This case-control study examines whether the general cognitive deficit observed across psychotic disorders is similarly associated with functional integrity of 2 brain networks widely implicated in supporting many cognitive domains.
Key Points
Questions
Is the efficiency of the cingulo-opercular network and frontoparietal network reduced across multiple psychotic disorders, and does lower efficiency predict impairments in generalized cognitive ability?
Findings
In this case-control study, cingulo-opercular network but not frontoparietal network efficiency was significantly reduced in patients with schizophrenia, schizoaffective disorder, and psychotic bipolar disorder compared with healthy control individuals. Lower cingulo-opercular network global efficiency was associated with worse general cognitive ability and mediates the association between psychotic disorder status and cognitive function.
Meaning
Reduced efficiency of information transfer within the cingulo-opercular network is a shared vulnerability across multiple psychotic disorders and represents a common mechanism that contributes to the generalized cognitive deficit.
Abstract
Importance
Cognitive impairment occurs across the psychosis spectrum and is associated with functional outcome. However, it is unknown whether these shared manifestations of cognitive dysfunction across diagnostic categories also reflect shared neurobiological mechanisms or whether the source of impairment differs.
Objective
To examine whether the general cognitive deficit observed across psychotic disorders is similarly associated with functional integrity of 2 brain networks widely implicated in supporting many cognitive domains.
Design, Setting, and Participants
A total of 201 healthy control participants and 375 patients with psychotic disorders from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium were studied from September 29, 2007, to May 31, 2011. The B-SNIP recruited healthy controls and stable outpatients from 6 sites: Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Detroit, Michigan; and Hartford, Connecticut. All participants underwent cognitive testing and resting-state functional magnetic resonance imaging. Data analysis was performed from April 28, 2015, to February 21, 2017.
Main Outcomes and Measures
The Brief Assessment of Cognition in Schizophrenia was used to measure cognitive ability. A principal axis factor analysis on the Brief Assessment of Cognition in Schizophrenia battery yielded a single factor (54% variance explained) that served as the measure of general cognitive ability. Functional network integrity measures included global and local efficiency of the whole brain, cingulo-opercular network (CON), frontoparietal network, and auditory network and exploratory analyses of all networks from the Power atlas. Group differences in network measures, associations between cognition and network measures, and mediation models were tested.
Results
The final sample for the current study included 201 healthy controls, 143 patients with schizophrenia, 103 patients with schizoaffective disorder, and 129 patients with psychotic bipolar disorder (mean [SD] age, 35.1 [12.0] years; 281 male [48.8%] and 295 female [51.2%]; 181 white [31.4%], 348 black [60.4%], and 47 other [8.2%]). Patients with schizophrenia (Cohen d = 0.36, P < .001) and psychotic bipolar disorder (Cohen d = 0.33, P = .002) had significantly reduced CON global efficiency compared with healthy controls. All patients with psychotic disorders had significantly reduced CON local efficiency, but the clinical groups did not differ from one another. The CON global efficiency was significantly associated with general cognitive ability across all groups (β = 0.099, P = .009) and significantly mediated the association between psychotic disorder status and general cognition (β = −0.037; 95% CI, −0.076 to −0.014). Subcortical network global efficiency was also significantly reduced in psychotic disorders (F3,587 = 4.01, P = .008) and positively predicted cognitive ability (β = 0.094, P = .009).
Conclusions and Relevance
These findings provide evidence that reduced CON and subcortical network efficiency play a role in the general cognitive deficit observed across the psychosis spectrum. They provide new support for the dimensional hypothesis that a shared neurobiological mechanism underlies cognitive impairment in psychotic disorders.
Introduction
Decades of research have revealed deficits in cognitive functioning across the psychosis spectrum. Furthermore, mounting evidence suggests common cognitive deficits across psychotic disorders, with patients with schizophrenia having the greatest impairment relative to healthy control participants (HCs), patients with psychotic bipolar disorder having the least impairment, and patients with schizoaffective disorder having an intermediate deficit. This dimensionality of cognitive impairment in psychosis is also related to the dimensionality of the diagnostic groups, such that cognitive function appears to decline as affective features become less dominant in the diagnostic criteria for psychotic disorders. Despite these behavioral patterns, the extent to which the observed cognitive deficits share common neurobiological correlates across disorders or are instead similar phenotypic byproducts of different underlying processes remains unclear.
The current study aims to address this question by examining functional connectivity as a common neurobiological mechanism that may influence cognitive function across the psychosis spectrum. An increasing body of literature suggests that functional connectivity abnormalities exist in psychotic disorders, including reduced functional connectivity in and between the frontoparietal network (FPN) and the cingulo-opercular network (CON). The FPN, which includes the dorsolateral prefrontal cortex as a core hub, and the CON, which includes the anterior insula and dorsal anterior cingulate cortex as core hubs, were first identified by their consistent pattern of increased blood oxygen level–dependent (BOLD) activity during the performance of 10 distinct cognitive tasks. These findings led to their specification as a core task-set system, with FPN nodes exhibiting increased BOLD activity during start cue and error feedback and CON demonstrating stably increased activity throughout the entire task epoch. Of importance, these 2 networks, which have been reproduced in multiple large-scale network analyses, are implicated in a range of cognitive processes and are considered to be domain-general functional networks that support many cognitive abilities. The involvement of these networks in multiple cognitive domains is particularly relevant given the often generalized nature of cognitive deficits observed in psychotic disorders. If reduced functional connectivity of CON and FPN are common correlates of general cognitive impairment, one would expect similar associations between cognitive ability and functional network characteristics in each diagnostic group, even if mean levels of cognition and connectivity differ.
Although many studies quantify a network’s functional connectivity by averaging connectivity strength, this approach is agnostic to the structure of the network, ignoring information regarding which nodes are interconnected. Network science quantifies properties of functional connectivity to provide potentially more sensitive metrics of network function. Global efficiency, for instance, measures the potential for information transfer and integration within a network and has been associated with IQ in healthy adults and general cognitive function in healthy individuals and patients with schizophrenia. Local efficiency measures the fault tolerance of a network in terms of local information processing and has also been associated with cognition.
In a large sample of HCs and patients with psychotic bipolar disorder, schizoaffective disorder, and schizophrenia, we hypothesized significantly reduced global and local efficiency of CON, FPN, and whole brain across all clinical groups compared with controls. On the basis of a dimensional hypothesis of the generalized cognitive impairment, we expected reductions in network efficiency to follow the pattern frequently observed in cognitive ability across the psychosis spectrum, with patients with psychotic bipolar disorder having network efficiency most similar to controls, patients with schizophrenia being the most impaired, and patients with schizoaffective disorder having intermediate deficits. We also hypothesized that network efficiency would predict general cognitive function across all groups with no significant interactions. If supported, these findings would provide evidence of a common dimensional neurobiological source associated with cognitive impairment across psychotic disorders.
Methods
Participants
Participants were identified from September 29, 2007, to May 31, 2011, as part of the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP), a multisite study focused on identifying intermediate phenotypes across the psychosis spectrum. With use of quality control procedures for the magnetic resonance imaging (MRI) data, the final sample for the current study included 201 HCs and 375 patients with psychotic disorders. All participants completed similar behavioral and MRI protocols across 6 sites (Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Detroit, Michigan; and Hartford, Connecticut), as reported in a previous article, and provided written informed consent before study enrollment. The study protocol was approved by the institutional review board at each local site (University of Maryland School of Medicine, Baltimore; Harvard Medical School, Boston, Massachusetts; University of Texas Southwestern Medical Center, Dallas; Yale University School of Medicine, New Haven, Connecticut; University of Illinois at Chicago; and Wayne State University, Detroit, Michigan). Data were deidentified, and data analysis was performed from April 28, 2015, to February 21, 2017.
As described in detail previously, diagnosis was determined using the Structured Clinical Interview of the DSM-IV, which was reviewed by at least 2 experienced research clinicians (C.A.T., G.D.P., M.S.K., J.A.S., and B.A.C.) to establish a consensus diagnosis. Patients were stable outpatients referred by mental health practitioners or recruited through the community. The HCs were recruited through community advertisements and research registries and had no history of a psychotic disorder or recurrent depression and no immediate family history of these disorders.
Cognitive and Clinical Measures
Cognitive ability was measured using the Brief Assessment of Cognition in Schizophrenia (BACS), a well-validated cognitive battery measuring working memory, executive functioning, processing speed, motor speed, verbal fluency, and verbal memory. All BACS scores were age adjusted and z scored using published norms, and z scores greater than 4.0 were truncated to minimize the effect of outliers. On the basis of research indicating a single cognition factor in BACS data from the B-SNIP data set, general cognition was defined as the factor score from an exploratory principal axis factor analysis that included all 6 BACS tasks. This single factor explained 54% of the variance in cognitive ability. Clinical symptoms were measured using the Positive and Negative Syndrome Scale, the Young Mania Rating Scale, and the Montgomery-Asberg Depression Rating Scale.
Imaging Data Acquisition and Processing
All participants underwent 5-minute resting-state functional MRI and T1-weighted structural imaging on a 3T scanner. Scanning factors differed slightly across sites (eTable 1 in the Supplement), and these differences were taken into account during preprocessing.
Data preprocessing was completed using in-house scripts at Washington University. Preprocessing included section timing correction, removal of the first 4 images from each run to allow data to reach a steady state, adjustment for odd and even section acquisition, rigid body motion correction, normalization of data to a whole-brain mode value of 1000, registration of structural images to Talairach space, and coregistration of functional volumes to atlas space using 3-mm cubic resampling in a 1-step interpolation. Frequency filtering (0.009-0.08 Hz) was applied after nuisance regression of 24 motion factors, whole brain, white matter, ventricle signals, and their temporal derivatives. See the eAppendix in the Supplement for additional details on preprocessing and graph creation.
After functional MRI and functional connectivity preprocessing, BOLD time courses were extracted from 264 regions of interest by using 6-mm spheres based on coordinates from the Power atlas. Global efficiency and local efficiency were computed on weighted, undirected graphs thresholded at 5% to 10% strongest positive connections for each participant by using algorithms from the Brain Connectivity Toolbox (additional information on thresholding is given in eFigure 1 and the eAppendix in the Supplement).
After thresholding of each participant’s whole-brain graph, nodes from the FPN and CON graphs were isolated from the whole-brain graph. Global and local efficiencies were calculated for each graph at each threshold. Global efficiency yields a single metric for the entire graph, whereas local efficiency is calculated on a nodal basis; therefore, local efficiency was averaged across all nodes in each network to yield a single metric.
The CON and FPN were selected a priori to be associated with cognitive ability; however, global and local efficiencies of the 10 other networks from the Power atlas were also analyzed to assess specificity of our findings (eFigure 2 in the Supplement).
Statistical Analysis
Analyses were performed using SPSS statistical software, version 23 (SPSS Inc). Group differences in demographic and clinical characteristics were analyzed using a 1-way analysis of variance and χ2 tests. Group differences in network metrics were calculated in 2 multivariate analyses of variance: global efficiency of our 4 networks and local efficiency of our 4 networks. Race, sex, age, B-SNIP site, and head motion were included as covariates.
Linear regression analysis was used to test associations between graph metrics and cognition. Regressions included cognitive ability as the dependent variable, with network metric, sex, motion, dummy codes for diagnostic group, site, and race as predictors. Interaction variables were included in a second block of regression models to assess interactions between group and network metrics. Bonferroni correction was determined for each a priori metric analysis, making our threshold P < .01, given 4 networks in each metric. Mediation analysis used the PROCESS macro for SPSS, with a 1000 bias-corrected bootstrap sample for significance testing. Mean functional connectivity of CON was calculated by averaging connectivity strength across all nodes and then averaged across 5% to 10% thresholds. Associations with head motion, symptom measures, BACS subdomains, covariates (eTable 2 in the Supplement), and sex differences are given in the eAppendix in the Supplement. Associations were tested using multivariate analysis of variance and Pearson correlation coefficient. Statistical significance was tested at 2-sided P < .05.
Results
Participant Characteristics
The final sample for the current study included 201 HCs, 143 patients with schizophrenia, 103 patients with schizoaffective disorder, and 129 patients with psychotic bipolar disorder (mean [SD] age, 35.1 [12.0] years; 281 male [48.8%] and 295 female [51.2%]; 181 white [31.4%], 348 black [60.4%], and 47 other [8.2%]). As described in a previous B-SNIP report, groups differed significantly on sex, race, age, personal educational level, and socioeconomic status but not parental educational level and on symptom scores across clinical groups (Table). As previously reported with the full B-SNIP sample, the patients with schizophrenia were the most cognitively impaired (Cohen d = 1.40), the patients with bipolar disorder the least (Cohen d = 0.83), and the patients with schizoaffective disorder were intermediate (Cohen d = 1.28) but statistically similar to schizophrenia.
Table. Demographic and Clinical Characteristicsa.
| Characteristic | Healthy Controls (n = 201) |
SCZ (n = 143) |
SCZAFF (n = 103) |
BP (n = 129) |
Omnibus Statistic | P Value | Post Hoc Tukey Significance |
|---|---|---|---|---|---|---|---|
| Age, y | 36.54 (11.68) | 33.39 (11.92) | 33.59 (11.19) | 35.71 (13.07) | F3,572 = 2.58 | .05 | NA |
| Sex, No. | |||||||
| Male | 86 | 103 | 49 | 43 | χ2 = 46.20 | <.001 | NA |
| Female | 115 | 40 | 54 | 86 | |||
| Race/ethnicity, No. | |||||||
| White | 58 | 61 | 36 | 26 | χ2 = 19.22 | .004 | NA |
| African American | 124 | 70 | 60 | 94 | |||
| Other | 19 | 12 | 7 | 9 | |||
| Educational level, y | |||||||
| Personal | 14.79 (2.31) | 13.08 (2.22) | 13.13 (2.17) | 14.23 (2.42) | F3,569 = 20.89 | <.001 | NA |
| Mother | 13.55 (3.56) | 13.91 (3.08) | 13.56 (4.32) | 14.32 (3.90) | F3,486 = 1.17 | .32 | NA |
| Father | 13.23 (3.23) | 13.58 (2.75) | 13.35 (3.34) | 14.09 (2.90) | F3,536 = 2.12 | .10 | NA |
| Socioeconomic statusb | 36.20 (14.56) | 52.11 (15.61) | 48.82 (15.10) | 43.03 (16.25) | F3,540 = 32.29 | <.001 | NA |
| PANSS score | |||||||
| Positive | NA | 16.30 (5.57) | 18.21 (5.13) | 12.48 (4.12) | F2,362 = 39.84 | <.001 | SCZ vs SCZAFF: P = .01; SCZ vs BP: P < .001; SCZAFF vs BP: P < .001 |
| Negative | NA | 16.34 (6.04) | 15.91 (4.83) | 12.08 (3.68) | F2,362 = 27.76 | <.001 | SCZ vs SCZAFF: P = .79; SCZ vs BP: P < .001; SCZAFF vs BP: P < .001 |
| General | NA | 31.06 (8.67) | 34.94 (9.10) | 28.49 (8.09) | F2,361 = 15.73 | <.001 | SCZ vs SCZAFF: P = .002; SCZ vs BP: P = .04; SCZAFF vs BP: P < .001 |
| Total | NA | 63.79 (17.02) | 68.99 (16.34) | 53.05 (13.46) | F2,361 = 31.07 | <.001 | SCZ vs SCZAFF: P = .03; SCZ vs BP: P < .001; SCZAFF vs BP: P < .001 |
| MADRS score | NA | 8.46 (7.53) | 14.33 (9.70) | 10.48 (8.79) | F2,367 = 13.89 | <.001 | SCZ vs SCZAFF: P < .001; SCZ vs BP: P = .14; SCZAFF vs BP: P = .002 |
| YMRS score | NA | 5.29 (5.80) | 7.80 (6.49) | 5.30 (5.98) | F2,365 = 6.33 | .002 | SCZ vs SCZAFF: P = .004; SCZ vs BP: P > .99; SCZAFF vs BP: P = .01 |
Abbreviations: BP, bipolar disorder; MADRS, Montgomery-Asberg Depression Rating Scale; NA, not applicable; PANSS, Positive and Negative Syndrome Scale; SCZ, schizophrenia; SCZAFF, schizoaffective disorder; YMRS, Young Mania Rating Scale.
Data are presented as mean (SD) unless otherwise indicated.
Socioeconomic status was measured using the Hollingshead Index on Social Position, in which higher scores indicate a lower social position.
Group Differences in Network Metrics
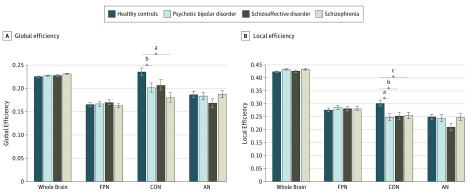
Multivariate analysis revealed a significant omnibus difference in global efficiency across all diagnostic groups (F4,575 = 2.62, P = .002) (Figure 1). Follow-up univariate tests revealed a significant difference in CON global efficiency (F3,577 = 6.76, P < .001) but no difference in whole brain (F3,577 = 2.08, P = .10), FPN (F3,577 = 0.44, P = .73), or auditory network (AN) (F3,577 = 1.03, P = .38). Post hoc tests revealed significantly reduced CON global efficiency (Cohen d = 0.36, P < .001) in patients with schizophrenia compared with HCs. Patients with bipolar disorder also had significantly reduced CON global efficiency compared with HCs (Cohen d = 0.33, P = .002). However, none of the clinical groups differed from each other (schizophrenia vs schizoaffective disorder: Cohen d = 0.19, P = .16; schizophrenia vs bipolar disorder: Cohen d = 0.01, P = .79; and schizoaffective disorder vs bipolar disorder: Cohen d = 0.18; P = .27).
Figure 1. Group Differences in Global and Local Efficiency of Functional Networks.
We observed an overall significant group difference in global efficiency (A) and local efficiency (B), controlling for sex, race/ethnicity, age, head motion, and Bipolar-Schizophrenia Network on Intermediate Phenotypes site. Cingulo-opercular network (CON) global efficiency and whole-brain global efficiency but not the frontoparietal network (FPN) or auditory network (AN) were significantly different across groups. Post hoc least significant difference tests revealed a significant reduction in CON global efficiency in the schizophrenia and bipolar disorder groups compared with healthy controls. A similar pattern was observed for local efficiency. A significant reduction in CON local efficiency was observed in all clinical groups compared with controls.
aP < .001.
bP < .01.
cP < .05.
Multivariate analysis of local efficiency also indicated statitically significant differences across all groups (F4,575 = 2.75, P = .001) (Figure 1). This omnibus difference was driven by a significant group difference in local efficiency of CON (F3,577 = 5.72, P = .001) with no difference for whole brain (F3,577 = 1.75, P = .16), FPN (F3,577 = 0.64, P = .59), or AN (F3,577 = 1.99, P = .12). The CON local efficiency was significantly higher in HCs when compared with all groups (schizophrenia: Cohen d = 0.23, P = .03; schizoaffective disorder: Cohen d = 0.27, P = .009; and bipolar disorder: Cohen d = 0.39, P < .001) but did not significantly differ between clinical groups (schizophrenia vs schizoaffective disorder: Cohen d = 0.04, P = .73; schizophrenia vs bipolar disorder: Cohen d = 0.17, P = .17; and schizoaffective disorder vs bipolar disorder: Cohen d = 0.12, P = .36).
Exploratory analysis of all Power atlas networks revealed significantly reduced global efficiency of the subcortical network in all psychotic disorder groups when controlling for race and sex (F3,587 = 4.01, P = .008). The somatosensory motor network, which includes only 5 nodes, also had significantly reduced global (F3,587 = 8.37, P < .001) and local efficiency (F3,587 = 6.86, P < .001) in psychotic disorders.
Network Efficiency and Cognition
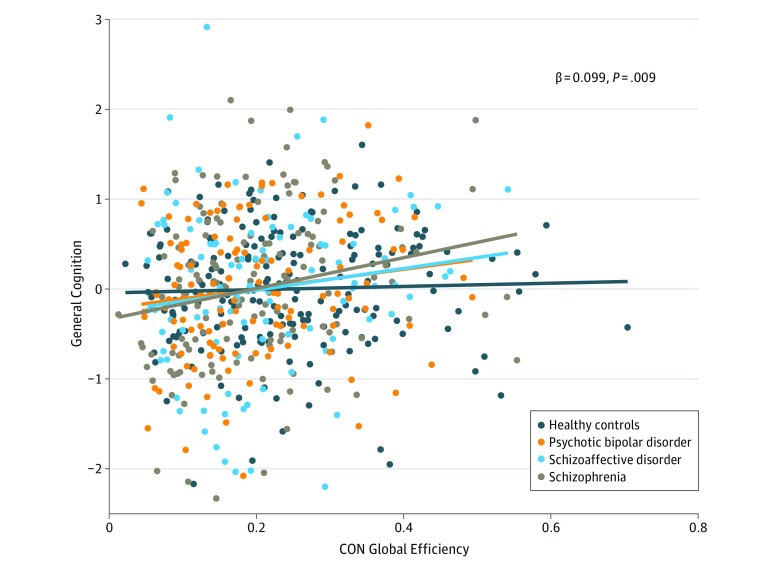
The CON global efficiency positively predicted general cognitive ability (standardized β = 0.099, P = .009). No interactions between group and CON global efficiency were observed for the schizoaffective or bipolar groups. A significant interaction was observed for the schizophrenia group (β = 0.195, P = .03), driven by a stronger association between general cognition and CON global efficiency in schizophrenia compared with HCs (Figure 2). Of interest, CON global efficiency continued to predict general cognition even when the mean CON functional connectivity was included as a predictor (β = 0.179, P = .05). Whole brain, FPN, and AN global efficiency did not significantly predict general cognition across all groups (whole brain: β = −0.029, P = .47; FPN: β = −0.004, P > .99; and AN: β = 0.039, P = .31). Follow-up analyses, including chlorpromazine equivalent values as a covariate, indicated a similar association between CON global efficiency and cognition (β = 0.125, P = .06), suggesting that this finding cannot be attributed to current antipsychotic therapy.
Figure 2. Association Between Cingulo-Opercular Network (CON) Global Efficiency and General Cognition.
Greater CON global efficiency predicted better general cognitive ability across all groups, suggesting that more globally efficient CON is related to better cognitive functioning across psychotic disorders. We observed a significant interaction for the schizophrenia and healthy control groups, reflecting a stronger association between CON global efficiency and general cognition in the schizophrenia group compared with the healthy control participants. No other significant interactions were observed, suggesting similar associations between cognition and CON global efficiency across groups. Diagonal lines represent the linear association between CON global efficiency and the residual of general cognitive ability after taking into account the diagnostic group.
No significant associations were observed between network local efficiency and cognitive ability for a priori networks: CON (β = 0.053, P = .16), whole brain (β = 0.009, P = .79), FPN (β = 0.001, P = .97), or AN (β = 0.032, P = .37) local efficiency.
Exploratory linear regressions predicting cognitive ability were performed for the networks that revealed significant group differences in efficiency. Subcortical global efficiency significantly positively predicted cognitive ability (β = 0.094, P = .009), with no significant group interactions (schizophrenia: β = 0.082, P = .37; schizoaffective disorder: β = 0.13, P = .24; and bipolar disorder: β = 0.01, P = .93). When both subcortical and CON global efficiency were included in the model, both predicted cognitive ability, suggesting independent contributions of each network to cognition (CON: β = 0.092, P = .02; subcortical: β = 0.079, P = .03). Somatosensory motor network global and local efficiency did not predict cognition (global efficiency: β = 0.037, P = .31; local efficiency: β = 0.037, P = .30).
Mediation Analysis
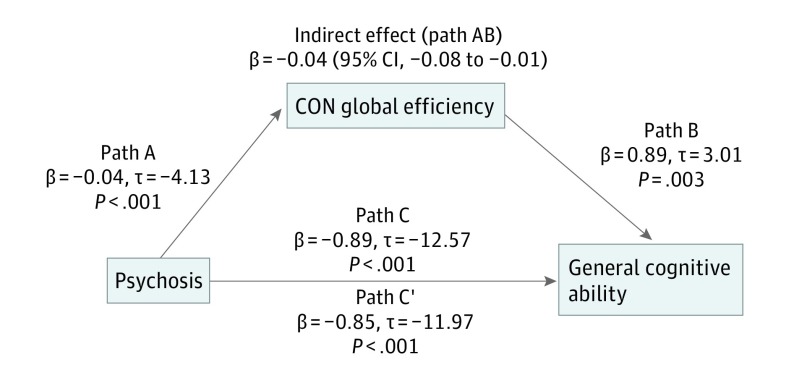
Given group differences in global efficiency and cognitive ability and the positive association between global efficiency and cognitive ability, we assessed whether CON and/or subcortical global efficiency significantly mediated the association between clinical status (patient or control) and cognition. We found that CON (β = −0.037; 95% CI, −0.076 to −0.014) (Figure 3) and subcortical (β = −0.022; 95% CI, −0.054 to −0.004) global efficiency significantly mediated group differences in cognition. When included in the same mediation model, CON (β = −0.029; 95% CI, −0.065 to −0.008) and subcortical (β = −0.018; 95% CI, −0.053 to −0.002) global efficiency continued to be significant mediators.
Figure 3. Cingulo-Opercular Network (CON) Global Efficiency Mediation.
The CON global efficiency significantly mediated the association between clinical status (patient/control) and general cognitive ability, providing further evidence that the reduced CON global efficiency in psychotic disorders may underlie deficits in general cognition. Path C represents the variance in psychosis status associated with general cognitive ability, and Path C’ represents the association between psychosis status and general cognition after taking into account CON global efficiency as a mediator. Path AB is the mediation effect and is significant at P < .05 based on confidence intervals from bias-corrected bootstrapping of 1000 samples.
Discussion
In the first study, to our knowledge, examining associations between functional network topology and cognition across the psychosis spectrum, we observed significant reductions in the efficiency of CON, a network that has been implicated in cognitive impairment and psychotic symptoms. Critically, we found behavioral relevance of reduced CON efficiency by revealing that lower CON global efficiency predicts greater impairment in general cognitive functioning—a dimension of impairment observed across psychotic disorders. The role of CON global efficiency in cognitive deficits in psychosis is further supported by its significant mediation of the association between psychotic disorder status and cognitive ability. Exploratory analyses revealed a similar role of the subcortical network in the generalized deficit, revealing significantly reduced global efficiency of the subcortical network in psychotic disorders, positive association between subcortical efficiency and cognitive ability, and subcortical global efficiency as a significant and independent mediator of psychosis and cognitive ability. These data add to an increasing body of literature implicating CON and subcortical structures in the pathophysiology of cognitive impairment in psychotic disorders and lend support to the dimensional nature of cognitive impairments across multiple psychiatric diagnoses.
The CON is a functional network that includes the anterior insula and dorsal anterior cingulate cortex (DACC). The CON is critically involved in cognitive ability and facilitates salience processing of goal-directed and environmental stimuli, relevant to the experience of psychosis. A recent transdiagnostic meta-analysis revealed reduced gray matter volume of the insula and DACC in a range of psychiatric disorders, and the volume of these nodes predicted executive functioning ability. Insula function is also abnormal in schizophrenia, revealing reduced effective connectivity with the dorsolateral prefrontal cortex, FPN, and default mode network and reduced functional connectivity with the DACC during information processing. The current study adds to this literature by revealing significantly reduced global and local efficiency of CON in psychotic disorders. These findings suggest that information transfer within this network is not optimally integrated in ways that contribute meaningfully to cognitive function. Given mounting evidence of functional connectivity abnormalities in the context of reduced brain volume in CON, future work looking at the role of structural connectivity would help further understanding of the abnormalities present in this network.
Exploratory analyses also revealed a significant role of the subcortical network in the association between psychotic disorders and the generalized deficit. The subcortical network includes nodes primarily within the thalamus and basal ganglia, which are critical for interacting with prefrontal regions to support cognitive ability. Therefore, these findings fit in an already impressive literature implicating thalamocortical connectivity in schizophrenia, conversion to psychosis, and improvement in cognitive ability after cognitive remediation. Although not initially hypothesized, these findings were robust when controlling for covariates of no interest (eg, motion, sex, race, and site) and appeared to predict cognitive ability above and beyond CON global efficiency. We therefore believe that these findings provide the first evidence of reduced global efficiency in the subcortical network in psychotic disorders and associations between subcortical global efficiency and cognitive ability.
It is widely recognized that individuals with psychosis experience cognitive impairments across many domains, and many researchers have argued that understanding the common substrate of this generalized deficit is as important as understanding the nature of specific impairments. We hypothesized that one neurobiological contribution to the generalized deficit was abnormal efficiency of functional brain networks. The CON global efficiency was related to general cognition above and beyond the mean CON connectivity, suggesting that the organization of nodal connections is important for understanding cognitive impairments. Critically, this study replicates previous findings, now in 3 distinct data sets, indicating a positive association between CON global efficiency and cognitive function in resting state and pseudoresting state data but extends this work across the spectrum of psychotic disorders. The association of cognitive deficits with network efficiency supports the hypothesis of a generalized impairment in cognition that is shared across psychotic disorders and is related to the efficiency of functional brain networks.
Finally, we did not observe significant reductions in FPN efficiency or a significant association between FPN efficiency and general cognition. This was surprising given the strong literature suggesting a role of FPN abnormalities in cognitive ability generally and psychiatric disorders specifically. Previous associations have been found between FPN efficiency and cognition; however, this was found in pseudoresting state data, which involved the regression of task-related BOLD signal. The FPN is composed of flexible hubs that rapidly update based on task demands. We speculate that pure resting state data may be less reflective of these flexible dynamics, and therefore FPN efficiency measured using resting state may be less sensitive to associations with cognition. However, follow-up studies that more directly compared resting state and pseudoresting state data would be needed to support this hypothesis.
Limitations
A limitation of the current study was that the amount of resting state data was relatively small (5 minutes) in an intermediate range of the time needed for stable resting state data estimates. Nonetheless, the consistency of our findings across multiple data sets provides evidence of convergent validity on the association between CON efficiency and cognition. In addition, most patients with psychotic disorders were taking antipsychotics, and the effect of medications on our findings cannot be determined. However, generalized cognitive deficits in psychotic disorders are not believed to be secondary to antipsychotics. Inclusion of a chlorpromazine equivalent dose as a covariate did not change the association between cognition and CON global efficiency.
Conclusions
Using a dimensional approach, we found that the generalized cognitive deficit is associated with reduced CON and subcortical network efficiency across psychotic disorders. Our findings add to an expanding literature implicating CON in the phenomenology of psychiatric disorders and support the utility of network science in understanding functional connectivity abnormalities in disease states. We revealed significant reductions in CON global and local efficiency and subcortical network global efficiency across psychotic disorders and a mediating role of CON and subcortical global efficiency in the association between psychotic disorder status and cognitive function. Further understanding of why CON and subcortical efficiency are reduced and how those connectivity differences interact with other brain systems will be critical to further elucidating the dimension of cognitive impairment in psychosis.
eTable 1. Scanning Factors
eTable 2. Associations Between Covariates and Variables of Interest
eAppendix. Supplementary Methods
eFigure 1. CON and FPN Global Efficiency Across Graph Thresholds
eFigure 2. Global and Local Efficiency in All Power Networks
eReferences
References
- 1.Lewandowski KE, Sperry SH, Cohen BM, Ongür D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med. 2014;44(15):3239-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833-858. [DOI] [PubMed] [Google Scholar]
- 3.Robinson LJ, Thompson JM, Gallagher P, et al. . A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93(1-3):105-115. [DOI] [PubMed] [Google Scholar]
- 4.Bora E, Yucel M, Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br J Psychiatry. 2009;195(6):475-482. [DOI] [PubMed] [Google Scholar]
- 5.Hill SK, Reilly JL, Keefe RS, et al. . Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170(11):1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodkind M, Eickhoff SB, Oathes DJ, et al. . Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55-61. [DOI] [PubMed] [Google Scholar]
- 8.Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JT, Holmes AJ, Masters GA, et al. . Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71(2):109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamah D, Barch DM, Repovš G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150(2):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69(10):967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123(2-3):105-115. [DOI] [PubMed] [Google Scholar]
- 13.Dosenbach NU, Visscher KM, Palmer ED, et al. . A core system for the implementation of task sets. Neuron. 2006;50(5):799-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power JD, Cohen AL, Nelson SM, et al. . Functional network organization of the human brain. Neuron. 2011;72(4):665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Buckner RL, Fox MD, et al. . Parcellating cortical functional networks in individuals. Nat Neurosci. 2015;18(12):1853-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun U, Schäfer A, Walter H, et al. . Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 2015;112(37):11678-11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold JM, Dickinson D. “Generalized cognitive deficit” in schizophrenia: overused or underappreciated? Schizophr Bull. 2013;39(2):263-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly JL, Sweeney JA. Generalized and specific neurocognitive deficits in psychotic disorders: utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr Bull. 2014;40(3):516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medaglia JD, Lynall ME, Bassett DS. Cognitive network neuroscience. J Cogn Neurosci. 2015;27(8):1471-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336-349. [DOI] [PubMed] [Google Scholar]
- 24.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29(23):7619-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheffield JM, Repovs G, Harms MP, et al. . Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamminga CA, Ivleva EI, Keshavan MS, et al. . Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170(11):1263-1274. [DOI] [PubMed] [Google Scholar]
- 27.Meda SA, Ruaño G, Windemuth A, et al. . Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A. 2014;111(19):E2066-E2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Non-Patient Edition (SCID-I/NP) Version 2.0. New York, NY: American Psychiatric Publishing; 1995. [Google Scholar]
- 29.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283-297. [DOI] [PubMed] [Google Scholar]
- 30.Keefe RS, Harvey PD, Goldberg TE, et al. . Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102(1-3):108-115. [DOI] [PubMed] [Google Scholar]
- 31.Hochberger WC, Hill SK, Nelson CL, et al. . Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophr Res. 2016;170(1):156-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989(7):59-67. [PubMed] [Google Scholar]
- 33.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [DOI] [PubMed] [Google Scholar]
- 35.Meda SA, Wang Z, Ivleva EI, et al. . Frequency-specific neural signatures of spontaneous low-frequency resting state fluctuations in psychosis: evidence from Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Consortium. Schizophr Bull. 2015;41(6):1336-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059-1069. [DOI] [PubMed] [Google Scholar]
- 37.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717-731. [DOI] [PubMed] [Google Scholar]
- 38.Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40(suppl 2):S131-S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? an emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5-6):655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79(4):814-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran LV, Tagamets MA, Sampath H, et al. . Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013;74(6):467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100(4):1740-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anticevic A, Haut K, Murray JD, et al. . Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72(9):882-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay IS, Nienow TM, MacDonald AW III. Increases in intrinsic thalamocortical connectivity and overall cognition following cognitive remediation in chronic schizophrenia. Biol Psychiatry. 2016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13(3):224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheffield JM, Kandala S, Burgess GC, Harms MP, Barch DM. Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(6):498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole MW, Repovš G, Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birn RM, Molloy EK, Patriat R, et al. . The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keefe RS, Bilder RM, Davis SM, et al. ; CATIE Investigators; Neurocognitive Working Group . Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Scanning Factors
eTable 2. Associations Between Covariates and Variables of Interest
eAppendix. Supplementary Methods
eFigure 1. CON and FPN Global Efficiency Across Graph Thresholds
eFigure 2. Global and Local Efficiency in All Power Networks
eReferences