Abstract
This invited review summarizes work presented in the Russell Ross lecture delivered at the 2012 proceedings of the American Heart Association. We begin with a brief overview of the structural, cellular, and molecular biology of Krüppel-like factors. We then focus on discoveries over the past decade implicating Krüppel-like factors as key determinants of vascular cell function in atherosclerotic vascular disease.
Keywords: Krüppel-like factor, atherosclerosis
Introduction to Krüppel-like factors
Krüppel-like factors (KLFs) are thusly named due to the amino acid sequence homology between their zinc finger (ZnF) domains and that found in the Drosophila transcriptional regulator Krüppel (Kr). In mammals, the KLF/SP family of transcription factors is characterized by 3 consecutive Cys2/His2 ZnF moieties located at their C-terminus and connected by a highly conserved seven residue interfinger sequence, TGEKP(Y/F)X 1–3 (Figure 1, panel A). To date 17 KLF genes and 9 Specificity Protein (SP) genes have been identified (with an 18th KLF recently predicted 4) forming a family of ZnF-containing transcription factors that bind to GC-, GT- and CACCC-box motifs found in gene promoters and other regulatory elements 5. The most distinguishing features that differentiate the KLF subfamily from SPs is the absence of both the SP box (located close to the N-terminus) and the SP hallmark, the Buttonhead (BTD) box (positioned just N-terminal to the ZnF domain). Using sequence analysis of the conserved 81-amino acid ZnF domain the KLF/SP family has been classified into subgroups of highly related genes; for informative cladograms and evolutionary trees see 4–6. Of import, homology between KLF family members is largely restricted to the DNA-binding zinc-finger region. They are highly divergent in their non-DNA binding regions — the domains that regulate transcriptional activation or repression —and it is proposed that the differing and sometimes opposing functions of different KLFs is a consequence of distinctive protein-protein interactions at these modulatory domains.
Figure 1.
(A) Schematic representation of common structure and functional domains for KLFs. The transactivation and transrepression domains are located at the N-terminal end. Three consecutive zinc finger moieties are located at the extreme C-terminus. A highly conserved 7-amino acid sequence, abbreviated TGEKP(Y/F)X, resides in the interfinger regions and contributes to DNA-binding. (B) Diagram illustrating one way the KLFs can have both transactivating and repressive functions. In this example, KLF2 or KLF4 interact with the cofactor p300 to augment transcription of eNOS and TM under basal conditions (left), or compete for binding to limiting amounts of the cofactor p300 to inhibit NF-kB-induced expression of proinflammatory genes (e.g. VCAM-1) (right).
KLF expression is differentially regulated by physiologic stimuli, during cell differentiation, and in response to inflammatory cytokines. This results in a restricted expression pattern for some KLFs but a wide tissue distribution for many. KLFs have been implicated in diverse cellular processes including growth and differentiation, metabolism, and homeostasis. KLFs are largely found in the cell nucleus; however cytoplasmic localization of KLFs has been documented in response to stimulation with cytokines or calcineurin inhibitors, post-translational modification, or isotypic variation and depending upon the cell type cytoplasmic localization of KLFs has been shown to effect maturation, quiescence, phenotypic switching, or oncogenic potential 7–11. Interestingly, accumulating evidence suggests that altered cellular function due to cytoplasmic localization of KLFs is not solely a consequence of the lack of access to nuclear contents, but that interaction of KLFs with cytoplasmic proteins can alter the function or stability of those proteins 12, 13.
Similar to Kr in Drosophila, the KLFs can act as either transcriptional activators or repressors, and exert their effects via either direct DNA-binding or through interaction with cofactors. There is accumulating evidence that context-dependent interactions are at the crux of the multifarious effects of KLFs. For example, in breast cancer cells KLF4 inhibits the expression of p53 (thus promoting cell proliferation) and also induces the cell-cycle inhibitor p21CIP1/WAF1. In the context of the loss of p21CIP1/WAF1 KLF4 is converted from a cell-cycle inhibitor to an oncoprotein 14. In endothelial cells (ECs) and monocytes, we have demonstrated that the “context” alters the result of KLF interaction with transcriptional cofactors 15, 16. Under basal (healthy) conditions in ECs, KLF2 and KLF4 interact with the cofactor p300 to augment transcription of eNOS and thrombomodulin. However, under inflammatory conditions, NF-κB translocates to the nucleus and interacts with p300 for maximal induction of target genes. By binding to p300 KLF2 or KLF4 can sequester this cofactor from NF-κB and thus attenuate NF-κB-dependent inflammatory gene expression 16. Thus, KLF interaction with p300 can result in either transactivation or repression, depending upon context (Figure 1, Panel B). Indeed, transcriptional regulation via interaction with p300 is thematic for several KLFs 15, 17–21.
Atherogenesis
Manifesting clinically as myocardial infarction, stroke and peripheral vascular disease with an enormous impact on health, atherosclerosis has garnered the greatest attention amongst the various pathologies afflicting vessels. The anatomic calling card of this disease is the co-optation of vessel walls by a fatty, inflammatory, calcific agglomeration. Cumulative clinical and experimental observations led Ross and Glomset to propose the “response to injury” hypothesis, published as a two-part series in the New England Journal of Medicine 22, 23. In its original incarnation, the theory proposed that endothelial desquamation allowed platelets to adhere to the subintima and thus initiate the disease. Repetition of this injury would lead to investment of additional cells (e.g. leukocytes) and progression of disease. While the hypothesis would undergo significant modification over the years, with increasing importance given to the centrality of endothelial dysfunction and importance of inflammation in disease pathogenesis, the model was critical in providing an envisaging framework for investigators in contemplation of this complex disease 24.
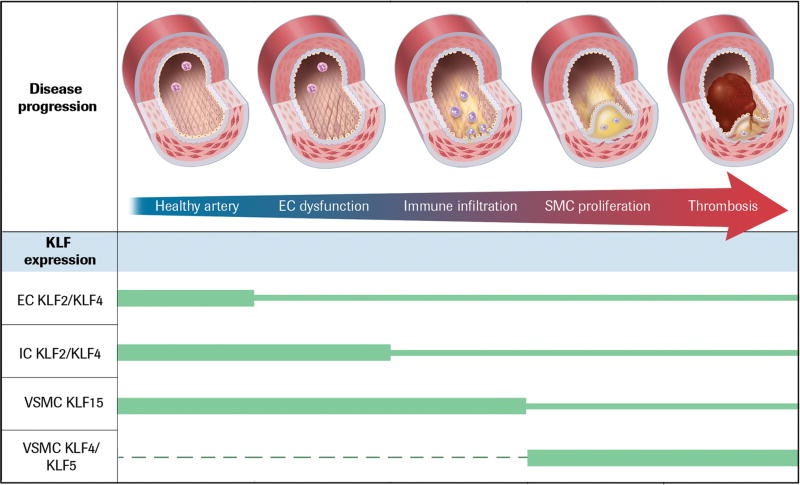
The convergence of cell and molecular tools over the past several decades have facilitated major advances in our understanding of nodal pathways operative within vessel-intrinsic and -extrinsic cells that control the development of atherosclerosis. In this review we focus on the discovery of a key role in atherogenesis for KLFs. For simplicity, we will consider atherogenesis as a multi-phase process that converts a healthy artery to one occluded by atherothrombosis by focusing on dysfunction of a particular cell type that distinguishes transition between each phase of this chronic disease (Figure 2). The consequences of altered KLF function at each phase will be discussed, followed by an outline of the current understanding of the mechanistic details.
Figure 2.
Development of an atherosclerotic lesion, from healthy vessel to artery occluded by atherothrombosis. Relative levels of cell-specific expression of KLFs during the process of atherogenesis are indicated by the size of the green bars. The three pop-up figures provide details of the molecular changes occurring in the vascular cells and refer to the 1st (left-most), the 2nd and 3rd, and the 4th and 5th vessel cut-away images, respectively.
Healthy artery → Endothelial Dysfunction
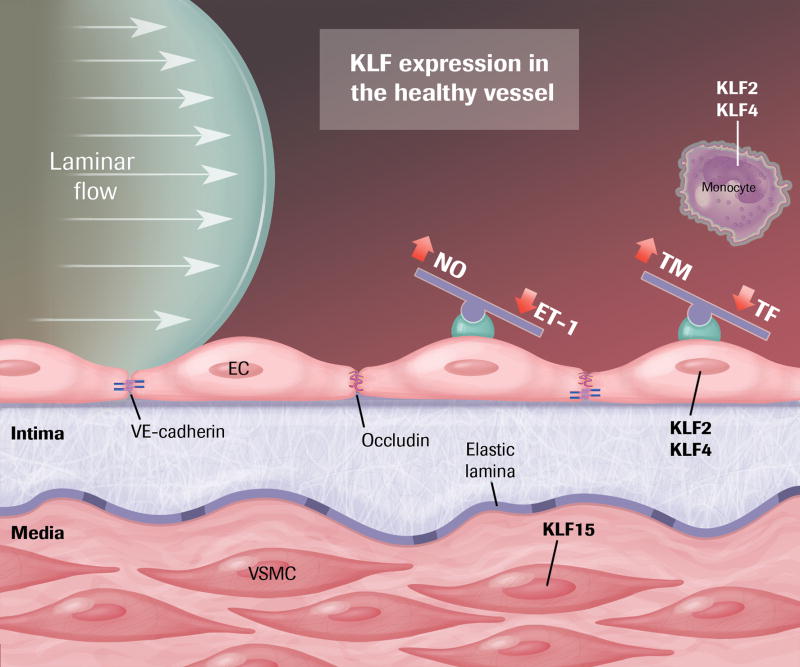
The healthy artery is lined by a confluent endothelial monolayer that elaborates substances that create a non-adhesive, non-thrombotic barrier between the flowing blood and underlying tissue. Interendothelial junctions tightly regulate permeability of fluid and macromolecules across the endothelial surface. Dynamic signaling between blood-borne humoral factors, metabolic products of underlying tissues, and chemical communication between the endothelium and the vascular smooth muscle cells of the medial layer of the vessel control vasomotor tone. Central to maintenance of this homeostasis is optimal endothelial function, and laminar shear stress (LSS) created by blood flowing over the endothelium is a highly potent effector in this respect. Evidence from our group and others demonstrates that members of the KLF family, particularly KLF2 and KLF4, are central players in this shear stress-mediated homeostasis.
KLF2 and KLF4 are highly expressed in ECs exposed to laminar flow and reduced in ECs at arterial branch points and other regions of turbulent flow, such as the inferior aspect of the aortic arch 16, 25–29. Anatomically, this correlates to regions long recognized as most prone to development of atherosclerotic lesions. In the last five years, in vivo animal experiments have demonstrated that EC deficiency of KLF2 and KLF4 also predispose to atherosclerosis. In mice deficient in apolipoprotein E and thus susceptible to diet-induced atherosclerosis (the “ApoE model”), KLF2 hemizygous mice have increased plaque burden 30. EC-specific loss of KLF4 also increases the severity of atherosclerotic lesion formation whereas EC-specific overexpression of KLF4 is protective 16. In vitro and in vivo data demonstrate that both KLF2 and KLF4 are potent inducers of eNOS and thrombomodulin expression and also inhibit cytokine-induced expression of adhesion factors-including VCAM-1, E-selectin- and other inflammatory mediators 26, 27. Recent studies demonstrate that KLF2 plays an essential role in maintaining endothelial barrier integrity, including protection from ischemic stroke, by differential regulation of key junction proteins (e.g. ZO-1 and occludin) 31, 32. In vivo permeability studies on KLF4 are limited thus far; however, KLF4 is likely to influence permeability via transcriptional regulation of VE-cadherin 33.
Determination of the precise mechanisms by which LSS induces KLF2 and KLF4 expression remains an active area of investigation for numerous laboratories. Primary EC cilia are postulated to be a shear stress sensor in areas of low or disturbed flow 34. ECs from mice with genetic loss of cilia fail to induce KLF2 and downregulate KLF4 in response to LSS. These non-ciliated cells do not express eNOS in response to shear; instead, they lose their cobblestone appearance and acquire a mesenchymal-transitional phenotype 35, 36. While fascinating, mechanotransduction via cilia may be most important during cardiovascular development or limited to areas of low shear stress as EC cilia are not found, nor required for response, in areas with high shear stress. Indeed, constitutive, endothelial-specific KLF2 null mice die at embryonic day 10.5 secondary to lack of vascular tone, bleeding and cardiac dysfunction 37, 38.
A molecular link between flow and its downstream effects is signaled, in part, by MAPK (mitogen-activated protein kinase) pathways that activate ERK. KLF2 and KLF4 are induced by ERK activation. The KLF2 promoter is upregulated by MEF2 binding downstream to both the MEK5/ERK5/MEF2 and the AMPK/ERK5/MEF2 flow pathways 39, 40. ERK5-dependent KLF4 induction confers the vasoprotective phenotype described by enhanced expression of anti-thrombotic, hemostatic, and vasodilatory genes 41. Post-transcriptional regulation also appears to play a role both in endogenous regulation of KLF2 and KLF4; two laboratories independently found that inhibition of miR-92a by atheroprotective flow allows for increased levels of EC KLF2 and KLF4 28, 42.
Other KLFs
KLF6 expression is induced in EC after vascular injury and, in cooperation with Sp1, induces several target genes involved in vascular remodeling, including endoglin, collagen α1, TGFβ1 43, 44, and activin receptor-like kinase-1 45. In in vitro scratch assays, overexpression of KLF6 leads to more rapid “wound healing” and enhanced EC migration 45, 46. KLF11 suppresses NF-κB-mediated EC activation, a role that may be particularly important in diabetes mellitus 47. Unproven as yet is the ability of KLF6 or KLF11 to alter atherogenesis, although it has been shown that KLF6 levels are upregulated in primary human monocytes after knockdown of the potent anti-inflammatory macrophage transcriptional regulator tristetraprolin 48. In regard to KLF2 and KLF4, however, the data is compelling for a central role in maintaining EC homeostasis and vascular health.
Endothelial Dysfunction → Immune Cell Infiltration
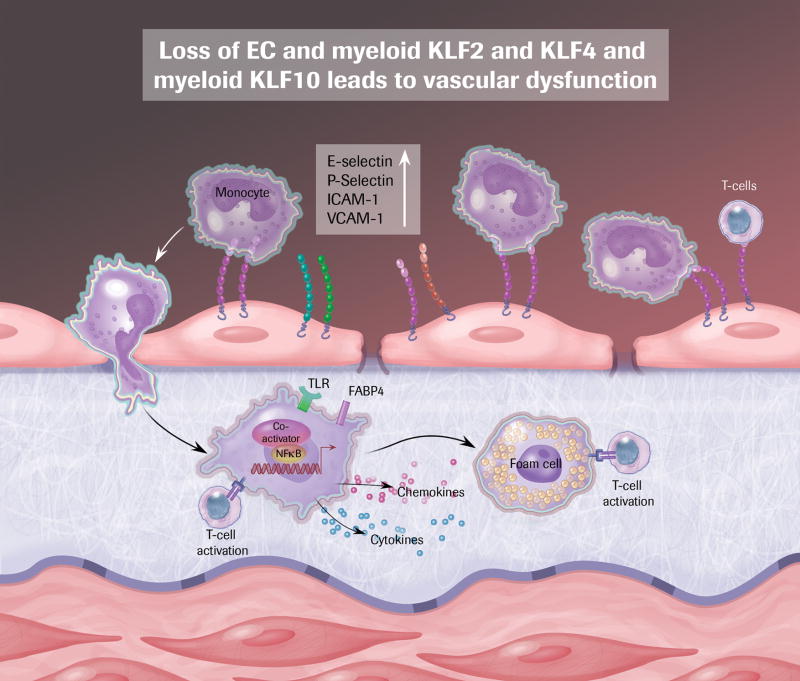
As a consequence of EC activation blood leukocytes adhere to the luminal surface of the vessel and transmigrate into the intima via dysregulated interendothelial junctions 49. Therein monocytes differentiate to macrophages, which then take up oxidized low-density lipoprotein (OxLDL) and become foam cells, an essential characteristic of the atherosclerotic lesion, manifesting early in disease as a “fatty streak” 50. Lipid-laden macrophages in turn further activate ECs, enhancing secretion of chemokines and expression of cell adhesion molecules, directing more monocytes and T-cells to move into the vessel wall, and thus leading to local activation of both innate and adaptive immunity and disease progression 49–51. Macrophages participate in atherogenesis by being central cellular effectors of both thrombotic and inflammatory signals 52. Arrested development of atherosclerosis is evident after depletion of monocyte/macrophages or manipulation of cytokine-mediated recruitment of these cells. 53–55. Several members of the KLF family, in particular KLF2 and 4, have emerged as important regulators of monocyte/macrophage biology.
Myeloid Cells
As factories of both cytokine and protein mediators of inflammation macrophages are central to the development atherosclerosis 56. There is strong data implicating KLFs in governance of myeloid activity during atherogenesis.
KLF2
KLF2 is a potent negative regulator of monocyte/macrophage proinflammatory activation and an essential regulator of the innate immune response 18, 21. While there is important data elucidating the role of KLF2 in acute inflammation (sepsis), due to space limitations we will focus here on chronic inflammation apropos of atherosclerosis. Patients with coronary artery disease have significantly lower KLF2 expression in circulating monocytes than do healthy subjects 18, 21. Animals with myeloid-specific deletion of KLF2 have elevated baseline plasma levels of proinflammatory molecules including as IL-1β and TNFα supporting the idea that KLF2 is a tonic repressor of myeloid activation 21. LDLR null mice with myeloid-specific KLF2 deletion develop a markedly greater atherosclerotic burden than controls 57. Mechanistically, there is data that supporting a role for myeloid KLF2 deficiency in enhancing macrophage adherence to ECs 57 and inducing OxLDL uptake 30, thus augmenting macrophage-derived foam cell formation.
KLF4
Gain- and loss-of-function studies show that KLF4, as a downstream target of PU.1, promotes monocyte differentiation 58. In vivo experiments in KLF4 −/− chimeric mice demonstrate a role of KLF4 in inflammatory monocyte differentiation, with KLF4 expression necessary for maturation of both Ly6Chi and Ly6Clo monocyte populations 59. Beyond developmental biology, KLF4 is also a mediator of macrophage subset specification by regulating macrophage M1/M2 polarization 15. In response to M2 stimuli (IL-4, IL-13) KLF4 expression is induced and expression remains high in the anti-inflammatory M2 macrophages. Conversely, KLF4 expression is suppressed by M1 polarization stimuli (LPS, IFNγ) and KLF4 levels in proinflammatory M1 macrophages are low. In line with these results, KLF4-deficient macrophages have increased proinflammatory gene expression and enhanced bactericidal effects. Pathophysiological effects including delayed wound healing, increased insulin resistance, and diet-induced obesity have been observed in animals with myeloid-specific deletion of KLF4. Mechanistically, KLF4 promotes the M2 phenotype by cooperating with STAT6 to promote M2 targets. KLF4 inhibits the M1 phenotype via inactivation of NF-κB by sequestration of the critical coactivators p300 and PCAF, as described above in ECs. KLF4 expression is downregulated in circulating monocytes of patients with coronary artery disease 60. A recent study from our group confirms that myeloid KLF4 has an atheroprotective effect. Myeloid KLF4-deficient animals on an ApoE null background develop significantly more vascular inflammation and atherosclerotic lesion burden than control mice 61. Collectively, these observations substantiated a major role of KLF4 in monopoiesis, macrophage polarization, and macrophage-driven pathology.
Other KLFs
Other KLFs including KLF1, KLF3 and KLF10 are expressed in myeloid cells and may participate in vascular inflammation and atherosclerosis 2, 62, 63.
T lymphocytes
Activation of naïve T cells leads to proliferation, enhancement of effector functions, and homing to areas of inflammation. This induction of adaptive immunity plays a role in progression of atherosclerosis 64. Our group and others have demonstrated a role for KLF2, 4, 10 and 13 in regulation of adaptive immunity, with implications for atherosclerosis.
KLF2
Gain-and loss-of function approaches have demonstrated a role of KLF2 in maintaining T-lymphocyte quiescence associated with the ability of KLF2 to inhibit c-Myc expression and upregulate cyclin-dependent kinase inhibitor p21CIP1/WAF1 65, 66. In addition, KLF2 serves a major role as a regulator of T- and B-cell survival and migration 67. Thymocytes deficient in KLF2 have diminished expression of surface receptors and trafficking molecules such as the sphingosine-1-phosphate (S1P) receptor S1P1R, L-selectin, CCR7, and integrin β7. T cells lacking KLF2 express proinflammatory chemokine receptors such as CXCR3, CCR3, CCR5 and CD69 on memory cells and activated thymocytes 68. These changes may lead to altered homing patterns of naïve T cells to nonlymphoid tissues and attenuation of T-cell proliferation. While not yet proven to effect atherosclerosis per se, an anti-inflammatory effect of KLF2 overexpression has been demonstrated via attenuation of a mouse model of T-cell dependent myocarditis 69.
KLF4
Differentiation of helper T cells type 17 (Th17) and the expression of IL-17 by these cells are regulated by KLF4 70, 71. While IL-17 has been shown to promote chronic inflammatory diseases such as arthritis, colitis, and autoimmune encephalitis, the role of the IL-17/Th17 in the development of atherosclerosis remains controversial. The presence of IL-17/Th17 has been demonstrated in both human and mouse atherosclerotic lesions, yet different mouse models have suggested either atheroprotective or proatherogenic roles 50, 72. KLF4-deficient mice have a significant reduction in the severity of autoimmune encephalomyelitis attributed to attenuation of Th17 responses and infiltration of leukocytes into the central nervous system 71. Taken together, these studies suggest a role of KLF4 in regulating T cell activation and proliferation, which may parlay into an effect on atherogenesis.
KLF10
Several studies have defined an atheroprotective role of regulatory T cells (Treg). KLF10 drives CD4+CD25− T cell activation and Treg differentiation and suppressor function 73. Overexpression of KLF10 in Treg induces expression of TGFβ1, an atheroprotective cytokine. Importantly, in an ApoE null, scid background, adoptive transfer of KLF10 deficient CD4+CD25− T cells accelerates atherosclerosis.
KLF13
KLF13 was initially identified as a transcription factor expressed in activated T lymphocytes 74. KLF13 enhances expression of the proinflammatory chemokine, RANTES, to promote T cell activation and attenuates promoter activity of a potent antiapoptotic factor, BCL-XL, to promote T cell and survival 75.
B lymphocytes
The role of B-cells in atherosclerosis is incompletely understood; however, B lymphocytes are found in the plaque and adventitia at areas of advanced atherosclerosis 64. Evidence supporting a role of KLF3 in innate and humoral immunity includes effects on B cell differentiation and quiescence, as well as regulation of the proliferative response to LPS via attenuation of the toll-like receptor signaling pathway 76, 77. KLF4 expression is found throughout all stages of B cell development and it has been demonstrated to take part in regulation of activation-induced B cell proliferation via induction of p21CIP1/Waf1 expression and downregulation of c-Myc and cyclin D2 78.
Immune Cell Infiltration → VSMC Proliferation
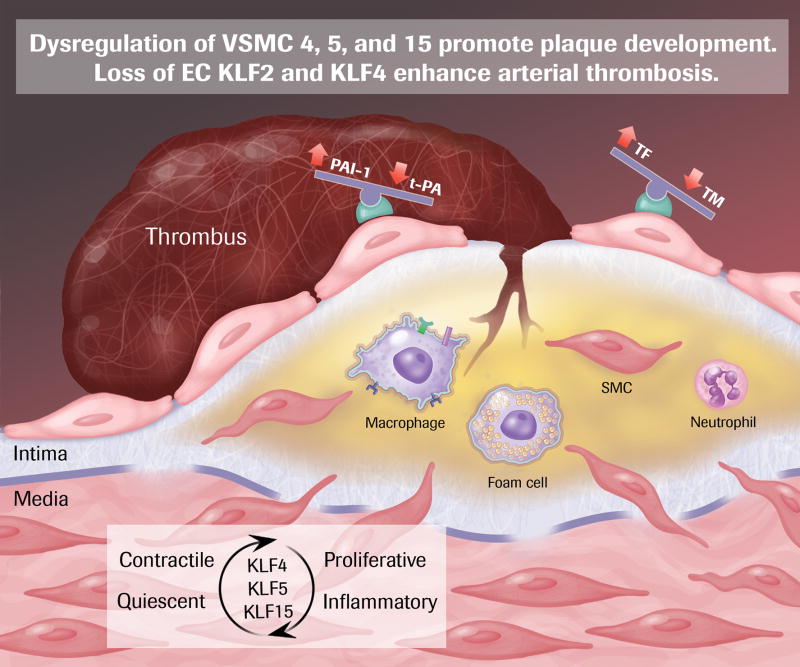
In a healthy artery, VSMC are located in the medial layer, separated from the ECs and intima by the internal elastic lamina. Their primary function is to respond to blood-borne, EC-derived, and tissue metabolic signals and relax or contract, controlling vasomotor tone. Synthetic activity and proliferative rate are very low, and they express markers of a contractile cellular phenotype, including SM a-actin, SM myosin heavy chain, h1-calponin, and smoothelin (reviewed in 79). During atherogenesis, intimal infiltration of activated leukocytes creates a state of continued cellular crosstalk that amplifies the inflammatory response and results in chronic inflammation. In response to growth factors and cytokine (and perhaps additional undefined mechanisms) VSMC migrate across the elastic lamina into the subendothelial space, and transition of the lesion from a fatty streak to a more complex, bulky atherosclerotic plaque that may impair blood flow enough to cause angina. Mature VSMC retain remarkable plasticity, and it is assumed that they undergo a “phenotypic switch” from a relatively quiescent contractile cell, to an inflammatory, proliferative cell during atherogenesis, though details of the changed expression profile have been rigorously documented in vitro 79. The relocated VSMC may proliferate (neointimal formation), take on characteristics of foam cells, elaborate inflammatory signals, and synthesize extracellular matrix proteins that lead to development of fibrous plaque cap 24, 80. There is evidence for KLF4, 5, and 15 as effectors of the VSMC response in atherogenesis.
KLF4
KLF4 is not expressed in VSMC of the healthy artery; however, after vascular injury expression is rapidly induced and corresponds to loss of VSMC differentiation markers that characterize the contractile phenotype. In KLF4-deficient mice VSMC neointimal proliferation enhanced 81. The Owens laboratory has demonstrated that OxLDL induces both phenotypic switching and enhanced VSMC migration in a KLF4-dependent fashion 82, 83. Control of VSMC proliferation by KLF4 is mediated via induction of p53 and p21CIP1/WAF1, reminiscent of mechanism in other cell types 81, 84. KLF4 inhibits expression of VMSC differentiation genes by interfering with expression and function of the potent SMC coactivator myocardin 85. Interestingly, KLF4 inhibits myocardin by binding to the promoter in cooperation with NF-κB 86. Thus, in contrast to ECs, KLF4 is pro-inflammatory by cooperating with NF-κB in activated. Direct evidence, via VSMC-specific genetic gain-and loss-of-function experiments, that altered VSMC KLF4 alters atherogenesis is lacking as yet; however, recent studies utilizing miRNA approaches are supportive. MicroRNA-145 is highly expressed in arteries, but is attenuated after vascular injury and in atherosclerosis 87, 88. Overexpression of miR-145 limits neointimal formation after vascular injury, regulating the VSMC phenotypic switch between contractile and proliferative states, and it significantly reduces KLF4 levels (amongst other targets) 87, 88. Of note, VSMC-specific lentiviral overexpression of miR-145 reduces KLF4 levels, limits atherosclerosis, and enhances plaque stability in the ApoE null mouse model89.
KLF5
KLF5 expression in VSMCs is induced after vascular injury and in atherosclerotic lesions 90–92. Genetic deficiency of KLF5 leads to baseline thinning of the medial and adventitial walls of arteries and inhibition of neointimal proliferation after vascular injury 93. A potential pro-atherogenic role for KLF5 is also suggested by the gene profile it activates. Targets include PDGF-A, TGFβ1, cyclin B, Egr-1, and PAI-1; genes that enhance proliferation, migration and vascular inflammation 94, 95. It will be of great interest to see the atherosclerotic phenotype of VSMC-specific KLF5 modulation.
KLF15
In contrast to KLF4 and KLF5, KLF15 is robustly expressed in VSMCs under basal conditions, but is attenuated after injury in mouse models and in human atherosclerotic tissue 96, 97. In mouse models global deficiency of KLF15 leads to enhanced susceptibility to both heart failure and aortic aneurysm and, supportive of translation to human disease, KLF15 levels are reduced in human aortic aneurysms 98. Indeed, VSMC-specific loss of KLF15 enhances atherosclerosis and vascular inflammation in the ApoE mouse model. True to our theme, KLF15 reduces activity of NF-κB on inflammatory gene promoters via direct interaction with p300 97. These observations provide the most stringent evidence to date implication a VSMC-intrinsic role in atherosclerosis for any KLF.
VSMC Proliferation → Atherothrombosis
Acute coronary syndrome results from plaque rupture with exposure of the blood to plaque lipids and tissue factor and thus initiation of the coagulation cascade, followed by platelet adherence and arterial thrombosis. Unfortunately, to date there are no reliable animal models that allow for quantitative assessment of plaque rupture and acute thrombosis, and thus data on the effect of KLFs on this aspect of atherosclerosis are limited. Indirect evidence does suggest however, that both EC KLF2 and KLF4 would be beneficial in protecting against atherothrombosis. KLF2 inhibits blood clotting in EC cultures 99. Ex vivo (fibrin clot) and in vivo (carotid injury) experiments demonstrate that KLF4 protects against thrombosis, even in the presence of inflammation 16. EC KLF2 and KLF4 increase thrombomodulin and tissue plasminogen activator expression (tPA) and decrease plasminogen activator inhibitor (PAI-1) and cytokine-stimulated tissue factor expression 27, 99, consistent with an anti-thrombotic effect. One can speculate that myeloid KLF2 and KLF4 might have a protective effect on plaque rupture-mediated thrombosis by inhibiting leukocyte expression of matrix metalloproteinases (MMPs) 21. Finally, as mentioned above, miR-145-mediated downregulation of VSMC KLF4 may enhance stability of atherosclerotic plaque 89.
Conclusion
Atherosclerosis is a complex, chronic, highly morbid disease; the leading cause of death in the United States as well as increasing parts of the rest of the world. Cardiovascular physicians have slew of powerful medical therapies that have attenuated the mortality of the disease, yet ~715,000 Americans suffer from myocardial infarction each year 100. Krüppel-like factors have potent effects on a broad range of vascular processes that contribute to atherogenesis (summarized in Table 1) and the cumulative data is sufficient to warrant their consideration as therapeutic targets. While exercise, healthy diet, and a tobacco-free lifestyle would likely be the most effective way to prevent atherosclerosis, adoption of these lifestyle changes has not proven reliable. Thus, although the benefits of these modalities are potentially mediated by vascular KLF expression—exercise increases laminar shear stress and thus EC KLF2 and KLF4 101, components of the “Mediterranean diet” including broccoli, grapes, red wine (resveratrol), and olive oil enhance KLF4 and KLF2 expression or reduce KLF6 102–106, and cigarette smoke increases VSMC KLF4 expression 107 —real-life conditions may be insufficient to effect change in vascular KLF levels and thus drug discovery studies are ongoing to find more specific, potent regulators. HMG CoA reductase inhibitors (statins) are commonly used for treatment of coronary artery disease and induce EC expression of KLF2 and KLF4 41, 69, 105, 108, 109. Other creative modes of altering vascular KLF expression are also being explored; groups are assessing whether stents coated with agents that regulate KLF2 or KLF4 may improve neointimal hyperplasia or stent thrombosis 110–112. Especially exciting is a recent study that used EC-derived extracellular vesicles to control gene expression in co-cultured VSMCs and reduce atherosclerotic lesion formation in ApoE null mice 113. Hergenreider et al. exposed ECs to shear stress or lentiviral-mediated overexpression of KLF2. Extracellular vesicles secreted from these cells were enriched in the mir143/145 and were atheroprotective. This is very exciting news for fans of the KLFs, and of great interest for all those fascinated by communication between ECs and VSMCs. We are confident Dr. Ross would continue to find this topic utterly enthralling.
Table 1. Summary of expression and function of KLFs in regard to atherosclerotic vascular disease.
See manuscript text for details, abbreviations, and references.
| Cell type | KLF | Function |
|---|---|---|
| Endothelial cell | KLF2 |
|
| KLF4 |
|
|
| KLF6 |
|
|
| KLF11 |
|
|
| Immune cell -Myeloid Cell | KLF2 |
|
| KLF4 |
|
|
| Immune cell -T lymphocyte | KLF2 |
|
| KLF4 |
|
|
| KLF10 |
|
|
| KLF13 |
|
|
| Immune cell -B lymphocyte | KLF3 |
|
| KLF4 |
|
|
| Vascular smooth muscle cell | KLF4 |
|
| KLF5 |
|
|
| KLF15 |
|
Acknowledgments
This work was performed with the support of NIH grants HL110630, HL097593, HL112486, HL086548, HL076754 (Mukesh K. Jain) and HL113570 (Anne Hamik).
Abbreviations
- EC
endothelial cell
- ET-1
endothelin-1
- FABP4
fatty acid binding protein 4
- IC
immune cell
- ICAM-1
intercellular adhesion molecule-1
- INFγ
interferonγ
- KLF
Krüppel-like factor
- Kr
Krüppel
- LPS
lipopolysaccharide
- LSS
laminar shear stress
- MMPs
matrix metalloproteinases
- NF-κB
nuclear factor κB
- NO
nitric oxide
- OxLDL
oxidized low-density lipoprotein
- PAI-1
plasminogen activator inhibitor-1
- PDGF
platelet-derived growth factor
- SP
Specificity Protein
- TF
tissue factor
- TLR
toll-like receptor
- TM
thrombomodulin
- TNFα
tumor necrosis factorα
- tPA
tissue plasminogen activator
- VCAM-1
vascular cell adhesion molecule-1
- VSMC
vascular smooth muscle cell
- ZnF
zinc finger
Footnotes
The authors report no conflict of interest.
References
- 1.Bieker JJ. Isolation, genomic structure, and expression of human erythroid kruppel-like factor (eklf) DNA and cell biology. 1996;15:347–352. doi: 10.1089/dna.1996.15.347. [DOI] [PubMed] [Google Scholar]
- 2.Turner J, Crossley M. Basic kruppel-like factor functions within a network of interacting haematopoietic transcription factors. The international journal of biochemistry & cell biology. 1999;31:1169–1174. doi: 10.1016/s1357-2725(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 3.Dang DT, Zhao W, Mahatan CS, Geiman DE, Yang VW. Opposing effects of kruppel-like factor 4 (gut-enriched kruppel-like factor) and kruppel-like factor 5 (intestinal-enriched kruppel-like factor) on the promoter of the kruppel-like factor 4 gene. Nucleic acids research. 2002;30:2736–2741. doi: 10.1093/nar/gkf400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pei J, Grishin NV. A new family of predicted kruppel-like factor genes and pseudogenes in placental mammals. PloS one. 2013;8:e81109. doi: 10.1371/journal.pone.0081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suske G, Bruford E, Philipsen S. Mammalian sp/klf transcription factors: Bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Bieker JJ. Kruppel-like factors: Three fingers in many pies. The Journal of biological chemistry. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Zheng B, Zhang XH, Nie CJ, Li YH, Wen JK. Localization and function of klf4 in cytoplasm of vascular smooth muscle cell. Biochemical and biophysical research communications. 2013;436:162–168. doi: 10.1016/j.bbrc.2013.05.067. [DOI] [PubMed] [Google Scholar]
- 8.Garvey SM, Sinden DS, Schoppee Bortz PD, Wamhoff BR. Cyclosporine up-regulates kruppel-like factor-4 (klf4) in vascular smooth muscle cells and drives phenotypic modulation in vivo. The Journal of pharmacology and experimental therapeutics. 2010;333:34–42. doi: 10.1124/jpet.109.163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du JX, Bialkowska AB, McConnell BB, Yang VW. Sumoylation regulates nuclear localization of kruppel-like factor 5. The Journal of biological chemistry. 2008;283:31991–32002. doi: 10.1074/jbc.M803612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.<klfs in cytoplasm cancer.Pdf>.
- 11.Behr R, Deller C, Godmann M, Muller T, Bergmann M, Ivell R, Steger K. Kruppel-like factor 4 expression in normal and pathological human testes. Molecular human reproduction. 2007;13:815–820. doi: 10.1093/molehr/gam064. [DOI] [PubMed] [Google Scholar]
- 12.Kawanami D, Mahabeleshwar GH, Lin Z, Atkins GB, Hamik A, Haldar SM, Maemura K, Lamanna JC, Jain MK. Kruppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. The Journal of biological chemistry. 2009;284:20522–20530. doi: 10.1074/jbc.M109.025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen B, Smith RS, Jr, Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. The Journal of biological chemistry. 2009;284:35471–35478. doi: 10.1074/jbc.M109.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland BD, Bernards R, Peeper DS. The klf4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nature cell biology. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 15.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, Jain MK. Kruppel-like factor 4 regulates macrophage polarization. The Journal of clinical investigation. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, Jain MK. Endothelial kruppel-like factor 4 protects against atherothrombosis in mice. The Journal of clinical investigation. 2012;122:4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. The Journal of biological chemistry. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 18.Das H, Kumar A, Lin Z, Patino WD, Hwang PM, Feinberg MW, Majumder PK, Jain MK. Kruppel-like factor 2 (klf2) regulates proinflammatory activation of monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnefond A, Lomberk G, Buttar N, Busiah K, Vaillant E, Lobbens S, Yengo L, Dechaume A, Mignot B, Simon A, Scharfmann R, Neve B, Tanyolac S, Hodoglugil U, Pattou F, Cave H, Iovanna J, Stein R, Polak M, Vaxillaire M, Froguel P, Urrutia R. Disruption of a novel kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 ins mutation found in neonatal diabetes mellitus. The Journal of biological chemistry. 2011;286:28414–28424. doi: 10.1074/jbc.M110.215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C. Kruppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. The Journal of biological chemistry. 2007;282:33994–34002. doi: 10.1074/jbc.M701847200. [DOI] [PubMed] [Google Scholar]
- 21.Mahabeleshwar GH, Kawanami D, Sharma N, Takami Y, Zhou G, Shi H, Nayak L, Jeyaraj D, Grealy R, White M, McManus R, Ryan T, Leahy P, Lin Z, Haldar SM, Atkins GB, Wong HR, Lingrel JB, Jain MK. The myeloid transcription factor klf2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts) The New England journal of medicine. 1976;295:420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 23.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts) The New England journal of medicine. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 24.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 25.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung kruppel-like factor (klf2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 26.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. Klf2 is a novel transcriptional regulator of endothelial proinflammatory activation. The Journal of experimental medicine. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. The Journal of biological chemistry. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Davies PF. Site-specific microrna-92a regulation of kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, Visvader JE, Jo H. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, Natesan V, Lin Z, Simon DI, Jain MK. Hemizygous deficiency of kruppel-like factor 2 augments experimental atherosclerosis. Circulation research. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi H, Sheng B, Zhang F, Wu C, Zhang R, Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, Wang Y, Chen J, Jain MK, Atkins GB. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. American journal of physiology. Heart and circulatory physiology. 2013;304:H796–805. doi: 10.1152/ajpheart.00712.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates ve-cadherin expression and endothelial barrier function. Circulation research. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 36.Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circulation research. 2011;108:1093–1101. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The lklf transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes & development. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Developmental cell. 2006;11:845–857. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Atkins GB, Jain MK. Role of kruppel-like transcription factors in endothelial biology. Circulation research. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 40.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, Ludwig S, Roth J, Goebeler M, Schmidt M. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of kruppel-like factor 4 (klf4) The Journal of biological chemistry. 2010;285:26199–26210. doi: 10.1074/jbc.M110.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of kruppel-like factor 2 is mediated by microrna-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McConnell BB, Yang VW. Mammalian kruppel-like factors in health and diseases. Physiological reviews. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botella LM, Sanchez-Elsner T, Sanz-Rodriguez F, Kojima S, Shimada J, Guerrero-Esteo M, Cooreman MP, Ratziu V, Langa C, Vary CP, Ramirez JR, Friedman S, Bernabeu C. Transcriptional activation of endoglin and transforming growth factor-beta signaling components by cooperative interaction between sp1 and klf6: Their potential role in the response to vascular injury. Blood. 2002;100:4001–4010. doi: 10.1182/blood.V100.12.4001. [DOI] [PubMed] [Google Scholar]
- 45.Garrido-Martin EM, Blanco FJ, Roque M, Novensa L, Tarocchi M, Lang UE, Suzuki T, Friedman SL, Botella LM, Bernabeu C. Vascular injury triggers kruppel-like factor 6 mobilization and cooperation with specificity protein 1 to promote endothelial activation through upregulation of the activin receptor-like kinase 1 gene. Circulation research. 2013;112:113–127. doi: 10.1161/CIRCRESAHA.112.275586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das A, Fernandez-Zapico ME, Cao S, Yao J, Fiorucci S, Hebbel RP, Urrutia R, Shah VH. Disruption of an sp2/klf6 repression complex by shp is required for farnesoid x receptor-induced endothelial cell migration. The Journal of biological chemistry. 2006;281:39105–39113. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- 47.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE. Kruppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-kappab signaling pathway. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2981–2988. doi: 10.1161/ATVBAHA.112.300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patino WD, Kang JG, Matoba S, Mian OY, Gochuico BR, Hwang PM. Atherosclerotic plaque macrophage transcriptional regulators are expressed in blood and modulated by tristetraprolin. Circulation research. 2006;98:1282–1289. doi: 10.1161/01.RES.0000222284.48288.28. [DOI] [PubMed] [Google Scholar]
- 49.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 50.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature immunology. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 51.Samson S, Mundkur L, Kakkar VV. Immune response to lipoproteins in atherosclerosis. Cholesterol. 2012;2012:571846. doi: 10.1155/2012/571846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Current opinion in hematology. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 53.Alaiti MA, Orasanu G, Tugal D, Lu Y, Jain MK. Kruppel-like factors and vascular inflammation: Implications for atherosclerosis. Current atherosclerosis reports. 2012;14:438–449. doi: 10.1007/s11883-012-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein e. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. The Journal of clinical investigation. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lingrel JB, Pilcher-Roberts R, Basford JE, Manoharan P, Neumann J, Konaniah ES, Srinivasan R, Bogdanov VY, Hui DY. Myeloid-specific kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circulation research. 2012;110:1294–1302. doi: 10.1161/CIRCRESAHA.112.267310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The kruppel-like factor klf4 is a critical regulator of monocyte differentiation. The EMBO journal. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, McDevitt M, Civin CI. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. Journal of immunology. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czepluch FS, Kuschicke H, Dellas C, Riggert J, Hasenfuss G, Schafer K. Atheroprotective kruppel-like factor 4 is downregulated in monocyte subsets of patients with coronary artery disease. Thrombosis and haemostasis. 2013:110. doi: 10.1160/TH13-05-0367. [DOI] [PubMed] [Google Scholar]
- 61.Sharma N, Lu Y, Zhou G, Liao X, Kapil P, Anand P, Mahabeleshwar GH, Stamler JS, Jain MK. Myeloid kruppel-like factor 4 deficiency augments atherogenesis in apoe−/− mice--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2836–2838. doi: 10.1161/ATVBAHA.112.300471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley M, Montaner LJ. Activation and repression of interleukin-12 p40 transcription by erythroid kruppel-like factor in macrophages. The Journal of biological chemistry. 2004;279:18451–18456. doi: 10.1074/jbc.M400320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wara AK, Foo S, Croce K, Sun X, Icli B, Tesmenitsky Y, Esen F, Lee JS, Subramaniam M, Spelsberg TC, Lev EI, Leshem-Lev D, Pande RL, Creager MA, Rosenzweig A, Feinberg MW. Tgf-beta1 signaling and kruppel-like factor 10 regulate bone marrow-derived proangiogenic cell differentiation, function, and neovascularization. Blood. 2011;118:6450–6460. doi: 10.1182/blood-2011-06-363713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circulation research. 2012;110:889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckley AF, Kuo CT, Leiden JM. Transcription factor lklf is sufficient to program t cell quiescence via a c-myc-dependent pathway. Nature immunology. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Lingrel JB. Klf2 inhibits jurkat t leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21CIP1/WAF1waf1/cip1. Oncogene. 2004;23:8088–8096. doi: 10.1038/sj.onc.1207996. [DOI] [PubMed] [Google Scholar]
- 67.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and t-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 68.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor klf2 regulates the migration of naive t cells by restricting chemokine receptor expression patterns. Nature immunology. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 69.Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, Maganto-Garcia E, Pepper-Cunningham Z, Jarolim P, Aikawa M, Garcia-Cardena G, Lichtman AH. Statin-induced kruppel-like factor 2 expression in human and mouse t cells reduces inflammatory and pathogenic responses. The Journal of clinical investigation. 2010;120:1961–1970. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lebson L, Gocke A, Rosenzweig J, Alder J, Civin C, Calabresi PA, Whartenby KA. Cutting edge: The transcription factor kruppel-like factor 4 regulates the differentiation of th17 cells independently of rorgammat. Journal of immunology. 2010;185:7161–7164. doi: 10.4049/jimmunol.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An J, Golech S, Klaewsongkram J, Zhang Y, Subedi K, Huston GE, Wood WH, 3rd, Wersto RP, Becker KG, Swain SL, Weng N. Kruppel-like factor 4 (klf4) directly regulates proliferation in thymocyte development and il-17 expression during th17 differentiation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:3634–3645. doi: 10.1096/fj.11-186924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen S, Crother TR, Arditi M. Emerging role of il-17 in atherosclerosis. Journal of innate immunity. 2010;2:325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Z, Wara AK, Icli B, Sun X, Packard RR, Esen F, Stapleton CJ, Subramaniam M, Kretschmer K, Apostolou I, von Boehmer H, Hansson GK, Spelsberg TC, Libby P, Feinberg MW. Kruppel-like factor klf10 targets transforming growth factor-beta1 to regulate cd4(+)cd25(−) t cells and t regulatory cells. The Journal of biological chemistry. 2009;284:24914–24924. doi: 10.1074/jbc.M109.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song A, Chen YF, Thamatrakoln K, Storm TA, Krensky AM. Rflat-1: A new zinc finger transcription factor that activates rantes gene expression in t lymphocytes. Immunity. 1999;10:93–103. doi: 10.1016/s1074-7613(00)80010-2. [DOI] [PubMed] [Google Scholar]
- 75.Zhou M, McPherson L, Feng D, Song A, Dong C, Lyu SC, Zhou L, Shi X, Ahn YT, Wang D, Clayberger C, Krensky AM. Kruppel-like transcription factor 13 regulates t lymphocyte survival in vivo. Journal of immunology. 2007;178:5496–5504. doi: 10.4049/jimmunol.178.9.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirberg J, Gschwendner C, Dangy JP, Ruckerl F, Frommer F, Bachl J. Proviral integration of an abelson-murine leukemia virus deregulates bklf-expression in the hypermutating pre-b cell line 18–81. Molecular immunology. 2005;42:1235–1242. doi: 10.1016/j.molimm.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 77.Vu TT, Gatto D, Turner V, Funnell AP, Mak KS, Norton LJ, Kaplan W, Cowley MJ, Agenes F, Kirberg J, Brink R, Pearson RC, Crossley M. Impaired b cell development in the absence of kruppel-like factor 3. J Immunol. 2011;187:5032–5042. doi: 10.4049/jimmunol.1101450. [DOI] [PubMed] [Google Scholar]
- 78.Klaewsongkram J, Yang Y, Golech S, Katz J, Kaestner KH, Weng NP. Kruppel-like factor 4 regulates b cell number and activation-induced b cell proliferation. Journal of immunology. 2007;179:4679–4684. doi: 10.4049/jimmunol.179.7.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular research. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circulation research. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circulation research. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 83.Cherepanova OA, Pidkovka NA, Sarmento OF, Yoshida T, Gan Q, Adiguzel E, Bendeck MP, Berliner J, Leitinger N, Owens GK. Oxidized phospholipids induce type viii collagen expression and vascular smooth muscle cell migration. Circulation research. 2009;104:609–618. doi: 10.1161/CIRCRESAHA.108.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wassmann S, Wassmann K, Jung A, Velten M, Knuefermann P, Petoumenos V, Becher U, Werner C, Mueller C, Nickenig G. Induction of p53 by gklf is essential for inhibition of proliferation of vascular smooth muscle cells. Journal of molecular and cellular cardiology. 2007;43:301–307. doi: 10.1016/j.yjmcc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. The Journal of biological chemistry. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida T, Yamashita M, Horimai C, Hayashi M. Smooth muscle-selective inhibition of nuclear factor-kappab attenuates smooth muscle phenotypic switching and neointima formation following vascular injury. Journal of the American Heart Association. 2013;2:e000230. doi: 10.1161/JAHA.113.000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. Microrna-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circulation research. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. Microrna expression signature and antisense-mediated depletion reveal an essential role of microrna in vascular neointimal lesion formation. Circulation research. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 89.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, Teoh H, Marsden PA, Verma S. Microrna-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 90.Hoshino Y, Kurabayashi M, Kanda T, Hasegawa A, Sakamoto H, Okamoto E, Kowase K, Watanabe N, Manabe I, Suzuki T, Nakano A, Takase S, Wilcox JN, Nagai R. Regulated expression of the bteb2 transcription factor in vascular smooth muscle cells: Analysis of developmental and pathological expression profiles shows implications as a predictive factor for restenosis. Circulation. 2000;102:2528–2534. doi: 10.1161/01.cir.102.20.2528. [DOI] [PubMed] [Google Scholar]
- 91.Ogata T, Kurabayashi M, Hoshino Y, Ishikawa S, Takeyoshi I, Morishita Y, Nagai R. Inducible expression of bteb2, a member of the zinc-finger family of transcription factors, in cardiac allograft arteriosclerosis. Transplantation proceedings. 2000;32:2032–2033. doi: 10.1016/s0041-1345(00)01544-x. [DOI] [PubMed] [Google Scholar]
- 92.Bafford R, Sui XX, Wang G, Conte M. Angiotensin ii and tumor necrosis factor-alpha upregulate survivin and kruppel-like factor 5 in smooth muscle cells: Potential relevance to vein graft hyperplasia. Surgery. 2006;140:289–296. doi: 10.1016/j.surg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 93.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor klf5/bteb2 is a target for angiotensin ii signaling and an essential regulator of cardiovascular remodeling. Nature medicine. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 94.Aizawa K, Suzuki T, Kada N, Ishihara A, Kawai-Kowase K, Matsumura T, Sasaki K, Munemasa Y, Manabe I, Kurabayashi M, Collins T, Nagai R. Regulation of platelet-derived growth factor-a chain by kruppel-like factor 5: New pathway of cooperative activation with nuclear factor-kappab. The Journal of biological chemistry. 2004;279:70–76. doi: 10.1074/jbc.M306621200. [DOI] [PubMed] [Google Scholar]
- 95.Nagai R, Suzuki T, Aizawa K, Miyamoto S, Amaki T, Kawai-Kowase K, Sekiguchi KI, Kurabayashi M. Phenotypic modulation of vascular smooth muscle cells: Dissection of transcriptional regulatory mechanisms. Annals of the New York Academy of Sciences. 2001;947:56–66. doi: 10.1111/j.1749-6632.2001.tb03930.x. discussion 66–57. [DOI] [PubMed] [Google Scholar]
- 96.Lu Y, Haldar S, Croce K, Wang Y, Sakuma M, Morooka T, Wang B, Jeyaraj D, Gray SJ, Simon DI, Jain MK. Kruppel-like factor 15 regulates smooth muscle response to vascular injury--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1550–1552. doi: 10.1161/ATVBAHA.110.207050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Y, Zhang L, Liao X, Sangwung P, Prosdocimo DA, Zhou G, Votruba AR, Brian L, Han YJ, Gao H, Wang Y, Shimizu K, Weinert-Stein K, Khrestian M, Simon DI, Freedman NJ, Jain MK. Kruppel-like factor 15 is critical for vascular inflammation. The Journal of clinical investigation. 2013 doi: 10.1172/JCI68552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haldar SM, Lu Y, Jeyaraj D, Kawanami D, Cui Y, Eapen SJ, Hao C, Li Y, Doughman YQ, Watanabe M, Shimizu K, Kuivaniemi H, Sadoshima J, Margulies KB, Cappola TP, Jain MK. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Science translational medicine. 2010;2:26ra26. doi: 10.1126/scitranslmed.3000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA, Jr, Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (klf2) regulates endothelial thrombotic function. Circulation research. 2005;96:e48–57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 100.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: Final data for 2009. National Vital Statistics Reports. 2011;60:1–116. [PubMed] [Google Scholar]
- 101.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 102.Traka MH, Chambers KF, Lund EK, Goodlad RA, Johnson IT, Mithen RF. Involvement of klf4 in sulforaphane- and iberin-mediated induction of p21CIP1/WAF1(waf1/cip1) Nutrition and cancer. 2009;61:137–145. doi: 10.1080/01635580802348641. [DOI] [PubMed] [Google Scholar]
- 103.Cui X, Liu X, Feng H, Zhao S, Gao H. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5′-amp activated protein kinase/surtuin 1-krupple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biological & pharmaceutical bulletin. 2012;35:2192–2197. doi: 10.1248/bpb.b12-00598. [DOI] [PubMed] [Google Scholar]
- 104.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of sirt1 by resveratrol induces klf2 expression conferring an endothelial vasoprotective phenotype. Cardiovascular research. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of klf4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochemical and biophysical research communications. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camargo A, Ruano J, Fernandez JM, Parnell LD, Jimenez A, Santos-Gonzalez M, Marin C, Perez-Martinez P, Uceda M, Lopez-Miranda J, Perez-Jimenez F. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC genomics. 2010;11:253. doi: 10.1186/1471-2164-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Starke RM, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez F, Hasan DM, Rosenwasser RH, Owens GK, Koch WJ, Dumont AS. Cigarette smoke modulates vascular smooth muscle phenotype: Implications for carotid and cerebrovascular disease. PloS one. 2013;8:e71954. doi: 10.1371/journal.pone.0071954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 109.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the klf2 transcription factor. The Journal of biological chemistry. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Zhao B, Zhang Y, Tang Z, Shen Q, Zhang Y, Zhang W, Du J, Chien S, Wang N. Kruppel-like factor 4 is induced by rapamycin and mediates the anti-proliferative effect of rapamycin in rat carotid arteries after balloon injury. British journal of pharmacology. 2012;165:2378–2388. doi: 10.1111/j.1476-5381.2011.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nie XM, Su LX, Xu RX, Guo YL, Zhou YJ, Li JJ. Kruppel-like factor 2 might mediate the rapamycin-induced arterial thrombosis in vivo: Implications for stent thrombosis in patients. Chinese medical journal. 2013;126:2636–2640. [PubMed] [Google Scholar]
- 112.Kwon JS, Ahn Y, Jeong MH, Song SJ, Kim YS, Cho DR. Abciximab-krueppel-like factor 4-plasmid-titanium dioxide coated coronary stent for inhibition of neotintimal hyperplasia. Eur Heart J Suppl. 2013;34:179–180. [Google Scholar]
- 113.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through mirnas. Nature cell biology. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]