Abstract
Filgrastim (Neupogen®, granulocyte-colony stimulating factor) is among the few countermeasures recommended for management of patients in the event of lethal total-body irradiation. Despite the plethora of studies using filgrastim as a radiation countermeasure, relatively little is known about the optimal dose schedule of filgrastim to mitigate radiation lethality. We evaluated the efficacy of filgrastim in improving 30-day survival of CD2F1 mice irradiated with a lethal dose (LD70/30) in the AFRRI cobalt-60 facility. We tested different schedules of 1, 3, 5,10 or 16 once-daily injections of filgrastim initiated one day after irradiation. Time optimization studies with filgrastim treatment were also performed, beginning 6–48 h postirradiation. Maximum survival was observed with 3 daily doses of 0.17 mg/kg filgrastim. Survival efficacy of the 3-day treatment was compared against the conventional 16-day filgrastim treatment after irradiation in four mouse strains with varying radiation sensitivities: C3H/HeN, C57BL/6, B6C3F1 and CD2F1. Blood indices, bone marrow histopathology and colony forming unit assays were also evaluated. Filgrastim significantly increased 30-day survival (P < 0.001) with a 3-day treatment compared to 16-day treatment. Filgrastim did not prevent cytopenia nadirs, but facilitated faster recovery of white blood cells, neutrophils, red blood cells, platelets, lymphocytes and hematocrits in all four strains. Accelerated hematopoietic recovery was also reflected in faster bone marrow reconstitution and significant increase in hematopoietic progenitors (P < 0.001) in all four mouse strains. These data indicate that prompt and abbreviated filgrastim treatment has potential benefit for triage in the event of a radiological incident for treating acute hematopoietic syndrome.
Introduction
Hematopoietic acute radiation syndrome (H-ARS) is characterized by dose- and time-dependent loss in circulating neutrophils and platelets resulting in severe myelo- and immunosuppression with subsequent hemorrhage and sepsis. Filgrastim [granulocyte-colony stimulating factor (G-CSF); Neupogen®] enhances recovery of neutrophils and reduces the duration of neutropenia in patients undergoing myelo-suppressive chemo or radiotherapy (1). The American Society of Clinical Oncology (ASCO) recommends the prompt administration of growth factors such as filgrastim in the event of accidental or intentional lethal total-body irradiation (TBI, 3–10 Gy in humans) resulting in bone marrow failure (2). Neupogen® has recently been approved by the U.S. Food and Drug Administration (FDA) for the treatment of neutropenia associated with acute radiation syndrome (ARS) after a radiological nuclear accident (3). It is the first FDA-approved radiation countermeasure under the criteria of the FDA “animal rule” and is currently being procured by the CDC’s Strategic National Stockpile for use in a radiological emergency. The scheduling of filgrastim administration is highly critical for favorable hematological recovery and cost-effective strategies. Despite conflicting interpretations arising from variability in dosing, time of administration in relationship to exposure and treatment schedules, the general consensus is that filgrastim treatment be initiated as soon as possible after TBI (within 24 h) and that treatment continue until the absolute neutrophil count (ANC) normalizes (4). In previous studies, researchers have evaluated the efficacy of 10–125 µg/kg filgrastim administered once a day for 10–16 days in mice (5), 14 days in canines (6) or up to 17–21 days in primates (7) after lethal irradiation, resulting in moderate increases in survival. Therefore, we posited that altering filgrastim administration (dose and dosing schedule) would improve survival of lethally irradiated mice and facilitate hematopoietic recovery.
Genetic variations in mice contribute to considerable disparity in the extent and response to radiation injury (8– 10). These studies underscore the intrinsic variability in response of the general population to ionizing radiation. Therefore, testing of radiation countermeasures requires more than one mouse strain to ensure their effectiveness in all populations irrespective of genetic variations (10, 11). To address the inherent variations in radiation sensitivities arising from genetic differences, we investigated the efficacy of filgrastim in mitigating lethality after irradiation in four mouse strains: C3H/HeN, C57BL/6, B6C3F1 and CD2F1. To our knowledge, this is the first reported study on the efficacy of filgrastim in four mouse strains with different radiation sensitivities. We determined that a 16 daily dosing (day 1–16 postirradiation) schedule did not demonstrate survival efficacy in all strains, however, an abbreviated schedule of 3 daily doses of filgrastim (day 1–3 postirradiation) had substantial survival advantage and facilitated faster regeneration of the irradiated hematopoietic progenitor cells, irrespective of the mouse strain.
Materials And Methods
Filgrastim
The test article, filgrastim (Neupogen) (Amgen Inc., Thousand Oaks, CA) and control, dextrose 5% in water (Baxter, Deerfield, IL), were purchased from the Uniformed Services University of the Health Sciences pharmacy (Bethesda, MD) and stored at 2–8°C upon receipt. Drug or vehicle was injected subcutaneously (s.c.) at the nape of the neck after TBI.
Animals
Male CD2F1, C3H/HeN and B6C3F1 mice (Harlant® Laboratories Inc., Dublin, VA), 8–9 weeks old, were maintained at the Armed Forces Radiobiology Research Institute (AFRRI, Bethesda, MD) vivarium. C57BL/6 mice (8–9 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). These animals underwent microbiological, serological and histopathological testing by the veterinary staff and were determined to be disease- and pathogen-free during the quarantine period. Prior to initiation of experimental procedures, animals were acclimatized for a two-week period. Healthy animals were housed 4 per box in conventional sterile polycarbonate boxes with filter covers (Microisolator; Lab Products Inc., Seaford, DE) and autoclaved hardwood chip bedding. Mice had access to Harlan Teklad Rodent diet 8604 (Purina Mills, St. Louis, MO) and acidified water (pH 2.5–3.0) ad libitum. The animal rooms were maintained at 21 ± 2°C and 50 ± 10% relative humidity with 10–15 cycles of fresh air hourly and a 12:12 h light:dark cycle. All animal procedures were reviewed and approved by the AFRRI Institutional Animal Care and Use Committee (IACUC) using the principles outlined in the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Irradiation
Unanesthetized mice were irradiated bilaterally at AFRRI’s cobalt-60 (Co-60) gamma irradiation facility. During irradiation, the animals were placed in well-ventilated Plexiglas® chambers made specifically for mouse irradiation. The midline dose to the animals was delivered at a dose rate of 0.6 Gy/min. An alanine/electron spin resonance (ESR) dosimetry system (American Society for Testing and Materials, Standard E 1607) was used to measure dose rates (to water) in the cores of acrylic mouse phantoms. Phantoms were 3 inches long and 1 inch in diameter and were located in all empty compartments of the exposure rack. ESR signals were measured with a calibration curve based on standard calibration dosimeters provided by the National Institute of Standards and Technology (NIST, Gaithersburg, MD). The accuracy of the calibration curve was verified by intercomparison with the National Physical Laboratory (London, UK). The corrections applied to the measured dose rates in phantoms were for decay of the Co-60 source and for a small difference in mass energy-absorption coefficients for water and soft tissue at the Co-60 energy. The radiation field was uniform within ±2% (12).
Selection of the Optimum Filgrastim Schedule in CD2F1 Mice
Male CD2F1 mice (12–14 weeks) were used in the preliminary dose and schedule optimization studies.
Optimization of schedule
Groups of 24 CD2F1 mice each were exposed to 9.25 Gy TBI (LD70/30 for CD2F1 mice) and treated as follows:
One dose, 0.17–0.68 mg/kg G-CSF, 1 day postirradiation.
Three doses, 0.17 or 0.34 mg/kg G-CSF, 1–3 days postirradiation.
Five doses, 0.17 or 0.34 mg/kg G-CSF, 1–5 days postirradiation.
Ten doses, 0.12 mg/kg, 0.17 or 0.34 mg/kg G-CSF, 1–10 days postirradiation.
Sixteen doses, 0.12 mg/kg or 0.34 mg/kg G-CSF, 1–16 days postirradiation.
Dose optimization studies
Based on data from the above studies, we selected the 3-dose schedule to optimize drug dose. The conventional drug dose of 0.125 mg/kg is equivalent to 2.5 µg/mouse. Using a dose escalation strategy, we increased the dose to 5, 10, 15 and 20 µg/mouse, which translated to 0.17, 0.34, 0.51 and 0.68 mg/kg in the CD2F1 mice. Groups of 24 mice each were irradiated with LD70/30 and injected with 0.17, 0.34, 0.51 or 0.68 mg/kg filgrastim on days 1, 2 and 3 after TBI, and survival monitored for 30 days. A separate cohort of irradiated mice injected s.c. with 5% dextrose on days 1, 2 and 3 after TBI served as vehicle control.
Time optimization studies
We selected three doses of 0.17 or 0.34 mg/kg filgrastim administered s.c. after TBI as our optimum schedule to test for maximum efficacy in relationship to time of administration after irradiation. We selected earlier time points of 6 and 12 h postirradiation, and delayed time points of 48 and 72 h postirradiation, to define the window of mitigation efficacy for filgrastim. Batches of 24 CD2F1 mice/group were irradiated with LD70/30 and injected with 0.17 or 0.34 mg/kg filgrastim starting at 6, 12, 24, 48 or 72 h after TBI, and survival monitored for 30 days. Vehicle controls were time-matched for each drug schedule.
Irradiated animals were observed 3–4 times daily (no more than 8 h between each observation), seven days per week for 30 days to monitor survival. Pain and distress were monitored using several criteria including unresponsiveness, abnormal posture, unkempt appearance, immobility and lack of coordination. A combination of symptoms was used to judge whether an animal had reached a moribund state informing euthanasia: inability of the mouse to right itself, limb paralysis, abdominal breathing or constant twitching, trembling or tremor that lasted for more than 10 s. Animals were euthanized according to American Veterinary Medical Association (AVMA) guidelines.
Establishing Radiation Sensitivities of the Different Strains and Validation of the Abbreviated Filgrastim Schedule
Probit curves for C3H/HeN, C57BL/6, B6C3F1 and CD2F1 mice were generated by irradiating naïve mice with different doses of radiation resulting in lethality ranging from LD5 to LD95 (Table 1). LD70/30 was determined from analysis of the probit curves for all four strains. Three-day regimen of filgrastim (0.17 mg/kg, s.c.) was compared to the conventional schedule of 16 daily dose of filgrastim (0.125 mg/kg, s.c.); 5% dextrose was used as vehicle control for both regimens.
Table 1.
Summary of Radiation Doses (Gy) Used in Developing Probit Curves for Different Mouse Strains
| Group no. | C3H/HeN | C57BL/6 | B6C3F1 | CD2F1 |
|---|---|---|---|---|
| 1 | 6 | 6 | 6.5 | 8.0 |
| 2 | 6.5 | 6.5 | 7 | 8.5 |
| 3 | 7 | 7 | 7.5 | 9.0 |
| 4 | 7.5 | 7.5 | 8 | 9.5 |
| 5 | 8 | 8 | 8.5 | 10.0 |
| 6 | 8.5 | 8.5 | 9 | 10.5 |
Studies on Hematological Recovery after Filgrastim Treatment in Irradiated Mice
Although all survival studies were conducted with LD70/30 for each strain, the hematological experiments used a high-sublethal dose that induced severe bone marrow myelosuppression without associated mortality; 6.5 Gy for C3H/HeN and C57BL/6 mice and 7 Gy for the B6C3F1 and CD2F1 mice. A sufficient number of mice survived at later postirradiation times (days 14–28), allowing for statistical evaluation.
Hematology
Mice (n = 6 per group) were administered either vehicle (5% dextrose) or 0.17 mg/kg filgrastim at days 1, 2, and 3 after TBI at nonlethal doses (6.5 Gy for C3H/HeN and C57BL/6 strains, and 7 Gy for B6C3F1 and CD2F1 strains). A group of age-matched, sham-irradiated mice for each strain were also treated with either vehicle or drug as controls.
Blood was collected at days 4, 7, 10, 14, 21 and 28 postirradiation (day 4 is the first day after the final filgrastim administration). Blood (0.6–1.0 ml) was obtained by cardiac puncture using a syringe with a 23g needle from mice deeply anesthetized with isoflurane (Hospira Inc., Lake Forest, IL). Samples were immediately transferred into ethylenediaminetetra-acetic acid (EDTA; Sigma-Aldrich® LLC, St. Louis, MO) tubes, and mixed gently on a rotary shaker until analysis. The tubes were analyzed for total red and white blood cell counts, absolute neutrophil counts, monocytes, lymphocytes, reticulocytes, hemoglobin, hematocrit and platelets using ADVIA® 2120 (Siemens Medical Solutions Diagnostics, Dublin, Ireland), and data generated using Microsoft® software version 5.9 (Microsoft Corp., Redmond, WA).
Sternum Marrow Histopathology
After blood collection, animals were euthanized, the sterna collected at days 4, 7, 10 and 14 after TBI. Sterna were fixed in a 20:1 volume of fixative (10% buffered formalin) to tissue for at least 24 h and up to 7 days. Fixed sterna were decalcified for 3 h in 12–18% sodium EDTA (pH 7.4–7.5) and specimens dehydrated using graded ethanol concentrations and embedded in paraffin. Longitudinal 5 µm sections were stained with regular hematoxylin and eosin (13). A board-certified veterinary pathologist conducted blinded histopathological evaluation of these samples. Bone marrow was evaluated in situ within sternebrae and graded (grade 1: <10%; grade 2: 11–30%; grade 3: 31–60%; grade 4: 61–89%; grade 5: >90%) for total cellularity, myeloid-to-erythroid (M:E) ratios, stromal changes and megakaryocyte numbers averaged per 10 high power fields (HPF) at 400× magnification using a BX41 microscope (Olympus, Minneapolis, MN). Images were captured with an Olympus DP70 camera and imported into Adobe Photoshop version CS4 (Adobe Systems Inc., San Jose, CA) for analysis.
Hematopoietic Progenitor Clonogenic Assay
After sternum collection from the euthanized animals, bone marrow was harvested from femurs. Clonogenicity of mouse bone marrow cells was quantified in standard semisolid cultures in triplicate using 1 ml of MethoCult™ GF+ system for mouse cells (STEMCELL™ Technologies Inc., Vancouver, Canada) according to the manufacturer’s instructions. Briefly, mouse bone marrow cells from pooled samples were washed twice with IMDM and seeded at 1–5 × 104 cells/dish in 35-cm cell culture dishes (BD Biosciences, San Jose, CA). Granulocyte-macrophage colony forming units (CFU-GM), granulocyte-erythrocyte-monocyte-macrophage CFU (CFU-GEMM) and erythroid burst-forming units (BFU-E) were identified and quantified following the manufacturer’s instructions. Colonies were counted 14 days after plating using a Nikon® TS100F microscope (Nikon Instruments Inc., Melville, NY). Fifty or more cells were considered one colony.
Statistical Analysis
All survival data were compared using Fisher’s exact test (one-tailed) and the generalized Savage (Mantel-Cox) procedure (BMDP Statistical Software Inc., Los Angeles, CA). Statistical software, GraphPad Prism version 6.01 (LaJolla, CA) was used to generate Kaplan-Meyer graphs. Probit analyses were done using PASW® Statistics 18 regression analyses (SPSS® Inc., Chicago, IL) and the LD50/30 for each strain determined from the mortality data. Means and standard errors were reported for blood cells, CFU assay and cell count data. Analysis of variance (ANOVA) was used to determine if there was a significant difference among the groups. For a given day, if there was a significant difference among the groups, a pairwise comparison was done using the Tukey-Kramer method. Significance was set at 5% for each test.
Results
A Case for Shorter Filgrastim Schedules (3 Doses after TBI)
Patchen et al. (14) reported that a filgrastim dose of 2.5 µg/mouse (equivalent to 0.12 mg/kg) injected 1–16 days postirradiation improved 30-day survival in C57BL/6 mice. However, in the CD2F1 mice irradiated with LD70/30 and treated with the same drug dose and schedule, we did not observe any improvement in 30 days, with merely 9% survival in the drug-treated group compared to 33% survival in the 5% dextrose-treated group (data not shown). Because filgrastim has been approved by the FDA for treatment of febrile neutropenia consequent to myeloablative chemotherapy (15) and as a countermeasure to mitigate lethality in the event of accidental exposure to radiation, we conducted a series of exploratory and systematic experiments to determine the optimum filgrastim schedule in increasing survival in CD2F1 mice.
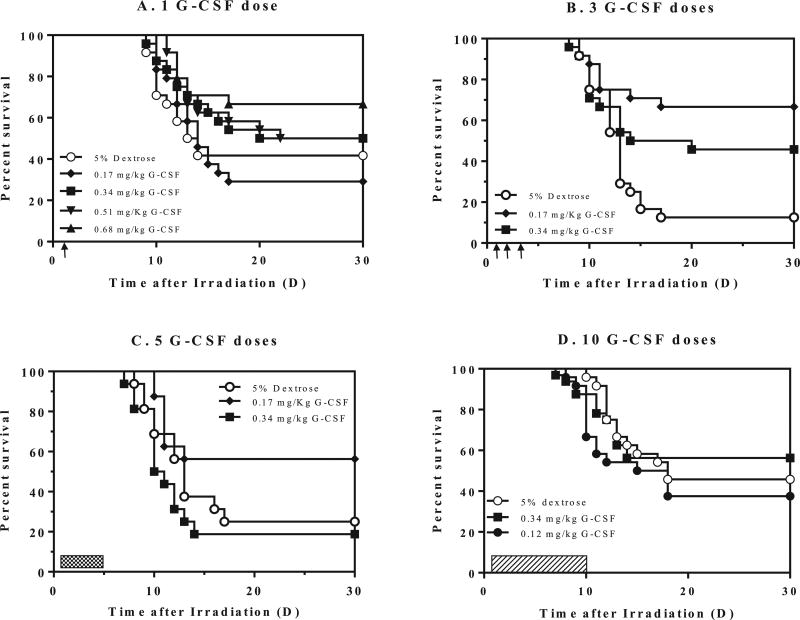
All mice received 9.25 Gy TBI and filgrastim administration was initiated 24 h postirradiation. Although the projected radiation dose is LD70/30, we observed some variation in survival of the vehicle-treated groups in these experiments. Survival for the vehicle groups at 30 days postirradiation ranged from 40% to 13% (Fig. 1A and B, respectively). However, it should be noted that these differences between vehicle-treated groups from different studies were not statistically different (P >0.05).
FIG. 1.
Survival of CD2F1 mice after 9.25 Gy TBI and therapy with different schedules of filgrastim (G-CSF). Panel A: A single dose of filgrastim [0.17–0.68 mg/kg, 1 day after TBI (single arrow)] compared to 5% dextrose. Panel B: Three daily doses (days 1–3) postirradiation (indicated by arrows), of 0.17 or 0.34 mg/kg filgrastim compared to 5% dextrose. Panel C: Five doses (cross-hatch) of either 5% dextrose or filgrastim, 0.17 or 0.34 mg/kg. Panel D: Ten doses (dashed bar) of either 5% dextrose or filgrastim, 0.12 or 0.17 mg/kg. Kaplan-Meier survival curves were plotted using GraphPad software; n = 24 mice/group and trend in survival is compared between vehicle and drug-treated groups.
CD2F1 mice injected with a single dose of filgrastim did not demonstrate improved survival for doses of 0.17 to 0.51 mg/kg; however, a single dose of 0.68 mg/kg filgrastim significantly increased survival (66%) compared to the vehicle-treated group (40%, P = 0.015) (Fig. 1A). CD2F1 mice treated with 3 doses of 0.17 or 0.34 mg/kg filgrastim showed significantly improved 30-day survival compared to the vehicle control (P < 0.05–0.002). The lower dose of 0.17 mg/kg filgrastim, with 66% survival (compared to 13% survival in the vehicle group) showed superior efficacy to the 0.34 mg/kg dose (45%), although the difference was not significant (Fig. 1B). Similarly, for 0.17 or 0.34 mg/kg of filgrastim administered to CD2F1 mice for 1–5 days postirradiation (Fig. 1C), the lower dose of 0.17 mg/kg afforded a modest increase in survival of 56% [25% survival in the vehicle-treated group (P = 0.054)], while increasing the dose to 0.34 mg/kg filgrastim resulted in 19% survival (not significant). However, in CD2F1 mice treated with either 0.12 mg/kg or 0.34 mg/kg filgrastim for 1–10 days, neither filgrastim schedule improved survival compared to the vehicle-treated group (Fig. 1D). In fact, filgrastim-treated mice began to die earlier than vehicle-treated mice, although mortality was not significantly higher than in vehicle-treated controls (P = 0.36).
Of note, a single dose of 0.68 mg/kg filgrastim, 3 doses of 0.17 or 0.34 mg/kg filgrastim and 5 doses of 0.17 mg/kg filgrastim all demonstrated significant and similar improvement in 30-day survival. Although comparable survival was observed with the 3- and 5-dose regimen, we opted for the 3-dose schedule since this would result in less handling stress to the mice and would be more practical for translation into higher mammal models.
Optimization of Filgrastim Dose and Time in Relationship to Exposure
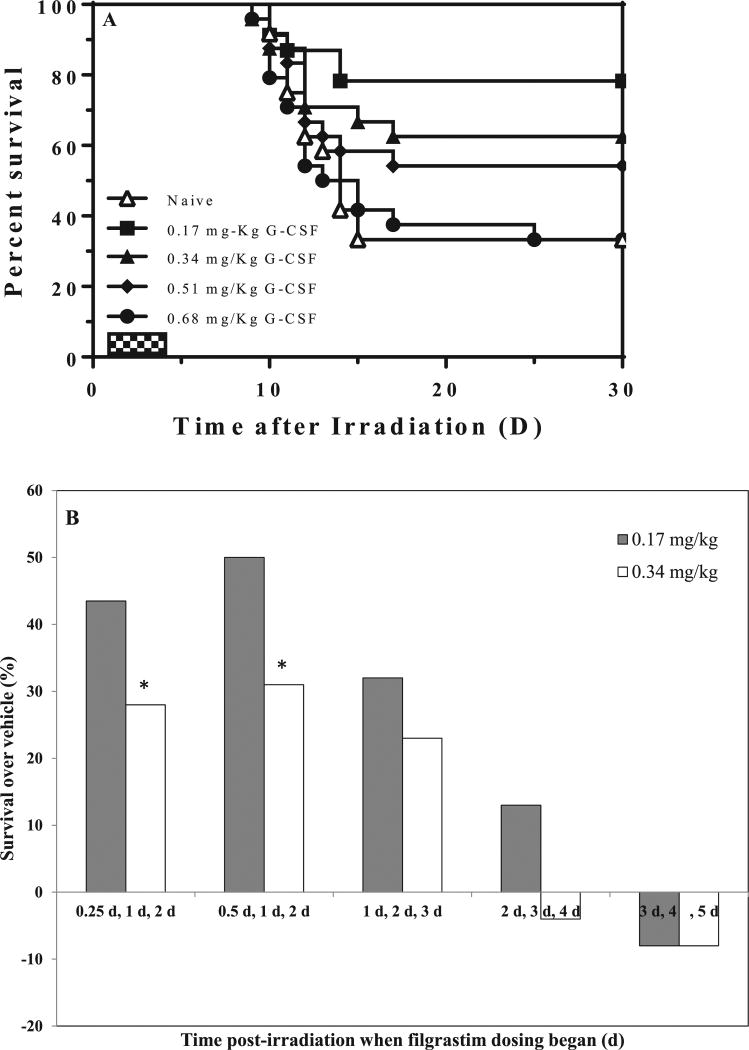
Based on these preliminary studies, we decided to proceed with three daily dosing as the preferred schedule. In an attempt to optimize the best drug dose, we administered three daily doses of 0.17, 0.34, 0.51 or 0.68 mg/kg (equivalent to 5, 10, 15 or 20 µg/mouse) filgrastim to irradiated mice on days 1, 2 and 3 after TBI. Thirty-day survival was not drug dose dependent; the lower doses of 0.17 and 0.34 mg/kg filgrastim significantly enhanced survival by 77% and 63%, respectively, compared to 33% survival in the vehicle-treated group, while survival in the 0.51 and 0.68 mg/kg filgrastim-treatment groups was not significant compared to vehicle (30-day survival = 54% and 33%, P = 0.43). An inverse relationship between survival and increasing filgrastim dose was observed consistently in two separate experiments (Fig. 2A); as filgrastim dose increased, survival decreased.
FIG. 2.
Optimization of filgrastim (G-CSF) dose to improve survival of lethally irradiated mice. Panel A. Effect of increasing filgrastim dose administered s.c. on days 1, 2 and 3 after 9.25 Gy TBI on 30-day mouse survival (n = 24/group). Three doses of 0.17 mg/kg filgrastim showed optimal mitigation, followed by 0.34 mg/kg and 0.51 mg/kg, while survival in the 0.68 mg/kg filgrastim-treated group was similar to the 5% dextrose-treated group (control). Panel B. Optimization of time of filgrastim administration in relationship to irradiation. Mice were irradiated and injected with 3 daily doses of either 0.17 or 0.34 mg/kg filgrastim beginning at 6 and 12 h, days 1, 2 or 3 after TBI. *P < 0.001 for 30-day survival of 0.17 mg/kg filgrastim-treated group compared to survival of 0.34 mg/kg group.
Using the 3-dose schedule, we investigated the effect on survival when drug administration was advanced or delayed in relationship to time of exposure. As with the initial studies, survival in the vehicle-treated groups ranged from 13–21%. For filgrastim administration initiated 6, 12, 24, 48 or 72 h after TBI, we observed an increase of 44%, 50%, 33%, 13% and 0% survival over vehicle (Fig. 2B). For each time schedule, 0.17 mg/kg filgrastim afforded higher survival benefit than 0.34 mg/kg, and was significantly higher when dosing was initiated at 6 and 12 h postirradiation (P < 0.05). Delaying drug administration beyond 24 h did not provide any survival benefit, a finding that supports rapid deployment of the drug under mass exposure scenarios.
Validation of Survival Efficacy with 3 Doses of Filgrastim in Four Strains of Mice with Different Radiosensitivities
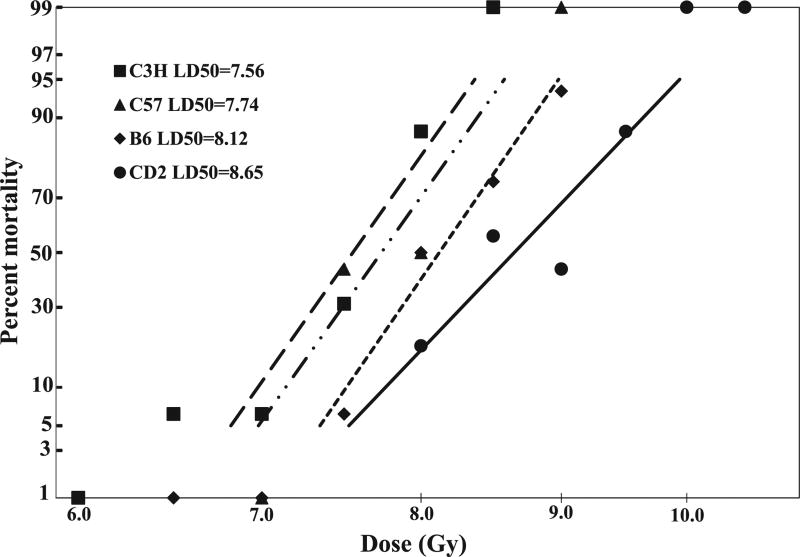
Prior to comparing the abbreviated filgrastim schedule with the conventional published schedule in mouse strains of varying radiation sensitivities, a probit curve was established for each of the selected strains. C3H/HeN, C57BL/6, B6C3F1 and CD2F1 mice were procured at the same time, and 16 mice/radiation dose/strain were irradiated with doses ranging from 6–10 Gy (Table 1), and 30-day survival monitored. From two experiments, the log dose of survival for each strain was plotted as a function of radiation dose, and the LD50/30 (dose of radiation resulting in 50% mortality 30 days after TBI) determined (Fig. 3). In order of increasing radiation resistance, the LD50/30 was C3H/HeN = 7.6 Gy (95% CI = 7.38–7.76 Gy), C57BL/6 = 7.75 Gy (95% CI = 7.48–7.95 Gy), B6C3F1 = 8.3 Gy (95% CI = 7.96–8.35 Gy) and CD2F1 = 8.8 Gy (95% CI = 8.37–8.92 Gy), respectively.
FIG. 3.
Comparison of the radiation sensitivities of four mouse strains exposed to different radiation doses. All mice were irradiated on the same day. There were 16 mice per group per dose per strain. Probit survival curves were generated for each strain exposed to LD5/30 to LD95/30 TBI. Mortality at 30 days postirradiation is plotted against dose for C3H/HeN mice (irradiated with 6–8.5 Gy), C57BL/6 mice (7–9 Gy), B6C3F1 mice (7.5–9 Gy) and CD2F1 mice (8.5–10.5 Gy).
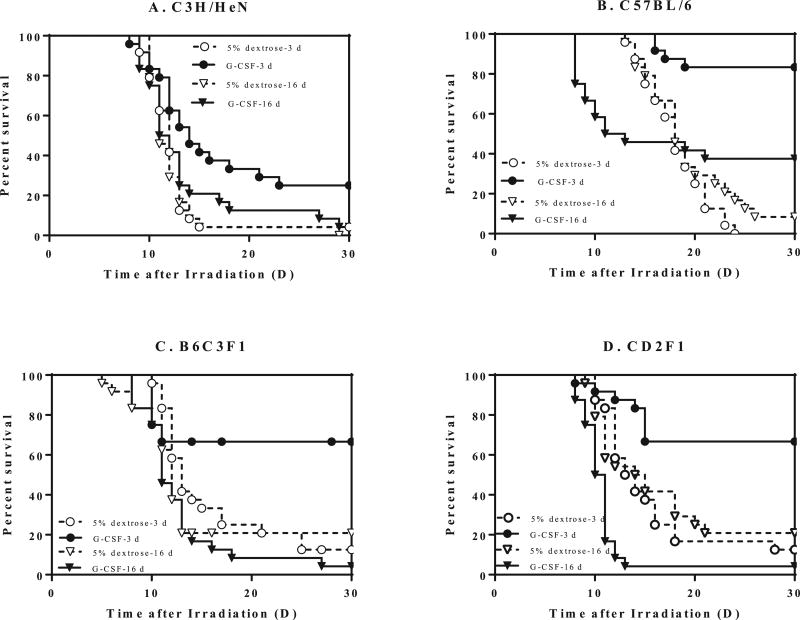
Based on these plots, the subsequent survival studies for countermeasure efficacy used the projected LD70/30 of 8.0 Gy for C3H/HeN, 8.2 Gy for C57BL/6, 8.5 Gy for B6C3F1 and 9.25 Gy for CD2F1 mice. The abbreviated 3-dose filgrastim treatment, beginning 24 h postirradiation, demonstrated significant survival benefit compared to the schedule-matched vehicle control (P < 0.001–0.0001) and the 16-dose filgrastim schedule (P < 0.001) in all mouse strains.
Irradiation of C3H/HeN mice with 7.8 Gy resulted in 4% survival in both vehicle-treated groups; no mice survived the 16-day filgrastim schedule, but the 3-day schedule resulted in 25% survival (log-rank test, P < 0.009) (Fig. 4A).
FIG. 4.
Mitigation of radiation lethality in four different mouse strains with 3 vs. 16 doses of filgrastim starting 24 h postirradiation. Male mice (12–14 weeks old) of each strain were exposed to LD70/30 TBI and treated with either 5% dextrose (3 or 16 doses) or filgrastim (0.17 mg/kg on days 1, 2 and 3 or 0.12 mg/kg filgrastim at days 1–16 postirradiation). Panel A: 8 Gy irradiated C3H/HeN mice. Panel B: 8.2 Gy irradiated C57BL/6 mice. Panel C: 8.5 Gy irradiated B6C3F1 mice. Panel D: 9.25 Gy irradiated CD2F1 mice. Log-rank test, P < 0.0001–0.009 (n = 24 mice per group).
C57BL/6 mice irradiated with 8 Gy exhibited the most favorable survival outcome for both schedules; 16 doses of filgrastim resulted in 38% survival (6% survival in vehicle group; P < 0.001), but the 3-dose schedule resulted in 83% survival compared to 0% in the 3-dose vehicle-treated group (P < 0.0001) (Fig. 4B). The 3-dose schedule also significantly increased survival compared to the 16-day schedule (83% vs. 38%, P < 0.001).
In the B6C3F1 mouse strain, 67% of the group survived (13% in control, P < 0.0001) after 3-day filgrastim treatment, while 4% survived after 16-day treatment compared to 20% in the vehicle-treated group (Fig. 4C).
There was a 67% survival in the 3-dose filgrastim-treated CD2F1 mice compared to 13% in the control (log-rank test, P < 0.0001) at day 30 postirradiation (Fig. 4D). However, only 21% of the animals survived after 16-day treatment in the control group compared to 4% in the 16-dose filgrastim-treated group.
Accelerated Recovery from Radiation-Induced Pancytopenia with 3 Doses of Filgrastim
Indications of acute hematopoietic syndrome, including reductions in peripheral blood counts, are seen after exposure to sublethal doses of 3–8 Gy (16). Hematological indices were evaluated in all four mouse strains (C3H/HeN, C57BL/6, B6C3F1 and CD2F1) after irradiation to a sublethal radiation dose and 3 daily injections of 5% dextrose or 0.17 mg/kg filgrastim on days 1, 2 and 3 postirradiation, resulting in hematopoietic syndrome but not mortality.
In all strains of mice, radiation induced severe depletion of circulating numbers of mature hematopoietic cells (Table 2, Supplementary Fig. S1; http://dx.doi.org/10.1667/RR14555.1.S1). Nadirs for white blood cells, neutrophils and lymphocytes were observed on day 4 postirradiation, and platelet nadir was observed on day 10 postirradiation, while red blood cell and hematocrit nadirs were observed on day 14 postirradiation (Table 2, Supplementary Fig. S1). There was no significant difference between vehicle- and filgrastim-treated groups at days 4, 7 and 10 in white blood cells, neutrophils, lymphocytes, red blood cells, hematocrit and platelet levels (Supplementary Fig. S1).
Table 2.
Filgrastim Accelerates Hematological Recovery in Peripheral Blood of Four Strains of Irradiated Mice
| Parameters for each mouse strain |
Nonirradiated vehicle |
Day 14
|
Day 21
|
Day 28
|
|||
|---|---|---|---|---|---|---|---|
| Vehicle | Filgrastim | Vehicle | Filgrastim | Vehicle | Filgrastim | ||
| C3H/HeN | |||||||
| Red blood cells (×106 cells/µl) | 8.57 ± 0.30 | 3.09 ± 0.14 | 4.34* ± 0.05 | 4.56 ± 0.34 | 7.02* ± 0.20 | 5.96 ± 0.23 | 5.99 ± 0.27 |
| White blood cells (×103 cells/µl) | 3.69 ± 0.24 | 0.16 ± 0.02 | 0.41* ± 0.02 | 1.16 ± 0.36 | 1.36 ± 0.20 | 3.29 ± 0.91 | 5.58 ± 1.43 |
| Platelets (×103 cells/µl) | 1072.5 ± 57.5 | 27.50 ± 2.14 | 90.00* ± 14.56 | 143.75 ± 39.11 | 342.92* ± 43.13 | 646.88 ± 70.94 | 677.50 ± 31.09 |
| Neutrophils (×103 cells/µl) | 1.15 ± 0.20 | 0.05 ± 0.01 | 0.20* ± 0.02 | 0.58 ± 0.24 | 0.73 ± 0.13 | 1.77 ± 0.44 | 3.30 ± 1.00 |
| Lymphocytes (×103 cells/µl) | 2.32 ± 0.03 | 0.08 ± 0.02 | 0.16* ± 0.02 | 0.49 ± 0.11 | 0.52 ± 0.07 | 0.96 ± 0.27 | 1.73 ± 0.37 |
| Hematocrit (%) | 41.06 ± 1.98 | 15.13 ± 0.66 | 22.29* ± 0.49 | 28.80 ± 2.90 | 40.04* ± 0.90 | 35.19 ± 1.18 | 31.71 ± 1.39 |
| C57BL/6 | |||||||
| Red blood cells (×106 cells/µl) | 8.57 ± 0.30 | 3.33 ± 0.29 | 5.55* ± 0.30 | 4.62 ± 0.69 | 7.58* ± 0.08 | 7.10 ± 0.20 | 7.78 ± 0.15 |
| White blood cells (×103 cells/µl) | 7.21 ± 0.95 | 0.15 ± 0.02 | 0.33* ± 0.06 | 0.70 ± 0.15 | 0.99 ± 0.14 | 2.25 ± 0.34 | 1.90 ± 0.18 |
| Platelets (×103 cells/µl) | 1184.0 ± 20.7 | 53.00 ± 10.34 | 308.50* ± 37.85 | 291.25 ± 50.18 | 460.42* ± 30.70 | 450.00 ± 79.17 | 594.17 ± 11.45 |
| Neutrophils (×103 cells/µl) | 0.53 ± 0.09 | 0.04 ± 0.01 | 0.11* ± 0.02 | 0.15 ± 0.04 | 0.24 ± 0.04 | 0.47 ± 0.10 | 0.30 ± 0.04 |
| Lymphocytes (×103 cells/µl) | 6.19 ± 0.94 | 0.07 ± 0.01 | 0.18 ± 0.03 | 0.46 ± 0.10 | 0.56 ± 0.09 | 1.14 ± 0.22 | 1.39 ± 0.16 |
| Hematocrit (%) | 43.00 ± 1.55 | 15.50 ± 1.46 | 27.33* ± 1.70 | 25.75 ± 4.35 | 40.25* ± 0.47 | 43.46 ± 0.89 | 43.83 ± 2.06 |
| B6C3F1 | |||||||
| Red blood cells (×106 cells/µl) | 8.89 ± 0.16 | 3.80 ± 0.23 | 5.23* ± 0.20 | 6.00 ± 0.71 | 8.09* ± 0.22 | 5.73 ± 0.12 | 6.35 ± 0.84 |
| White blood cells (×103 cells/µl) | 9.64 ± 1.98 | 0.15 ± 0.02 | 0.42* ± 0.05 | 3.52 ± 0.66 | 3.30 ± 1.00 | 4.93 ± 1.30 | 4.56 ± 0.65 |
| Platelets (×103 cells/µl) | 1205.6 ± 23.7 | 22.00 ± 3.01 | 88.00 ± 20.78 | 403.00 ± 101.2 | 502.08 ± 62.58 | 491.88 ± 108.8 | 594.58 ± 48.22 |
| Neutrophils (×103 cells/µl) | 2.83 ± 0.73 | 0.08 ± 0.01 | 0.17* ± 0.02 | 1.87 ± 0.29 | 1.65 ± 0.50 | 2.98 ± 0.86 | 2.05 ± 0.38 |
| Lymphocytes (×103 cells/µl) | 6.48 ± 1.04 | 0.05 ± 0.01 | 0.20 ± 0.03 | 1.54 ± 0.18 | 1.33 ± 0.41 | 1.81 ± 0.39 | 1.94 ± 0.42 |
| Hematocrit (%) | 44.25 ± 1.10 | 18.38 ± 1.01 | 25.75* ± 1.06 | 38.85 ± 1.95 | 43.13 ± 1.11 | 31.63 ± 0.67 | 32.83 ± 4.31 |
| CD2F1 | |||||||
| Red blood cells (×106 cells/µl) | 9.42 ± 0.14 | 6.49 ± 0.20 | 8.09* ± 0.16 | 7.63 ± 0.20 | 8.42 ± 0.40 | 8.93 ± 0.22 | 9.22 ± 0.11 |
| White blood cells (×103 cells/µl) | 7.59 ± 0.50 | 0.23 ± 0.04 | 0.63* ± 0.07 | 0.84 ± 0.19 | 1.23 ± 0.09 | 1.38 ± 0.13 | 1.91* ± 0.08 |
| Platelets (×103 cells/µl) | 1211.0 ± 69.6 | 59.58 ± 11.15 | 183.33* ± 27.20 | 277.50 ± 34.95 | 412.92* ± 34.04 | 495.00 ± 75.92 | 612.50 ± 46.18 |
| Neutrophils (×103 cells/µl) | 1.43 ± 0.19 | 0.05 ± 0.01 | 0.23* ± 0.03 | 0.39 ± 0.09 | 0.63 ± 0.08 | 0.54 ± 0.02 | 0.81* ± 0.06 |
| Lymphocytes (×103 cells/µl) | 5.74 ± 0.27 | 0.17 ± 0.03 | 0.35 ± 0.06 | 0.35 ± 0.08 | 0.49 ± 0.03 | 0.74 ± 0.12 | 0.94 ± 0.08 |
| Hematocrit (%) | 44.15 ± 0.69 | 25.75 ± 3.84 | 37.67* ± 0.77 | 38.54 ± 1.24 | 40.46 ± 2.06 | 43.46 ± 0.89 | 43.83 ± 2.06 |
Notes. Values are mean ± SEM, n = 6/group. Data were analyzed using Student’s t test.
P < 0.005–0.05 compared to matched 5% dextrose-treated group.
Consistent with the myelopoietic effect reported earlier (29), filgrastim-treated mice demonstrated faster hematopoietic recovery for all blood indices beginning at day 14 postirradiation, compared to irradiated vehicle (P < 0.05) (Table 2). Only PLT counts in filgrastim-treated groups were significantly higher than the vehicle-treated group at day 21 in all strains except the B6C3F1 mice (Table 2).
Filgrastim Increases Recovery of Bone Marrow Injury in the Irradiated Mouse Strains
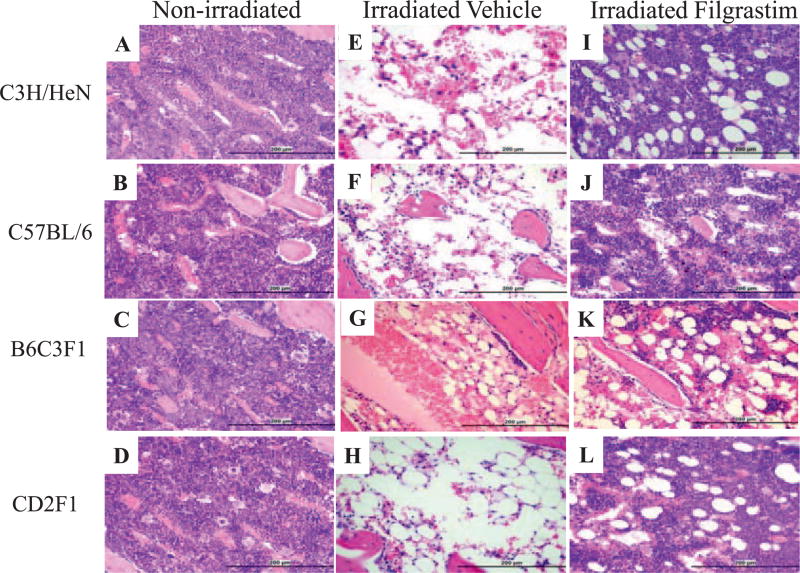
In the nonirradiated vehicle-treated group, histological evaluation of the bone marrow revealed cellularity > 90% (grade 5) and normal morphology in all mouse strains (Fig. 5A – D). The megakaryocytic numbers/HPF were variable among all the strains, with the highest numbers/HPF occurring in the CD2F1 strain. The B6C3F1 strain demonstrated the least number/HPF of megakaryocytes. The M:E was 1:1 in all controls.
FIG. 5.
Photomicrographs show regeneration in representative sections of sternal bone marrow after filgrastim treatment in C3H/HeN, C57BL/6, B6C3F1 and CD2F1 mice exposed to a sublethal dose of radiation. Panels A–D: Nonirradiated mice injected with 3 doses of 5% dextrose. Panels E–H: Irradiated mice 14 days after exposure injected with 3 doses of 5% dextrose (vehicle) on days 1, 2 and 3 after TBI, showing bone marrow atrophy, infiltration by adipocytes and rare megakaryocytes. Panels I–L: Mice at 14 days after TBI, s.c. injected with 0.17 mg/kg filgrastim on days 1, 2 and 3 postirradiation, show increased cellularity and myeloid cells. All sections were routinely H&E stained (200× magnification).
In the irradiated vehicle-treated group, the bone marrow cellularity in all strains ranged between 11–30% (grade 2), indicative of severe bone marrow suppression (Fig. S2; http://dx.doi.org/10.1667/RR14555.1.S1). Expsoure to radiation ablated the hematopoietic tissue, resulting in profound hypocellularity (5% in irradiated mice compared to >90% in controls) (Fig. 5E – H), a reduction of megakaryocytes (1/HPF from 35–42/HPF in controls), disruption of the M:E ratio to 10:1, and a striking increase in adipocytes, cell debris, sinusoidal blood congestion and reactive osteoblasts in sternum stroma. When bone marrow cellularity (myeloid, erythroid and megakaryocytic cells) decreased, the numbers of adipocytes filling the marrow cavity and osteoblasts lining the marrow cavity and trabeculae increased. Treatment with filgrastim after irradiation stimulated recovery of the ablated bone marrow tissue by day 14 after TBI in all strains (Fig. 5I – L). Filgrastim treatment restored marrow cellularity to grade 4 (61–89%), increased the megakaryocytes to 25–30/HPF and restored the appearance of the stroma to near normal, although the M:E ratio remained in the 3:1 to 5:1 range, favoring myelopoiesis.
Filgrastim Accelerates Colony Formation after Irradiation
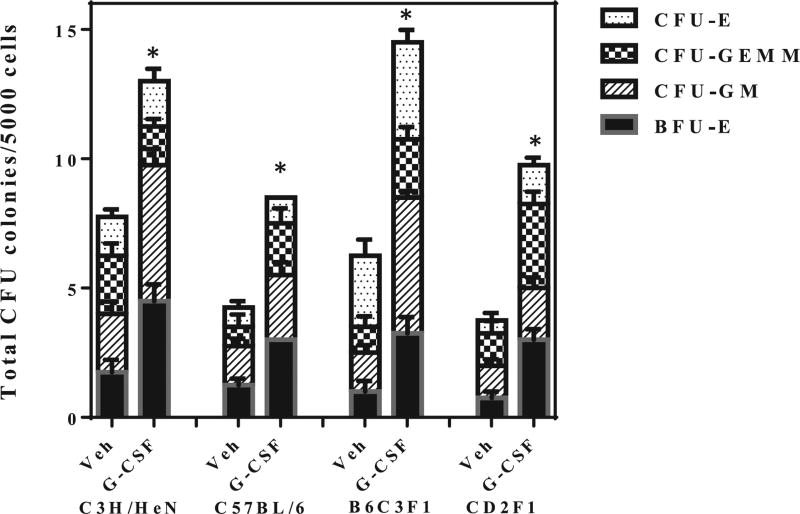
In addition to depleting the circulating end cells, exposure to radiation also decreased the number and viability of the hematopoietic progenitor cells (HPCs) as evaluated by CFU assay. In the nonirradiated group, plating 1,000 cells resulted in 200–250 colonies per plate. Expsoure to radiation decreased the bone marrow progenitor cells in all strains from day 4–28 after TBI (P < 0.001). Filgrastim treatment did not prevent insult to the HPCs, with prominent reduction in CFU counts between days 4–10 postirradiation; since the number of colonies scored were 0– 15/plate, these data are not shown. However, at day 14 after TBI, filgrastim treatment showed evidence of accelerated recovery of HPCs among all four strains (Fig. 6), but did not achieve nonirradiated control levels even at day 28 (60% of control values, not shown). Total CFU was significantly (P < 0.01) increased in all four mouse strains by day 14 after TBI and filgrastim treatment compared to vehicle-treated mice (Fig. 6). Filgrastim-treated mice showed significant increase (P ≤ 0.002) in BFU-E and CFU-GEMM compared to their vehicle controls. In the case of CFU-GM, specific to the myelogenous lineage, significant increase was observed in C57BL/6, C3H/HeN and B6C3F1 mice (P = 0.027 for C57BL/6, P < 0.0001 for C3H/HeN and B6C3F1 mice), and CD2F1 mice (P < 0.001). Significant enhancement (P < 0.0001) was seen in CFU-E only in the B6C3F1 mice both at days 4 and 14 postirradiation. These data demonstrate that in addition to the myelogenous effect, filgrastim also enhances multilineage hematopoietic recovery.
FIG. 6.
Filgrastim accelerates hematopoietic progenitor recovery after a nonlethal radiation dose in all four mouse strains. CD2F1, C57BL/6, C3H/HeN and B6C3F1 mice (n = 6 per group) were irradiated with a sublethal dose and treated with filgrastim on days 1, 2 and 3 after TBI. Filgrastim treatment after irradiation enhanced survival of bone marrow hematopoietic stem and progenitor cells. Clonogenic potential of bone marrow cells was assessed by a CFU assay on days 4, 7, 10 and 14 postirradiation. Cells from two femurs were pooled, counted and each sample plated in triplicate to be scored 12 days after plating. Data are expressed as mean ± SEM for day 14 only, since earlier time points did not yield statistically quantifiable data. Statistical significance was determined between irradiated control and filgrastim-treated groups as described. *P < 0.001 compared to irradiated control. When compared with the vehicle-treated group, filgrastim-treated animals showed significant increase (P ≤ 0.002) in BFU-E and CFU-GEMM on day 14 in all four mouse strains. Similarly, for CFU-GM, significant increase was obtained (P < 0.05–0.00) on day 14 after TBI in all strains. However, significant CFU-E increase (P < 0.0001) was observed only in B6C3F1 mice on day 14 postirradiation.
Discussion
The past decade witnessed a dramatic upsurge in research focused on identifying novel radiation mitigators or therapeutics in the event of nuclear/radiological threats. The wide range of pharmacological approaches demonstrate varying degrees of efficacy, including anti-apoptotic cytokines, immunomodulators, growth factors that stimulate hematopoiesis, modulators of signal transduction pathways, thrombopoietin receptor agonist, antibiotics and key effectors of survival pathways (17–23). Granulocyte colony stimulating factor is one of the earliest and most frequently evaluated radiation mitigators and the first to obtain FDA licensure for the treatment of ARS. The efficacy of filgrastim (a human G-CSF produced by recombinant DNA technology) in improving survival and hematopoietic recovery in multiple species (dogs, primates, minipigs, mouse) is well documented (24–27). However, optimization studies with filgrastim are limited to a handful of publications from murine models after chemotherapy (1, 28), or focus only on neutrophil recovery after irradiation (27). Scheduling of filgrastim is important for improving hematologic recovery after exposure, as well as developing the most economic strategy for the implementation of filgrastim treatment in a mass casualty event. The current practice of initiating filgrastim treatment 20 h after TBI and continuing daily until neutrophil counts stabilize has serious implications for stress arising from repeated injections (20 or more in primates) and cost-effective strategies.
Our studies led to these conclusions: 1. Frequent and long dosing schedules are not beneficial in the CD2F1 model; 2. Abbreviated filgrastim schedule (3 doses instead of 10 or 16) demonstrated significant survival benefit even when the first dose was delayed to 24 h postirradiation; 3. Survival was not drug dose-dependent for the 3-dose schedule; 4. Filgrastim administered earlier and less frequently (3 vs. 16 doses) improved survival significantly in four strains of mice with different radiosensitivities; and 5. Filgrastim mitigated radiation-induced hematotoxicity and accelerated cell repopulation in blood and bone marrow, while stimulating proliferation of hematopoietic stem and progenitor cells in different strains of mice.
Previous reports of G-CSF dosing schedules have been conflicting. Patchen et al. (14) reported a dose reduction factor of 1.06 for 16 injections of G-CSF (2.5 µg/mouse/day/s.c.). Plett et al. (29) showed increased 30-day survival in mice treated with 125 µg/kg filgrastim, s.c., for 1–16 days after TBI in C57BL/6 mice (62% survival compared to 35% survival in the vehicle-treated group). Conversely, Tanikawa et al. (28) reported that a twice daily dose of 1 µg/mouse administered intraperitoneally on days 0–13 after TBI did not increase survival of irradiated BDF1 mice; death in this group was attributed to bleeding resulting from multiple injections while in a thrombocytopenic state. Our 16 daily doses of filgrastim also did not improve survival, but hemorrhagic occurrences at the s.c. site were minimal, and we do not attribute mortality to bleeding. Rather, the different mouse strains used in these studies account for varying survival (discussed in detail later).
In earlier published studies, single administration of G-CSF or murine GM-CSF did not provide a survival benefit when administered to lethally irradiated mice (30, 31), unless administered within 2 h of irradiation (32). However, in the current studies, we observed a dose-dependent increase in survival with a single dose, 3 doses or 5 doses, even when filgrastim was administered 24 h after TBI. Although significant survival was observed with 1, 3 and 5 doses of filgrastim, maximal efficacy was observed with 3 doses; this dosing regimen is uniquely different from other published studies that demonstrate daily dosing of G-CSF from 1–16 days after TBI improved survival (14). The conventional 16-day schedule is designed to dose past the neutrophil nadir. Yet, our data demonstrate that G-CSF is a potent mitigator even if administered for just 1–3 days after TBI; prolonging the dosing schedule appears to negate the survival benefit in three of the four strains studied and significantly reduced survival in C57BL/6 mice. One potential reason for lack of efficacy with the 16-dose regimen can be due to the handling stress during days 8–14 postirradiation, which coincides with the peak morbid period and overt radiation sickness in mice. In preliminary studies in primates, our group reported accelerated neutrophil and platelet recovery with 3 doses of filgrastim in irradiated primates.2 The recommended treatment of radiological victims is the prompt and continuous administration of growth factors like filgrastim, with therapy being halted when the absolute neutrophil count reaches a level of greater than 1.0 × 109 cells/l, after recovery from the nadir (33), which necessitates injecting 15–24 doses of filgrastim to the irradiated population. Our data have significant implications for medical preparedness, both for improving hematological outcome postirradiation as well as developing a cost-effective strategy for implementing filgrastim therapy.
Furthermore, our study strongly suggests that the window for optimal efficacy of filgrastim in mice is within 24 h after TBI; survival reduced dramatically when the first filgrastim dose was delayed up to 48 or 72 h postirradiation. Optimal survival was observed when filgrastim treatment was initiated at 6 or 12 h postirradiation; this is in keeping with the ASCO recommendation of prompt administration of hematopoietic growth factors after lethal TBI (2). The current studies highlight the fact that advancing filgrastim therapy to times earlier than 24 h postirradiation does not reduce drug efficacy. However, delay of filgrastim treatment up to 48 or 72 h after TBI resulted in diminished survival. Schuening et al. (34) initiated G-CSF 7 days after TBI in canines and did not observe efficacy. More aligned with the current study, Farese et al. (35) highlighted the necessity of initiating filgrastim within 24 h after TBI, and reported that filgrastim, administered 48 h postirradiation, did not improve survival in primates. The authors opined that the deficiency could be associated with mechanisms of action of filgrastim on cellular components, specifically stimulation, proliferation and differentiation of hematopoietic progenitor cells (HPC), enhanced mitotic activity of immature neutrophils, a reduced transit time into circulation from marrow and amplification of marrow-derived neutrophil compartment. Our study, demonstrating a lack of survival benefit due to delayed filgrastim administration, is consistent with these observations. Given the possibility that the pool of filgrastim-target cells is exhausted 24 h postirradiation, filgrastim administered closer to time of exposure may provide optimal benefit by abrogating cytotoxic/cytostatic effects of radiation, decreasing cell-cycle arrest times and accelerating marrow regeneration.
Radiation can cause a general injury/recovery response, however, the kinetics of the response, as well as the degree of injury that informs viability of the individual, is highly varied. This variability is due to inherent genotypic diversity, and one model or strain is insufficient to reflect the probable response of different populations to injury.
To this end, we extended our 3-dose filgrastim schedule to other mouse strains with different radiation sensitivities. We demonstrated that an abbreviated 3-dose filgrastim schedule had significant survival benefit over the conventional 14–16 days of filgrastim therapy in four strains of lethally irradiated mice: C3H/HeN, C57BL/6, B6C3F1 and CD2F1. Genetic differences influence the response of different mouse strains to ionizing radiation (36–38). Grahn and Hamilton described the impact of genetic variations to acute lethal response of four mouse strains exposed to TBI (39). Roderick et al. described survival of 27 inbred mice exposed to daily low-dose X rays (10). Some of these strains were resistant to radiation-induced death while others were highly susceptible. These differences were attributed to inherited genes, which reflect the general human population and influence individual response to ionizing radiation. C57BL/6J and C3H/HeN were the recommended strains of choice for testing radiation countermeasures, since they have shown divergence in many tissue responses after irradiation (11). The B6C3F1 strain was recommended as the most popular strain used in several toxicological studies (40). All radiation counter-measure screenings at AFRRI are performed on the CD2F1 strain (12, 22, 41). Drugs that are effective radiation countermeasures in some strains do not demonstrate the same efficacy in CD2F1 mice. This was reflected in our preliminary studies, in which irradiated CD2F1 mice were administered 16 doses of filgrastim, that failed to achieve efficacy, whereas the study described by Plett et al. demonstrated efficacy of the 16-day filgrastim regimen in C57BL/6 mice (29). However, when we compared the efficacy of 16 vs. 3 daily doses in C3H/HeN, C57BL/6, B6C3F1 and CD2F1 mice, there was a consistent and significantly higher survival observed with the 3-day filgrastim dose, whereas the conventional 16-day dosing regimen failed to improve survival in three of the four strains tested. Furthermore, in C57BL/6 mice, we observed a 30% improvement in survival with the conventional 16-day schedule, which is strikingly similar to the published data by Plett et al. (29). However, the abbreviated schedule significantly increased survival to 83% in a head-to-head comparison. Interestingly, the efficacy of filgrastim therapy does not appear to correlate with the inherent radiation sensitivity of the strains; C3H/HeN mice, the most radiation sensitive (LD50/30 = 7.5 Gy) among the four strains tested here, received the least benefit. However, C57BL/6, closest to the C3H/HeN strain in terms of radiation sensitivity (LD50/30 = 7.7 Gy), profited the most with the abbreviated filgrastim treatment. B6C3F1 and CD2F1, with the highest radiation resistance, had modest and comparable survival. These data validate the existing filgrastim efficacy studies, and lend emphasis for the need to optimize medical interventions.
As alluded to in earlier published studies, filgrastim is a potent stimulator of myelopoiesis. It increases granulocyte counts in normal (42) as well as myelosuppressed animals (14, 24, 43, 44), enhances resistance to infections (45) and stimulates hematopoiesis. The radiation dose selected results in severe injury to hematopoietic tissue, which is comparable to published data for LD70/30 in C57BL/6 mice (29). In addition, the radiation dose does not result in mortality, allowing for adequate powering between the control and filgrastim-treated groups for statistical significance and reduction in the number of animals used, as requested by the IACUC. Our data are in concordance with established literature. Since there was a 24 h gap between the insult and filgrastim therapy, nadirs in all blood indices were observed for both vehicle- and filgrastim-treated groups. Filgrastim accelerated regeneration of granulocytes and platelets, increased hematopoietic progenitors and recovery of the bone marrow, leading to repopulation of circulating end cells. Observed increase in platelets, red blood cells and neutrophils, with concurrent increase in CFU and improvement of bone marrow histopathology, supports the concept that filgrastim has effects on cell differentiation and amplified hematopoiesis extending to lineages other than myeloid alone in all four strains of mice, irrespective of their genetic differences.
In Summary, to the best of our knowledge we have shown for the first time that an abbreviated 3-day (starting 24 h after TBI) schedule of filgrastim is beneficial over an extended 10- or 16-day schedule in four strains of mice exposed to lethal-dose radiation. It is crucial to extend our study to explicate the mechanisms of actions to encompass effects on extrinsic microenvironmental signals and cellular responses, as well as to extend the regimen in higher animal models. One valuable outcome of these studies is the provision of a suitable lower mammal model utilizing the concept of “medical management”, which includes administration of filgrastim, for identifying successful polypharmaceutical approaches in treating radiation injury. If investigators at the preliminary drug development stages continue to use the 16-dose schedule, the emergence of a successful polypharmaceutical approach may be adversely affected by either a lack of efficacy in some strains or ambiguous results for other possible countermeasures.
Also, given the scarcity of data on filgrastim dosing and dosing schedule in the irradiation models for larger mammals, there are urgent requirements for detailed drug dose and schedule optimization studies, as well as a strongly powered pivotal trial to assess drug efficacy at drug concentration and schedule comparable to that shown in the rodents.
Supplementary Material
Acknowledgments
This study was supported by funding from the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Satyamitra MM, Kumar V, Biswas S, Cary L, Dickson L, Ghosh SP, et al., Abbreviated dosing with filgrastim improves survival following total body irradiation. Presented at 61st Annual Meeting of the Radiation Research Society; 2015; Orlando, FL.
References
- 1.Crawford J, Kreisman H, Garewal H, Jones SE, Shoemaker D, Pupa MR, et al. The impact of filgrastim schedule variation on hematopoietic recovery post-chemotherapy. Ann Oncol/ESMO. 1997;8:1117–1124. doi: 10.1023/a:1008271804151. [DOI] [PubMed] [Google Scholar]
- 2.Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. FDA approves radiation medical countermeasure. 2015 http://bit.ly/1Tpsbov.
- 4.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5:202–212. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Guo M, Sun X, Sun W, Hu H, Wei L, et al. Effects of recombinant human granulocyte colony-stimulating factor on central and peripheral T lymphocyte reconstitution after sublethal irradiation in mice. J Radiat Res. 2013;54:83–91. doi: 10.1093/jrr/rrs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuening F, Storb R, Goehle S, Graham T, Appelbaum F, Hackman R, et al. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1989;74:1308–1313. [PubMed] [Google Scholar]
- 7.Farese AM, Cohen MV, Stead RB, Jackson W, 3rd, Macvittie TJ. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res. 2012;178:403–413. doi: 10.1667/RR2900.1. [DOI] [PubMed] [Google Scholar]
- 8.Kallman RF, Kohn HI. The influence of strain on acute x-ray lethality in the mouse. I. LD50 and death rate studies. Radiat Res. 1956;5:309–317. [PubMed] [Google Scholar]
- 9.Mori N, Okumoto M, Yonezawa M, Nishikawa R, Takamori Y, Esaki K. Factors related to resistance to hematopoietic death in mice. J Radiat Res. 1994;35:1–10. doi: 10.1269/jrr.35.1. [DOI] [PubMed] [Google Scholar]
- 10.Roderick TH. The response of twenty-seven inbred strains of mice to daily doses of whole-body X-irradiation. Radiat Res. 1963;20:631–639. [PubMed] [Google Scholar]
- 11.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh SP, Perkins MW, Hieber K, Kulkarni S, Kao TC, Reddy EP, et al. Radiation protection by a new chemical entity, Ex-Rad: efficacy and mechanisms. Radiat Res. 2009;171:173–179. doi: 10.1667/RR1367.1. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–747. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 14.Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Therapeutic administration of recombinant human granulocyte colony-stimulating factor accelerates hemopoietic regeneration and enhances survival in a murine model of radiation-induced myelosuppression. Int J Cell Cloning. 1990;8:107–122. doi: 10.1002/stem.5530080204. [DOI] [PubMed] [Google Scholar]
- 15.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. New Eng J Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 16.Hall E, Giaccia AJ. Radiobiology for the radiologist. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 17.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinan EC, Barbon CM, Kalish LA, Parmar K, Kutok J, Mancuso CJ, et al. Bactericidal/permeability-increasing protein (rBPI21) and Fluoroquinolone mitigate radiation-induced bone marrow aplasia and death. Sci Translat Med. 2011;3:110ra8. doi: 10.1126/scitranslmed.3003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 22.Satyamitra M, Lombardini E, Graves J, 3rd, Mullaney C, Ney P, Hunter J, et al. A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice. Radiat Res. 2011;175:746–758. doi: 10.1667/RR2462.1. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Deoliveira D, Kang Y, Choi SS, Li Z, Chao NJ, et al. Insulin-like growth factor 1 mitigates hematopoietic toxicity after lethal total body irradiation. Int J Oncol. 2013;85:1141–1148. doi: 10.1016/j.ijrobp.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nothdurft W, Selig C, Fliedner TM, Hintz-Obertreis P, Kreja L, Krumwieh D, et al. Haematological effects of rhGM-CSF in dogs exposed to total-body irradiation with a dose of 2.4 Gy. Int J Radiat Biol. 1992;61:519–531. doi: 10.1080/09553009214551281. [DOI] [PubMed] [Google Scholar]
- 25.Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroni M, Ngudiankama BF, Christensen C, Olsen CH, Owens R, Lombardini ED, et al. The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int J Oncol. 2013;86:986–992. doi: 10.1016/j.ijrobp.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Weaver AL, Wan XS, Diffenderfer ES, Lin L, Kennedy AR. Kinetics of neutrophils in mice exposed to radiation and/or granulocyte colony-stimulating factor treatment. Radiat Res. 2013;180:177–188. doi: 10.1667/RR3055.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanikawa S, Nose M, Aoki Y, Tsuneoka K, Shikita M, Nara N. Effects of recombinant human granulocyte colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–449. [PubMed] [Google Scholar]
- 29.Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, et al. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neta R. Cytokines in radioprotection and therapy of radiation injury. Biotherapy. 1988;1:41–45. doi: 10.1007/BF02170134. [DOI] [PubMed] [Google Scholar]
- 31.Neta R, Oppenheim JJ, Douches SD. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol. 1988;140:108–111. [PubMed] [Google Scholar]
- 32.Sureda A, Valls A, Kadar E, Algara M, Ingles-Esteve J, Bigas A, et al. A single dose of granulocyte colony-stimulating factor modifies radiation-induced death in B6D2F1 mice. Exp Hematol. 1993;21:1605–1607. [PubMed] [Google Scholar]
- 33.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 34.Schuening FG, Appelbaum FR, Deeg HJ, Sullivan-Pepe M, Graham TC, Hackman R, et al. Effects of recombinant canine stem cell factor, a c-kit ligand, and recombinant granulocyte colony-stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1993;81:20–26. [PubMed] [Google Scholar]
- 35.Farese AM, Brown CR, Smith CP, Gibbs AM, Katz BP, Johnson CS, et al. The ability of filgrastim to mitigate mortality following LD50/60 total-body irradiation is administration time-dependent. Health Phys. 2014;106:39–47. doi: 10.1097/HP.0b013e3182a4dd2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhard MC, Mirand EA, Goltz HL, Hoffman JG. Mouse-strain differences in response to radiation. Proc Soc Exp Biol Med. 1954;85:367–370. doi: 10.3181/00379727-85-20883. [DOI] [PubMed] [Google Scholar]
- 37.Frolen H, Luning KG, Ronnback C. The effect of x-irradiation on various mouse strains due to their genetic background. I. Lethality after acute irradiation. Radiat Res. 1961;14:381–393. [PubMed] [Google Scholar]
- 38.Grahn D. Acute radiation response of mice from a cross between radiosensitive and radioresistant strains. Genetics. 1958;43:835–843. doi: 10.1093/genetics/43.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grahn D, Hamilton KF. Genetic variation in the acute lethal response of four inbred mouse strains to whole body X-irradiation. Genetics. 1957;42:189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King-Herbert A, Thayer K. NTP workshop: animal models for the NTP rodent cancer bioassay: stocks and strains-should we switch? Toxicol Pathol. 2006;34:802–805. doi: 10.1080/01926230600935938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, et al. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int J Radiat Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 42.Tamura M, Hattori K, Nomura H, Oheda M, Kubota N, Imazeki I, et al. Induction of neutrophilic granulocytosis in mice by administration of purified human native granulocyte colony-stimulating factor (G-CSF) Biochem Biophys Res Comm. 1987;142:454–460. doi: 10.1016/0006-291x(87)90296-8. [DOI] [PubMed] [Google Scholar]
- 43.Shimamura M, Kobayashi Y, Yuo A, Urabe A, Okabe T, Komatsu Y, et al. Effect of human recombinant granulocyte colony-stimulating factor on hematopoietic injury in mice induced by 5-fluorouracil. Blood. 1987;69:353–355. [PubMed] [Google Scholar]
- 44.Welte K, Bonilla MA, Gillio AP, Boone TC, Potter GK, Gabrilove JL, et al. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987;165:941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto M, Matsubara S, Matsuno T, Tamura M, Hattori K, Nomura H, et al. Protective effect of human granulocyte colony-stimulating factor on microbial infection in neutropenic mice. Infect Immun. 1987;55:2715–2720. doi: 10.1128/iai.55.11.2715-2720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.