ABSTRACT
Nitidine chloride (NC) has been reported to exert its anti-tumor activity in various types of human cancers. However, the molecular mechanism of NC-mediated tumor suppressive function is largely unclear. In the current study, we used several approaches such as MTT, FACS, RT-PCR, Western blotting analysis, invasion assay, transfection, to explore the molecular basis of NC-triggered anti-cancer activity. We found that NC inhibited cell growth, induced cell apoptosis, caused cell cycle arrest in ovarian cancer cells. Emerging evidence has demonstrated that Skp2 plays an important oncogenic role in ovarian cancer. Therefore, we also explored whether NC exerts its biologic function via downregulation of Skp2 in ovarian cancer cells. We observed that NC significantly inhibited the expression of Skp2 in ovarian cancer cells. Notably, overexpression of Skp2 abrogated the anti-cancer activity induced by NC in ovarian cancer cells. Consistently, downregulation of Skp2 expression enhanced the sensitivity of ovarian cancer cells to NC treatment. Thus, inactivation of Skp2 by NC could be a novel strategy for the treatment of human ovarian cancer.
KEYWORDS: Apoptosis, invasion, nitidine chloride, ovarian cancer, proliferation, Skp2
Introduction
Ovarian cancer is one of common and aggressive gynecological malignancies, which remains the second leading cause of cancer-related deaths among females.1 Patients with ovarian cancer often exhibit the lack of symptoms in early stage of ovarian cancer.1 Current available detection approaches in clinic failed to diagnose ovarian cancer patients in early stages. Therefore, the majority of ovarian cancer patients present with the advanced stages. The current standard therapies include surgery, platinum-based chemotherapy and immune therapy.2 The overall 5-year survival rate sof ovarian cancer patients is less than 50 % due to lack of early detection.3 The emergence of drug resistance is also a key reason for low survival rate in patients with ovarian cancer. Therefore, to achieve better treatment outcome, it is pivotal to develop or discover new agents for treating ovarian cancer patients.4
NC, a natural bioactive phytochemical alkaloid, has been initially found to exhibit antioxidant, antifungal, anti-inflammatory, and analgesic functions.5 Further studies revealed that NC has anti-tumor potential in several types of human cancers.6 For example, it has been reported that NC inhibited cell migration and invasion via suppression of c-Src/FAK associated signaling pathway in breast cancer.6 Moreover, NC inhibited the angiogenesis and cell growth through inhibition of STAT3 signaling pathway in human gastric cancer.7 In line with this, NC inhibited cell growth in vivo via suppression of JAK1/STAT3 signaling pathway in hepatocellular carcinoma.8 Furthermore, NC was found to induce apoptosis, cell cycle arrest and synergistic cytotoxicity with doxorubicin in breast cancer cells.9 Kang et al. found that NC inhibited cell growth and triggered apoptosis via upregulation of p53 in nasopharyngeal carcinoma cells.10 Similarly, NC induced apoptosis and inhibited cell growth through suppression of ERK signaling pathway in renal cancer11 and colorectal cancer.12 Although these studies dissect the mechanism of NC-mediated antitumor activity, the underlying molecular basis has not fully elucidated.
Skp2 (S-phase kinase associated protein 2) plays an important role in the development and progression of human cancers including ovarian cancer.13-15 Skp2 has been known as one of the well-characterized F-box proteins, which functions as the substrate-recruiting component of the SCF (Skp1-Cullin1-F-box complex) type of E3 ubiquitin ligase complex.15 Skp2 has been characterized as an oncoprotein that targets its substrates including p27,16,17 p21,18 p57,19 and FOXO1 (Forkhead box protein O1).20,21 In the current study, we explore whether NC could exhibit its anti-tumor activity in ovarian cancer cells. Moreover, we explore whether NC exerts its biologic function via downregulation of Skp2 in ovarian cancer cells. We observed that NC suppressed cell growth, induced cell apoptosis, inhibited cell invasion, and caused cell cycle arrest in part through downregulation of Skp2 in ovarian cancer cells. Therefore, inactivation of Skp2 by NC could be a useful approach for the treatment of human ovarian cancer patients.
Results
NC inhibited ovarian cancer cell proliferation
NC has been exhibited anti-tumor activities in various types of human cancers. In this study, we aimed to determine whether NC could suppress the cell proliferation in SKOV3 ovarian cancer cells. Ovarian cancer cells were treated with 2.5 μM, 5.0 μM, 7.5 μM, and 10 μM NC for 24 h, 48 h, and 72 h, respectively. Our MTT results demonstrated that NC significantly inhibited cell proliferation in a time- and dose-dependent manner in both ovarian cancer cell lines (Fig. 1A). Specifically, 2.5 μM and 5 μM NC treatments of 72 h led to about 40% and 60% of cell proliferation inhibition in SKOV3 and OVCAR3 ovarian cancer cells (Fig. 1A). Therefore, 2.5 μM and 5 μM of NC were selected for the following studies. Altogether, NC suppressed cell proliferation in ovarian cancer cells.
Figure 1.
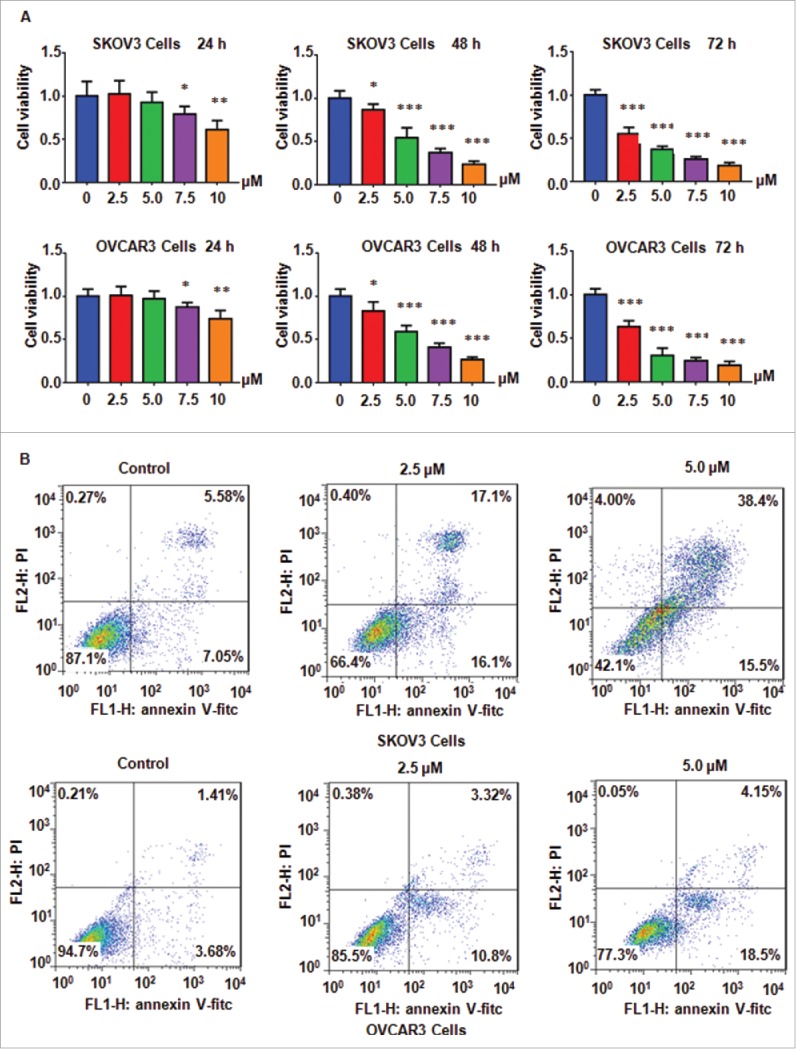
NC inhibited ovarian cancer cell proliferation and induced apoptosis. (A) Ovarian cancer cell viability was detected after treatment with NC for 24 h, 48 h and 72 h, respectively, using MTT assay. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups treated with DMSO. (B) NC-induced ovarian cancer cell apoptosis was accessed by Flow cytometry.
NC induced cell apoptosis in ovarian cancer cells
It has been known that NC induced cell apoptosis in human cancer cells. Next, we investigated whether NC could promote cell apoptosis in ovarian cancer cells. Annexin V-FITC/PI assay was performed to measure cell apoptosis in cells treated with different concentrations of NC for 48 h. Our data showed that NC induced cell apoptosis in both ovarian cancer cell lines (Fig. 1B). Briefly, 2.5 μM and 5 μM NC treatments led to cell apoptotic death from 12.6 % of control group to 33.2 % and 53.9 % in SKOV3 cells (Fig. 1B). These data indicated that NC promoted cell apoptosis in ovarian cancer cells.
NC induced cell cycle arrest in ovarian cancer cells
Cell cycle analysis was performed by PtdIns staining and flow cytometry in both ovarian cancer cells after NC treatments. We found that NC treatment caused cell cycle at G2/M phase in SKOV3 cells and OVCAR3 (Fig. 1C). Specifically, 2.5 μM and 5.0 μM NC treatment resulted in G2/M phase from 4.11 % in control group to 23.78 % and 33.07 % in SKOV3 cells, respectively (Fig. 2A). Similar results were observed in OVCAR3 cells (Fig. 2B). Our results clearly demonstrated that NC induced cell cycle at G2/M phase in ovarian cancer cells.
Figure 2.
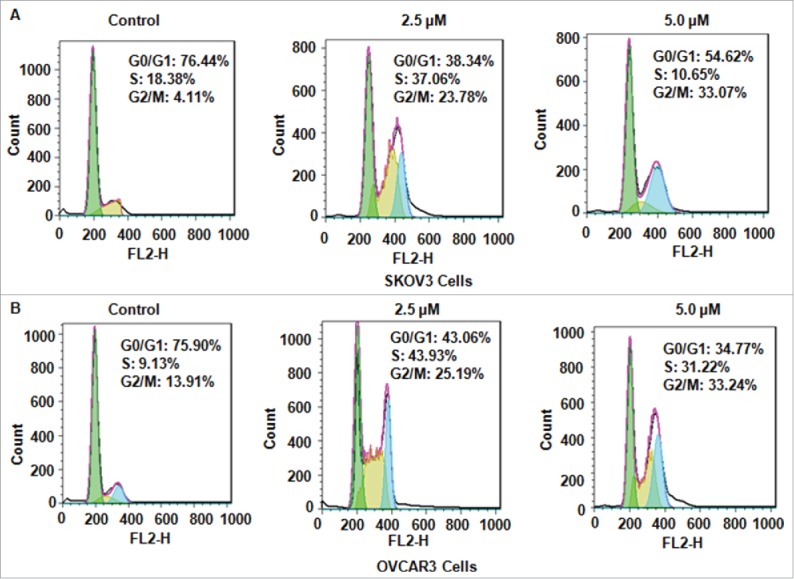
NC induced cell cycke arrest in ovarian cancer cells. (A)NC-triggered cell cycle arrest was measured by Flow cytometry in SKOV3 ovarian cancer cells. (B) Cell cycle analysis was performed in OVCAR3 cells treated with NC.
NC inhibited cell migration and invasion in ovarian cancer cells
To further investigate whether NC have effects on ovarian cancer cell motility, Transwel assay was conducted in SKOV3 and OVCAR3 cells after NC treatment. We found that cell migration was remarkably inhibited by NC treatment in ovarian cancer cells (Fig. 3A and B). In line with this, our invasion assay showed that NC retarded cell invasion ability in ovarian cancer cells (Fig. 3A and B). These findings suggest that NC treatment retarded cell motility in ovarian cancer cells.
Figure 3.
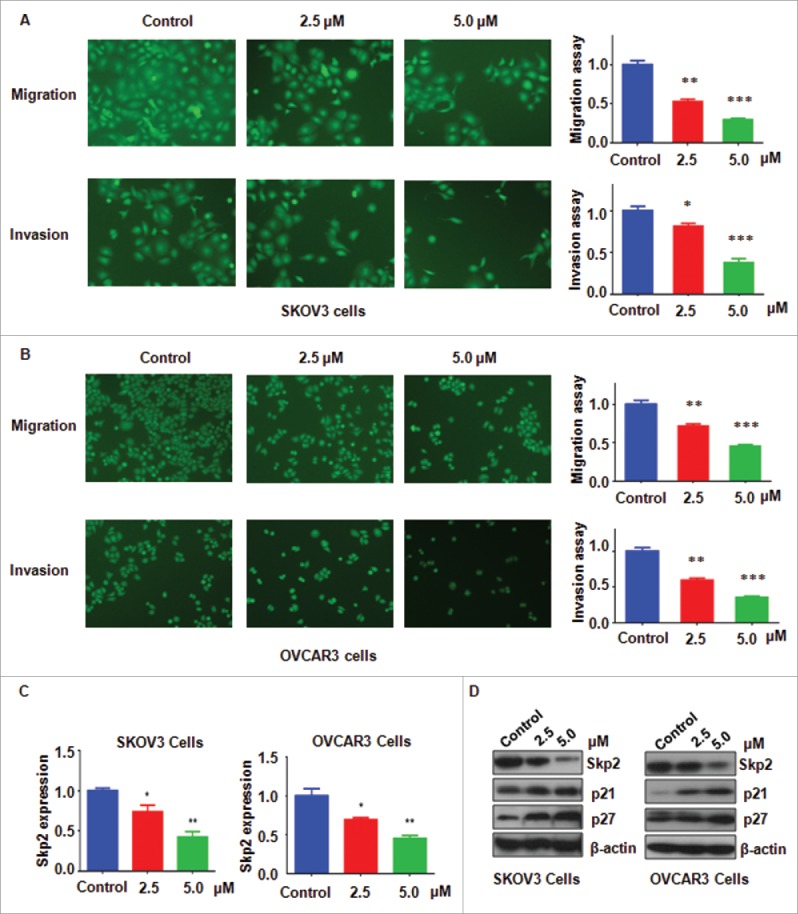
Inhibitory effects of NC on ovarian cancer cell migration and invasion. (A) Left panel: The inhibitory effect of NC on cell migration and invasion was detected using Transwell chambers assay in SKOV3 cells. Right panel: Quantitative results were illustrated for panel A. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups treated with DMSO. (B) Left panel: Cell migration and invasion were measured using Transwell chambers assay in OVCAR3 cells. Right panel: Quantitative results were illustrated for left panel. **P < 0.01, ***P < 0.001 compared with the control groups treated with DMSO. (C) The mRNA expression of Skp2 was measured by real-time RT-PCR in NC-treated ovarian cancer cells. (D) The expression of Skp2, p21 and p27 was detected by Western immunobloting analysis in NC-treated ovarian cancer cells.
NC suppressed Skp2 expression in ovarian cancer cells
Since Skp2 has been considered as a key oncoprotein in ovarian cancer, we hypothesized whether NC exerts its antitumor function partly due to inhibition of Skp2. Q-PCR was used to measure the Skp2 mRNA levels in ovarian cancer cells after NC treatment of 72 hours. We found that NC reduced the mRNA levels of Skp2 in both ovarian cancer cell lines (Fig. 3C). Western blot analysis was further used to detect the Skp2 protein levels in ovarian cancer cells with NC treatment. We observed that NC dramatically decreased Skp2 protein level in ovarian cancer cells (Fig. 3D). It has been known that p21 and p27 are 2 downstream targets of Skp2. In keeping with this note, we observed that NC treatment increased the levels of p21 and p27 in ovarian cancer cells (Fig. 3D). Therefore, NC exerts its tumor suppressive function in part due to downregulation of Skp2 and subsequent upregulation of p21 and p27 in ovarian cancer cells.
Over-expression of Skp2 rescued NC-induced cell growth inhibition and apoptosis
To further validate whether NC mediated cell growth inhibition via downregulation of Skp2 in ovarian cancer cells, SKOV3 cells were transfected with Skp2 cDNA or pcDNA3.1 as control. We found that Skp2 cDNA transfection blocked the inhibition of Skp2 induced by NC treatment in ovarian cell lines (Fig. 4A and B), Moreover, overexpression of Skp2 abrogated NC-mediated cell growth inhibition in ovarian cancer cell lines (Fig. 4C). Similarly, overexpression of Skp2 decreased percentage of G2/M phase induced by NC treatments (Fig. 4D). We also investigated whether NC triggered cell apoptosis partly due to inhibition of Skp2 in ovarian cancer cells. We found that overexpression of Skp2 reduced NC-induced cell apoptosis in ovarian cancer cells (Fig. 5A).
Figure 4.
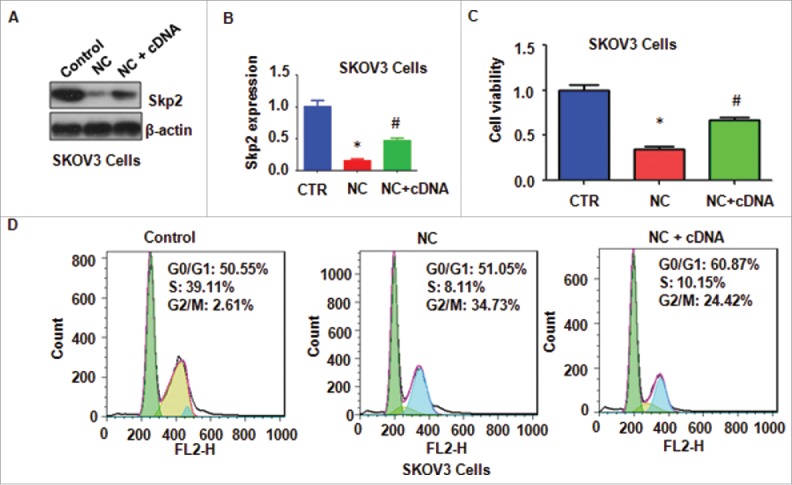
Overexpression of Skp2 abrogated NC-induced cell growth inhibition and cell cycle arrest. (A) The expression of Skp2 was detected by western immunobloting analysis in ovarian cancer cells after Skp2 overexpression plus NC treatment. NC: Nitidine Chloride; NC+cDNA: NC plus Skp2 cDNA transfection. (B) Quantitative results were illustrated for panel A. CTR: Control (C) Ovarian cancer cell growth was measured by MTT assay after Skp2 overexpression in combination with NC treatment. *P < 0.05, vs control; #P < 0.05 vs NC treatment. CTR: Control (D) Cell cycle was accessed by Flow cytometry after Skp2 overexpression in combination with NC treatment.
Figure 5.
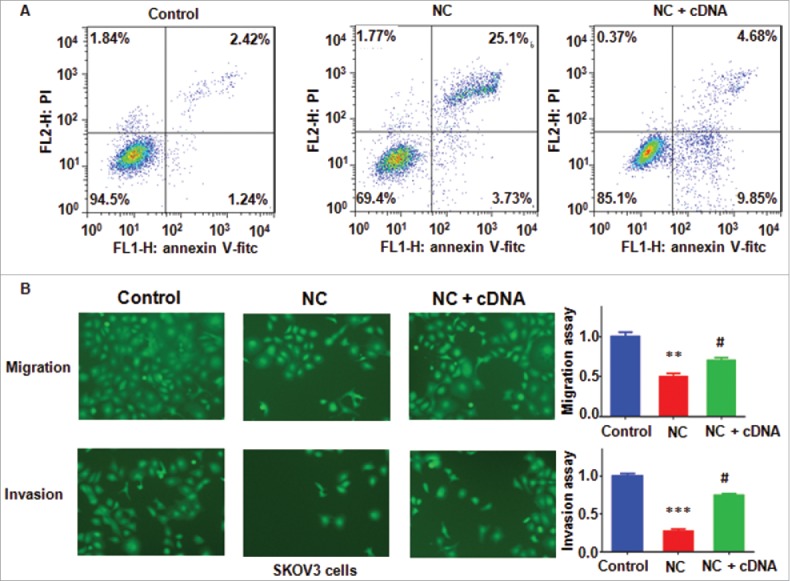
Overexpression of Skp2 abrogated NC-induced apoptosis and invasion inhibition in ovarian cancer cells. (A) Apoptotic cells were accessed by Flow cytometry after Skp2 overexpression in combination with NC treatment. NC: Nitidine Chloride; NC + cDNA: NC plus Skp2 cDNA transfection. (B), Left panel: Cell invasion were detected by Transwell chambers assay in ovarian cancer cells after Skp2 overexpression in combination with NC treatment. Right panel: Quantitative results were illustrated for left panel. **P < 0.01, vs control; #P < 0.05 vs NC treatment.
Over-expression of Skp2 abolished NC-mediated cell motility inhibition
To investigate whether overexpression of Skp2 regulates cell motility and invasiveness in ovarian cancer cells, Transwell assay was conducted to measure the migration and invasion in SKOV3 cells after Skp2 cDNA transfection. We observed that overexpression of Skp2 abrogated the inhibitory effects of NV on cell motility in ovarian cancer cells (Fig. 5B). These findings suggest that NC exhibits anti-cancer function partly through downregulation of Skp2 in ovarian cancer cells.
Depletion of Skp2 promoted NC-induced anti-tumor activities
To further dissect the role of Skp2 in ovarian cancer cells, Skp2 siRNA oligonucleotides was used to down-regulate Skp2 level in ovarian cancer cells after NC treatment. We found that Skp2 siRNA transfection plus NC treatment resulted in less expression of Skp2 compared with siRNA treatment alone (Fig. 6A and B). We found downregulation of Skp2 plus NC treatment enhanced cell growth inhibition to a greater degree compared with NC treatment alone (Fig. 6C). Furthermore, depletion of Skp2 enhanced NC-induced G2/M cell cycle arrest (Fig. 6D). Moreover, downregulation of Skp2 promoted NC-induced apoptosis in ovarian cancer cells (Fig. 7A). Notably, Skp2 siRNA transfection plus NC treatment led to cell migration and invasion suppression to a greater degree compared with NC alone (Fig. 7B). Taken together, our findings indicated that NC exhibited antitumor activity via inhibition of Skp2 in ovarian cancer cells.
Figure 6.
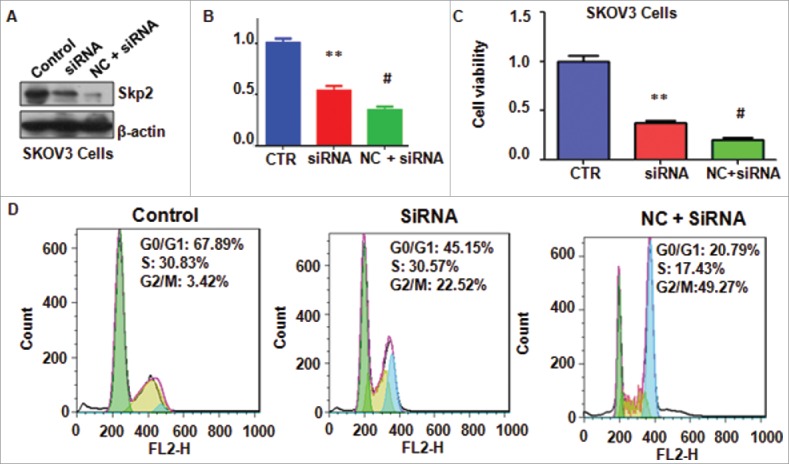
Knockdown of Skp2 enhanced NC-mediated cell proliferation inhibition and cell cycle arrest. (A) The expression of Skp2 was detected by Western blotting analysis in ovarian cancer cells after Skp2 downregulation plus NC treatment. siRNA: Skp2 siRNA; NC+siRNA: NC plus Skp2 siRNA transfection. (B) Quantitative results were illustrated for panel A. CTR: Control. *P < 0.05, vs control; #P < 0.05 vs Skp2 siRNA transfection. (C) MTT assay was performed to detect the effect of Skp2 downregulation in combination with NC treatment on ovarian cancer cell growth. *P < 0.05, vs control; #P < 0.05 vs Skp2 siRNA transfection. (D) Cell cycle was accessed by Flow cytometry after Skp2 downregulation in combination with NC treatment.
Figure 7.
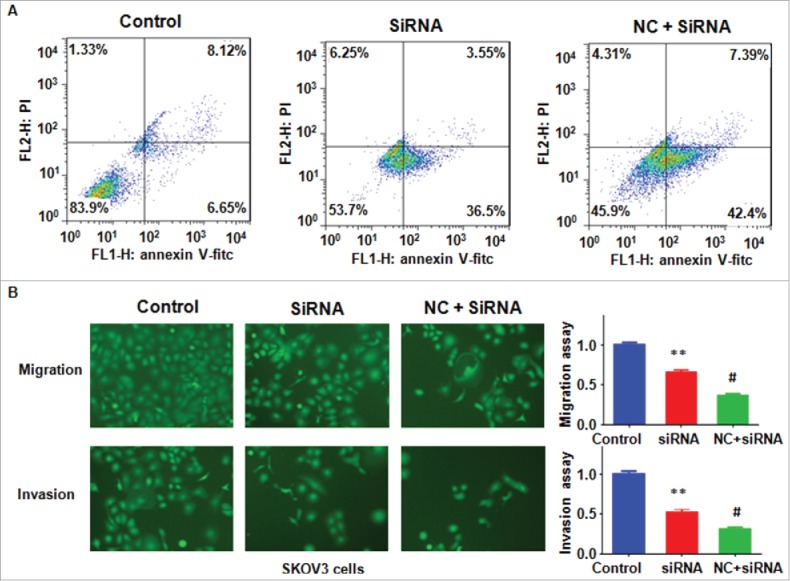
Knockdown of Skp2 enhanced NC-induced cell apoptosis and migration inhibition. (A) Apoptotic cells were detected by Flow cytometry in ovarian cancer cells after Skp2 siRNA transfection and NC treatment. siRNA: Skp2 siRNA; NC+siRNA: NC plus Skp2 siRNA transfection. (B) Left panel: Cell invasion were detected by Transwell chambers assay in ovarian cancer cells after Skp2 siRNA transfection in combination with NC treatment. Right panel: Quantitative results were illustrated for left panel. **P < 0.01, vs control; #P < 0.05 vs Skp2 siRNA treatment.
Discussion
Emerging evidence has demonstrated that Skp2 plays an oncogenic role in ovarian cancer. It has been reported that Skp2 overexpression is an independent prognostic factor in patients with ovarian adenocarcinoma.22 Another study also identified that Skp2 expression is significantly associated with malignancy in epithelial ovarian carcinomas.23 Moreover, the combined evaluation of Skp2/Jab1/p27 proteins could be a better approach to diagnose prognosis on patients with ovarian cancer.23 Similarly, Skp2/p27 pathway is associated with advanced ovarian cancer in Saudi patients.24 Lu et al. revealed that Skp2 and Foxo3a may be considered to be important prognoses in ovarian cancer.25 Recently, one study suggests that Skp2, cyclin E, and stathmin may be valuable biomarkers for metastasis in ovarian cancer patients.26 Xu et al. reported that the oncoprotein hepatitis B X-interacting protein promoted the cell invasion via upregulation of Skp2 in ovarian cancer cells.27 These studies implied that inactivation of Skp2 could be helpful for treating ovarian cancer.
NC has been validated to inhibit cancer cell growth and induced apoptosis and suppressed cancer angiogenesis. For instance, Lin et al. found that NC suppressed the activation of STAT3, ERK and SHH pathways.28 Moreover, this group observed that NC altered the expression of multiple genes such as Bcl-2, Bax, Cyclin D1, CDK4, VEGF-A and VEGFR2 in hepatic cancer cells.28 In support of this report, Ou et al. also found that NC induced apoptosis via regulation of p53, p21, Bax and Bcl-2 in hepatocellular carcinoma cells.29 NC inhibited cell metastasis through inhibition of Akt signaling pathway in renal cancer.30 Consistently, NC inhibited the malignant behavior of glioblastoma cells through targeting the PI3K/Akt/mTOR signaling pathway.31 Furthermore, NC was reported to suppress the EMT (epithelial-mesenchymal transition) and migration and invasion through regulation of Akt/GSK-3β/Snail signaling pathway in osteosarcoma cells.32 Strikingly, NC induced inhibition of EMT and cancer stem cells-like properties via regulation of hedgehog pathway in breast cancer cells.33 Moreover, NC inhibited cell migration and invasion via suppression of MMP-2 and MMP-9 production in ovarian cancer cells.34 Notably, NC inhibited cell growth and induced apoptosis via regulation of Akt pathway in ovarian cancer cells.35 Importantly, NC exhibited a synergistic inhibitory effect with dosorubicin on the viability of ovarian cancer cells.35 In the present study, we found that NC significantly inhibited the expression of Skp2 in ovarian cancer cells.
Since Skp2 is an oncoprotein in ovarian cancer development and progression, inhibition of Skp2 could be a useful way for the treatment of ovarian cancer. Indeed, several compounds have been identified as Skp2 inhibitors. SZL-P1–41 could completely prevent Skp2-Skp1 interaction and subsequently block Skp2 biologic function.36 Bortezomib-mediated expression of p27 is through Skp2 degradation on ovarian cancer.37 Dihydrotestosterone induced p27 degradation through direct binding Skp2 in ovarian cancer.38 One study demonstrated that salinomycin induced cell death via inhibition of Skp2 in ovarian cancer cells.39 Rottlerin was found to exert its antitumor effect via inhibition of Skp2 in pancreatic and breast cancer cells.40,41 Curcumin was also considered as a Skp2 inhibitor in several cancer cell lines.42-45 Moreover, lycopene and quercetin were reported to reduce the expression of Skp2 in breast cancer cells.43 Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition partly via downregulation of Skp2 in prostate cancer cells.46 Recently, matrine derivative YF-18 inhibited lung cancer cell proliferation and migration through downregulation of Skp2.47 Here, we validated NC as a new Skp2 inhibitor in ovarian cancer cells. Without a doubt, further investigation is required to explore whether NC exerts its tumor suppressive activity via inactivation of Skp2 in animal models.
Materials and methods
Reagents
Anti-Skp2 and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-p21 and anti-p27 antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). NC was obtained from Tauto Biotech Company (Shanghai, China) and dissolved in DMSO (dimethyl sulfoxide). MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Transwell inserts and Matrigel were purchased from BD Biosciences. Lipofectamine 2000 reagent was provided by Invitrogen (Waltham, MA USA). Annexin apoptosis assay kit was bought from Beyotime Biotechnology (Shanghai, China).
Cell culture
The human ovarian cancer SKOV3 and OVCAR3 cells were obtained from ATCC company (Manassas, VA, USA) and cultured in RPMI 1640 medium (Gibro Invitrogen) supplemented with 10 % fetal bovine serum (FBS), 2 mM glutamine, 1 mM sodium pyruvate, 100 μg/ml streptomycin and 100 U/ml penicillin. These cells were maintained in a 5 % CO2 atmosphere at 37°C.
MTT assay
Ovarian cancer cells were seeded in 96-well plates. After overnight incubation, cells were treated with different concentrations of NC or Skp2 siRNA/cDNA or combination for 48 and 72 hours. MTT was conducted to measure the cell viability in ovarian cancer cells after different treatments. Briefly, after treated cells were incubated for 48 h and 72 h, 10 μl MTT solution (0.5 mg/ml) was added and incubated for 4 h in 5% CO2 atmosphere at 37°C. Then, we removed the supernatant in each well and added 100 μl DMSO. The absorption was measured at 490 nm.
Cell apoptosis assay
Ovarian cells were seeded in 6-well plates. After 24 h incubation, cells were treated with NC or Skp2 siRNA/cDNA or combination for 48 h. Then, cells were trypsinized and harvested by centrifugation. Cells were washed by PBS and subsequently suspended in 500 μl binding buffer with 5 μl annexin V-FITC and 5 μl Propidium iodide (PtdIns) for 15 minutes at room temperature in the dark. Cell apoptosis was measured by a FACScalibur flow cytometer (BD, Franklin Lakes, NJ, USA) and analyzed by FlowJo software.
Cell cycle analysis
The treated ovarian cells as mentioned above were cultured for 48 h in RPMI 1640 medium. Then, after cells were harvested and washed with PBS, 7% cold alcohol was used to fix cells for overnight at 4°C. Subsequently, washed cells and suspended them with PBS, incubated with 100 μg/ml RNase and 40 mg/ml PtdIns for 30 minutes at 4°C. Cell cycle was determined by a FACScalibur flow cytometer (BD, USA).
Transwell migration and invasion assay
The capacities of cell migration and invasion were determined by Transwell assay according to the manufacturer protocol. The cells with different treatments were seeded into an upper chamber of inserts with serum free medium in a 24-well plate. The complete medium was added in bottom chamber of inserts. For cell invasion assay, the Transwell inserts were precoated with Matrigel. After cells were incubated for 20 h, the upper cells in chamber were removed by the cotton buds and the bottom surface cells were stained with 4 μg/ml Calcein AM in Hanks buffered saline at 37°C for 1 h. These fluorescently labeled invasive cells were photographed by a fluorescent microscope.
Quantitative real-time reverse transcription-PCR (Q-PCR) analysis
The total RNA was extracted with Trizol reagent and reversed-transcribed into cDNA by RevertAid First Strand cDNA Synthesis Kit. Real-time Q-PCR was performed using Power SYBR Green PCR Master Mix and the results were calculated by 2-⊿⊿Ct method.41 The primers for Skp2 were as follows: forward primer: 5′– GCT GCT AAA GGT CTC TGG TGT -3′; and reverse primer 5′- AGG CTT AGA TTC TGC AAC TTG -3′. And primers for GAPDH: forward primer: 5′- ACC CAG AAG ACT GTG GAT GG -3′; reverse primer: 5′- CAG TGA GCT TCC CGT TCA G- 3′.
Western blotting analysis
The cells were harvested and lysed in RIPA buffer with protease inhibitors and phosphatase inhibitors (EMD Millipore, Billerica, MA, USA). The protein concentrations were measured by the Pierce bicinchoninic acid protein assay. The equal quantity of proteins were loaded on SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and subsequently transferred to PVDF (polyvinylidene fluoride) membranes. The membranes were blocked with 5% fat-free milk for 1 hour at room temperature and then incubated with primary antibodies overnight at cold room. Anti-Skp2 (1:2000), anti-p21 (1:1000), anti-p27 (1:1000), and anti-β-actin (1:5000) antibodies were used. The membrane was washed with TBST and incubated with second antibody for 1 hour at room temperature. ECL (enhanced chemiluminescene) reagents were used for detection of the protein bands as described before.48 ImageJ software was used for densitometric quantification of the protein blots.
Transfection
Ovarian cancer cells (3 × 105 cells/well) were cultured in 6-well plates, and transfected with Skp2 cDNA or Skp2 siRNA or empty vector using lipofectamine 2000 following the manufacturer's instructions.49 The Skp2 siRNA was purchased from GenePharma Company (Shanghai, China). Skp2 siRNA: sense 5′-GGA GUG ACA AAG ACU UUG UTT-3′; antisense 5′-ACA AAG UCU UUG UCA CUC CTT-3′.
Statistical analysis
The data were statistical analyzed to determine the differences between control group and transfection group by ANOVA using GraphPad Prism 4.0 (Graph pad Software, La Jolla, CA). p < 0.05 was considered to indicate statistically significant.
Disclosure of potential conflicts of interest
There is no conflict of interest.
Acknowledgment
We thank our colleagues for their critical comments and kind help.
References
- [1].Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 2016; 13:255-61; PMID:26787282; https://doi.org/ 10.1038/nrclinonc.2015.224 [DOI] [PubMed] [Google Scholar]
- [2].Alipour S, Zoghi S, Khalili N, Hirbod-Mobarakeh A, Emens LA, Rezaei N. Specific immunotherapy in ovarian cancer: a systematic review. Immunotherapy 2016; 8:1193-204; PMID:27605068; https://doi.org/ 10.2217/imt-2016-0034 [DOI] [PubMed] [Google Scholar]
- [3].Ren F, Shen J, Shi H, Hornicek FJ, Kan Q, Duan Z. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta 2016; 1866:266-75; PMID:27717733 [DOI] [PubMed] [Google Scholar]
- [4].Nielsen FC, van Overeem Hansen T, Sorensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 2016; 16:599-612; PMID:27515922; https://doi.org/ 10.1038/nrc.2016.72 [DOI] [PubMed] [Google Scholar]
- [5].Hu J, Zhang WD, Liu RH, Zhang C, Shen YH, Li HL, Liang MJ, Xu XK. Benzophenanthridine alkaloids from Zanthoxylum nitidum (Roxb.) DC, and their analgesic and anti-inflammatory activities. Chem Biodivers 2006; 3:990-5; PMID:17193331; https://doi.org/ 10.1002/cbdv.200690108 [DOI] [PubMed] [Google Scholar]
- [6].Pan X, Han H, Wang L, Yang L, Li R, Li Z, Liu J, Zhao Q, Qian M, Liu M, et al.. Nitidine Chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett 2011; 313:181-91; PMID:21959111; https://doi.org/ 10.1016/j.canlet.2011.09.001 [DOI] [PubMed] [Google Scholar]
- [7].Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, Yi Z, Chen Y, Pang X, Liu M. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther 2012; 11:277-87; PMID:22203730; https://doi.org/ 10.1158/1535-7163.MCT-11-0648 [DOI] [PubMed] [Google Scholar]
- [8].Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu DB, Peng J. Nitidine chloride inhibits hepatocellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway. Int J Mol Med 2013; 32:79-84; PMID:23613111 [DOI] [PubMed] [Google Scholar]
- [9].Sun M, Zhang N, Wang X, Cai C, Cun J, Li Y, Lv S, Yang Q. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells. Tumour Biol 2014; 35:10201-12; PMID:25027404; https://doi.org/ 10.1007/s13277-014-2327-9 [DOI] [PubMed] [Google Scholar]
- [10].Kang M, Ou H, Wang R, Liu W, Tang A. The effect of nitidine chloride on the proliferation and apoptosis of nasopharyngeal carcinoma cells. Journal of BUON 2014; 19:130-6; PMID:24659654 [PubMed] [Google Scholar]
- [11].Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W, Xu Z, Liu Z. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food and chemical toxicology 2014; 66:210-6; PMID:24508476; https://doi.org/ 10.1016/j.fct.2014.01.049 [DOI] [PubMed] [Google Scholar]
- [12].Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G, Zhang Y, Sui M, Liu H, Jiang L. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Molecular medicine reports 2016; 13:2536-42; PMID:26847477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chan CH, Morrow JK, Zhang S, Lin HK. Skp2: a dream target in the coming age of cancer therapy. Cell Cycle 2014; 13:679-80; PMID:24526126; https://doi.org/ 10.4161/cc.27853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fu HC, Yang YC, Chen YJ, Lin H, Ou YC, Chien CC, Huang EY, Huang HY, Lan J, Chi HP, et al.. Increased expression of SKP2 is an independent predictor of locoregional recurrence in cervical cancer via promoting DNA-damage response after irradiation. Oncotarget 2016; 7:44047-61; PMID:27317767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer 2014; 14:233-47; PMID:24658274; https://doi.org/ 10.1038/nrc3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1999; 1:193-9; PMID:10559916; https://doi.org/ 10.1038/7022110.1038/12013 [DOI] [PubMed] [Google Scholar]
- [17].Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9:661-4; PMID:10375532; https://doi.org/ 10.1016/S0960-9822(99)80290-5 [DOI] [PubMed] [Google Scholar]
- [18].Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A 1998; 95:11324-9; PMID:9736735; https://doi.org/ 10.1073/pnas.95.19.11324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A 2003; 100:10231-6; PMID:12925736; https://doi.org/ 10.1073/pnas.1831009100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A 2005; 102:1649-54; PMID:15668399; https://doi.org/ 10.1073/pnas.0406789102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang H, Cui J, Bauzon F, Zhu L. A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle 2010; 9:1021-2; PMID:20160512; https://doi.org/ 10.4161/cc.9.5.10916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shigemasa K, Gu L, O'Brien TJ, Ohama K. Skp2 overexpression is a prognostic factor in patients with ovarian adenocarcinoma. Clin Cancer Res 2003; 9:1756-63; PMID:12738731 [PubMed] [Google Scholar]
- [23].Sui L, Dong Y, Watanabe Y, Yamaguchi F, Sugimoto K, Tokuda M. Clinical significance of Skp2 expression, alone and combined with Jab1 and p27 in epithelial ovarian tumors. Oncol Rep 2006; 15:765-71; PMID:16525656 [PubMed] [Google Scholar]
- [24].Hafez MM, Alhoshani AR, Al-Hosaini KA, Alsharari SD, Al Rejaie SS, Sayed-Ahmed MM, Al-Shabanah OA. SKP2/P27Kip1 pathway is associated with Advanced Ovarian Cancer in Saudi Patients. Asian Pac J Cancer Prev 2015; 16:5807-15; PMID:26320455; https://doi.org/ 10.7314/APJCP.2015.16.14.5807 [DOI] [PubMed] [Google Scholar]
- [25].Lu M, Zhao Y, Xu F, Wang Y, Xiang J, Chen D. The expression and prognosis of FOXO3a and Skp2 in human ovarian cancer. Med Oncol 2012; 29:3409-15; PMID:22714061; https://doi.org/ 10.1007/s12032-011-9919-710.1007/s12032-012-0275-z [DOI] [PubMed] [Google Scholar]
- [26].Lv H, Shi Y, Zhang L, Zhang D, Liu G, Yang Z, Li Y, Fei F, Zhang S. Polyploid giant cancer cells with budding and the expression of cyclin E, S-phase kinase-associated protein 2, stathmin associated with the grading and metastasis in serous ovarian tumor. BMC cancer 2014; 14:576; PMID:25106448; https://doi.org/ 10.1186/1471-2407-14-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu F, Zhu X, Han T, You X, Liu F, Ye L, Zhang X, Wang X, Yao Y. The oncoprotein hepatitis B X-interacting protein promotes the migration of ovarian cancer cells through the upregulation of S-phase kinase-associated protein 2 by Sp1. Int J oncology 2014; 45:255-63; PMID:24788380 [DOI] [PubMed] [Google Scholar]
- [28].Lin J, Shen A, Chen H, Liao J, Xu T, Liu L, Peng J. Nitidine chloride inhibits hepatic cancer growth via modulation of multiple signaling pathways. BMC Cancer 2014; 14:729; PMID:25266147; https://doi.org/ 10.1186/1471-2407-14-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ou X, Lu Y, Liao L, Li D, Liu L, Liu H, Xu H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol Rep 2015; 33:1264-74; PMID:25530218 [DOI] [PubMed] [Google Scholar]
- [30].Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W, Shi B, Xu Z, Liu Z. Nitidine chloride inhibits renal cancer cell metastasis via suppressing AKT signaling pathway. Food Chem Toxicol 2013; 60:246-51; PMID:23911800; https://doi.org/ 10.1016/j.fct.2013.07.062 [DOI] [PubMed] [Google Scholar]
- [31].Liu M, Wang J, Qi Q, Huang B, Chen A, Li X. Nitidine chloride inhibits the malignant behavior of human glioblastoma cells by targeting the PI3K/AKT/mTOR signaling pathway. Oncol Rep 2016; 36:2160-8; PMID:27498786 [DOI] [PubMed] [Google Scholar]
- [32].Cheng Z, Guo Y, Yang Y, Kan J, Dai S, Helian M, Li B, Xu J, Liu C. Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3beta/Snail signaling pathway. Oncol Rep 2016; 36:1023-9; PMID:27279040 [DOI] [PubMed] [Google Scholar]
- [33].Sun M, Zhang N, Wang X, Li Y, Qi W, Zhang H, Li Z, Yang Q. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci 2016; 6:44; PMID:27313840; https://doi.org/ 10.1186/s13578-016-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun X, Lin L, Chen Y, Liu T, Liu R, Wang Z, Mou K, Xu J, Li B, Song H. Nitidine chloride inhibits ovarian cancer cell migration and invasion by suppressing MMP-2/9 production via the ERK signaling pathway. Mol Med Rep 2016; 13:3161-8; PMID:26935265 [DOI] [PubMed] [Google Scholar]
- [35].Ding F, Liu T, Yu N, Li S, Zhang X, Zheng G, Lv C, Mou K, Xu J, Li B, et al.. Nitidine chloride inhibits proliferation, induces apoptosis via the Akt pathway and exhibits a synergistic effect with doxorubicin in ovarian cancer cells. Mol Med Rep 2016; 14:2853-9; PMID:27485415 [DOI] [PubMed] [Google Scholar]
- [36].Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al.. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013; 154:556-68; PMID:23911321; https://doi.org/ 10.1016/j.cell.2013.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Uddin S, Ahmed M, Hussain AR, Jehan Z, Al-Dayel F, Munkarah A, Bavi P, Al-Kuraya KS. Bortezomib-mediated expression of p27Kip1 through S-phase kinase protein 2 degradation in epithelial ovarian cancer. Lab Invest 2009; 89:1115-27; PMID:19636294; https://doi.org/ 10.1038/labinvest.2009.75 [DOI] [PubMed] [Google Scholar]
- [38].Shi P, Zhang Y, Tong X, Yang Y, Shao Z. Dihydrotestosterone induces p27 degradation via direct binding with SKP2 in ovarian and breast cancer. Int J Mol Med 2011; 28:109-14; PMID:21503567 [DOI] [PubMed] [Google Scholar]
- [39].Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee CH, Kim YN. Salinomycin induces cell death via inactivation of Stat3 and downregulation of Skp2. Cell Death Dis 2013; 4:e693; PMID:23807222; https://doi.org/ 10.1038/cddis.2013.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X, Zhou X, Wang Z. Rottlerin exhibits anti-cancer effect through inactivation of S phase kinase-associated protein 2 in pancreatic cancer cells. Am J Cancer Res 2016; 6:2178-91; PMID:27822410 [PMC free article] [PubMed] [Google Scholar]
- [41].Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X, Zhao Z, Zhou X, Li Y, Wang Z. Rottlerin exerts its anti-tumor activity through inhibition of Skp2 in breast cancer cells. Oncotarget 2016; 7:66512-24; PMID:27582552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015; 6:18027-37; PMID:26046466; https://doi.org/ 10.18632/oncotarget.4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem 2011; 59:6765-75; PMID:21598989; https://doi.org/ 10.1021/jf201096v [DOI] [PubMed] [Google Scholar]
- [44].Jia T, Zhang L, Duan Y, Zhang M, Wang G, Zhang J, Zhao Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int 2014; 14:126; PMID:25530715; https://doi.org/ 10.1186/s12935-014-0126-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Su J, Zhou X, Wang L, Yin X, Wang Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am J Cancer Res 2016; 6:1949-62; PMID:27725901 [PMC free article] [PubMed] [Google Scholar]
- [46].Lin HP, Lin CY, Huo C, Hsiao PH, Su LC, Jiang SS, Chan TM, Chang CH, Chen LT, Kung HJ, et al.. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget 2015; 6:6684-707; PMID:25788262; https://doi.org/ 10.18632/oncotarget.3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wu L, Wang G, Wei J, Huang N, Zhang S, Yang F, Li M, Zhou G, Wang L. Matrine derivative YF-18 inhibits lung cancer cell proliferation and migration through down-regulating Skp2. Oncotarget 2017; 8:11729-38; PMID:28036296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang Y, Zhao M, Xu H, Wang K, Fu Z, Jiang Y, Yao Z. RASAL2 down-regulation in ovarian cancer promotes epithelial-mesenchymal transition and metastasis. Oncotarget 2014; 5:6734-45; PMID:25216515; https://doi.org/ 10.18632/oncotarget.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X, Zhou X, Zhou L, Wang Z. Rottlerin inhibits cell growth and invasion via down-regulation of Cdc20 in glioma cells. Oncotarget 2016; 7:69770-82; PMID:27626499 [DOI] [PMC free article] [PubMed] [Google Scholar]


