18162551; https://doi.org/ 10.1073/pnas.0707277105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ang SF, Zhao ZS, Lim L, Manser E. DAAM1 is a formin required for centrosome re-orientation during cell migration. PloS One 2010; 5:pii: e13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoffmann AK, Naj X, Linder S. Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages. FASEB J 2014; 28:3075-89; PMID:24696301; https://doi.org/ 10.1096/fj.13-247049 [DOI] [PubMed] [Google Scholar]
- [23].Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006; 131:193-205; PMID:16452714; https://doi.org/ 10.1530/rep.1.00847 [DOI] [PubMed] [Google Scholar]
- [24].Yi K, Rubinstein B, Li R. Symmetry breaking and polarity establishment during mouse oocyte maturation. Philos Trans R Soc Lond B Biol Sci 2013; 368:20130002; PMID:24062576; https://doi.org/ 10.1098/rstb.2013.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aspenstrom P, Luo W, Lieu ZZ, Manser E, Bershadsky AD, Sheetz MP. Formin DAAM1 organizes actin filaments in the cytoplasmic nodal actin network. PloS One 2016; 11:e0163915; PMID:27760153; https://doi.org/ 10.1371/journal.pone.0163915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, et al.. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem 2008; 283:8746-55; PMID:18218625; https://doi.org/ 10.1074/jbc.M707839200 [DOI] [PubMed] [Google Scholar]
- [27].Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 2006; 133:957-66; PMID:16469972; https://doi.org/ 10.1242/dev.02266 [DOI] [PubMed] [Google Scholar]
- [28].Yasunaga T, Hoff S, Schell C, Helmstadter M, Kretz O, Kuechlin S, Yakulov TA, Engel C, Muller B, Bensch R, et al.. The polarity protein Inturned links NPHP4 to Daam1 to control the subapical actin network in multiciliated cells. J Cell Biol 2015; 211:963-73; PMID:26644512; https://doi.org/ 10.1083/jcb.201502043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ju R, Cirone P, Lin S, Griesbach H, Slusarski DC, Crews CM. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis. Proc Natl Acad Sci U S A 2010; 107:6906-11; PMID:20351293; https://doi.org/ 10.1073/pnas.1001075107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Colombo A, Palma K, Armijo L, Mione M, Signore IA, Morales C, Guerrero N, Meynard MM, Perez R, Suazo J, et al.. Daam1a mediates asymmetric habenular morphogenesis by regulating dendritic and axonal outgrowth. Development 2013; 140:3997-4007; PMID:24046318; https://doi.org/ 10.1242/dev.091934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sun SC, Kim NH. Molecular mechanisms of asymmetric division in oocytes. Microsc Microanal 2013; 19:883-97; PMID:23764118; https://doi.org/ 10.1017/S1431927613001566 [DOI] [PubMed] [Google Scholar]
- [32].Holubcova Z, Howard G, Schuh M. Vesicles modulate an actin network for asymmetric spindle positioning. Nat Cell Biol 2013; 15:937-47; PMID:23873150; https://doi.org/ 10.1038/ncb2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jia RX, Duan X, Song SJ, Sun SC. LIMK1/2 inhibitor LIMKi 3 suppresses porcine oocyte maturation. PeerJ 2016; 4:e2553; PMID:27761340; https://doi.org/ 10.7717/peerj.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duan X, Liu J, Dai XX, Liu HL, Cui XS, Kim NH, Wang ZB, Wang Q, Sun SC. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol Reprod 2014; 90:37; PMID:24429217; https://doi.org/ 10.1095/biolreprod.113.113522 [DOI] [PubMed] [Google Scholar]
- [35].Wang F, Zhang L, Zhang GL, Wang ZB, Cui XS, Kim NH, Sun SC. WASH complex regulates Arp2/3 complex for actin-based polar body extrusion in mouse oocytes. Sci Rep 2014; 4:5596; PMID:24998208; https://doi.org/ 10.1038/srep05596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang QC, Liu J, Wang ZB, Zhang Y, Duan X, Cui XS, Kim NH, Sun SC. Dynamin 2 regulates actin-mediated spindle migration in mouse oocytes. Biol Cell 2014; 106:193-202; PMID:24735075; https://doi.org/ 10.1111/boc.201400007 [DOI] [PubMed] [Google Scholar]
- [37].Wang ZB, Jiang ZZ, Zhang QH, Hu MW, Huang L, Ou XH, Guo L, Ouyang YC, Hou Y, Brakebusch C, et al.. Specific deletion of Cdc42 does not affect meiotic spindle organization/migration and homologous chromosome segregation but disrupts polarity establishment and cytokinesis in mouse oocytes. Mol Biol Cell 2013; 24:3832-41; PMID:24131996; https://doi.org/ 10.1091/mbc.E13-03-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang ZB, Ma XS, Hu MW, Jiang ZZ, Meng TG, Dong MZ, Fan LH, Ouyang YC, Snapper SB, Schatten H, et al.. Oocyte-specific deletion of N-WASP does not affect oocyte polarity, but causes failure of meiosis II completion. Mol Hum Reprod 2016; 22:613-21; PMID:27401749; https://doi.org/ 10.1093/molehr/gaw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Obermann H, Raabe I, Balvers M, Brunswig B, Schulze W, Kirchhoff C. Novel testis-expressed profilin IV associated with acrosome biogenesis and spermatid elongation. Mol Hum Reprod 2005; 11:53-64; PMID:15591451; https://doi.org/ 10.1093/molehr/gah132 [DOI] [PubMed] [Google Scholar]
- [40].Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, Bathe M. Structure, evolutionary conservation, and conformational dynamics of Homo sapiens fascin-1, an F-actin crosslinking protein. J Mol Biol 2010; 400:589-604; PMID:20434460; https://doi.org/ 10.1016/j.jmb.2010.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol 2004; 16:590-6; PMID:15363811; https://doi.org/ 10.1016/j.ceb.2004.07.009 [DOI] [PubMed] [Google Scholar]


