Abstract
Background
A retrospective analysis was conducted of the early and long-term outcomes after surgery for infective endocarditis (IE).
Material/Methods
We included 360 patients with IE operated upon between 1993 and 2012. The primary endpoint was overall cumulative postoperative survival at 30 days. Secondary endpoints were early postoperative outcomes and complication rates. Factors associated with 30-day mortality were analyzed.
Results
Mean age was 58.7±14.7 years and 26.9% (n=97) were female. The mean follow-up was 4.41±4.53 years. Postoperative survival was 81.7% at 30 days, 69.4% at 1 year, 63.3% at 5 years, and 63.3% at 10 years. Non-survivors were significantly older (p=0.014), with higher NYHA Class (p=0.002), had higher rates of preoperative diabetes mellitus (p=0.005), renal failure (p=0.001), and hepatic disease (p=0.002). Furthermore, non-survivors had higher baseline alanine aminotransferase (ALT, p=0.048), aspartate transaminase (AST, p=0.027), bilirubin (p=0.013), white cell count (WCC, p=0.034), and CRP (p=0.049). Factors associated with 30-day mortality were longer duration of surgery, CPB, and aortic cross-clamping times (p<0.001, p<0.001, and p=0.003, respectively), as well as higher RBC, FFP, and platelet transfusion requirements (p<0.001, p=0.005, and p<0.001, respectively). Multivariate logistic regression analysis revealed liver cirrhosis (OR 4.583, 95-CI: 1.096–19.170, p=0.037) and longer CPB time (OR 1.025, 95-CI 1.008–1.042, p=0.004) as independent predictors of 30-day mortality.
Conclusions
Surgical treatment of IE shows satisfactory early, midterm, and long-term results. Multivariate logistic regression analysis revealed cirrhosis and longer CPB time as independent predictors of 30-day mortality.
MeSH Keywords: Cardiac Surgical Procedures, Endocarditis, Heart Valve Diseases
Background
Although medical and surgical treatment of infective endocarditis (IE) has drastically improved, it is considered a severe clinical picture, with potential mortality approaching 50%. Surgical intervention has significantly improved survival, compared to isolated medical treatment [1]. Common indications for surgery include refractory heart failure, disseminating infection with periannular extension, multi-resistant organisms, recurrent embolic events, and the presence of prosthetic material [2,3].
Moreover, there are a number of controversial opinions in terms of the timing of surgical treatment of such patients. The American College of Cardiology/American Heart Association (ACC/AHA) endocarditis guidelines recommend early surgery for patients with recurrent emboli and persistent vegetation (class IIa) [4], while the European guidelines recommend early surgery as a class-IIb indication for patients with vegetations >1.5 cm [5].
Progress in echocardiography has led to earlier diagnosis and hence a more aggressive approach to surgical management. Recent series, including the French registry [6] and the Euro Heart Survey data [7], included more than 50% of patients with active disease.
This study sought to analyze clinical characteristics associated with early mortality after surgery for IE in order to improve preoperative management and decrease postoperative mortality.
Material and Methods
Study design and patient cohort
After approval by the Ethics Committee of the Medical Faculty, University of Heidelberg, 360 patients who underwent surgery for IE from January 1993 to December 2012, were included in this study. Demographic, pre-, intra- and post-operative clinical data were collected from electronic medical charts. Adverse clinical events, postoperative valve-related complications, and transesophageal echocardiography data were documented.
The primary endpoint was postoperative 30-day mortality. The secondary endpoints were early postoperative outcomes and adverse events. Demographics and preoperative clinical characteristic of patients who survived 30 days after surgery were compared with those of non-survivors to identify factors predictive of 30-day mortality.
Diagnosis and indications for surgery
A transesophageal echocardiography (TEE) was mandatorily performed in patients with positive clinical and laboratory results, and the modified Duke criteria were assessed for diagnosis of IE [8,9]. Indications for surgery in the active phase of IE included increasing cardiac failure, therapy refractory infections, recurrent embolic events, acute kidney failure, and prosthetic valve endocarditis.
Surgical technique
The standard procedures for IE included the use of cardio-pulmonary bypass with mild hypothermia. Cold crystalloid cardioplegia was administered in antegrade fashion to protect myocardial function of the heart. Radical resection of affected tissue followed by a topical application of antibiotic agent was routinely performed in all patients. The debridement of paravalvular infected structures was done despite potential damage to the conduction system or myocardial/fibrous tissue. A careful examination of the valvular annulus and surrounding structures was carried out for potential extensive abscess resection. In some patients, ventricular septal defects that resulted in extension of abscess cavities were closed using Dacron patches. In cases with annular destruction and periannular invasion with aortic root involvement, the reconstruction was performed using allografts. The use of artificial material was kept as low as possible. The type of valve implanted was generally determined by the preference of each surgeon and according to patient characteristics.
Postoperative care
Apart from patient stabilization in terms of hemodynamics and weaning from respirator, strict monitoring of the local and systemic infective process was the primary target of early therapy in our intensive care unit (ICU). The standard regimen of postoperative antibiotic therapy consisted of a triple-combination with vancomycin, meropenem, and rifampicin. The administration of rifampicin was carried out over 10 days, whereas the other 2 antibiotics were given for 6 weeks intravenously. Deviating from this, the adapted antibiotic treatment was conducted according to the microorganism isolated and the corresponding resistogram. Transesophageal echocardiography was performed at close intervals postoperatively to exclude recurrent endocarditis.
Statistical snalysis
Data are presented as continuous or categorical variables. All continuous variables were analyzed with the t test and expressed as the mean ± standard deviation. Pearson’s χ2 or Fisher exact tests were used for categorical data, dependent on the minimum expected count in each cross-tabulation. The categorical data are expressed as total numbers and percentages. ANOVA with repeated measurements was applied for the comparison of survivors and non-survivors regarding perioperative inflammatory markers. A Kaplan-Meier actuarial survival estimate was generated to analyze survival of the entire cohort. Multivariate logistic regression analysis was performed on univariate predictors for 30-days mortality with an entry criterion of p<0.05. All data were analyzed using the Statistical Package for Social Sciences, version 21.0 (SPSS Inc., Chicago, IL).
Results
Perioperative outcome
The mean age of the present cohort was 58.7±14.7 years and 26.9% (n=97) were female. Twenty patients (5.6%) were current IVDUs and presented with symptoms of progressive heart failure with the mean NYHA stage of 3.4±0.9. Twelve patients (3.3%) had past medical history of valve endocarditis, in which cases all valves were replaced. Fifty-three patients (14.7%) from the present cohort had undergone previous valve surgery for various reasons: 11.1% (n=40) aortic valve surgery, 1.7% (n=6) mitral valve surgery, 1.4% (n=5) combined aortic und mitral valve surgery, and 0.6% (n=2) combined mitral and tricuspid surgery. Patients’ preoperative NYHA status in this study was distributed as follows: 3.1% (n=11) had NYHA class I, 12.2% (n=44) had NYHA class II, 39.4% (n=142) had NYHA class III and 34.4% (n=124), and 10.8% (n=39) had NYHA stadium IVa and IVb. Other characteristics included diabetes mellitus (23.3%, n=84), hepatitis (7.8%, n=28), liver cirrhosis (5.6%, n=20), and other hepatic disorders (17.5%, n=63). Renal insufficiency was present in 39.2% of patients: 27.2% (n=98) had chronic kidney disease, 2.5% (n=9) had acute renal failure, 7.5% (n=27) required dialysis preoperatively, and 1.9% (n=7) had undergone previous renal transplantation. Furthermore, 3 patients were HIV-positive and 27.2% (n=98) had previous cerebrovascular events.
Most valves affected in this study were aortic (45.6%, n=164), followed by mitral (31.1%, n=112), tricuspid (4.4%, n=16), and pulmonary (0.3%, n=1) valves. A relatively large number of patients with 2 or more affected valves were also included in this observation: 13.3% (n=48) with combined aortic and mitral valves, 2.2% (n=8) with aortic and tricuspid valves, and 1.1% (n=4) each with aortic, mitral, and tricuspid as well as mitral and tricuspid valves. The remaining 3 patients had aortic, mitral, and pulmonary valves; aortic and pulmonary valves; and mitral and pulmonary valves affected, respectively. Of them, 86.1% (n=310) were native valve endocarditis and 13.9% (n=50) were prosthetic valve endocarditis. Interestingly, only 3 cases were performed electively, whereas 50.3% (n=181) were urgent, 43.9% (n=158) were emergencies, and 5.0% (n=18) received surgery as a last resort.
The mean operation duration was 260±117.0 min, while mean CPB time was 151.0±82.7 min with a cross-clamp time of 94.0±46.3 min. Patients received 1902±1405 ml red blood cells, 687±715 ml fresh frozen plasma, and 338±336 ml platelets. Intraoperatively, a valve reconstruction could only be performed in 8 cases (2.2%), whereas most patients underwent valve replacement using prosthetic (60.6%, n=218), biologic (36.4%, n=131), or combined (0.8%, n=3) material. In 5.3% (n=19) of cases, no vegetations were found in situ, whereas all other patients had vegetations of various size, ranging from 0–5 mm to >20 mm (Table 1).
Table 1.
Presence of vegetations and their size found during surgical treatment of endocarditis.
| No vegetations | 24 (6.7%) |
| 0–5 mm | 8 (2.1%) |
| 5–10 mm | 40 (11.3%) |
| 10–15 mm | 75 (20.8%) |
| 15–20 mm | 85 (23.6%) |
| >20 mm | 128 (35.6%) |
The mean ICU stay was 13.9±24.3 days. The mean stay from the day of surgery until discharge to general ward was 20.1±22.8 days, and the mean postoperative total in-hospital stay was 40.4±25.3 days.
During the postoperative hospital stay, 48.1% (n=173) developed renal dysfunction, with 24.4% (n=88) requiring hemodialysis. Liver toxicity, presented as >5-fold elevation in aspartate transaminase (AST) or alanine aminotransferase (ALT) from the baseline value, developed in 16.1% (n=58) and 10.3% (n=37), respectively. Increased bilirubin (>1.0 mg/dL) was observed in 68.1% (n=245) of patients postoperatively. Further adverse events included atrioventricular block (grade I – 5.1%, n=18; grade II – 1.1%, n=4; grade III – 10.4%, n=37, with requirement for pace maker implantation in – 11%, n=39), coagulopathy in 17.7%, (n=63), need for rethoracotomy for bleeding 7.9% (n=28), and new-onset cerebrovascular events in 2.5% (n=9).
There was a wide range of pathogens responsible for infective endocarditis in our patient cohort, with Staphylococcus species found in 40.0% (n=144), Streptococcus species in 23.6% (n=85), Enterococcus species in 12.8% (n=46), and other pathogens in the remaining cases. In 18.3% (n=66) of the patients, no pathogens could be isolated. A detailed distribution of the microorganisms is presented in Table 2.
Table 2.
Microorganisms isolated from patients with endocarditis.
| No pathogen isolated | 66 (18.3%) |
| Enterococci | |
| Enterococcus faecalis | 40 (11.1%) |
| Enterococcus species | 6 (1.7%) |
| Staphylococci | |
| Staphylococcus aureus | 89 (24.7%) |
| Staphylococcus capitis | 1 (0.3%) |
| Staphylococcus caprae | 1 (0.3%) |
| Staphylococcus epidermidis | 23 (6.4%) |
| Staphylococcus haemolyticus | 1 (0.3%) |
| Staphylococcus hominis | 6 (1.7%) |
| Staphylococcus intermedius | 1 (0.3%) |
| Staphylococcus coag.-neg. | 5 (1.4%) |
| Staphylococcus lugdunensis | 5 (1.4%) |
| Staphylococcus xylosus | 1 (0.3%) |
| MRSA | 14 (3.9%) |
| Streptococci | |
| Streptococcus agalactiae | 5 (1.4%) |
| Streptococcus anginosus | 3 (0.8%) |
| Streptococcus bovis | 22 (6.1%) |
| Streptococcus constellatus | 2 (0.6%) |
| Streptococcus intermedius | 1 (0.3%) |
| Streptococcus mitis | 10 (2.8%) |
| Streptococcus mutans | 5 (1.4%) |
| Streptococcus oralis | 7 (1.9%) |
| Streptococcus parasanguis | 1 (0.3%) |
| Streptococcus pneumonia | 2 (0.6%) |
| Streptococcus salivarius | 1 (0.3%) |
| Streptococcus sanguinis | 6 (1.7%) |
| Streptococcus viridans | 9 (2.5%) |
| Streptococcus haemolyticus group B | 10 (26%) |
| Other microorganisms | |
| Abiotropia species | 1 (0.3%) |
| Aggregatibacter actinomycetemcomitans | 1 (0.3%) |
| Aggregatibacter aphrophilus | 1 (0.3%) |
| Bacillus cemus | 1 (0.3%) |
| Citrobacter kosteri | 1 (0.3%) |
| Corynebacterium jeikeium | 1 (0.3%) |
| E. coli | 5 (1.4%) |
| Enterobacter cloacae | 1 (0.3%) |
| Kocuria palustris | 1 (0.3%) |
| Lactococcus plantamm | 1 (0.3%) |
| Neisseria meningitides | 1 (0.3%) |
| Peptostreptococcus micros | 1 (0.3%) |
| Pseudomonas aeruginosa | 1 (0.3%) |
The mean follow-up was 1611±1651 days (4.41±4.53 years), with the longest follow-up 7399 days (>20 years). The postoperative survival was 81.7% at 30 days, 69.4% at 1 year, 65.7% at 2 years, 63.3% at 5 years, 63.3% at 10 years, and 48.3% at 20 years (Figure 1). Causes of death were sepsis in 32 patients (8.9%), followed by multi-organ failure in 18 patients (5.0%) and death related to cardiac complications in 16 patients (4.4%). A detailed analysis of causes of death is presented in Table 3.
Figure 1.
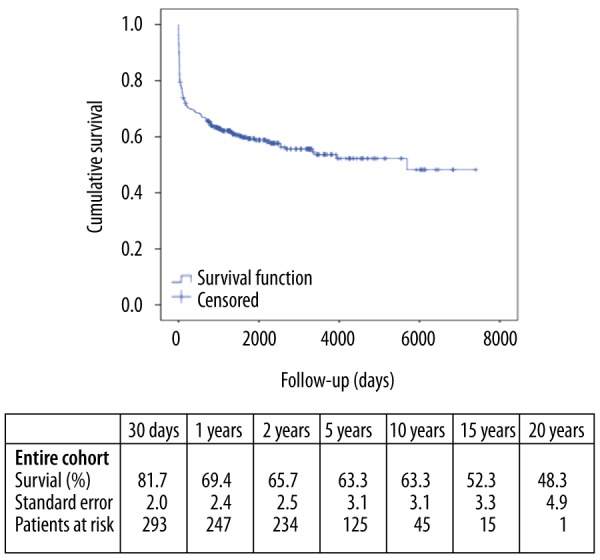
Kaplan-Meier Survival estimate for patients with infective endocarditis who underwent surgical treatment between September 1993 and December 2012. Patients were censored at the cut-off of the study.
Table 3.
Postoperative causes of death in patients with infective endocarditis.
| Unknown | 48 (13.3%) |
| Sepsis | 32 (8.9%) |
| Multi organ failure | 18 (5%) |
| Cardiac complications | 16 (4.4%) |
| Cerebral complications | 8 (2.2%) |
| Sudden cardiac death | 2 (0.6%) |
| Bleeding | 2 (0.6%) |
| Embolism | 1 (0.3%) |
| Haemodynamic collapse | 19 (5.3%) |
| Other | 5 (1.4%) |
Univariate and multivariate predictors of early mortality
A subgroup analysis of 30-day survivors and non-survivors in terms of analyzing factors associated with early mortality is presented in Tables 4 and 5. Non-survivors were significantly older (p=0.014), had significantly higher incidence of preoperative diabetes mellitus (p=0.005), higher NYHA class (p=0.002), higher incidence of renal failure (p=0.001), and more liver cirrhosis (p=0.002). Furthermore, non-survivors had significantly higher baseline alanine aminotransferase (ALT, p=0.048), aspartate transaminase (AST, p=0.027), bilirubin (p=0.013), white cell count (WCC, p=0.034), and CRP (p=0.049). Further factors associated with 30-day mortality were longer operating time (p<0.001), longer CPB time (p<0.001), longer aortic cross-clamp time (p=0.003), higher RBC, FFP and platelet transfusion requirement (p<0.001, p=0.005 and p<0.001, respectively), postoperative renal failure (p<0.001), and coagulation disorder (p=0.020).
Table 4.
Preoperative demographics in 30-day survivors vs. non-survivors.
| 30-d survivors | 30-d non-survivors | p-value | |
|---|---|---|---|
| Age (yrs) | 57.8±14.9 | 62.6±13.5 | 0.014 |
| Female | 79 (27.1%) | 18 (26.1%) | 0.858 |
| BMI | 26.3±5.1 | 26.9±5.0 | 0.370 |
| Diabetes mellitus | 59 (20.3%) | 25 (36.2%) | 0.005 |
| NYHA class | 0.002 | ||
| Class I | 9 (3.1%) | 2 (2.9%) | |
| Class II | 42 (14.4%) | 2 (2.9%) | |
| Class III | 122 (41.9%) | 20 (29.0%) | |
| Class IVa | 92 (31.6%) | 32 (46.4%) | |
| Class IVb | 26 (8.9%) | 13 (18.8%) | |
| Renal insufficiency | 0.001 | ||
| No renal failure | 189 (64.9%) | 30 (43.5%) | |
| Compensated renal failure | 76 (26.1%) | 22 (31.9%) | |
| Dialysis | 15 (5.2%) | 12 (17.4%) | |
| Previous renal transplant | 5 (1.7%) | 2 (2.9%) | |
| Acute renal failure | 6 (2.1%) | 3 (4.3%) | |
| Hepatic disease | 0.002 | ||
| No hepatic disease | 207 (71.1%) | 42 (60.9%) | |
| Hepatitis | 25 (8.6%) | 3 (4.3%) | |
| Cirrhosis | 10 (3.4%) | 10 (14.5%) | |
| Other | 49 (16.8%) | 14 (20.3%) | |
| Previous valve surgery | 0.232 | ||
| No previous valve surgery | 253 (86.9%) | 54 (78.3%) | |
| Aortic valve surgery | 27 (9.3%) | 13 (18.8%) | |
| Mitral valve surgery | 5 (1.7%) | 1 (1.4%) | |
| Aortic and mitral valve surgery | 4 (1.4%) | 1 (1.4%) | |
| Mitral and tricuspid valve surgery | 2 (0.7%) | 0 | |
| HIV | 2 (0.7%) | 1 (1.4%) | 0.473 |
| Previous CVA | 76 (26.1%) | 22 (31.9%) | 0.333 |
| Arrhythmias | 196 (67.4%) | 52 (80.0%) | 0.045 |
| Previous endocarditis | 11 (3.8%) | 1 (1.4%) | 0.475 |
| IVDU | 16 (5.5%) | 4 (5.8%) | 1.000 |
| Pathogen isolated | 138 (70.4%) | 24 (54.5%) | 0.042 |
| ALT (U/L) | 40.4±80.8 | 215.7±705.3 | 0.048 |
| AST (U/L) | 49.3±114.5 | 366.2±1137.5 | 0.027 |
| Bilirubin (mg/dL) | 1.15±2.77 | 2.02±2.40 | 0.013 |
| Positive preoperative blood culture | 239 (82.1%) | 55 (79.7%) | 0.640 |
| Prosthetic valve endocarditis | 36 (12.4%) | 14 (20.3%) | 0.087 |
| Recent prosthetic valve endocarditis | 13 (4.5%) | 5 (7.2%) | 0.232 |
| AV-block | 0.415 | ||
| No AV-block | 239 (82.1%) | 53 (81.5%) | |
| Grade I | 17 (5.8%) | 1 (1.5%) | |
| Grade II | 3 (1.0%) | 1 (1.5%) | |
| Grade III | 29 (10.0%) | 8 (12.3%) | |
| Vegetations | 0.656 | ||
| No vegetations | 13 (5.6%) | 6 (11.5%) | |
| 0–5 mm | 5 (2.2%) | 1 (1.9%) | |
| 5–10 mm | 28 (12.1%) | 4 (7.7%) | |
| 10–15 mm | 47 (20.3%) | 12 (23.1%) | |
| 15–20 mm | 55 (23.7%) | 12 (23.1%) | |
| >20 mm | 84 (36.2%) | 17 (32.7%) | |
| Laboratory parameters | |||
| WCC | 12.3±5.7 | 14.6±8.7 | 0.034 |
| CRP | 84.7±73.0 | 105.2±71.4 | 0.049 |
Table 5.
Intraoperative data and postoperative outcomes in 30-day survivors vs. non-survivors.
| 30-d survivors | 30-d non-survivors | p-value | |
|---|---|---|---|
| Urgency of the procedure | <0.001 | ||
| Elective | 3 (1.0%) | 0 | |
| Urgent | 152 (52.2) | 29 (42.0%) | |
| Emergency | 129 (44.3%) | 29 (42.0%) | |
| Salvage procedure | 7 (2.4%) | 11 (15.9%) | |
| Bypass time (min) | 139.2±70.4 | 202.2±108.3 | <0.001 |
| Cross clamp time (min) | 89.7±42.1 | 112.3±57.5 | 0.003 |
| Operation duration (Min) | 245.4±106.4 | 323.5±138.1 | <0.001 |
| Type of valve surgery | |||
| Mechanical prosthesis | 181 (62.2%) | 37 (53.6%) | |
| Biological prosthesis | 101 (34.7%) | 30 (43.5%) | |
| Combined | 2 (0.7%) | 1 (1.4%) | |
| Reconstruction | 7 (2.4%) | 1 (1.4%) | |
| Site of valve surgery | 0.466 | ||
| Aortic | 135 (46.4%) | 29 (42.0%) | |
| Mitral | 88 (30.2%) | 24 (34.8%) | |
| Tricuspid | 14 (4.8%) | 2 (2.9%) | |
| Aortic and mitral | 38 (13.1%) | 10 (14.5%) | |
| Aortic and tricuspid | 7 (2.4%) | 1 (1.4%) | |
| Mitral and tricuspid | 4 (1.4%) | ||
| Aortic, mitral and tricuspid | 2 (0.7%) | 2 (2.9%) | |
| Aortic, mitral and pulmonary | 0 | 1 (1.4%) | |
| Pulmonary | 1 (0.3%) | 0 | |
| Mitral and pulmonary | 1 (0.3%) | 0 | |
| Aortic and pulmonary | 1 (0.3%) | 0 | |
| RBC (mL) | 1743±1276 | 2570±1710 | <0.001 |
| FFP (mL) | 619±618 | 977±980 | 0.005 |
| Platelets (mL) | 305±327 | 477±339 | <0.001 |
| Permanent pacemaker | 32 (11.0%) | 7 (10.8%) | 0.958 |
| Renal failure | <0.001 | ||
| Normal renal function | 178 (61.2%) | 9 (13.8%) | |
| Conservative treatment | 65 (22.3%) | 16 (24.6%) | |
| Dialysis | 11 (3.8%) | 2 (3.1%) | |
| Hemofiltration | 37 (12.7%) | 38 (58.5%) | |
| Coagulation disorder | 45 (15.5%) | 18 (27.7%) | 0.020 |
| Reopening for bleeding | 22 (7.6%) | 6 (9.2%) | 0.651 |
| CVA | 5 (1.7%) | 4 (6.2%) | 0.062 |
RBC – red blood cells; FFP – fresh frozen plasma; CVA – cerebrovascular accident.
Figures 2 and 3 represent the patients’ inflammatory status prior to surgery, without statistical differences. Multivariate logistic regression analysis revealed cirrhosis (OR 4.583, 95% CI 1.096–19.170, p=0.037) and longer CPB time (OR 1.025, 95% CI 1.008–1.042, p=0.004) as the only independent predictors of 30-day mortality after surgery for IE.
Figure 2.
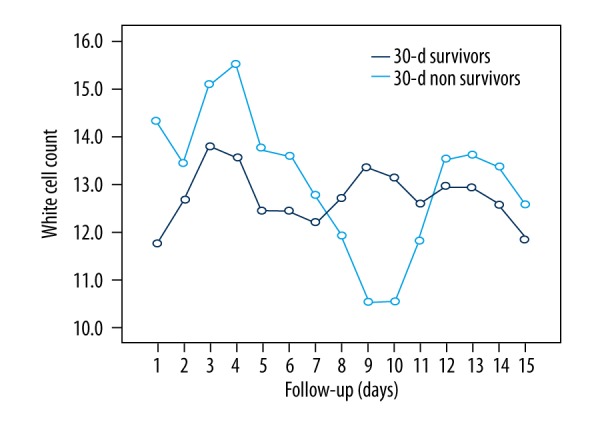
Postoperative white cell count (WCC) course in 30-day survivors vs. 30-day non-survivors. There are no statistically significant differences between the two groups (p=0.788).
Figure 3.
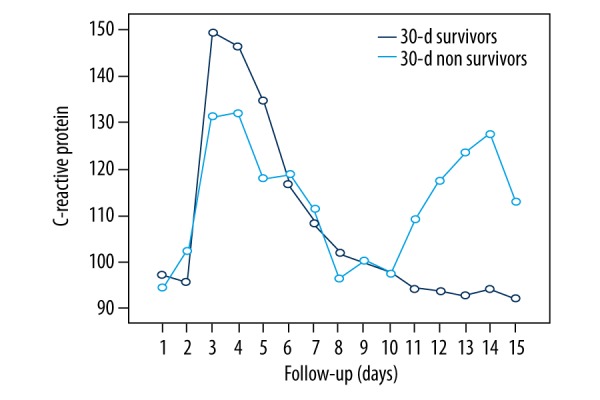
Postoperative CRP course in 30-day survivors vs. 30-day non-survivors. There are no statistically significant differences between the two groups (p=0.704).
Discussion
In the current study, higher age, preoperative diabetes mellitus, higher NYHA class, renal failure, and hepatic disease were associated with 30-day mortality following surgery for IE. Moreover, non-survivors had significantly higher baseline liver enzymes and infection parameters. Further factors associated with 30-day mortality were longer operating time, CPB time, and aortic cross-clamp time. Additionally, higher blood product transfusion requirement was identified as a surrogate parameter associated with postoperative mortality.
Pang et al. [10] found that preoperative impaired creatinine clearance and stroke at the time of presentation were independent risk factors for in-hospital mortality. In this cohort, renal insufficiency was present in 39.2% of patients and 27.2% had previous cerebrovascular events. Survival after surgery for IE is significantly impaired once cerebral embolism has occurred [11]. Other studies showed that up to 40% of patients with IE experience cerebrovascular events [12–14]. However, in a study by Sonneville et al. [15], most patients who underwent urgent cardiac surgery with pre-existing neurologic complications survived and had good functional outcomes. In the International Collaboration of Endocarditis (ICE) prospective cohort study, it was shown that the highest risk of embolism and death occurred during the first week after diagnosis of IE [16]. Additionally, our current data show that almost half of the patients suffered from advanced heart failure symptoms at the time of surgery (NYHA IV). Considering the impact of preoperative congestive heart failure and preoperative cerebral embolism on survival following surgery for IE, early intervention should be recommended, potentially reducing early and late mortality [17,18].
Our data demonstrate that liver cirrhosis and increased infection parameters are significant contributors to mortality in the setting of IE. End-stage liver disease (ESLD) is often complicated by infections, taking a significant toll on overall outcome [19]. Recurrent hospitalizations and the increased need of medical interventions make these patients susceptible to developing complex infections with multidrug-resistant pathogens [20,21]. Bloodstream infections are significantly more common in cirrhotic than in non-cirrhotic patients [22,23] and infection-related mortality is reported to be as high as 66% within the first year. Hence, systemic infection and liver cirrhosis are considered important prognostic markers in patients with IE.
IE has shifted from being a disease linked to dental interventions to being a healthcare-associated problem, with a high incidence of Staphylococcus aureus. S. aureus has repeatedly been shown to be an independent predictor of mortality in IE [24–26]. This species is characterized by high virulence, giving rise to large vegetations and increased potential of abscess formation. In our patient cohort, Staphylococcus species were the most frequent pathogens, found in 40.0% (n=144) of all cases, followed by Streptococci and Enterococci species. Previous studies have shown that S. aureus has replaced Streptococci as the most common pathogen in IE [24,25]. Others found that mortality is independently associated with S. aureus IE, demonstrating a 5-year survival of less than 50%, while the survival rate of patients with IE resulting from other pathogens was over 80% [27]. Jung et al. performed a study on active infective native mitral valve endocarditis, reporting 5-year survival rates of 97.5% and 89.5% for patients who had undergone mitral valve repair and replacement, respectively [28]. However, the rate of S. aureus infection in their study was 18%, compared to 40% in the present study. In a study by Fowler et al. [26] including patients with left- and right-sided IE, in addition to higher mortality, patients with S. aureus-associated IE also presented with higher event rates of stroke, systemic embolization, and persistent bacteremia, compared to patients with non-S. aureus IE. The aggressive nature of S. aureus-associated IE and the resulting morbidity are reflected in the 2014 AHA/ACC Guidelines for the Management of Patients with Valvular Heart Disease, where early surgery is recommended in IE caused by S. aureus [4].
Previous studies report the incidence of culture-negative IE at between 2.5% and 31%, with negative impact on outcome, because of subsequent delay of diagnosis and treatment [29]. Our cohort included 66 patients (18.3%) with culture-negative IE, which were significantly more prevalent in the non-survivors group, possibly due to prior empiric antibiotic therapy before acquiring blood cultures or infection by fastidious organisms [3,5].
The presence of large valvular vegetations has been reported as a risk factor for adverse events and even as a predictor of stroke in the case of mitral valve endocarditis [30–33]. Macroscopic vegetations usually contain high concentrations of pathogens, which seldom regress under antibiotic treatment and potentially give rise to embolic events [34]. Moreover, some studies have shown a correlation between vegetation size and outcome [30–32], yet this observation was not sustained in our current cohort. In our study, we used TEE routinely in the diagnosis of IE to maximize the diagnostic sensitivity, which enabled us to assess vegetation size and to detect other surgical indication such as perivalvular infection with abscess, fistula, or pseudoaneurysm formation, thus influencing the clinical management and indirectly affecting the outcome.
Additionally, 50 patients (13.9%) had prosthetic valve endocarditis (PVE) at the time of admission, which in itself is a recognized predictor of long-term mortality, as already shown by Pang et al. [10]. PVE affects 1–6% of prosthetic valve patients and is considered the most serious form of endocarditis [37]. Although surgical treatment for PVE is usually indicated, it remains a demanding procedure with high recurrence rates. Wang et al. [35] reported that the prognosis of PVE is worse than that of native valve IE. In this patient cohort, however, PVE was not associated with early mortality.
In the current cohort, 11% of patients were in need of permanent pacemaker implantation, which is comparable to the previously published range of between 12% and 24% [36,37]. Conduction abnormalities are usually the result of infective expansion involving the membranous interventricular septum, often in cases of aortic valve endocarditis.
The occurrence of major end-organ complications is of utmost importance because prolonged operating time, CPB time, and aortic cross-clamp time, with resulting coagulation disorders and massive transfusions, were predictors of mortality in our cohort. These findings call for further improvements of intraoperative strategies and highlight the importance of (peri-) operative blood conservation protocols and the important role of excessive bleeding.
The long-term survival for patients in our study was comparable to data reported in other studies, with 10-year survival of 63.3% in our study versus 52–73% in other studies [38–40].
Conclusions
Despite progress in antibiotic therapy and surgical management, treatment of IE remains demanding due to potential complications further aggravating comorbidities such as embolic stroke and renal failure. Thus, adequate antibiotic therapy and timely surgical intervention may be key factors for improving outcome.
Footnotes
Partially presented at the 44th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery, Freiburg, Germany, 9–11 February 2015
Source of support: Departmental sources
References
- 1.Netzer RO, Altwegg SC, Zollinger E, et al. Infective endocarditis: Determinants of long term outcome. Heart. 2001;88:61–66. doi: 10.1136/heart.88.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniel WG, Mügge A, Martin RP, et al. Improvement in the diagnosis of abscesses associated with endo- carditis by transesophageal echocardiography. N Engl J Med. 1991;324:795–800. doi: 10.1056/NEJM199103213241203. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease) Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 5.Habib G, Lancellotti P, Antunes MJ, et al. Authors/Task Force Members. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European association for cardio-thoracic surgery (EACTS), the european association of nuclear medicine (EANM) Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 6.Hoen B, Alla F, Selton-Suty CH, et al. Changing profile of infective endocarditis. Results of a 1 year survey in France. JAMA. 2002;288:75–81. doi: 10.1001/jama.288.1.75. [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Baron G, Butchart E, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on valvular heart disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 8.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis Offic Publ Infect Dis Soc Am. 2000;30:633–38. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 10.Pang PY, Sin YK, Lim CH, et al. Surgical management of infective endocarditis: An analysis of early and late outcomes. Eur J Cardiothorac Surg. 2015;47(5):826–32. doi: 10.1093/ejcts/ezu281. [DOI] [PubMed] [Google Scholar]
- 11.Misfeld M, Girrbach F, Etz CD, et al. Surgery for infective endocarditis complicated by cerebral embolism: A consecutive series of 375 patients. J Thorac Cardiovasc Surg. 2014;147:1837–44. doi: 10.1016/j.jtcvs.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 12.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 13.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eishi K, Kawazoe K, Kuriyama Y, et al. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg. 1995;110:1745–55. doi: 10.1016/S0022-5223(95)70038-2. [DOI] [PubMed] [Google Scholar]
- 15.Sonneville R, Mirabel M, Hajage D, et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: The ENDOcardite en REAnimation prospective multicenter study. Crit Care Med. 2011;39:1474–81. doi: 10.1097/CCM.0b013e3182120b41. [DOI] [PubMed] [Google Scholar]
- 16.Dickerman SA, Abrutyn E, Barsic B, et al. The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: An analysis from the ICE prospective cohort study (ICE-PCS) Am Heart J. 2007;154:1086–94. doi: 10.1016/j.ahj.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Gelsomino S, Maessen JG, van der Veen F, et al. Emergency surgery for native mitral valve endocarditis: The impact of septic and cardiogenic shock. Ann Thorac Surg. 2012;93:1469–76. doi: 10.1016/j.athoracsur.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Revilla A, Lopez J, Vilacosta I, et al. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J. 2007;28:65–71. doi: 10.1093/eurheartj/ehl315. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56(Suppl 1):S1–12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: A prospective study. Hepatology. 2012;55:1551–61. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 21.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–85. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Seminars Resp Critical Care Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- 23.Thulstrup AM, Sorensen HT, Schonheyder HC, et al. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357–61. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 24.Selton-Suty C, Celard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin Infect Dis. 2012;54:1230–39. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 25.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The international collaboration on endocarditis-prospective cohort study. Arch Intern Med. 2009;169:463–73. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: A consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 27.Ragnarsson S, Sjögren J, Stagmo M, et al. Clinical presentation of native mitral valve infective endocarditis determines long-term outcome after surgery. J Card Surg. 2015;30:669–76. doi: 10.1111/jocs.12591. [DOI] [PubMed] [Google Scholar]
- 28.Jung SH, Je HG, Choo SJ, et al. Surgical results of active infective native mitral valve endocarditis: Repair versus replacement. Eur J Cardiothorac Surg. 2011;40:834–39. doi: 10.1016/j.ejcts.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Lamas CC, Eykyn SJ. Blood culture negative endocarditis: Analysis of 63 cases presenting over 25 years. Heart. 2003;89:258–62. doi: 10.1136/heart.89.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mugge A, Daniel WG, Frank G, Lichtlen PR. Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol. 1989;14:631–38. doi: 10.1016/0735-1097(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 31.Sanfilippo AJ, Picard MH, Newell JB, et al. Echocardiographic assessment of patients with infectious endocarditis: Prediction of risk for complications. J Am Coll Cardiol. 1991;18:1191–99. doi: 10.1016/0735-1097(91)90535-h. [DOI] [PubMed] [Google Scholar]
- 32.Cabell CH, Pond KK, Peterson GE, et al. The risk of stroke and death in patients with aortic and mitral valve endocarditis. Am Heart J. 2001;142:75–80. doi: 10.1067/mhj.2001.115790. [DOI] [PubMed] [Google Scholar]
- 33.Vilacosta I, Graupner C, San Roman JA, et al. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol. 2002;39:1489–95. doi: 10.1016/s0735-1097(02)01790-4. [DOI] [PubMed] [Google Scholar]
- 34.Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:407–16. doi: 10.1056/NEJM199608083350607. [DOI] [PubMed] [Google Scholar]
- 35.Wang A, Athan E, Pappas PA, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. 2007;297:1354–61. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 36.Delay D, Pellerin M, Carrier M, et al. Immediate and long-term results of valve replacement for native and prosthetic valve endocarditis. Ann Thorac Surg. 2000;70:1219–23. doi: 10.1016/s0003-4975(00)01887-7. [DOI] [PubMed] [Google Scholar]
- 37.Jassal DS, Neilan TG, Pradhan AD, et al. Surgical management of infective endocarditis: Early predictors of short-term morbidity and mortality. Ann Thorac Surg. 2006;82:524–29. doi: 10.1016/j.athoracsur.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser SP, Melby SJ, Zierer A, et al. Long-term outcomes in valve replacement surgery for infective endocarditis. Ann Thorac Surg. 2007;83:30–35. doi: 10.1016/j.athoracsur.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 39.David TE, Gavra G, Feindel CM, et al. Surgical treatment of active infective endocarditis: A continued challenge. J Thorac Cardiovasc Surg. 2007;133:144–49. doi: 10.1016/j.jtcvs.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 40.Alexiou C, Langley SM, Stafford H, et al. Surgery for active culture-positive endocarditis: Determinants of early and late outcome. Ann Thorac Surg. 2000;69:1448–54. doi: 10.1016/s0003-4975(00)01139-5. [DOI] [PubMed] [Google Scholar]


