Abstract
Background
This study examined whether the densities of stem- and enteroendocrine cell progenitors are abnormal in the ileum of patients with irritable bowel syndrome (IBS), and whether any abnormalities in ileal enteroendocrine cells are correlated with abnormalities in stem cells and enteroendocrine cell progenitors.
Methods
One hundred and one IBS patients covering all IBS subtypes were recruited, and 39 non-IBS subjects were included as a control group. The patients and controls underwent standard colonoscopies, during which biopsy specimens were obtained from the ileum. The biopsy specimens were stained with hematoxylin-eosin and immunostained for Musashi-1 (Msi-1), neurogenin 3 (NEUROG3), chromogranin A (CgA), serotonin, peptide YY (PYY), oxyntomodulin (enteroglucagon), pancreatic polypeptide, and somatostatin. The immunoreactive cells were quantified by computerized image analysis.
Results
The densities of Msi-1, NEUROG3, CgA, and serotonin cells were reduced in all IBS patients and in patients with diarrhea-predominant IBS (IBS-D), mixed-diarrhea-and-constipation IBS (IBS-M), and constipation-predominant (IBS-C) relative to the control subjects. While the PYY cell density was increased in IBS-C relative to controls, it did not differ between control subjects and IBS-D and IBS-M patients. The densities of Msi-1 and NEUROG3 cells were strongly correlated with that of CgA cells.
Conclusions
The abnormalities in the ileal enteroendocrine cells appear to be caused by two mechanisms: (1) decreases in the clonogenic activity of the stem cells and in the endocrine-cell progenitors differentiating into enteroendocrine cells, and (2) switching on the expression of PYY and switching off the expression of certain other hormones in other types of the enteroendocrine cells.
Keywords: Enteroendocrine cells, IBS, Ileum, Immunohistochemistry, Mushasi-1, Neurogenin 3, PYY, Serotonin
Background
Irritable bowel syndrome (IBS) is a chronic gastrointestinal condition with a prevalence of 5–20% of the world adult population [1–9]. IBS is reportedly also present in in about 50% of patients with ulcerative colitis patients and Crohn’s disease in remission [10, 11]. Furthermore, 38% of patients with celiac disease suffer from IBS symptoms despite consuming a gluten-free diet [12].
The etiology of IBS is unclear, but certain factors appear to play a pivotal role in its pathophysiology, including genetic factors, intestinal bacterial flora, diet, and chronic low-grade intestinal inflammation [13, 14]. Abnormally low densities of endocrine cells in the stomach, duodenum, ileum, colon, and rectum have been reported in patients with IBS [15–29], which could explain the dysmotility, visceral hypersensitivity, and abnormal secretion seen in IBS patients [30]. These observations have prompted suggestions that the abnormalities in the gut endocrine cells play a significant role in the pathophysiology of IBS [13, 14].
Musashi-1 (Msi-1) is localized in gastrointestinal stem cells and their early progenitors, and neurogenin 3 (NEUROG3) is a marker for early intestinal endocrine-cell progenitors [31–33]. It has been reported recently that the densities of Msi-1 and NEUROG3 cells are reduced in the duodenum of patients with IBS, with these decreases being associated with reductions in duodenal endocrine cells, which could in turn be caused by decreases in stem cells and their proliferation progeny into endocrine cells [34].
The types and densities of endocrine cells differ between the distal and proximal parts of the small intestine, which is probably due to the quite different functions of these two sections of the gastrointestinal tract [35, 36]. Whereas the proximal small intestine contains serotonin, secretin, cholecystokinin, gastric inhibitory polypeptide (GIP), and somatostatin cells, the distal small intestine contains serotonin, peptide YY (PYY), pancreatic polypeptide (PP), oxyntomodulin (enteroglucagon), and somatostatin cells [35, 36].
This study aimed at examining whether the densities of stem- and endocrine-cell progenitors as manifested by Msi-1 and NEUROG3 are affected in the ileum of patients with IBS. Furthermore, the densities of ileal endocrine cells were measured with the aim of establishing their correlation with possible abnormalities in stem- and endocrine-cell progenitors.
Methods
Patients and controls
One hundred and one patients with IBS according to Rome III criteria [37, 38] were recruited at Stord Hospital, Stord, Norway. These patients comprised 80 females and 21 males with a mean age of 40 years (age range 18–65 years). Thirty-five of the patients had diarrhea-predominant IBS (IBS-D), 34 had mixed-diarrhea-and-constipation IBS (IBS-M), and 32 had constipation-predominant IBS (IBS-C). The IBS symptoms had been present in all of the patients for many years, and their onset was not identified as being associated with gastrointestinal infection. All of the patients underwent a physical examination and were investigated by blood tests to exclude inflammatory, endocrine, liver, kidney, and systemic diseases. Furthermore, celiac disease was excluded by performing histopathological examinations of duodenal biopsy samples obtained during gastroscopy.
A control group was included that comprised 39 non-IBS subjects (27 females and 12 males; mean age 38 years, age range 18–64 years) who had undergone colonoscopies because of (1) gastrointestinal bleeding that was found to be due to hemorrhoids (n = 24) or angiodysplasia (n = 3), or (2) health worries resulting from a relative being diagnosed with colon carcinoma (n = 12).
The study was performed in accordance with the Declaration of Helsinki and was approved by the Regional Committee for Medical and Health Research Ethics West, Bergen, Norway. All subjects gave both oral and written consents to participate.
Colonoscopy, histopathology, and immunohistochemistry
A standard colonoscopy was performed on both the patients and controls, with segmental biopsy specimens taken from the colon and rectum, and four biopsy samples taken from the ileum of each subject. The biopsy samples were fixed overnight in 4% buffered paraformaldehyde, embedded in paraffin, and cut into 5-μm-thick sections. The sections were stained with hematoxylin-eosin, and immunostained using the ultraView Universal DAB Detection Kit (version 1.02.0018, Venata Medical Systems, Basel, Switzerland) and the BenchMark Ultra IHC/ISH staining module (Venata Medical Systems). The sections were incubated with the primary antibodies for 30 min at 37 °C. Details of the primary antibodies used are given in Table 1.
Table 1.
Primary antibodies used for immunohistochemical staining
| Antibody target | Source | Code number | Working dilution | Type of antibody |
|---|---|---|---|---|
| Msi-1 | Novus Biologicals Europe (Abingdon, UK) | NB100–1759 | 1:100 | Polyclonal, raised in rabbit against residues 5–21 [APQPGLASPDSPHDPCK] of human, mouse, and rat Msi-1 |
| NEUROG3 | ThermoFisher Scientific (Oslo, Norway) | BT-B56180 | 1:50 | Polyclonal, raised in rabbit against KLH-conjugated synthetic peptide at 40–69 amino acids from the N-terminal region of human NEUROG3 |
| CgA | Dako (Glostrup, Denmark) | M869 | 1:1000 | Monoclonal, raised in mouse against the N-terminal of purified CgA |
| Serotonin | Dako | 5HT-209 | 1:1500 | Monoclonal, raised in mouse against serotonin |
| PYY | Alpha-Diagnostica (San Antonio, TX, USA) | PYY 11A | 1:1000 | Polyclonal, raised in rabbit against PYY |
| Oxyntomodulin (enteroglucagon) | Acris Antibodies (Herford, Germany) | BP508 | 1:800 | Polyclonal, raised in rabbit against porcine glicentin/glucagon |
| PP | Diagnostic Biosystems (Pleasanton, CA, USA) | #114 | 1:400 | Polyclonal, raised in rabbit against synthetic human PP |
| Somatostatin | Dako | A566 | 1:200 | Polyclonal, raised in rabbit against synthetic human somatostatin |
Computerized image analysis
Morphometric measurements were performed by applying imaging software (version 1.7, cellSens, Olympus) to images obtained with the aid of a computer linked to a microscope (type BX 43, Olympus) with a camera (DP 26, Olympus). The number of immunoreactive cells and the area of the epithelial cells were measured. The numbers of Msi-1, NEUROG3, and endocrine cells in each field were counted manually by pointing and clicking the computer mouse. The area of epithelial cells was determined by manually drawing an enclosed region using the computer mouse. A × 40 objective was used, which resulted in each frame (field) on the monitor representing a tissue area of 0.14 mm2. The density of endocrine cells was expressed as the number of endocrine cells per square millimeter of epithelium, the density of Msi-1 cells was expressed as the number of immunoreactive cells per crypt, and the density of NEUROG3 cells was expressed as the number of immunoreactive cells per field. Quantification was performed in ten randomly chosen microscopic fields. The measurements were made by the same person (M.E-S.) who was blind to the identities of the slides.
Statistical analysis
Differences in the sex and age distributions between patients and controls were tested using Fisher’s exact test and the Mann-Whitney nonparametric test, respectively. Differences between control subjects, all IBS patients (IBS-total), and IBS-D, IBS-M, and IBS-C patients were tested using the Kruskal-Wallis nonparametric test with Dunn’s posttest. The Kruskal-Wallis test is a nonparametric test that compares three or more unmatched groups, while Dunn’s multiple-comparisons test compares the difference in the sum of ranks between two columns with the expected average difference. Correlations were assessed using the Spearman nonparametric test. The data are presented as mean ± SEM values, and differences for which P < 0.05 were considered to be statistically significant.
Results
Sex and age characteristics of patients and controls
The sex and age distributions did not differ significantly between the patients and controls (P = 0.27 and P = 0.67, respectively).
Endoscopy, histopathology, and immunohistochemistry
The ileum, colon, and rectum of both the patients and control subjects were macroscopically normal. The findings of histopathological examinations of the ileum, colon, and rectum were also normal in both the patients and controls.
Cells that were immunoreactive for Msi-1, NEUROG3, chromogranin A (CgA), serotonin, PYY, PP, oxyntomodulin (enteroglucagon), and somatostatin were found in the ileum of all of the subjects (i.e., both patients and controls), mostly in the crypts. These cells were basket- or flask-shaped, and sometimes had a basal cytoplasmic process.
Computerized image analysis
The densities of Msi-1, NEUROG3, CgA, serotonin, and PYY cells are summarized in Table 2 and illustrated in Figs. 1, 2, 3, 4, 5 and 6. Few of the cells in the examined biopsy material were immunoreactive for PP, enteroglucagon, or somatostatin, which made it impossible to reliably quantify these types of cells.
Table 2.
Densities of immunoreactive cells in control subjects, IBS-total patients, and IBSsubtype patients
| Cell type | Controls | IBS-total | IBS-D | IBS-M | IBS-C |
|---|---|---|---|---|---|
| Msi-1 | 6.2 ± 0.2 | 4.8 ± 0.2*** | 4.3 ± 0.3*** | 4.8 ± 0.2** | 5.1 ± 0.2* |
| NEUROG3 | 16.8 ± 0.8 | 7.6 ± 0.3*** | 8.0 ± 0.6*** | 6.7 ± 0.5*** | 8.1 ± 0.7*** |
| CgA | 64.3 ± 3.6 | 28.8 ± 2.11*** | 29.2 ± 3.5*** | 25.7 ± 3.6*** | 30.3 ± 3.7*** |
| Serotonin | 40.8 ± 3.5 | 12.0 ± 1.3*** | 10.7 ± 2.0*** | 11.1 ± 2.0*** | 13.9 ± 2.5*** |
| PYY | 26.6 ± 1.6 | 33.5 ± 1.4* | 27.6 ± 1.4 | 33.5 ± 2.4 | 41.9 ± 2.3** |
*P < 0.05, **P < 0.01, ***P < 0.001 vs. controls (Dunn’s multiple-comparisons test)
Fig. 1.
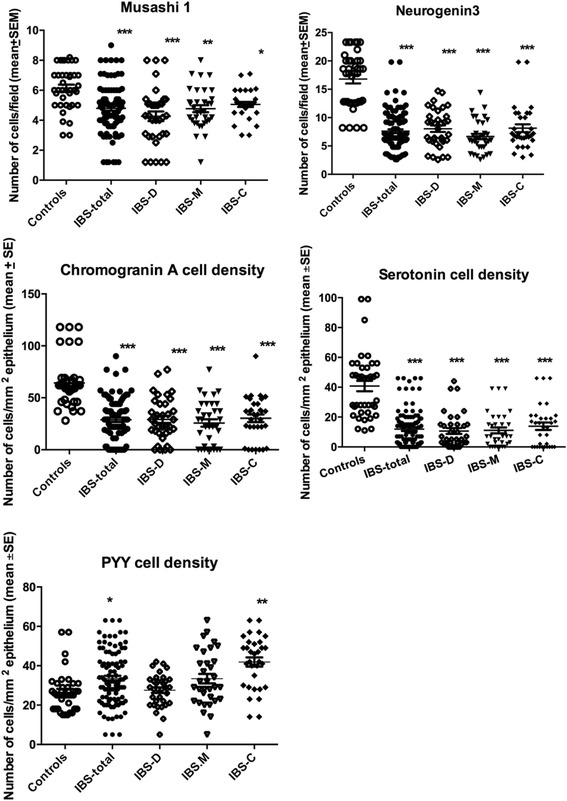
Densities of Msi-1, NEUROG3, CgA, serotonin, and PYY cells in the ileum of control subjects and IBS-total, IBS-D, IBS-M, and IBS-C patients. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. controls (Dunn’s multiple-comparisons test)
Fig. 2.
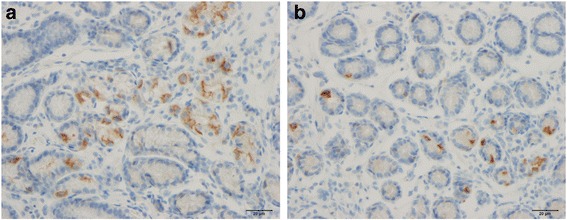
Ileal Msi-1 cells in a control subject (a) and an IBS patient (b)
Fig. 3.
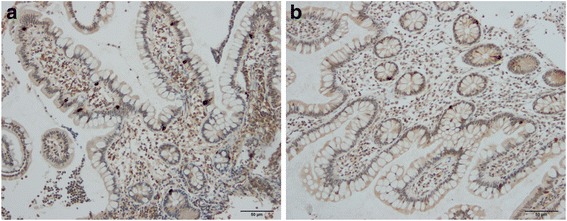
Ileal NEUROG3 cells in a control subject (a) and an IBS patient (b)
Fig. 4.
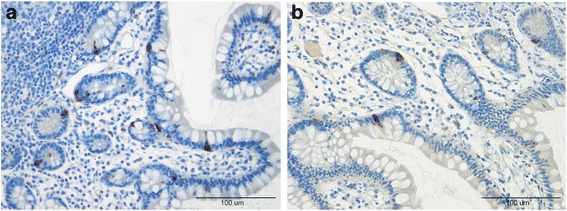
Ileal CgA cells in a control subject (a) and a patient with IBS (b)
Fig. 5.
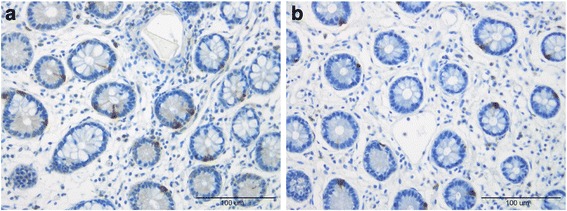
Ileal serotonin cells in a control subject (a) and a patient with IBS (b)
Fig. 6.
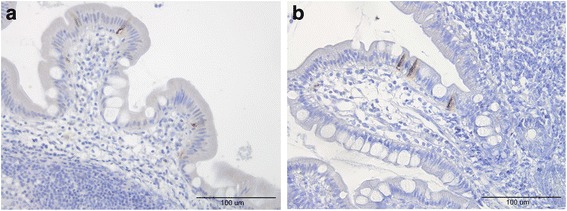
Ileal PYY cells in a control subject (a) and a patient with IBS-C (b)
The Msi-1 cell density was significantly lower in IBS-total, IBS-D, IBS-M, and IBS-C patients than in the control subjects (P < 0.001, P < 0.001, P < 0.01, and P < 0.05, respectively). Dunn’s posttest showed that the NEUROG3 cell density was lower in IBS-total, IBS-D, IBS-M, and IBS-C patients than in the controls (P < 0.001 for all).
The Kruskal-Wallis nonparametric test showed significant differences in the CgA and serotonin cell densities between control subjects, IBS-total patients, and IBS-subtype patients (P < 0.0001 for both cell types). The CgA and serotonin cell densities were lower in IBS-total, IBS-D, IBS-M, and IBS-C patients than in controls (P < 0.001 for both cell types). The Kruskal-Wallis test revealed significant differences in PYY cell density between controls and IBS-total, IBS-D, IBS-M, and IBS-C patients (P < 0.0001 for all). Dunn’s posttest showed that the PYY cell density was significantly higher in IBS-total and IBS-C patients than in the control subjects (P < 0.02 and P < 0.01, respectively), while it did not differ significantly between IBS-D and IBS-M patients and the control subjects (P = 0.2 and P = 0.5, respectively).
The densities of Msi-1 and NEUROG3 cells were strongly correlated with that of CgA cells (P < 0.0001 and r = 0.47, and P < 0.0001 and r = 0.40, respectively) (Fig. 7).
Fig. 7.
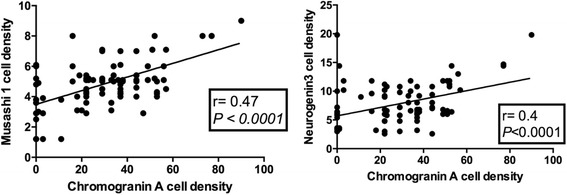
Correlations between the density of CgA cells and the densities of Msi-1 and NEUROG3 cells (Spearman nonparametric test)
Discussion
Each crypt in the intestine contains four to six stem cells. Stem cells either divide into 2 new stem cells (clonogeny) or differentiate into mucosal epithelial cells (differentiation progeny) [33, 39–52]. The stem cell differentiation comprises two lineages: secretory and absorptive. The secretory lineage ends to goblet, endocrine, and Paneth cells, and the absorptive lineage leads to absorptive enterocytes [33, 39–52]. Msi-1 occurs in both intestinal stem cells and early progenitors [33, 53–55], while NEUROG3 is localized in intestinal endocrine-cell progenitors [32, 56, 57]. In the present study the densities of both Msi-1 and NEUROG3 cells in the ileum were lower in patients with IBS than in controls, which is similar to previous findings in the proximal small intestine [34].
CgA occurs in all types of enteroendocrine cells [58–60], with CgA cells density reflecting the total density of the ileal endocrine cells. Similar to the situation in the proximal small intestine [34], the CgA cells density in the ileum was reduced in the present IBS patients, which is consistent with previous observations that we have made in another cohort of patients [61]. The reduction in CgA cell density observed herein was significantly correlated with the reductions in Msi-1 and NEUROG3 cells. A reduction in NEUROG3 was associated with a decrease in the number of intestinal endocrine cells in patients with congenital malabsorptive diarrhea [31], in patients with small-intestine allograft rejection [32], and in NEUROG3-knockout mice [62]. It is tempting to speculate that the decreased density of ileal endocrine cells observed in the present IBS patients is caused by the reductions in Msi-1 and NEUROG3 cells.
Serotonin cells are the main endocrine cell type in the ileum [36]. The decreased cell density of CgA cell density found here and in our previous cohort of IBS patients [29] may be caused by the decreases in the serotonin cell density. Serotonin stimulates small-, and large intestine motility, and activates the submucosal enteric nervous system that transports sensation from the gastrointestinal tract to the central nervous system [30].
While the density of ileal PYY cells was similar to the control subjects in the IBS-D and IBS-M patients, it was higher in IBS-C patients, which is consistent with previously results obtained in another cohort of IBS patients [29]. It is well known that enteroendocrine cell can express two hormones, such as glucagon-like peptide-1 and GIP in the small intestine, and PYY and oxyntomodulin in the distal small and large intestines [63–65]. It has been shown further that mature enteroendocrine cells are able of synthetize up to seven different hormones [66–68]. The increase in the density of PYY cells in IBS-C is probably caused by switching off the expression of other hormones and switching on the expression of PYY. PYY regulates the so-called ileal brake and increases the absorption of water and electrolytes [69–74]. It also inhibits vasoactive intestinal polypeptide and prostaglandin E2, both of which stimulate intestinal fluid secretion [75–77]. It is possible that the increase in the PYY cell density in IBS-C patients would slow the intestinal transit by strengthening the ileal brake, increasing the absorption of water, and decreasing the secretion of the intestinal fluid, and thereby also result in constipation.
It is difficult to conclude whether the changes in stem and enteroendocrine cells observed in here are primary or secondary to IBS. However, it was hypothesized recently that factors known to play a part in the pathophysiology of IBS—including genetics, diet, the intestinal microbiome, and low-grade inflammation—exert their effects by acting on stem cells and differentiation progeny toward enteroendocrine cells [78]. In other words, the changes found in the present study are believed to be caused by other factors engaged in the pathophysiology of IBS.
Conclusion
The present study confirms the finding of a previous study involving another cohort of IBS patients that the density of enteroendocrine cells is reduced in the ileum [36]. This reduction in the enteroendocrine cells seems to be caused by decreases in the clonogenic activity of the stem cell and in the differentiation into enteroendocrine cells from stem-cell progenitors. The changes in the proportions of the endocrine cell types appear to result from switching on and switching off the expressions of certain hormones by the enteroendocrine cells [66–68].
Acknowledgements
Not applicable.
Funding
This study was supported by grants from Helse-Vest, Norway (grant no. 911978) and Helse-Fonna (grant no. 40415).
Availability of data and materials
All data generated or analyzed during the study are included in this article. The subjects included in the study were assigned a code, and all data were kept in a secure database to maintain anonymity. Relevant raw data from this study can be provided upon reasonable request to the authors.
Abbreviations
- CgA
Chromogranin A
- GIP
Gastric inhibitory polypeptide
- IBS
Irritable bowel syndrome
- IBS-C
Constipation-predominant IBS
- IBS-D
Diarrhea-predominant IBS
- IBS-M
Mixed-diarrhea-and-constipation IBS
- Msi-1
Musashi-1
- NEUROG3
Neurogenin 3
- PP
Pancreatic polypeptide
- PYY
Peptide YY
Authors’ contributions
ME-S designed the study, performed the research, analyzed the results, and wrote the manuscript; OHG contributed to the study design and the analysis of the results, and commented on the manuscript. Both authors have approved the submitted version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Regional Committee for Medical and Health Research Ethics West, Bergen, Norway. All subjects gave both oral and written consents to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Magdy El-Salhy, Phone: +47-53491000, Email: magdy.el-salhy@helse-fonna.no, Email: magdy.elsalhy@helse-fonna.no.
Odd Helge Gilja, Email: odd.helge.gilja@helse-bergen.no.
References
- 1.Thompson WG: A world view of IBS In: Irritable bowel syndrome: diagnosis and treatment. edn. Edited by Camilleri M, Spiller RC. Philadelphia and London: Saunders; 2002;17–26.
- 2.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 3.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc.; 2012. [Google Scholar]
- 4.Ford AC, Vandvik PO. Irritable bowel syndrome. Clin Evid (Online). 2012;2012 [PMC free article] [PubMed]
- 5.Quigley EM, Locke GR, Mueller-Lissner S, Paulo LG, Tytgat GN, Helfrich I, Schaefer E. Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther. 2006;24:411–419. doi: 10.1111/j.1365-2036.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- 6.Vandvik PO, Lydersen S, Farup PG. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand J Gastroenterol. 2006;41:650–656. doi: 10.1080/00365520500442542. [DOI] [PubMed] [Google Scholar]
- 7.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 8.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, Ghatei MA, Bloom SR. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–2695. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 9.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 10.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;2:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Salhy M. Recent advances in the diagnosis of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2015;9:1161–1174. doi: 10.1586/17474124.2015.1067138. [DOI] [PubMed] [Google Scholar]
- 12.Sainsbury A, Sanders DS, Ford AC: Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin Gastroenterol Hepatol 2013;11:359–365 e351qqq. [DOI] [PubMed]
- 13.El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:7621–7636. doi: 10.3748/wjg.v21.i25.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–5163. doi: 10.3748/wjg.v18.i42.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Salhy M, Lillebo E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 16.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, Hausken T. Relative importance of abnormalities of CCK and 5-HT (serotonin) in giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–891. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 17.El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–3513. doi: 10.1007/s10620-010-1169-6. [DOI] [PubMed] [Google Scholar]
- 18.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pep. 2014;188:60–65. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.13.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection--an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 26.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin a as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. doi: 10.3109/00365521.2010.503965. [DOI] [PubMed] [Google Scholar]
- 28.El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin a cell density in the rectum of patients with irritable bowel syndrome. Mol Med Report. 2012;6:1223–1225. doi: 10.3892/mmr.2012.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Cortina G, Wu SV, Tran R, Cho JH, Tsai MJ, Bailey TJ, Jamrich M, Ament ME, Treem WR, et al. Mutant neurogenin-3 in congenital malabsorptive diarrhea. N Engl J Med. 2006;355:270–280. doi: 10.1056/NEJMoa054288. [DOI] [PubMed] [Google Scholar]
- 32.Fishbein TM, Novitskiy G, Lough DM, Matsumoto C, Kaufman SS, Shetty K, Zasloff M. Rejection reversibly alters enteroendocrine cell renewal in the transplanted small intestine. Am J Transplant. 2009;9:1620–1628. doi: 10.1111/j.1600-6143.2009.02681.x. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Salhy M, Hatlebakk JG, Hausken T. The reduction in duodenal endocrine cells in IBSis associated with stem cell abnormalities. World J Gastroenterol. 2015;21:9577–9587. doi: 10.3748/wjg.v21.i32.9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 36.El-Salhy M, Mazzawi T, Hausken T, Hatlebakk JG. Interaction between diet and gastrointestinal endocrine cells. Biomedical reports. 2016;4:651–656. doi: 10.3892/br.2016.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 38.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 40.Darlington GJ. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678–682. doi: 10.1016/S0955-0674(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 41.Fausto N, Campbell JS, Riehle KJ: Liver regeneration. Hepatology (Baltimore, Md). 2006;43:S45–53. [DOI] [PubMed]
- 42.Rawlins EL, Hogan BL. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 44.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 45.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 48.Fontaine J, Le Lievre C, Le Douarin NM. What is the developmental fate of the neural crest cells which migrate into the pancreas in the avian embryo? Gen Comp Endocrinol. 1977;33:394–404. doi: 10.1016/0016-6480(77)90055-7. [DOI] [PubMed] [Google Scholar]
- 49.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 50.Rawdon BB, Andrew A. Origin and differentiation of gut endocrine cells. Histol Histopathol. 1993;8:567–580. [PubMed] [Google Scholar]
- 51.Hoffman J, Kuhnert F, Davis CR, Kuo CJ: Wnts as essential growth factors for the adult small intestine and colon. Cell cycle (Georgetown, Tex) 2004;3:554–557. [PubMed]
- 52.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 53.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71(1):28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 54.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/S0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 55.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- 57.Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 59.Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58:95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- 60.Deftos LJ. Chromogranin a: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12:181–187. doi: 10.1210/edrv-12-2-181. [DOI] [PubMed] [Google Scholar]
- 61.El-Salhy M, Wendelbo I, Gundersen D. Reduced chromogranin a cell density in the ileum of patients with irritable bowel syndrome. Mol Med Report. 2013;7:1241–1244. doi: 10.3892/mmr.2013.1325. [DOI] [PubMed] [Google Scholar]
- 62.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spangeus A, Forsgren S, el-Salhy M. Does diabetic state affect co-localization of peptide YY and enteroglucagon in colonic endocrine cells? Histol Histopathol. 2000;15:37–41. doi: 10.14670/HH-15.37. [DOI] [PubMed] [Google Scholar]
- 64.Pyarokhil AH, Ishihara M, Sasaki M, Kitamura N. The developmental plasticity of colocalization pattern of peptide YY and glucagon-like peptide-1 in the endocrine cells of bovine rectum. Biomed Res. 2012;33:35–38. doi: 10.2220/biomedres.33.35. [DOI] [PubMed] [Google Scholar]
- 65.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi: 10.1016/S0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 66.Svendsen B, Holst JJ. Regulation of gut hormone secretion. Studies using isolated perfused intestines. Peptides. 2016;77:47–53. doi: 10.1016/j.peptides.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Torang S, Rehfeld JF, Poulsen SS, Holst JJ. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156:847–857. doi: 10.1210/en.2014-1710. [DOI] [PubMed] [Google Scholar]
- 68.Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maljaars PW, Keszthelyi D, Masclee AA. An ileal brake-through? Am J Clin Nutr. 2010;92:467–468. doi: 10.3945/ajcn.2010.30180. [DOI] [PubMed] [Google Scholar]
- 70.Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep. 2006;8:367–373. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 71.Lin HC, Zhao XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- 72.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-O. [DOI] [PubMed] [Google Scholar]
- 73.Spiller RC, Trotman IF, Adrian TE, Bloom SR, Misiewicz JJ, Silk DB. Further characterisation of the 'ileal brake' reflex in man--effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 1988;29:1042–1051. doi: 10.1136/gut.29.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spiller RC, Trotman IF, Higgins BE, Ghatei MA, Grimble GK, Lee YC, Bloom SR, Misiewicz JJ, Silk DB. The ileal brake--inhibition of jejunal motility after ileal fat perfusion in man. Gut. 1984;25:365–374. doi: 10.1136/gut.25.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goumain M, Voisin T, Lorinet AM, Ducroc R, Tsocas A, Roze C, Rouet-Benzineb P, Herzog H, Balasubramaniam A, Laburthe M. The peptide YY-preferring receptor mediating inhibition of small intestinal secretion is a peripheral Y(2) receptor: pharmacological evidence and molecular cloning. Mol Pharmacol. 2001;60:124–134. doi: 10.1124/mol.60.1.124. [DOI] [PubMed] [Google Scholar]
- 76.Souli A, Chariot J, Voisin T, Presset O, Tsocas A, Balasubramaniam A, Laburthe M, Roze C. Several receptors mediate the antisecretory effect of peptide YY, neuropeptide Y, and pancreatic polypeptide on VIP-induced fluid secretion in the rat jejunum in vivo. Peptides. 1997;18:551–557. doi: 10.1016/S0196-9781(97)00069-7. [DOI] [PubMed] [Google Scholar]
- 77.Whang EE, Hines OJ, Reeve JR, Jr, Grandt D, Moser JA, Bilchik AJ, Zinner MJ, McFadden DW, Ashley SW. Antisecretory mechanisms of peptide YY in rat distal colon. Dig Dis Sci. 1997;42:1121–1127. doi: 10.1023/A:1018869116284. [DOI] [PubMed] [Google Scholar]
- 78.El-Salhy M, Hausken T, Gilja OH, Hatlebakk JG. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2017;11:139–148. doi: 10.1080/17474124.2017.1269601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this article. The subjects included in the study were assigned a code, and all data were kept in a secure database to maintain anonymity. Relevant raw data from this study can be provided upon reasonable request to the authors.


