Abstract
In their classic 1976 paper, Page & Schroeder described the histopathologic events and the types of myeloid cells and lymphocytes involved in the initiation and progression of inflammatory periodontal disease. The staging of periodontal disease pathogenesis as ‘initial’, ‘early’, ‘established’ and ‘advanced’ lesions, productively guided subsequent research in the field and remains fundamentally valid. However, major advances regarding the cellular and molecular mechanisms underlying the induction, regulation, and effector functions of immune and inflammatory responses necessitate a reassessment of their work and its integration with emerging new concepts. We now know that each type of leukocyte is actually represented by functionally distinct subsets with different or even conflicting roles in immunity and inflammation. Unexpectedly, neutrophils, traditionally regarded as merely anti-microbial effectors in acute conditions and protagonists of the ‘initial’ lesion, are currently appreciated for their functional versatility and critical roles in chronic inflammation. Moreover, an entirely new field of study, osteoimmunology, has developed and shed light on the impact of immunoinflammatory events on the skeletal system. These developments and the molecular dissection of crosstalk interactions between innate and adaptive leukocytes, as well as between the immune system and local homeostatic mechanisms, offer a more nuanced understanding of the host response in periodontitis with profound implications for treatment. At the same time, deeper insights have generated new questions many of which remain unanswered. In this review, forty years after Page & Schroeder proposed their model, we summarize enduring and emerging advances in periodontal disease pathogenesis.
The plaque-induced forms of periodontal disease, gingivitis and periodontitis, are extremely prevalent chronic inflammatory conditions affecting distinct components of the periodontium (8). In gingivitis, the more benign of the two, the inflammatory process is limited to the gingival epithelium and connective tissue. In its most severe form, the clinical manifestations of gingivitis include breakdown of the epithelial and connective tissue attachment of the gingiva to the teeth and the formation of gingival pockets. In contrast, the hallmark of periodontitis is an immunoinflammatory infiltrate of the deeper compartments of the periodontium resulting in destruction of the tooth-supporting tissues (cementum, periodontal ligament and alveolar bone), tooth mobility and ultimately, tooth loss. Furthermore, moderate-to-severe periodontitis is associated with increased risk for certain systemic disorders (e.g., atherosclerosis and rheumatoid arthritis) (83).
In 1976, Roy Page & Hubert Schroeder published their classic paper ‘Pathogenesis of chronic inflammatory periodontal disease: A summary of the current work’ (213). Based upon evaluation of tissue obtained from both human and animal models of naturally occurring and experimentally-induced disease, the authors provided a histopathologic ‘roadmap’ of the events leading to clinically detectable gingivitis and periodontitis. In describing the host response to the accumulation of dental plaque, they defined four lesions. The initial, early and established lesions exemplified distinct stages of gingivitis, while the advanced lesion was equated with overt periodontitis. Limited by the available knowledge regarding the induction and regulation of inflammatory and immunologic reactions, the paper provided a description of the sequential cellular (stromal and infiltrating) and structural events progressing from periodontal health to disease. These included: (i) increasing complexity of the composition of the cellular infiltrate from one dominated by neutrophils (initial lesion) to one containing large numbers of macrophages and T cells (early lesion) and finally, a preponderance of plasma cells (established and advanced lesions); (ii) proliferation of basal cells and eventual apical migration of the junctional epithelium; (iii) cytopathologic changes in fibroblasts and increasing loss of collagen in the gingival connective tissue; and (iv) bone loss in the advanced lesion.
Although there was limited knowledge regarding the identities and roles of periodontitis-associated bacterial species at the time that Page & Schroeder co-authored their landmark paper, they were quick to realize that the bacteria were an essential but not a sufficient cause of periodontitis. They observed that the established lesion (moderate-to-severe gingivitis) did not necessarily progress to periodontitis but could remain stable indefinitely leading them to conclude that the host response to the bacterial challenge plays a key role in determining the extent and severity of disease (213).
Capitalizing on the concept that periodontitis results from host-microbe interactions, Page along with Kenneth Kornman and Maurizio Tonetti, published a manuscript in 1997 entitled ‘The host response to the microbial challenge in periodontitis: assembling the players’ (137). The authors utilized the analogy of putting on a theatrical production and described how the stage changes as the story line progresses from health through disease progression. They proposed four ‘scenes’, each of which included its histologic features, predominant cell types and listing of key molecular players. As with the original paper, these authors stressed that progression from one scene to the next was not an inevitable process. Rather, they suggested that bacteria and/or their products as well as intrinsic and extrinsic factors that modify an individual’s response to the bacterial challenge collectively determine progression (or not) from one scene to the next. The four scenes were: (i) Acute bacterial challenge phase (representing the response to the early colonizers of the acquired pellicle); (ii) Acute inflammatory phase (a mild inflammatory response representing a reactive host response to the bacterial challenge); (iii) Immune response phase (activation of numerous types of mononuclear cells that mediate and regulate the local and systemic immune response); and (iv) Regulation and resolution phase (representing the point at which a normal protective host response may deviate towards a destructive chronic immuno-inflammatory process). This interpretation further underscored the complexity of the interactions between the bacterial challenge, host response and modifying factors that contribute to the ultimate outcome, disease protection or progression. Recent microbiome and mechanistic studies revealed that (i) the periodontitis-associated microbiota is much more diverse and complex than traditionally thought and (ii) its role in disease involves polymicrobial synergistic and dysbiotic interactions with the host (4, 49, 52, 90, 143). Despite the emerging understanding that periodontitis represents dysbiosis rather than infection, the original concept that bacteria are necessary but not sufficient for periodontitis (thus requiring a susceptible host) is still valid.
Although much of what Page & Schroeder proposed in 1976 (213) has stood the test of time, advances that occurred in the fields of basic and periodontal immunology necessitate a reassessment of their work as well as its integration with emerging new concepts. Major advances were made regarding the cellular and molecular mechanisms underlying the induction, regulation, and effector functions of immune and inflammatory responses. Chief amongst these are major developments in our understanding of mechanisms of innate immunity and how they interface with acquired immune responses. We now recognize that innate immunity is not simply a series of physical, chemical and physiologic barriers but an extremely complex and highly regulated network of cells and molecules (proinflammatory and regulatory cytokines and chemokines, adhesion molecules and their ligands/counter-receptors) that function over and beyond an initial obstacle to bacterial challenge (66, 76, 84, 202). The various components of the innate response determine if and when an immunologic response is necessary and thus play both an inductive and regulatory role in these host responses. With respect to the adaptive immune system itself, specialized functional subsets of dendritic cells, macrophages, T cells and B cells have been identified that have revolutionized traditional concepts on the roles of antigen-presenting cells and lymphocytes as well as the mechanisms regulating the collective response (191, 203). Moreover, it is now appreciated that neutrophils, historically regarded as simply anti-microbial effector cells, exhibit significant functional versatility including regulation of other leukocytes (93, 241). During this time, it was also demonstrated that the junctional epithelium can initiate and modulate numerous aspects of plaque-induced gingival inflammation (22). At the same time, we came to appreciate that local tissues have a ‘regulatory say’ over the host inflammatory response through several mechanisms including local production of homeostatic molecules (84, 172, 262). In addition, an entirely new field of study, osteoimmunology, has developed as we have come to acknowledge the impact of immunoinflammatory events on the cells that mediate bone formation and resorption (194) (Fig. 1).
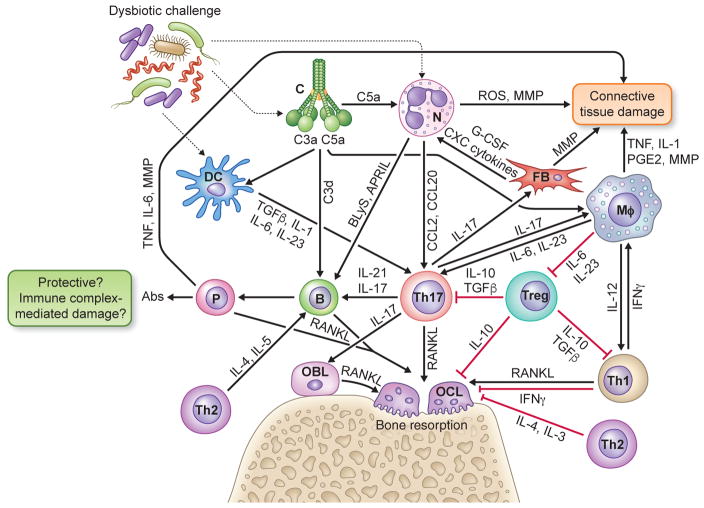
Fig. 1. Innate-adaptive immune interplay leading to inflammatory tissue damage and bone loss in periodontitis.
Periodontitis arises from complex interactions between the host and the subgingival dysbiotic microbiota that lead to excessive or dysregulated inflammatory responses involving elements of both innate (complement, phagocytes) and adaptive immunity (regulatory and effector lymphocytes). Shown is a simplified view of cytokine- and chemokine-mediated cross-talk interactions between innate and adaptive immune cells leading to destruction of connective tissue and bone in periodontitis. See text with figure-1 callouts for details. Abbreviations in the figure: Abs, antibodies; APRIL, a proliferation-inducing ligand; B, B cell; BLyS, B lymphocyte stimulator; C, complement; DC, dendritic cell; FB, fibroblast; G-CSF, granulocyte-colony stimulating factor; Mφ, macrophage; MMP, matrix metalloproteinases; IL, interleukin; IFN, interferon; N, neutrophil; OBL, osteoblast; OCL, osteoclast, P, plasma cell; RANKL, receptor activator of nuclear factor-κB ligand; ROS, reactive oxygen species; TGFβ, transforming growth factor-β; TNF, tumor necrosis factor; Treg, T regulatory cell; Th-1,-2,-17, T helper type -1,-2, -17 cells.
The goal of this review, therefore, is to update the current state of our knowledge regarding the role of the host response in periodontal disease pathogenesis by discussing these new concepts in the context of the lesions described by Page & Schroeder 40 years ago.
Neutrophils
Recent developments in neutrophil biology
Healthy human gingiva displays coordinated gradients of chemokines and adhesion molecules that are thought to provide chemotactic and haptotactic cues for the directed migration of neutrophils to the gingival crevice. The concentration of these molecules increases from the basal cells of the junctional epithelium toward its outer surface that forms the floor of the crevice and is therefore closer to the bacterial challenge (79, 276, 299). The coordinated and regulated recruitment of neutrophils is vital for periodontal tissue homeostasis. This notion is supported by clinical observations in individuals with neutrophil deficiencies affecting absolute cell numbers (chronic or cyclic neutropenia) or trafficking capacity (leukocyte adhesion deficiencies); these individuals display increased susceptibility to and severity of periodontitis (80, 187). However, neutrophils can also be involved in periodontal tissue destruction if their recruitment is not properly regulated or the microbial challenge in the periodontium cannot be controlled. According to the Page & Schroeder model, the periodontal lesion is initiated as acute inflammation characterized by increased numbers of neutrophils migrating into the gingival crevice through the junctional epithelium (213). However, besides hallmarking acute inflammation, neutrophils are now increasingly appreciated as important players in chronic inflammatory disorders, including rheumatoid arthritis, psoriasis, atherosclerosis, inflammatory bowel disease, diabetes, and cancer (62, 134, 173, 241, 279). Indeed, research in the past 10–15 years has shown that neutrophils can greatly extend their lifespan and exhibit remarkable functional versatility and hitherto unsuspected roles, including regulatory interactions with both innate and adaptive immune leukocytes (19, 182, 241). Rather than being rapidly exhausted at peripheral tissues, neutrophils are currently considered capable of migrating to lymph nodes where they can interact with dendritic cells to modulate antigen presentation, thereby participating in the regulation of adaptive immunity (167).
In addition to stored granule-derived antimicrobial molecules and enzymes, neutrophils are now acknowledged for their de novo biosynthetic capacity for chemokines and cytokines with proinflammatory, anti-inflammatory, or immunoregulatory properties (241). This previously underappreciated ability of neutrophils facilitates their networking with tissue resident cells and other leukocytes. For instance, by releasing the chemokines CCL2 and CCL20, neutrophils can induce the recruitment of interleukin-17–producing CD4-positive T helper (Th17) cells to sites of infection or inflammation (219). By secreting the cytokines B-lymphocyte stimulator and a proliferation-inducing ligand (APRIL), neutrophils can promote the survival, proliferation, and development of B cells into antibody-secreting plasma cells (105, 240) (Fig. 1). Activated neutrophils (e.g., from the synovial fluid of patients with active rheumatoid arthritis) were additionally shown to express membrane-bound receptor activator of nuclear factor-κB ligand (RANKL), a key osteoclastogenic cytokine (16, 194), and thereby able to induce osteoclastic bone resorption (31). Emerging evidence also suggests that the neutrophil population may not be as homogeneous as traditionally thought but, rather, it is composed of cells that display substantial functional plasticity to form subsets with specialized cytotoxic or regulatory functions (38, 61, 64, 118, 167, 223).
These recent concepts suggest that neutrophils could contribute to periodontitis not only by initiating the lesion but also by participating in its progression, e.g., by recruiting Th17 cells or promoting the accumulation of B and plasma cells in the established and advanced lesions. Moreover, since neutrophils dwell and become activated within the gingival connective tissue under severe inflammatory conditions (212), these cells have the opportunity to contribute to bone demineralization and matrix degradation by releasing matrix metalloproteinases, such as collagenase, and expressing RANKL (31, 103, 147). Intriguingly, in aggressive forms of periodontitis associated with neutrophil-related primary immunodeficiencies, the disease may not actually be the result of impaired neutrophil immune surveillance but may – alternatively or additionally – derive from disruption of homeostatic mechanisms that are normally performed by neutrophils (188). In other words, neutrophils can potentially contribute to periodontitis through both their presence and absence, suggesting that neutrophil homeostasis is key to periodontal health. Below we discuss in greater detail recent developments that have promoted our understanding on the fascinating roles of neutrophils in periodontal inflammation and bone loss.
Neutrophil persistence in periodontitis
In response to the tooth-associated biofilm, neutrophils exit the gingival plexus of the postcapillary venules and are chemotactically recruited to the gingival crevice via the junctional epithelium, which, under inflammatory conditions, is largely occupied by transiting neutrophils (48). Neutrophils are actually the predominant leukocyte type (≥95%) in the gingival crevice (48), where they form a ‘defense wall’ against the biofilm, ostensibly to block bacterial invasion into the underlying gingival connective tissue (236). Although neutrophils provide homeostatic immunity in clinically healthy gingiva, their defense mechanisms appear to be insufficient to control a dysbiotic microbial community despite their persistent recruitment and capacity to elicit immune and inflammatory responses (81, 236). This implies that periodontitis-associated bacteria can largely escape neutrophil-mediated killing in an inflammatory environment, that is, without causing generalized immunosuppression. In fact, the latter would not be in ‘their best interest’ since periodontal dysbiotic communities rely on inflammation to secure nutrients, such as degraded collagen and heme-containing compounds derived from inflammatory tissue breakdown (82).
A possible mechanism whereby periodontal bacteria can uncouple neutrophil-mediated clearance from neutrophil-facilitated inflammation was dissected using Porphyromonas gingivalis as a model organism. This unique bacterium is a keystone pathogen that can manipulate innate immunity in ways that promote the conversion of a symbiotic community into a dysbiotic one (83, 90). Porphyromonas gingivalis can co-activate complement C5a receptor-1 and Toll-like receptor-2 in human neutrophils resulting in signaling crosstalk that leads to the ubiquitylation and proteasomal degradation of the Toll-like receptor-2 adaptor myeloid differentiation primary response protein-88, thereby suppressing a host-protective antimicrobial response. Moreover, this C5a receptor-1–Toll-like receptor-2 crosstalk utilizes another Toll-like receptor-2 adaptor (the myeloid differentiation primary response protein-88-like adaptor protein) and activates phosphoinositide 3-kinase, which blocks phagocytosis through the inhibition of RhoA GTPase and actin polymerization, while at the same time stimulating the production of inflammatory cytokines (162). In mice, oral colonization of P. gingivalis leads to the emergence of a dysbiotic and inflammation-provoking microbiota in the periodontium, but pharmacological blockade of C5a receptor-1 or Toll-like receptor-2 leads to near elimination of P. gingivalis and reversal of inflammation (162).
The data from this model suggest a periodontal host-microbe interplay that perpetuates chronic inflammation and recruitment of neutrophils that are unable to control the dysbiotic challenge. Sufficient clinical evidence indicates that neutrophils mediate a substantial portion of periodontal tissue destruction (101, 147) and that their numbers correlate positively with the severity of the disease (144). Furthermore, owing to persistent inflammation, patients with chronic periodontitis have longer-lived neutrophils in the oral tissues compared with healthy individuals (140).
Supernumerary and dysregulated neutrophils in periodontitis
The extravasation of circulating neutrophils is tightly regulated and proceeds via the leukocyte adhesion cascade, a sequence of low- and high-affinity adhesive interactions between the neutrophils and the vascular endothelium (84, 222, 283). The first step involves transient rolling interactions between the neutrophils and the endothelium mediated by the binding of selectin ligands on neutrophils to P- or E-selectin on endothelial cells. This rolling-dependent slowing down of neutrophils facilitates transition to firm adhesion on the endothelium, which is followed by intraluminal crawling to identify appropriate sites for extravasation. Firm adhesion and crawling are largely mediated by activated β2-integrins (CD11/CD18 heterodimers) and endothelial counter-receptors, such as intercellular adhesion molecules-1 and -2 (179, 222, 283). Whereas the neutrophil recruitment cascade has long been established, only recently have endogenous mechanisms that downregulate the process been identified. These mechanisms can block distinct steps of the leukocyte adhesion cascade, such as (i) rolling, (ii) activation, and (iii) firm adhesion. The regulatory molecules that mediate these effects are locally expressed by the endothelium or by the recruited neutrophils upon their interaction with endothelial cells (84). For instance, neutrophil-derived pentraxin-3 blocks rolling by binding P-selectin on the endothelium and thus interfering with its interaction with P-selectin glycoprotein ligand-1 on neutrophils (46). The paired immunoglobulin-like type 2 receptor-α on neutrophils mediates immunoreceptor tyrosine-based inhibitory motif signaling, which inhibits β2-integrin activation in response to chemoattractants (286). Endothelial cell-derived developmental endothelial locus-1 is a homeostatic molecule that binds the β2-integrin lymphocyte function-associated antigen-1 (CD11a/CD18) and antagonizes its interaction with endothelial intercellular adhesion molecule-1, thereby inhibiting firm neutrophil adhesion to the endothelium (35). It is therefore feasible that the severity of periodontal inflammation and tissue breakdown is influenced by the relative abundance of these endogenous negative regulators of neutrophil recruitment.
In this regard, developmental endothelial locus-1–deficient mice develop spontaneous periodontitis characterized by excessive neutrophil infiltration and interleukin-17–mediated inflammatory bone loss (59). This observation suggests that normal developmental endothelial locus-1 expression is crucial for appropriate regulation of neutrophil recruitment and the local host inflammatory response. Interestingly, the expression of developmental endothelial locus-1 and interleukin-17 are inversely correlated in both human and murine periodontal tissue, with developmental endothelial locus-1 dominating in healthy gingiva and interleukin-17 in periodontitis-involved gingiva (59). Moreover, developmental endothelial locus-1 mRNA and protein expression declines in the gingiva of aged mice (18-months old or older), correlating with excessive neutrophil recruitment and interleukin-17–dependent inflammatory bone loss (59, 253). Accordingly, intragingival administration of recombinant developmental endothelial locus-1 in old mice inhibits neutrophil infiltration and bone loss (59). Similar results were obtained by treating non-human primates with recombinant human developmental endothelial locus-1 (254).
Although it is not currently known whether humans experience decline of developmental endothelial locus-1 expression in old age as seen in mice, recruited neutrophils in old individuals might be more likely to cause collateral tissue damage owing to age-related cell-intrinsic defects. In this regard, neutrophils isolated from elderly subjects (60 years of age or older) display unfocused or inaccurate chemotaxis during which they release excessive amounts of proteases (238). Mechanistically, this defect was linked to elevated constitutive phosphoinositide 3-kinase activity, as compared to neutrophils from young controls (238). In a related context, neutrophils from patients with chronic periodontitis have decreased chemotactic accuracy as compared to healthy controls (231). Therefore, the recruitment of increased numbers of neutrophils with imprecise chemotaxis could exacerbate neutrophil-mediated bystander tissue damage.
In addition to elevated numbers, neutrophil hyperactivity or hyper-reactivity can also enhance bystander tissue damage through the release of inflammatory and cytotoxic mediators and enzymes (32, 40, 236, 257). For instance, peripheral blood neutrophils from chronic periodontitis patients release higher levels of pro-inflammatory cytokines (e.g., tumor necrosis factor, interleukin-1β, and interleukin-8) than healthy controls in response to several stimuli. Interestingly, this hyper-inflammatory phenotype persisted even following successful periodontal therapy (155). Therefore, although of uncertain genetic or other mechanistic basis, this hyper-reactivity appears to be spontaneous rather than secondary to local periodontal inflammation. However, long-lasting epigenetic effects on the phenotype cannot be ruled out. Peripheral blood neutrophils from chronic periodontitis patients also show higher release of reactive oxygen species than those from healthy individuals, even in the absence of exogenous stimulation. Similar to the hyper-inflammatory phenotype, this reactive oxygen species-related hyperactivity was not corrected by successful periodontal treatment (171).
Neutrophil-dependent regulatory functions
A recent study has identified a subset of suppressor neutrophils at sites of gram-negative bacteria-induced inflammation, such as the periodontal pockets of patients with chronic periodontitis, but not at sites of aseptic inflammation (e.g., the cerebrospinal fluid of patients with neuromyelitis optica) (149). Specifically, these investigators showed that human neutrophils produce interleukin-10 following direct contact with lipopolysaccharide-stimulated regulatory T cells (Tregs). This interaction is mediated by the β2-integrin Mac-1 (CD11b/CD18) on neutrophils and intercellular adhesion molecules-1 on Tregs (149). The ability of human neutrophils to express interleukin-10 was unexpected given that an earlier study showed that the IL10 genomic locus in human neutrophils stimulated with lipopolysaccharide and other pro-inflammatory stimuli remains in an inactive state, in stark contrast to autologous monocytes that can readily be induced to express interleukin-10 (269). These authors, nevertheless, did not preclude as-yet-undefined conditions under which the human interleukin-10 genomic locus could undergo major reorganization rendering it permissive to activation (269). In this regard, lipopolysaccharide-stimulated Tregs appear to induce chromatin modifications (H3K4me3, H3AcLys4) specific for transcriptional activation of the interleukin-10 gene in human neutrophils (149). The biological significance of the interleukin-10–producing neutrophils detected in periodontal pockets is currently uncertain. One possibility is that gram-negative bacteria evade host immunity by programming Tregs to suppress immune and antimicrobial responses of neutrophils. Alternatively, interleukin-10–producing neutrophils may represent a subset involved in the mitigation of destructive inflammation and/or in the promotion of the resolution of inflammation.
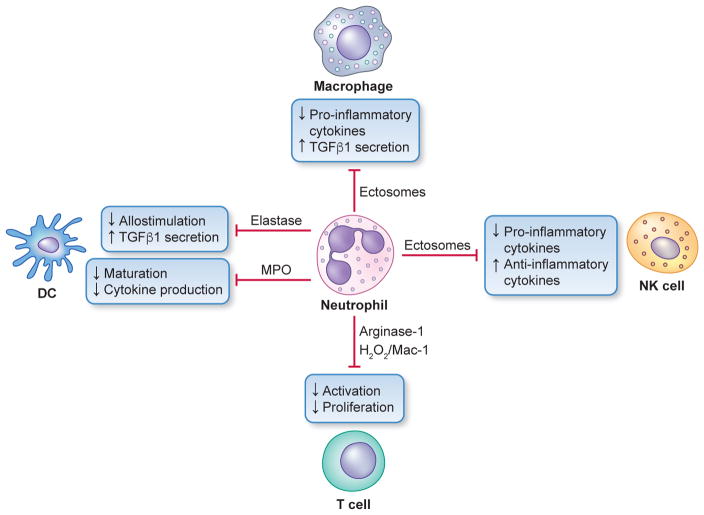
Additional mechanisms have been described whereby neutrophils can downregulate the host immune response. Indeed, neutrophils can directly suppress T cell responses by releasing arginase-1 which depletes arginine (required for T cell activation) (189, 235) or indirectly by releasing myeloperoxidase or elastase, which inhibit the antigen-presenting function of dendritic cells (163, 204) (Fig. 2). Ectosomes (extracellular vesicles with immunosuppressive properties) released by human neutrophils were shown to inhibit activation and promote an anti-inflammatory phenotype in several innate immune cells, including macrophages and natural killer cells (69, 237, 241) (Fig. 2), which are thought to contribute to destructive periodontal inflammation (290). Moreover, it was recently shown that a subset of human mature neutrophils (CD16bright/CD62Ldim) inhibits T cell activation by delivering H2O2 into the immunological synapse in a Mac-1–dependent manner (223) (Fig. 2). It is conceivable, therefore, that the presence of neutrophils may be required for restraining excessive and potentially harmful T cell activation in periodontitis.
Fig. 2. Regulatory neutrophil cross-talk with other leukocyte types.
Neutrophils can potentially suppress T cell activation by releasing arginase-1 (depletes arginine required for T cell activation) or by delivering H2O2 into the immunological synapse in a Mac-1 integrin–dependent manner. Neutrophils can also indirectly suppress T cell activation through myeloperoxidase- or elastase-dependent mechanisms that inhibit dendritic cell (DC) function. Furthermore, neutrophils can release ectosomes that can down-regulate the inflammatory activity of innate immune cells, such as macrophages and natural killer (NK) cells.
Other recent developments do indicate a regulatory function for neutrophils in human periodontal disease, and specifically in the context of an aggressive form of the disease associated with a primary immunodeficiency, leukocyte adhesion deficiency Type I–associated periodontitis. The extent and severity of attachment loss observed in subjects affected by this condition constitutes unequivocal evidence that neutrophils are required for the maintenance of periodontal health (45, 96, 187, 285). Leukocyte adhesion deficiency Type I is an autosomal recessive immunodeficiency owing to mutations in the ITGB2 gene that encodes for the common subunit CD18 of β2-integrins (96, 249). This deficiency in β2-integrins impedes normal neutrophil adhesion and extravasation to peripheral tissues including the periodontium (96, 249). Consequently, neutrophils are absent or rarely found in extravascular sites in leukocyte adhesion deficiency Type I patients, who exhibit neutrophilia (elevated neutrophil counts in blood), suffer from frequent infections at mucosal or skin surfaces, and develop severe periodontitis in childhood (45, 96, 187, 285). Given the established antimicrobial activities of neutrophils and the observation that the periodontal tissue of leukocyte adhesion deficiency Type I patients is specifically devoid of neutrophils, this form of periodontitis has been historically attributed to defective neutrophil surveillance of the periodontal bacterial infection (44, 45, 129, 145, 201, 258, 285). A recent mechanistic study that examined periodontitis in leukocyte adhesion deficiency Type I patients and relevant mouse models has linked impaired neutrophil transmigration to dysregulated overexpression of predominantly T-cell-derived interleukin-17 (187), a pro-inflammatory and bone-resorptive cytokine (298) (Fig. 3). Local antibody-mediated neutralization of interleukin-17 in lymphocyte function-associated antigen-1–deficient mice, which mimic the human leukocyte adhesion deficiency Type I phenotype, was shown to diminish periodontal inflammation and bone loss and, moreover, reduce the microbial burden (187). These findings are consistent with disruption of the ‘neutrostat’, a homeostatic mechanism that senses neutrophil recruitment and clearance in tissues and regulates neutrophil production through a negative-feedback loop involving a granulopoietic cascade of cytokines, specifically the interleukin-23–interleukin-17–granulocyte-colony stimulating factor axis (260). When neutrophils cannot transmigrate to peripheral tissues, as in leukocyte adhesion deficiency Type I, this regulatory circuit breaks down leading to unrestrained expression of interleukin-23 and the downstream cytokines interleukin-17 and granulocyte-colony stimulating factor. Whereas the overproduction of granulocyte-colony stimulating factor explains the increased granulopoiesis and blood neutrophilia in leukocyte adhesion deficiency Type I patients, the local overproduction of interleukin-17 causes inflammation that leads to bone loss and bacterial outgrowth (187).
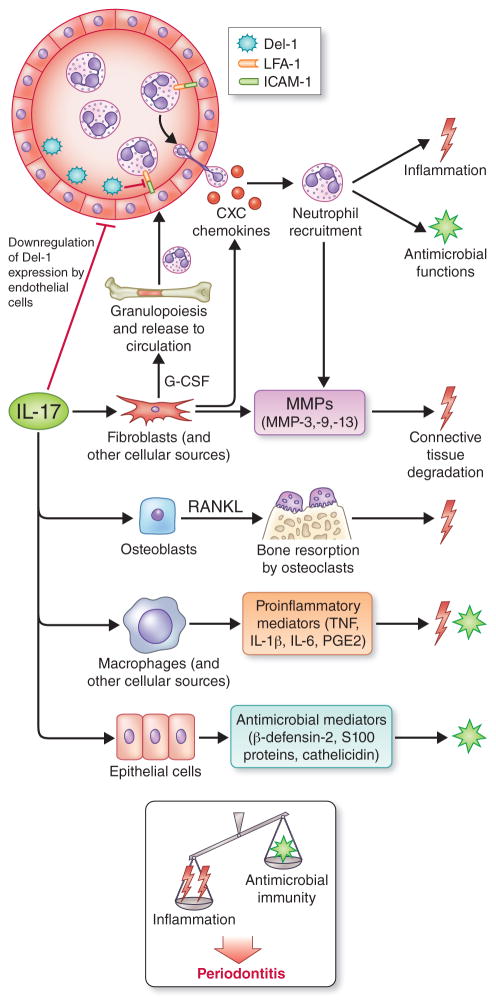
Fig. 3. Biological functions of interleukin-17 and their potential impact on periodontitis.
Interleukin-17 acts predominantly on innate immune and stromal cells (e.g., fibroblasts, endothelial and epithelial cells) to promote innate immunity, especially neutrophil-mediated antimicrobial and inflammatory responses. In this regard, interleukin-17 promotes granulopoiesis and neutrophil recruitment by up-regulating granulocyte-colony stimulating factor and CXC chemokines and by down-regulating developmental endothelial locus-1, an endogenous inhibitor of neutrophil adhesion and extravasation. Interleukin-17 can moreover induce the production of epithelial cell-derived antimicrobial molecules. On the other hand, interleukin-17 can contribute to the destruction of both connective tissue and the underlying bone by stimulating the production of matrix metalloproteinases and RANKL from the indicated stromal cell types. Interleukin-17 is thus an immunological double-edged sword with both protective and destructive functions. As indicated, the current burden of evidence from human and animal model studies suggests that the net effect of interleukin-17 signaling promotes periodontal disease development. Abbreviations in the figure: developmental endothelial locus-1; developmental endothelial locus-1; G-CSF, granulocyte-colony stimulating factor; MMPs; matrix metalloproteinases; RANKL, receptor activator of nuclear factor-κB ligand. From Zenobia and Hajishengallis [ref. (298)]; used by permission.
In this respect, it should be noted that inflammation can promote the growth of ‘inflammophilic’ bacteria that thrive on inflammatory tissue breakdown products (82). The finding that microbial outgrowth in leukocyte adhesion deficiency Type I periodontitis can be controlled by inhibiting inflammation, despite the absence of the presumed protective effects of neutrophils, questions earlier assumptions that the absence of neutrophils promotes disease due to defective surveillance of the periodontal microbiota. This concept also offers a new insight into the long-standing puzzle of why individuals with chronic granulomatous disease, who are susceptible to infections elsewhere in the body, do not exhibit increased susceptibility to periodontitis relative to the general population (201, 257). Although the neutrophils in patients with chronic granulomatous disease have defective oxygen-dependent killing (due to mutations in the genes encoding for components of the nicotinamide adenine dinucleotide oxidase), their recruitment to peripheral tissues such as the gingiva is intact. Therefore, normal neutrophil recruitment may be more critical for the homeostasis of the periodontium than is the ability of neutrophils to kill bacteria, a function that could be compensated for by other innate immune cells, such as macrophages. The notion that leukocyte adhesion deficiency Type I periodontitis does not represent an uncontrolled infection is also consistent with the fact that this form of periodontitis is not responsive to antibiotics and/or mechanical removal of the bacterial biofilm (44, 45, 187). This does not imply that the bacteria do not participate in the pathogenesis of leukocyte adhesion deficiency Type I periodontitis. In fact, the tooth-associated microbial community serves as the local stimulus to unleash the already dysregulated interleukin-23–interleukin-17 axis through translocation of released bacterial lipopolysaccharide into the underlying gingival connective tissue (92, 185).
As discussed above, developmental endothelial locus-1–deficient mice display excessive neutrophil recruitment leading to inflammatory bone loss (59). Therefore, with respect to neutrophil infiltration in the periodontium, lymphocyte function-associated antigen-1–deficient mice and developmental endothelial locus-1-deficient mice display opposite phenotypes (59, 91, 187). However, both lymphocyte function-associated antigen-1–deficient mice and developmental endothelial locus-1–deficient mice develop spontaneous periodontitis, whereas wild-type littermate controls or even their corresponding doubly deficient mice maintain a healthy periodontium (59, 187). These findings suggest that periodontal tissue homeostasis requires the presence of ‘normal’ numbers of neutrophils; neither ‘too few’ nor ‘too many’.
Neutrophil subsets in the periodontium
Mounting evidence suggests the existence of distinct neutrophil subsets with diverse roles in infection, inflammation and cancer pathogenesis. In addition to subsets of neutrophils that perform regulatory functions as discussed above (e.g., the CD16bright/CD62Ldim subset that inhibits T cell activation (223)), there is precedent for the polarization of tumor-associated neutrophils to phenotypes with antitumor or protumorigenic activities (64). It is thought that tumor-associated neutrophils can be polarized towards a transforming growth factor-β-dependent protumoral ‘N1’ or antitumoral ‘N2’ state that mirror M1 and M2 macrophages, respectively (64, 167). The presence of tumor-associated neutrophils with proangiogenic and protumoral functions (N2) was also shown by an independent study (110). With regards to infection control, an investigation conducted in mice has demonstrated three distinct neutrophil subsets that differ in their Toll-like receptor, integrin, and cytokine expression pattern and exhibit dissimilar susceptibilities to methicillin-resistant Staphylococcus aureus (280). More recently, it was shown that human oral neutrophils associated with mucosal health have a different phenotype from those derived from inflamed mucosae (61). Specifically, in addition to resting/naive circulatory neutrophils, the authors demonstrated the presence of distinct neutrophil subsets in the oral cavity: intermediately activated para-inflammatory neutrophils in healthy oral tissues and pro-inflammatory neutrophils associated with pathologic inflammation, as in chronic periodontitis. Para-inflammation is a stress response that displays some but not all of the characteristics of inflammation; in other words, it is an intermediate state between the basal (homeostatic) and inflammatory states (37). Pro-inflammatory neutrophils could be distinguished from para-inflammatory cells by their elevated degranulation, phagocytosis, reactive oxygen species production, formation of neutrophil extracellular traps, and display of a characteristic pattern of cell surface activation markers (61).
At present, it is uncertain whether the above-discussed neutrophil subsets are derived from separate lineages of neutrophils, develop from a single neutrophil precursor with considerable plasticity or, represent different stages of neutrophil development/maturation. Regarding plasticity, it is possible that neutrophils adaptively change their phenotypes in the course of their response to stressors, such as tissue injury, inflammation, and infection. For instance, even in clinically healthy periodontium, the constant presence of a tooth-associated biofilm would necessitate the presence of para-inflammatory neutrophils. Such cells could ostensibly handle the relatively mild microbial challenge without having to become fully activated and risking the integrity of the host tissue.
Macrophages
Macrophages in periodontitis: clinical and mechanistic evidence
Macrophages are relatively few in healthy gingiva but their numbers are increased in gingival tissue biopsies from patients with gingivitis or chronic periodontitis (137). By responding to a variety of microbial conserved structures (e.g., bacterial lipopolysaccharide, lipoproteins, and nucleic acids that are abundantly present in the periodontium (126)), macrophages constitute an important source of pro-inflammatory and potentially tissue-destructive molecules (interleukin-1, tumor necrosis factor, matrix metalloproteinases, and prostaglandin E2) that are elevated in the gingival tissue and gingival crevicular fluid of chronic periodontitis patients (11, 76, 137) (Fig. 1). Therefore, macrophages have been assumed by Page & Schroeder (213, 214) to participate in the pathogenesis of periodontitis, a notion that is supported – albeit indirectly – by clinical evidence. For instance, in a study that examined healthy controls, patients with gingivitis, and patients with moderate or severe periodontitis, CD68-positive macrophages were positively correlated with collagen breakdown and the severity of periodontal disease (250, 295). Moreover, an independent study showed that the numbers of macrophages were significantly increased in sites associated with disease progression (≥ 2mm change in probing attachment levels) as compared to contralateral sites that remained stable (297). More recently, CD68-positive macrophages were detected in abundance in deep periodontal lesions where they express interleukin-23, a cytokine that supports the proliferation of Th17 cells, which are also abundantly present in the lesions along with CD20-positive B cells (5).
In line with the clinical studies, P. gingivalis-induced periodontitis in mice is associated with recruitment of monocytes/macrophages to the gingival tissue, whereas clodronate liposome-mediated depletion of monocytes/macrophages results in decreased inflammatory bone loss (142, 261). Interestingly, macrophage depletion also leads to significant reduction in the levels of P. gingivalis colonization of the periodontal tissue (142). The association of macrophages with elevated periodontal inflammation and P. gingivalis colonization is consistent with findings that P. gingivalis manipulates Toll-like receptor-2 signaling in macrophages to escape clearance while promoting a nutritionally favorable inflammatory response (82, 151, 287). In this regard, moreover, macrophage-specific Toll-like receptor-2 signaling was linked to inflammatory periodontal bone loss (215). Specifically, adoptive transfer of Toll-like receptor-2-expressing macrophages to Toll-like receptor-2-deficient mice enabled P. gingivalis to cause bone loss in the recipient Toll-like receptor-2-deficient mice (215), which are normally resistant to P. gingivalis-induced periodontitis (23). Experiments in the subcutaneous chamber model (89) have suggested additional mechanisms whereby P. gingivalis may benefit from the presence of macrophages, and explicitly from their monocytic precursors. Specifically, it was found that the presence of Ly6Clow/CCR2low/CX3CR1high (but not of Ly6Chigh/CCR2high/CX3CR1low) monocytes was associated with reduced neutrophil-mediated phagocytosis of P. gingivalis in the chamber environment (261). Consistent with this observation, the absence of the CX3CR1high subset in the gingiva of CX3CR1-deficient mice was associated with significantly decreased P. gingivalis-induced dysbiosis and bone loss as compared to the same parameters in wild-type mice that displayed normal recruitment of CX3CR1high monocytes (261).
Macrophage subsets
Mature macrophages display heterogeneity and functional versatility (‘plasticity’) although the role of specific macrophage subsets in periodontitis is largely unexplored. Macrophages can alter their functional activities in response to local microenvironmental factors, and this plasticity allows them to function appropriately in distinct conditions (255, 263). In this context, macrophages can undergo M1 (classical) or M2 (alternative) activation (138, 177, 255). M1 macrophages can be induced by microbial agents (e.g., lipopolysaccharide) or type 1 cytokines (e.g., interferon-γ). Relative to M2 macrophages, cells in the M1 subset display an increased ability to phagocytose microbes, enhanced expression of proinflammatory cytokines, costimulatory molecules (e.g., CD86) and antimicrobial molecules (e.g., reactive oxygen species and nitric oxide) but a decreased capacity to phagocytose apoptotic cells (efferocytosis). M1 macrophages are therefore important for protection against microbial pathogens and are early players in the course of infection or inflammation. On the other hand, M2 macrophages can be induced by diverse agonists including type 2 cytokines (interleukin-4 and interleukin-13) and secrete high levels of interleukin-10 and transforming growth factor-β1 relative to M1 cells. Thus, they have immunoregulatory properties and promote cell proliferation and tissue regeneration. In fact, the M1 and M2 subsets represent extremes of a continuum of different activation states (184, 190, 255). Consequently, the M1/M2 categorization, though conceptually useful, oversimplifies and detracts from the important roles that macrophages play in an ever-changing environment, especially in mucosal tissues, such as the periodontium, that are constantly under microbial challenge (107, 124, 184). It has also been argued that the M1/M2 classification has largely resulted from in vitro experiments, whereas in vivo macrophage phenotypes at sites of inflammation may not be as clearly defined (24). For instance, a subset of macrophages associated with resolution of inflammation (‘resolving macrophages’) in models of acute inflammation, such as chemical peritonitis, share properties of both M1 and M2. This macrophage subset expresses the M1-associated molecules inducible nitric oxide synthase and cyclooxygenase-2 while at the same time expressing mannose receptor (CD206), arginase-1, transforming growth factor-β and high levels of interleukin-10, all of which are associated with M2 macrophages (24, 259). Moreover, the phenotype of macrophages involved in inflammation resolution may be tailored to both the characteristics of the tissue microenvironment and the particular inflammatory and microbial stimuli encountered (24, 248).
In the above-discussed P. gingivalis-induced periodontitis model, M1 macrophages (defined as CD86-positive) were shown to predominate over M2 macrophages (defined as CD206-positive), suggesting that the M1 macrophages likely represent a periodontitis-associated subset (142). Currently there are no clinical studies on the association of different macrophage subsets with periodontal disease. However, evaluation of gingival gene expression profiles as indicators of macrophage variation in non-human primates showed that M1 macrophage gene transcription patterns increased significantly with aging and with development of periodontitis, whereas changes in M2 gene profiles were relatively modest (75). Moreover, a recent study utilizing another murine model of periodontitis (induced by placing ligatures impregnated with P. gingivalis) showed that periodontal inflammation is associated with increased presence of both M1- and M2-like macrophage phenotypes (relative to control mice) (296). Nonetheless, because the ratio of inducible nitric oxide synthase-expressing (M1-like) to CD206-positive (M2-like) macrophages was significantly greater in the experimental periodontitis group as compared to the control group, the authors suggested that a phenotypic switch to M1 might contribute to disease in this model.
Although macrophages have been investigated in the context of homeostatic immunity (induction of an immune response with timely resolution of inflammation and restoration of tissue integrity) and as effectors of resolution in various inflammatory diseases and relevant models (191, 259), they have received little attention yet in the periodontal research field. The ability of the host to resolve the inflammatory response (to infection or injury) in a timely manner is critical since non-resolving inflammation underlies the pathogenesis and is the common denominator of many chronic diseases, including periodontitis (251). The successful resolution of inflammation involves a distinct series of processes, including down-regulation of proinflammatory mediators, up-regulation of anti-inflammatory and pro-resolving mediators, termination of neutrophil recruitment, clearance of apoptotic neutrophils by tissue phagocytes (efferocytosis), and tissue repair (267). Macrophages play a central role in the resolution of inflammation. In this regard, efficient efferocytosis of apoptotic cells by macrophages not only prevents secondary necrosis and inflammation but also reprograms the transcriptional profile of the efferocytic macrophage (termed resolving macrophage). Specifically, upon efferocytosis, macrophages are re-programmed to reduce the expression of proinflammatory cytokines and increase the expression of regulatory cytokines, including transforming growth factor-β and interleukin-10 (210, 227, 266). Macrophage plasticity is therefore crucial for successful resolution of inflammation and merits intensive investigation in both human periodontitis and animal models of the disease. Further research may reveal novel protective roles by resolving or other as-yet uncharacterized subsets of macrophages in periodontitis.
Osteoimmunology: crosstalk between the immune and bone systems
Osteoimmunology is a relatively new field of study that focuses on the molecular understanding of the crosstalk between the immune and the bone systems (194). This field has provided important insights into how inflammatory events regulate osteoclasts (Fig. 1), the cells that along with osteoblasts are responsible for bone metabolism. Whereas osteoblasts are involved in bone formation, osteoclasts can resorb bone by demineralizing it and degrading its organic matrix. Under homeostatic conditions, bone resorption is followed by osteoblast-mediated bone formation in a process that involves osteoclast-osteoblast communication and is known as ‘coupling’ (78). A persisting inflammatory environment, however, can ultimately disrupt bone homeostasis resulting in a net loss of bone (bone resorption exceeding bone formation). The fundamental mechanism governing bone resorption involves the regulation of osteoclastogenesis by a triad of proteins of the tumor necrosis factor/tumor necrosis factor-receptor family. This group of proteins includes RANKL, its functional receptor RANK, and its soluble decoy receptor osteoprotegerin (194). While osteoclast differentiation and activation are promoted by the binding of RANKL (expressed by osteoblasts and activated T cells and B cells) to RANK on osteoclast precursors, osteoprotegerin acts to block the RANKL-RANK interaction, thereby restraining osteoclastogenesis.
A unique feature of the periodontium compared with other mucosal sites, such as the gastrointestinal and respiratory tracts, is that it consists of both mucosal and bone tissue. Thus, it is clear that the cellular and molecular cross-talk between the periodontal immune and bone systems plays a critical role in the regulation of bone homeostasis. Proinflammatory cytokines that can induce RANKL expression in osteoblasts, such as interleukin-1β, tumor necrosis factor, and interleukin-17, are abundant in the inflamed periodontium. Therefore, osteoblasts can contribute to the increase of the RANKL/osteoprotegerin ratio. Interestingly, RANKL and osteoprotegerin were shown to be reciprocally regulated by several stimuli. For instance, interleukin-17 induces RANKL and inhibits osteoprotegerin expression in human periodontal ligament cells (153), which are also thought to be important in the regulation of osteoclastogenesis (119). However, adaptive immune cells appear to be the major source of RANKL in human periodontitis. In this regard, approximately 50% of T cells and 90% of B cells in diseased gingival tissues of periodontitis patients express RANKL (122).
RANKL is produced in two different versions, a membrane-bound form and a soluble form (195). Osteoblasts and bone marrow stromal cells express predominantly membrane-bound RANKL, which induces osteoclastogenesis through cell contact with osteoclast precursors. On the other hand, activated T cells and B cells produce both the membrane-bound and soluble forms of RANKL (Fig. 4). Soluble RANKL can induce osteoclastogenesis independently of direct contact between infiltrating lymphocytes and osteoclast precursors on the bone surface. Nevertheless, RANKL-expressing Th17 cells, but not Th1 cells, were shown to activate osteoclasts also by direct cell-cell contact (125).
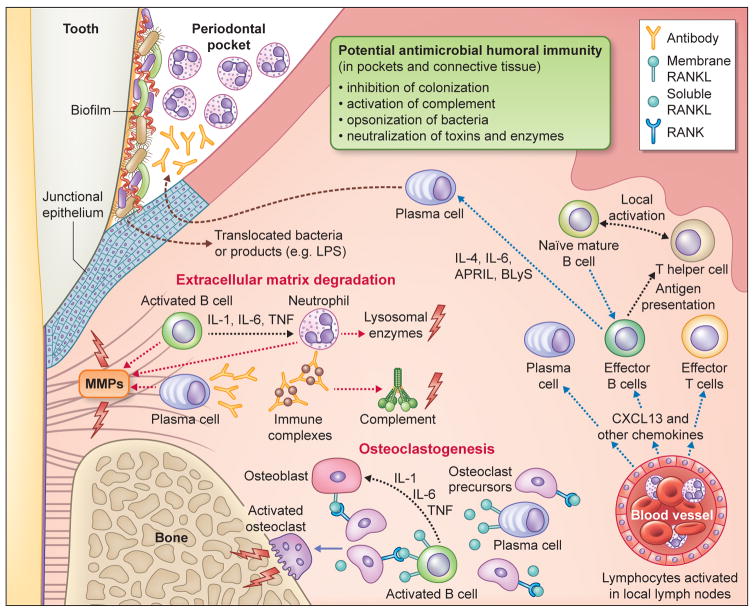
Fig. 4. Protective and destructive functions of B-lineage cells within the periodontium.
The junctional epithelium is situated in close proximity to the bacterial biofilm that develops on teeth. Quantitative and compositional changes of the microbiota (dysbiosis) induce innate immune signaling pathways that lead to the development of an adaptive immune response within the gingival connective tissue. Regarding the humoral component of the response, pathogen-specific antibody that diffuses into the gingival sulcus (or pocket) or remains in the connective tissue can, in principle, inhibit the bacterial challenge via a number of potential mechanisms (indicated). However, the antibody response has not been associated with protection in clinical studies. In fact, antibody-mediated activation of complement and innate immune cells can enhance gingival inflammation and contribute to tissue breakdown. Moreover, recent evidence has demonstrated the potential for B-lineage cells to express proinflammatory cytokines, matrix metalloproteinases, and RANKL. B-lineage cells therefore directly and indirectly participate in the degradation of the soft and hard tissue components of the periodontium, as indicated. The details are discussed in the text. Abbreviations in the figure: APRIL, a proliferation-inducing ligand; BLyS, B lymphocyte stimulator; MMP, matrix metalloproteinases; IL, interleukin; LPS, lipopolysaccharide; RANK; receptor activator of nuclear factor-κB; RANKL, receptor activator of nuclear factor-κB ligand; TNF, tumor necrosis factor.
T lymphocytes
T cells and homeostatic immunity
To maintain periodontal tissue homeostasis, the immune system proactively patrols the periodontium, which is in constant contact with the tooth-associated microbiota (54). Healthy human gingiva contains a predominantly T cell-rich inflammatory infiltrate and a network of antigen-presenting cells (dendritic cells and macrophages) that orchestrate local immunity. In contrast, minimal numbers of B cells and plasma cells are present. Neutrophils are also found in healthy gingiva (although at lower levels than in gingivitis and periodontitis) and can potentially interact with adaptive immune cells. A small population of innate lymphoid cells can also be seen at the gingival mucosal barrier (54). These cells belong to the lymphoid lineage, play important roles in protective immunity and maintenance of tissue homeostasis, but do not respond in an antigen-specific manner (106). The majority of the T cells in healthy gingiva are CD4-positive T helper (Th), but also present are cytotoxic CD8-positive T cells and a small population of γδ T cells, unconventional CD3-positive T cells involved in local immune surveillance and tissue homeostasis (54). Their potentially protective role notwithstanding, T cells are also implicated in the pathogenesis of periodontal disease.
T cells and antigen-presenting cells in periodontitis
In the Page & Schroeder model, a dense infiltrate of lymphocytes and other mononuclear cells is associated with gingivitis (‘early lesion’) but not necessarily with periodontitis. Indeed, this stage, as well as the plasma cell-rich ‘established lesion’, could remain stable without inevitably progressing to bone loss, the hallmark of the ‘advanced lesion’ (213). This key observation is consistent with the concept that periodontitis requires a susceptible host, although we do not fully understand the mechanisms that tip the fine host-microbe balance from a controlled immuno-inflammatory state to dysbiotic inflammation sufficient to cause irreversible damage (bone loss) to the periodontium (143). The association of T cells expressing the αβ T-cell antigen receptor with periodontitis probably represents a cause-and-effect relationship as suggested by animal model experiments. Studies in the 1990s using the murine oral gavage model of P. gingivalis-induced periodontitis indicated that the periodontal adaptive immune response is potentially destructive in periodontitis. Specifically, severe combined immunodeficient mice, which lack both T and B cells, develop less P. gingivalis-induced bone loss than immunocompetent controls (14). In the same model, major histocompatibility complex class II-restricted CD4-positive T cell-deficient mice exhibit reduced P. gingivalis-induced bone loss as compared to mice deficient in major histocompatibility complex class I-restricted CD8-positive T cells or normal controls, thus implicating CD4-positive T cells in periodontal tissue destruction (13).
In part due to our enhanced appreciation of the innate-adaptive immune cell cross-talk (Fig. 1), we currently know more about CD4-positive T cells and their functionally distinct subsets and how they are instructed by antigen-presenting mononuclear cells to elicit their immune responses (203, 256). Prior to the discovery of Toll-like receptors, innate immunity was viewed as a non-specific, transient and expedient reaction to ‘buy time’ until the activation of adaptive immunity, the system of B and T lymphocytes that express antigen receptors of exquisite specificity. We now recognize that Toll-like and other pattern-recognition receptors endow innate immunity with adequate specificity to distinguish between different types of pathogens. Most importantly, it has become apparent that innate immune mechanisms are sophisticated enough to make ‘judgments’ that instruct the initiation and progression of the adaptive immune response (174). In contrast to pattern-recognition receptors, the exquisite specificity of the adaptive immune receptors is not the result of co-evolution with microbes but the outcome of randomly generated gene recombination. Thus, although the overall B and T cell receptor repertoires collectively can bind virtually any antigenic structure (epitope), the cells are essentially ignorant of the biologic context of their antigen-specificity. In other words, they know what antigen they can respond to but do not have a clue about whether and how to respond. This information is predominantly provided by dendritic cells, which mediate between detection of infection and induction of the adaptive response. For instance, in the oral mucosa, dendritic cells encounter and capture oral microbes, then migrate to the regional lymph nodes where they provide costimulatory signals and present processed antigen to CD4-positive T cells, thereby regulating their activation and differentiation into different subsets (43, 291) (Fig. 5). Given the importance of T cells in periodontitis, it becomes apparent that dendritic cells can influence the outcome of this oral disease in either protective or destructive directions, and both outcomes have been described in animal models (6, 58, 292). Langerhans cells are a specialized subset of dendritic cells phenotypically hallmarked by their expression of the C-type lectin receptor langerin (CD207). They populate skin epidermis as well as oral and mucosal epithelia, survey their environment for foreign antigens and can regulate T cell responses (120, 291). Consistent with an immunoregulatory role, in vivo ablation of langerin-expressing cells in a mouse model of P. gingivalis-induced periodontitis resulted in increased infiltration of B and T cells and enhanced bone loss (7). However, langerin is also expressed by a population of dendritic cells known as langerin+ dendritic cells, which are present in the mouse gingiva according to an independent study (20). In fact, the latter study showed that specific ablation of Langerhans cells (thus retaining langerin+ dendritic cells) had no effect on P. gingivalis-induced bone loss (20). Therefore, it is plausible that the observed immunoregulatory effects (7) are attributable to langerin+ dendritic cells or require the presence of both Langerhans cells and langerin+ dendritic cells.
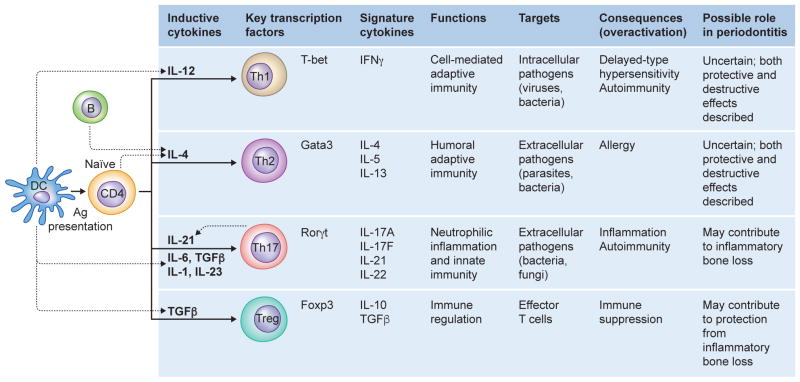
Fig. 5. Developmental pathways of CD4-positive T cells, their functions and associations with particular diseases.
Naïve CD4-positive Th cells differentiate into distinct lineages depending on the indicated cytokine milieu during activation by antigen-presenting cells. As shown, each of these effector or regulatory T cell subsets has a characteristic cytokine expression pattern and hence distinct functions and roles in autoimmune or inflammatory diseases, including periodontitis. Ag, antigen; B, B cell; DC, dendritic cell.
T cell subsets
Upon activation by antigen-presenting cells such as dendritic cells, naive CD4-positive T cells can be polarized into distinct effector T helper (Th) cell subsets; Th1, Th2, Th17 and regulatory T (Treg) cells, depending on the local cytokine milieu. Each of these T cell subsets has a characteristic cytokine expression pattern and hence distinct functions (136, 288) (Fig. 5). Th1 cells secrete interferon-γ and are responsible for cell-mediated immunity to intracellular pathogens but are also implicated in inflammatory and delayed-type hypersensitivity reactions. Th2 cells secrete interleukin-4, interleukin-5, and interleukin-13 and mediate humoral immunity (including IgE) and mast cell activation, and are also involved in allergic reactions. Th17 cells secrete interleukin-17A, interleukin-17F, interleukin-21, and interleukin-22 and mediate responses that recruit neutrophils and activate immunity to extracellular bacterial and fungal infections. Moreover, these cells have been implicated in autoimmune, inflammatory, and bone resorptive disorders. CD4-positive, forkhead box P3-expressing Tregs secrete interleukin-10 and transforming growth factor-β and play important regulatory roles in the maintenance of self-tolerance and suppression of unwarranted inflammation by downregulating Th1, Th2, and Th17 effector functions (136, 288). The differentiation of each of these effector T cell subsets is driven by distinct cytokines and is controlled by different sets of transcription factors (Fig. 5). The differentiation of Th1 and Th2 subsets is driven by interleukin-12 and interleukin-4, respectively. Whereas interleukin-12 can be derived from innate immune sources, such as an antigen-presenting dendritic cells, interleukin-4 can be provided by B cells or the naive CD4-positive T cells themselves. The key transcription factors controlling the differentiation of Th1 and Th2 cells are T-box expressed in T-cells and GATA sequence-binding protein-3, respectively. Transforming growth factor-β, interleukin-6, interleukin-1, and interleukin-21 are involved in the differentiation of Th17 cells, whereas interleukin-23 is important for Th17 cell expansion and survival. All these cytokines can be derived from innate immune cell sources, except for interleukin-21, which feeds back on the developing Th17 cells and amplifies the differentiation process. The retinoic acid-related orphan receptor-γt is the key transcription factor driving the differentiation of Th17 cells. Tregs share a reciprocal developmental pathway with Th17 cells since transforming growth factor-β is also required for the differentiation of naive T cells into Tregs, whereas forkhead box P3 controls their development and function (136, 288).
The Th1/Th2 paradigm
The Th1/Th2 paradigm, which was established in the late 1980s, provided a useful conceptual framework to understand T cell involvement in immunity and inflammation, although diseases of immunological etiology were occasionally pigeonholed into Th1 or Th2 categories without adequate supportive evidence (66). Similarly, periodontitis cannot be readily described in simple Th1 vs. Th2 dichotomous terms despite over two decades of intensive investigation. Studies in humans and animal models have described both protective and destructive effects associated with either subset [reviewed in refs. (66, 68, 71, 256, 273)]. In some reports, Th1-type cytokines (interferon-γ and interleukin-12) were negatively correlated with periodontal disease severity, ostensibly attributed to the ability of these cytokines to promote cell-mediated immunity and inhibit osteoclastogenesis. Other investigations, however, attributed destructive effects to interferon-γ and Th1 cells in periodontitis, consistent with the ability of activated Th1 cells to express the osteoclastogenic factor RANKL. Certain studies showed that the presence of Th2 cells was associated with protective effects attributed to the ability of Th2 cells to secrete interleukin-4 and interleukin-13, which can restrain osteoclastogenesis. Contrasting results linked Th2 cells to the pathogenesis of periodontitis, owing to their capacity to support destructive B cell responses [for details see dedicated reviews (66, 68, 70, 256, 273)] (Fig. 4). Under the Th1/Th2 paradigm, an interesting model was proposed according to which Th1 cells predominate in stable early lesions (i.e., when there is a homeostatic balance between the host and the periodontal microbiota), whereas Th2 cells are associated with disease progression beyond the established lesion, featuring an inflammatory infiltrate that is particularly rich in B cells and antibody-secreting plasma cells (70). However, as alluded to above, this and other disease models under the Th1/Th2 paradigm are consistent with only a subset of the clinical and experimental data.
Th17 cells
The subsequent discovery of the Th17 and Treg subsets has re-invigorated the interest on the role of T cell subsets in inflammatory diseases including periodontitis. The discovery of Th17 cells is particularly relevant in the context of osteoimmunology, a field of study developed well after the Page & Schroeder model was proposed. We now have a relatively good understanding of how inflammatory events regulate osteoclasts through the osteoprotegerin-RANKL-RANK axis (194) (see above for details). The bone-protective effect of osteoprotegerin is however diminished in periodontal disease, as the osteoprotegerin/RANKL ratio decreases with increasing periodontal inflammation often dominated by interleukin-17-expressing Th17 cells (5, 16, 27, 34, 207).
Th17 cells constitute a significant source of interleukin-17 production in the periodontal tissue (54, 187). Interleukin-17 potentiates innate immunity, especially neutrophil-mediated host defenses (123) by promoting granulopoiesis and orchestrating neutrophil recruitment via up-regulation of granulocyte-colony stimulating factor and chemokines (284, 294) and down-regulation of developmental endothelial locus-1, an endogenous inhibitor of the leukocyte adhesion cascade (59, 161) (Fig. 3). However, interleukin-17 also has potent osteoclastogenic properties, in part due to its capacity to stimulate the expression of RANKL in osteoblasts and other stromal cells (139). Moreover, interleukin-17 can mediate destruction of connective tissue by inducing the expression of matrix metalloproteinases in fibroblasts (178) (Fig. 3). In addition to secreting interleukin-17, Th17 cells express RANKL and function as a dedicated osteoclastogenic subset that links T-cell activation to osteoclast activation and inflammatory bone destruction (Fig. 1). Although this concept is derived mostly from studies in rheumatoid arthritis (178), multiple investigations have shown increased levels of locally produced interleukin-17 in human periodontitis as compared with healthy periodontal tissue (5, 15, 27, 53, 55, 108, 114, 148, 186, 207, 208, 252, 268, 282, 301). Although these correlative studies in humans do not prove a cause-and-effect relationship, antibody-mediated neutralization of interleukin-17 in different murine periodontitis models (where the disease is induced spontaneously or upon placement of ligatures) was shown to block periodontal inflammation and bone loss (59, 187). It should be noted that interleukin-17 is also produced by a variety of adaptive and immune cell types, including CD8-positive T cells, γδ T cells, natural killer T cells, and innate lymphoid cells (42, 167, 265, 270). Therefore, the protective effects of anti-interleukin-17 interventions do not necessarily target Th17 cells exclusively.
Interestingly, P. gingivalis-induced periodontitis in Langerhans cell-deficient mice suppresses the numbers of P. gingivalis-specific interleukin-17A–producing CD4+ T cells but elevates P. gingivalis-specific interferon-γ–producing CD4+ T cells (as compared to wild-type mice), although the induced bone loss is comparable in Langerhans cell-deficient and wild-type mice (20). This suggests that both Th17 (interleukin-17) and Th1 (interferon-γ) responses can mediate destructive periodontal inflammation, although another possibility is that interleukin-17 was produced by other cellular sources in Langerhans cell-deficient mice, such as γδ T cells, which are abundant in the mouse periodontium and other mucosal sites (59, 230).
At least in principle, the ability of Th17 cells to secrete interleukin-17 and interleukin-22 could exert a protective effect in periodontitis. As mentioned earlier, interleukin-17 can stimulate granulocyte-colony stimulating factor–dependent granulopoiesis and chemotactic recruitment and activation of neutrophils (109, 284) (Fig. 3) and, moreover, both interleukin-17 and interleukin-22 can induce epithelial cell production of antimicrobial peptides (136). However, this protective scenario seems more plausible in the case of acute infections. However, the persistence of Th17 cells at sites of inflammation provides prolonged interleukin-17 signaling which can turn an acute inflammatory response into chronic immunopathology (156). Moreover, Th17 cells have been implicated in promoting B-cell responses in autoimmune and inflammatory conditions (60, 102, 180). In this regard, Th17 cells were shown to act as B-cell helpers by inducing a robust proliferative response of B cells in vitro and by triggering antibody production with IgG class switch in vivo (180). The same study showed that inhibition of interleukin-17 signaling leads to a significant decrease in both the number and size of B cell germinal centers (180). In a similar context, Th17-derived interleukin-21 synergizes with B-lymphocyte stimulator in stimulating plasma cell differentiation (60) (Fig. 1). From a pathologic viewpoint, Th17 cells can provide B cell help in autoantibody-induced arthritis whereas neutralization of interleukin-17 delays the onset of disease (102). The possibility of similar mechanisms occurring in the setting of periodontitis has not been investigated, although whether periodontitis includes an autoimmune component remains uncertain (more in the following section).
Tregs
Tregs have been identified in chronic inflammatory periodontal lesions (28, 193). These regulatory cells express forkhead box P3, a transcription factor that induces expression of genes required for their immune suppressive functions. Tregs are classified into natural Tregs (nTregs) and inducible Treg cells (iTregs) based on the site of maturation. The nTregs are generated in and released from the thymus to the periphery as a functionally distinct lineage that already expresses forkhead box P3. The iTregs originate from naïve T cells and are induced in the periphery by antigen presentation from tolerogenic dendritic cells in the presence of transforming growth factor-β and the absence of interleukin-6 and/or interleukin-21. interleukin-2 and transforming growth factor-β are required for induction of forkhead box P3 in iTregs (200). Since the differentiation of Th17 cells is induced by transforming growth factor-β in combination with interleukin-6 and/or other cytokines such as interleukin-21, the development of Tregs and Th17 is reciprocally regulated. In fact, interleukin-6 and interleukin-21 inhibit the expression of forkhead box P3 (by activating signal transducer and activator of transcription-3) and promote (transforming growth factor-β-induced) retinoic acid-related orphan receptor-γt expression to induce Th17 cells. In other words, the cytokines controlling the forkhead box P3/retinoic acid-related orphan receptor-γt balance give rise to Tregs or Th17 cells, respectively. In a related context, interleukin-23–activated γδ T cells were shown to restrain Tregs and tip the balance in favor of effector T helper cells (221). Moreover, interleukin-23 can induce expansion and stabilization of the Th17 subset and stimulate their interleukin-17 production (288). It is, therefore, plausible that factors that regulate the balance between Th17 and Tregs can in principle influence the pathogenesis of periodontitis.
In both human periodontitis and animal models of the disease, the number of forkhead box P3-positive Tregs increases in the inflamed periodontium in comparison to healthy tissue (28, 53, 133, 193). These findings imply a homeostatic mechanism aiming to mitigate collateral tissue damage caused by the overactivation of adaptive immunity. Mechanistically, immunosuppressive cytokines, such as interleukin-10, produced by Tregs can inhibit the induction of RANKL expression by activated T cells (Fig. 1). Additionally, interleukin-10 may suppress RANKL-mediated differentiation and activation of osteoclasts (183, 217) (Fig. 1). It should be noted, however, that Tregs have a substantial degree of plasticity and may lose their suppressive function in an inflammatory environment, implying that an increase in the number of Tregs does not necessarily mean that these cells will eventually control development of inflammatory disease (200). In this regard, forkhead box P3 was shown to be over-expressed in active periodontal lesions (along with RANKL, interleukin-17, and other inflammatory cytokines) as compared with inactive lesions (53). Furthermore, it has been suggested that Tregs may convert into interleukin-17-producing Th17 cells in human periodontitis lesions (208). In this study interleukin-17/forkhead box P3 double-positive cells were detected in periodontitis lesions, intimating an intermediate stage in the process of Treg-to-Th17 conversion (208). In contrast, another investigation failed to detect interleukin-17/forkhead box P3 double-positive cells in the inflammatory infiltrate of human periodontal lesions (216)..
Studies in animal models of periodontitis have provided important insights into the possible function of Tregs in this oral disease. In murine models of pathogen-induced periodontitis, Tregs appear in high numbers after the peak emergence of RANKL-expressing CD4-positive T cells (133), whereas their depletion (by anti-glucocorticoid-inducible tumour necrosis factor receptor) exacerbates inflammation and bone loss (68). Conversely, recruitment of Tregs upon local treatment of mice or dogs with a Treg-recruiting chemokine (CCL22) formulation blocks experimental periodontitis in these models (73). Therapeutic approaches that promote the presence of Tregs, therefore, have the potential to mitigate inflammatory tissue damage in periodontitis.
Our knowledge on the T cell response in periodontitis has come a long way since the Page & Schroeder model was proposed but the roles of T cells are still enigmatic. Although the discovery of the Th17 and Treg subsets has offered enhanced insights into the pathogenesis of periodontitis, the dissection of the protective and destructive aspects of the periodontal T cell response remains a complex and challenging endeavor. This may have to do with the very nature of periodontal disease, a polymicrobial community-induced dysbiotic malady (88), where a potentially protective antimicrobial response could be offset by bystander tissue damage. In summary, while Th17 cells and interleukin-17 have the capacity to mediate protective responses, the current burden of evidence from human and animal model studies suggests that their net effect promotes disease development, whereas the opposite effect (protection) appears to be facilitated by Tregs (5, 28, 53, 59, 66, 68, 73, 109, 152, 178, 193, 284).
B-Lineage Cells
Abundance of B lymphocytes and plasma cells in periodontitis
In their classic paper, Page & Schroeder described the initial appearance of B cells and plasma cells as a minor component of the lymphoid infiltrate detected within the early lesion (213). Upon progression of the disease, however, a dramatic increase in the numbers of these cell types was observed such that the “distinguishing feature of the established lesion is the predominance of B-lymphocytes and of plasma cells within the affected connective tissue at a stage prior to extensive bone loss” (213). They reported a further increase in the proportion of B-lineage cells within the infiltrate yielding the advanced lesion that was associated with overt bone loss. From a histopathologic perspective, plasma cells represented the predominant cell type within periodontitis lesions. Since the lesions were described on the basis of morphometric and ultrastructural observations, it is critical to appreciate the limitations of these approaches relative to distinguishing B and T cells from one another.
Soon thereafter, immunohistochemical and immunofluorescent approaches were utilized by a number of other groups to better define the composition of the inflammatory infiltrate in gingivitis and periodontitis lesions. With advancing knowledge regarding the expression of unique structures on the surfaces of T, B and plasma cells, specific reagents were developed that allowed investigators to not only more accurately distinguish these cell types from one another but to also identify the distribution of lymphocyte subsets within diseased gingival tissue. A majority of these studies have confirmed the observations of Page & Schroeder demonstrating the predominance of B lineage cells in clinical scenarios analogous to the established and advanced lesions (17, 18, 113, 127, 181, 274, 297). B-lineage cells were estimated to comprise around 60% of the total leukocytes that are present in periodontal lesions associated with bone loss (274). We will therefore limit our comments to studies communicating unique findings.
Utilizing CD19 as a marker for B-lineage cells, it was shown that B cells and plasma cells collectively represented the most common lymphoid cells within the inflammatory infiltrate of tissue from patients with severe chronic periodontitis (50). Interestingly, moreover, B cells outnumbered both plasma cells and T cells (50). Furthermore, a majority of the B cells were also found to express CD5. This observation was taken to suggest that these cells were analogous to the previously described CD5-positive murine B-1a subset of B cells. In a number of murine models, the B-1a subset was shown to exhibit significant autoimmune activity. Thus, the presence of large numbers of CD5-positive cells in the periodontitis lesions was interpreted to indicate that autoantibody production has a role in the pathogenesis of periodontitis (18). Unlike ‘conventional’ B cells (B-2) that have a hematopoietic origin, B-1a cells develop from peritoneal precursors during the fetal and neonatal phases of life. They typically respond to common pathogen-associated carbohydrates and tend to produce broadly reactive but low-affinity IgM antibodies thought to be involved in initial responses to certain bacteria and viruses. It has recently been shown that only a small percentage of CD5-expressing human B cells exhibit the functional phenotype of B-1a cells (CD5 is expressed on multiple human B cell populations) (234). Thus, it is uncertain that the CD5-expressing cells detected in periodontitis lesions are truly B-1a cells, at least not in their majority. Moreover, conclusive mechanistic studies implicating an autoimmune component in periodontitis are lacking and the role of autoantibodies in the pathogenesis of periodontitis remains ambiguous but cannot be ruled out at present.
Another investigation utilized an immunohistochemical approach to evaluate the lymphocyte composition of inflamed human connective tissue within long-standing gingivitis and periodontitis lesions (274). B-lineage cells (CD20-expressing B cells and CD138-expressing plasma cells) were found in higher numbers in periodontitis lesions relative to gingivitis lesions and in both situations exceeded the number of CD3-positive T cells. Moreover, an independent study showed that the numbers of plasma cells were significantly elevated in sites associated with periodontal disease progression (≥ 2mm change in probing attachment levels) in comparison to contralateral sites that remained stable (297). Recently, another group sought to describe the make-up of the immune cell populations present in clinically healthy human gingiva (54). As the focus of the study was on homeostatic immunity, they evaluated tissue obtained from 50 healthy subjects and only 6 chronic periodontitis patients, for comparative reasons. Utilizing flow cytometric analysis of collagenase-digested tissue, they found significant numbers of T cells but very few B cells in healthy samples, an observation consistent with Page & Schroeder’s findings regarding the lymphocyte population within the initial lesion. Although the numbers of CD19-positive B cells were increased in periodontitis relative to health, their analysis did not include plasma cells. Therefore, the overall abundance of B-lineage cells was not estimated and consequently no comparison could be made with the abundance of T cells.
As discussed in an earlier section of this review, significant effort has gone into determining the contribution of distinct T cell subsets to the pathogenesis of the plaque-induced periodontal diseases. In contrast, very little is known regarding the distribution of B-lineage cells belonging to well-defined subsets within healthy and diseased periodontal tissues. Even less understood is the role of the cells belonging to distinct B cell subsets in the maintenance of gingival tissue homeostasis, disease initiation or disease progression. In order to shed light on these issues, a very recent study evaluated the phenotypes of B-lineage cells detected in samples of healthy, diseased (gingivitis and periodontitis) and treated (resolved-periodontitis) human gingiva (164). Utilizing flow cytometry, naïve B cells, memory B cells, plasmablasts and plasma cells were identified based upon the differential expression of a panel of relevant cell surface molecules (CD19, CD27, CD38, CD138, HLA-DR, IgG and IgA). As one would predict, the percentage of B-lineage cells was highest in cell suspensions prepared from tissue affected by periodontitis. Very few naïve B cells were detected in any of the samples while plasma cells, as expected, were the predominant B-lineage cell in periodontitis samples. The majority of B cells observed in healthy, gingivitis and treated samples exhibited the memory cell phenotype. When intact healthy tissue was examined via immunohistochemistry, the memory B cells were localized adjacent to the junctional epithelium leading the investigators to speculate that the cells are involved in maintaining gingival homeostasis. The mechanism(s) through which this occurs was not delineated.
Potential mechanisms of B and plasma cell involvement in periodontitis
As discussed above, an overwhelming literature supports the original observations of Page & Schroeder regarding the predominance of B-lineage cells within the inflammatory infiltrate of periodontitis lesions [reviewed in ref. (17)]. Over the ensuing four decades, researchers have attempted to address a number of questions incited by the observations of Page & Schroeder. Amongst others, these included: (i) What are the mechanisms underlying the predominance of B-lineage cells in periodontitis lesions? (ii) What is the function of the B-lineage cells in periodontal pathogenesis? (iii) Are B-lineage cells requisite for alveolar bone loss in periodontitis? As these questions are actually intertwined, we will attempt to review the current knowledge in a systematic fashion.
Our understanding of the cellular and molecular mechanisms underlying the recruitment, activation and differentiation of B-lineage cells in peripheral lymphoid and non-lymphoid tissues has increased dramatically since the description of the four lesions by Page & Schroeder. Typically, mature B cells that exit the bone marrow are activated and induced to undergo differentiation into antibody-secreting plasma cells or memory cells in an antigen-dependent fashion within secondary lymphoid tissues. For most microbial antigens (T-dependent) this requires engagement of the B-cell antigen receptor (cell-surface bound immunoglobulin) and secondary signals derived from T cells. A small number of antigens (T-independent) with highly repetitive structures, most notably bacterial polysaccharides, can cross-link multiple antigen receptors and stimulate B cells in the absence of T cells. Additionally, innate signals (such as lipopolysaccharide and other conserved microbial structures) may enhance antigen-mediated activation by binding to Toll-like receptors (Toll-like receptor-4, Toll-like receptor-7 and Toll-like receptor-9) expressed by human B cells (104). It is well established that the inflammatory infiltrate within gingivitis and periodontitis lesions represents the host response to the polymicrobial challenge provided by the plaque biofilm. These organisms express well-defined T-dependent and T-independent antigens that are likely to induce local activation and differentiation of B cells into plasma cells within gingival connective tissue. Additionally, bacterial antigens can be transported via antigen-presenting cells to local lymph nodes leading to the generation of activated B cells and plasma cells that selectively home to the site of bacterial challenge.
CXCL13 (also known as B lymphocyte chemoattractant) is a chemokine expressed by several cell types (follicular dendritic cells, endothelial cells, fibroblasts, and macrophages) that binds CXCR5 on the surface of mature B cells and serves to selectively recruit these cells to sites such as the follicular component of lymph nodes (57) To examine whether CXCL13 can serve a similar purpose in the periodontium (i.e., to help recruit B cells), the expression of this chemokine in gingival connective tissue from subjects with gingivitis or periodontitis was evaluated (192). Significantly increased numbers of CXCL13-expressing cells were detected in periodontitis as compared to gingivitis. These cells were localized to the connective tissues underlying the pocket epithelium and, importantly, the numbers of CXCL13-expressing cells positively correlated with the number of CD19-positive cells. It was concluded that enhanced CXCL13 expression was in part responsible for the recruitment to and distribution of B cells within the diseased tissue (Fig. 4).
A large body of literature currently exists on the role of cytokines in the pathogenesis of periodontal disease. Specifically related to B-lineage cells, increased expression/production of multiple molecules relevant to their activation, proliferation, differentiation, function and survival has been detected in diseased gingival tissue. Classically, most notable are interleukin-4, interleukin-5, and interleukin-6, which are involved in B cell differentiation into antibody-secreting plasma cells (170), and, furthermore, are upregulated in periodontitis (65). More recently, two cytokines belonging to the tumor necrosis factor superfamily, APRIL (also known as tumor necrosis factor ligand superfamily member 13) and B-lymphocyte stimulator (also known as B cell-activating factor or tumor necrosis factor ligand superfamily member 13B) have been linked to the survival and maturation of human B cells (25, 157). Interleukin-6 and APRIL play important roles in maintaining the survival of long-lived plasma cells within the bone marrow (135). Epithelial cells stimulated by Toll-like receptor ligands constitute a rich source of APRIL (100), while activated myeloid cells (e.g., neutrophils, macrophages, and dendritic cells) express B-lymphocyte stimulator (242). It was recently reported that expression and production of both APRIL and B-lymphocyte stimulator is enhanced in gingival tissue from periodontitis patients relative to healthy controls (1), and these findings were confirmed by a subsequent independent study (164). The abundance of the above-discussed cytokines in periodontitis (relative to health) can, at least in part, account for the maintenance and predominance of B cells and plasma cells in periodontitis lesions.
Despite their abundance in advanced periodontal lesions, the precise role of B-lineage cells in the pathogenesis of periodontitis remains quite enigmatic, although several mechanisms are plausible (Fig. 4). By mediating humoral immunity, B cells/plasma cells could, in principle, have a protective function in periodontitis. For instance, the antibody response could contribute to the control of the polymicrobial dysbiotic communities in periodontal pockets as well as prevent bacterial invasion into the gingival connective tissue, thereby limiting inflammation and disease. A large number of studies have addressed this issue and the topic has been extensively reviewed (71, 243, 289) In general, antibody levels have typically been found to be elevated in diseased individuals relative to healthy controls. Surprisingly, the high titer of antibody found in patients does not appear to ameliorate the disease. It has been suggested that antibody responses to periodontal bacteria in periodontitis patients are of low affinity and/or poor opsonophagocytic capacity (71, 243, 289). In fact, individuals with the highest titers of antibody tend to exhibit severe alveolar bone loss relative to low-titer controls (130). Conceptually, antibody, particularly IgG, has the capacity to exacerbate inflammatory reactions and tissue degradation via a number of mechanisms, including activation of complement and Fc receptor-mediated activation of innate immune cells. In this regard, advanced periodontitis is associated with increased deposition of IgG immune complexes along with complement activation fragments (117, 199, 278). Overall, whether antibodies specific for periodontitis-associated bacteria play a protective, destructive or innocuous role in the pathogenesis of periodontitis, and the precise mechanisms involved, remain to be clarified.
Since the early 1970s, it has become increasingly obvious that B-lineage cells have immune and inflammatory functions that extend well beyond simply antibody production, such as antigen presentation to T cells, expression of proinflammatory/osteoclastogenic cytokines and matrix metalloproteinases, thereby having the potential to induce both soft and hard tissue degradation. Thus, it is feasible that these cells participate in the immunopathogenesis of periodontitis via functions that are independent of their role in humoral immune responses (18, 74). Two of the hallmarks of the established and advanced lesion described by Page & Schroeder are collagen degradation and alveolar bone resorption. Owing to the then-limited information, they were unaware of the potential of B-lineage cells to actively influence both of these processes. The association of elevated numbers of plasma cells with an increase in collagen breakdown (113) is consistent with their ability to release (in addition to interleukin-1, tumor necrosis factor and interleukin-6) matrix-degrading enzymes including collagenase (18, 71, 111). B-lineage cells also constitute a major source of both membrane-bound and secreted RANKL in the bone resorptive lesions of human periodontitis (122). Indeed, more than 90% of B cells express RANKL in periodontitis lesions, whereas RANKL-positive B cells in healthy gingiva are negligible (122). It has recently been demonstrated that B cells and plasma cells in diseased human periodontal tissue constitute a significant source of the so-called secreted osteoclastogenic factor of activated T cells (112), a cytokine that induces osteoclast differentiation via a RANKL-independent pathway (229). It is therefore plausible that B-lineage cells may participate in the induction of pathological bone loss in periodontitis via both RANKL–dependent and –independent effects on osteoclast precursors.
Mechanistic studies in animal models and clinical intervention
The notion that B-lineage cells mediate bone loss in periodontitis is supported by findings from animal model studies. Adoptive transfer of bacterial antigen-specific B cells into rats followed by local oral exposure to the same bacterium (Aggregatibacter actinomycetemcomitans) leads to RANKL-dependent periodontal bone loss (98) even when the recipient rats are congenitally athymic and hence T-cell deficient (94). This suggests that B cells can contribute to periodontal bone loss independently of any possible co-operation with T cells. IgD-deficient mice (which have delayed B cell affinity maturation (232)) exhibit diminished B cell activation and are protected against bone loss induced by oral gavage with P. gingivalis as compared to wild-type controls (12). The role of B cells in periodontitis was addressed more recently in studies utilizing wild-type and B-cell knockout mice; these mice, also known as μMT, do not express membrane-bound IgM and consequently B cell development is blocked at the pro-B stage (132). Utlilizing an oral gavage model, one study implicated B cells in periodontal bone loss in normal mice as the results showed that C57BL/6J B-cell knock-out mice are protected from P. gingivalis-induced bone loss relative to wild-type mice, which developed modest bone loss as compared to sham-infected controls (209). Another study reported that lean, normoglycemic C57BL/6J B-cell knock-out mice raised on a low-fat diet were not protected from P. gingivalis-induced bone loss, while obese, insulin-resistant B-cell knock-out animals were completely protected (302). The investigators interpreted the findings to suggest that B cells are “able to promote periodontitis only if the hosts are primed by obesity”. The discrepancy between the two studies is not clear but different experimental conditions, use of different P. gingivalis strains and frequency of their oral inoculation, might have contributed to the different outcome. Apparently, the experimental protocol used in the latter study did not readily support the development of a B-cell infiltrate in lean mice that is characteristic of human periodontitis, consistent with the authors’ comment that “later phases of periodontal disease may be better studied in a model characterized by B cell-dominated lesions that mimic long-term human disease” (302).
An alternative to the oral gavage model is the ligature-induced periodontitis model. The placement of a ligature facilitates rapid accumulation of a heavy biofilm around the teeth and represents a model of accelerated inflammation and bone loss, which is more robust than bone loss seen in the oral gavage model and hence more sensitive to detect subtle differences (2, 77). This model involves early and strong activation of complement and expression of high levels of interleukin-6, as well as APRIL and B-lymphocyte stimulator (1, 3, 158), which are all factors that promote B cell activation, proliferation and differentiation (25, 29, 33, 157). Accordingly, in a ligature-induced periodontitis study, gingival B cells and plasma cells were detected in significant numbers 5 days after initiation of periodontitis in wild-type mice and their numbers increased through day 10, correlating with increased expression of IgG during the same time interval (1). Consistent with the absence of substantial numbers of B-lineage cells in wild-type mice during the first 5 days of the study, wild-type mice developed bone loss comparable to that experienced by B-cell knock-out mice at the same time interval (1). In stark contrast, however, wild-type mice developed significantly more bone loss than B-cell knock-out mice at day 10 when B/plasma cells were abundant, suggesting that B-lineage cells contribute to a late phase of ligature-induced alveolar bone loss (1). In the same study, neutralizing monoclonal antibodies to APRIL and B-lymphocyte stimulator diminished the number of B cells in the gingival tissue of wild-type mice and protected them from bone loss. Both antibodies failed to inhibit bone loss in B-cell knock-out mice, indicating that the observed effects of anti-APRIL and anti-B-lymphocyte stimulator were mediated through action on B cells. In summary, B cells and B cell-stimulatory cytokines are temporally and causally linked to periodontal bone loss.
Although the animal experiments described above provide compelling evidence that B cells are involved in the induction of alveolar bone loss, there is minimal corroborating data from investigations on humans. A very recent study evaluated the periodontal status of two groups of rheumatoid arthritis patients treated with Rituximab (anti-CD20 monoclonal antibody) (39). Based on the evaluation of a number of clinical periodontal parameters, they showed that the majority of the subjects exhibited an improvement in their periodontal status compared to that before initiation of Rituximab therapy. It was concluded that B cells have “a major role” in the immunopathogenesis of periodontitis. Taken together with relevant clinical observations discussed above and animal model-based mechanistic studies, B cells and plasma cells do appear to contribute to the breakdown of soft and/or hard tissue components of the periodontium probably via several mechanisms.
In conclusion, the observations reported by Page & Schroeder 40 years ago provided the impetus for investigators to evaluate the functional significance of the accumulation of B lineage cells in periodontitis lesions. It is now clear that these cells are anything but innocent antibody-producing bystanders within inflamed gingival connective tissue. They in fact have the capacity to take an active role in the immunopathogenesis of the disease (Fig. 4).
Complement
Page & Schroeder only briefly mentioned complement in their landmark paper (213). Complement was suspected to play a role in the initial lesion, as it was “probably present in the extravascular gingival tissues” but there was “insufficient evidence to determine the role, if any, that these substances may play at this stage in the pathogenesis” (213). Regarding the established lesion, they cited “evidence for the presence of complement and antigen-antibody complexes, especially around the blood vessels” but similarly did not expand on its possible biological significance. However, soon after their publication, several groups showed that gingival crevicular fluid from periodontitis patients contains complement cleavage products at significantly higher levels than in gingival crevicular fluid samples from healthy controls (10, 245–247). Moreover, an abundance of complement components and cleavage products was demonstrated in chronically inflamed gingiva, whereas complement was undetectable or present at lower levels in gingival biopsy samples from healthy controls (41, 141, 277). Therefore, five years later, Page & Schroeder acknowledged that complement may play an important role in periodontitis, although “the extent to which complement participation is protective in contrast to destructive has not been resolved” (214). They discussed complement as a source of endogenous chemotactic substances (C5a) for the recruitment of neutrophils, noting that its effects may not necessarily be destructive since complement could also play a protective role (promotion of bacterial phagocytosis through C3b opsonization) (214).
Subsequently, it was shown that experimental gingivitis in human volunteers induces progressive complement activation (as determined by C3 conversion) that correlates with elevated clinical inflammatory parameters (218). Conversely but consistently, the resolution of periodontal inflammation in patients during successful therapy is associated with reduced complement activation (198). More recently, a study using an integrative gene prioritization method and databases from genome-wide association and microarray approaches has identified the central complement component C3 amongst the top 21 most promising candidate genes associated with periodontal disease (300).
Also in recent years, complement started to be appreciated as a central system of immune surveillance and homeostasis and a key link between innate and adaptive immunity (228). Specifically, it was realized that complement is not restricted to a linear cascade of events but rather involves a network of interactions with other immune and physiological systems, which collectively coordinate host responses to infection or tissue injury. For instance, complement activation can amplify immune and inflammatory responses by synergizing with Toll-like receptors (86), form a barrier against the spread of invading bacteria by augmenting local clotting (168), and regulate antigen-presenting cells and the activation and differentiation of T-cell subsets (51). These developments greatly facilitated recent complement studies in periodontitis.
As also acknowledged by Page & Schroeder, clinical periodontal observations are correlative and do not necessarily establish a cause-and-effect relationship between complement activation and periodontitis. Evidence implicating a destructive role of complement in periodontitis was generated by recent intervention studies in relevant animal models, including non-human primates. Briefly, it has been shown that complement is exploited by periodontal bacteria in ways that promote the dysbiotic transformation of the microbial community and, moreover, complement signaling pathways synergize with Toll-like receptors in eliciting inflammatory response that lead to alveolar bone destruction (3, 87, 90, 158, 162, 287). It should be noted that, despite enhanced complement-dependent inflammation in periodontitis, important disease-associated bacteria (such as, P. gingivalis Tannerella forsythia, Treponema denticola, and Prevotella intermedia) are equipped with several mechanisms to protect themselves against complement-mediated opsonophagocytosis or lysis, including inhibition of opsonization and ability to capture and co-opt physiological inhibitors of complement (e.g., factor H or C4b-binding protein) (116, 166, 176, 224, 225, 244). On the basis of mouse studies that C3-dependent inflammation is critical for long-term sustenance of periodontal dysbiotic communities and for maximal induction of alveolar bone loss (158), translational studies were undertaken in non-human primates. These studies showed that local C3 inhibition (using the drug compstatin Cp40) blocks both inducible and naturally occurring periodontitis in cynomolgus monkeys, thus unequivocally linking complement activation to periodontal tissue destruction (158, 160).
Local tissue control of inflammation in periodontitis
This review has focused on the pathogenic role of leukocytes infiltrating the periodontium, as the main objective was to revisit the Page & Schroeder model of the involvement of the myeloid and lymphoid cell involvement in inflammatory periodontal disease. That said, it is becoming increasingly apparent that local tissues may have greater control (than traditionally acknowledged) over the initiation and properties of the developing immune response, by virtue of their ability to communicate with and instruct the immune system through tissue-derived (stromal/parenchymal) signals, such as, cytokines, chemokines, antimicrobial peptides, growth factors, and recently identified locally acting homeostatic molecules (84, 172, 262). In other words, tissues are not passive recipients of immune surveillance. In this context, tissue-derived ‘alarm’ signals (e.g., locally produced cytokines or chemokines) can educate and appropriately activate antigen-presenting cells to promote a particular type of host response (172). Conversely, when no longer needed, the recruitment of inflammatory leukocytes can be suppressed or arrested by local homeostatic or ‘health’ signals (e.g., endothelial-cell derived developmental endothelial locus-1 or locally produced resolvins) to promote resolution of inflammation and tissue regeneration (59, 172, 179, 251, 262). Stromal cells in the periodontium, such as junctional epithelial cells, gingival fibroblasts, periodontal ligament cells, and vascular endothelial cells normally contribute to periodontal tissue homeostasis. However, under an inflammatory challenge, they appear to have the requisite plasticity to alter their phenotypes and contribute to the host response, which, however, has significant potential to cause tissue damage (63, 115, 145). The notion that local tissues have a ‘regulatory say’ over the host inflammatory response is consistent with evidence that immune-privileged sites (e.g., the brain and the eye) have built-in mechanisms to promote the appropriate class of immune response that can potentially protect the tissue against infection without destructive inflammation (172, 197, 264). In this context, the spontaneous periodontal inflammatory phenotype of developmental endothelial locus-1–deficient mice suggests that developmental endothelial locus-1–expressing tissues have the potential to control the local host inflammatory response. Intriguingly, immune-privileged tissues/organs display abundant expression of developmental endothelial locus-1 at even higher levels than peripheral tissues (e.g., lung or the gingiva) (35, 59), and developmental endothelial locus-1 deficiency in mice promotes experimental autoimmune encephalomyelitis, a model of multiple sclerosis (36). Therefore, tissues and organs appear to have evolved local homeostatic mechanisms for protection against the destructive potential of the immune system. Identification of the tissue-specific promoters and inhibitors of leukocyte recruitment, as well as the mechanisms that regulate them, is key to further our understanding of the pathogenesis of plaque-induced periodontal diseases and developing novel approaches to treat them.
The ‘double-edged sword’ nature of the periodontal host response
Much of the uncertainty on the roles of the various cell types in periodontitis is likely due to the ‘double-edged sword’ nature of the immune system and the complex etiology of periodontitis. The notion that the host response can be intimately involved in the destructive process has been conclusively demonstrated in animal models. For instance, the inhibition of the host response through various approaches (e.g., blocking complement activation, prostaglandin synthesis, and proinflammatory/osteoclastogenic cytokines, such as tumor necrosis factor, interleukin-1, interleukin-17, and RANKL) was shown to reduce inflammatory bone loss in several rodent and non-human primate models of inducible or naturally-occurring periodontitis (47, 59, 150, 154, 158, 159, 205, 272). On the other hand, an optimally regulated host response can provide homeostatic immunity and thus be protective. In this context, Page & Schroeder proposed that stable gingivitis may represent a protective host response that prevents transition to periodontitis (213). Ample evidence exists that elements of innate or adaptive immunity can prevent or ameliorate disease severity. For instance, immunization of rats or non-human primates against P. gingivalis protects against bone loss induced by inoculation of this bacterium (72, 121, 211, 220, 226). Similar protective effects were observed after adoptive transfer of T helper lymphocytes in a rat model of periodontitis (293). Moreover, both humans and mice with defective ability to recruit neutrophils to the periodontal tissue (e.g., due to leukocyte-adhesion deficiency) fail to properly regulate the host response and develop an aggressive form of periodontitis early in life (187).
There is a fine balance between homeostatic immunity and immune pathology and it is possible that the protective versus pathogenic actions of leukocytes in periodontitis reflect appropriate versus aberrant operation of regulatory mechanisms. This in turn is likely influenced, to a significant extent, by genetic, epigenetic, and environmental factors (128, 146, 206) as well as the immune subversive capacity of the periodontal microbial communities (88). Moreover, the ‘double-edged sword’ behavior of a given leukocyte type could be attributed to the activation of different subsets with functionally distinct roles that could be protective or destructive depending on context. In this regard, the recent concept of immune plasticity has led to the realization that the function of recruited leukocytes can be tailored to the requirements of the tissue through intimate crosstalk with tissue-derived factors; this adaptation can contribute critically to immune homeostasis and resolution of inflammation (21, 26, 67, 255, 263). However, when the tissue environment is deficient in critical homeostatic molecules, this becomes a predisposing factor for leukocyte-driven inflammatory tissue destruction (59). In general, important advances have been made in the periodontal research field that can potentially provide insights as to when leukocyte recruitment serves homeostatic immunity as opposed to pathology or when inflammation resolution fails and proceeds to a chronic stage (56, 59, 93, 175, 187, 208, 251, 281). Page & Schroeder proposed their periodontal pathogenesis model in the absence of this recent knowledge (immune plasticity, specialized functional leukocyte subsets, local homeostatic mechanisms etc). Yet, their model was one that could be improved upon with emerging knowledge but not one that could be ignored or appreciated only for its historical interest.
Conclusions and future directions
As alluded to above, the Page & Schroeder model remains fundamentally relevant with regard to the main cellular players involved and their order of appearance during the development of periodontitis. What has changed tremendously in our understanding of this disease is the elucidation (albeit still incomplete) of the cross-interactions and roles of the various players and that the host response develops in a setting of dysbiosis rather than infection. In comparison to current knowledge, relatively little was known in the 1970s about crosstalk between leukocytes via ‘products of activated lymphocytes’ (cytokines) at the time Page & Schroeder proposed their model of periodontal disease pathogenesis (213), or even five years later in a subsequent review by the same authors (214). We now have an improved understanding of how different leukocyte types communicate between themselves and with humoral systems, such as complement, to stage immune and inflammatory responses in the periodontium, and this knowledge has offered promising therapeutic targets (18, 66, 68, 81, 256, 271). Furthermore, we now appreciate that many of the infiltrating cells exhibit functions that extend well beyond their classically accepted roles in the disease process. For example, the contribution of neutrophils is not restricted to the initial lesion; rather, neutrophils appear to be significantly involved in the chronic stages of periodontitis and may potentially contribute to the regulation of both T and B lymphocytes (85) (Figs. 1 and 2), as shown in other disease settings (239). The lymphocytes, in turn, come in different ‘flavors’, that is, different functional subsets each one of which may play distinct roles in periodontal disease pathogenesis (Fig. 5). Tregs, for instance, may have a protective role by preventing or mitigating excessive inflammation, whereas T helper cells, especially Th17 cells, can become destructive as they are important sources of potent proinflammatory cytokines and osteoclastogenic factors, including tumor necrosis factor, interleukin-17, and RANKL; the last is also produced in large amounts by activated B and plasma cells (122, 298) (Fig. 4).
Despite the progress made, there are significant gaps in our knowledge on the interrelationships of the periodontitis-involved leukocyte types and how the four lesions evolve. Thus, there are many exciting challenges ahead in the periodontal field. For example, Th17 cells are now implicated as effective B cell helpers for antibody responses linked to autoimmune and inflammatory conditions, such as arthritis (60, 102, 180), although their involvement in the establishment of the B/plasma cell-rich lesions of periodontitis has not been addressed yet. Similar to T lymphocytes, mature macrophages also display heterogeneity and functional versatility (‘plasticity’). However, the role of specific macrophage subsets in periodontitis is largely unexplored. In functional analogy to Tregs, certain populations of B cells, collectively termed regulatory B cells, can inhibit immunopathology by suppressing the expansion of pathogenic T cell subsets such as Th1 and Th17 cells (30, 233). Moreover, another newly discovered subset of B cells in mice and humans, termed age-associated B cells, is thought to represent a memory subset induced by nucleic acid-containing antigens in the context of an inflammatory cytokine environment (196). Age-associated B cells accumulate progressively with age, can have both protective and pathogenic effects (e.g., in autoimmunity) and appear to favor T helper cell polarization to a Th17 profile (97, 196). The existence of age-associated B cells or regulatory B cells (let alone their function) in the periodontium has yet to be addressed. Whereas it has long been known that the numbers of mast cells are elevated in active periodontal lesions (297), they were recently implicated in the pathogenesis of experimental mouse periodontitis (165). As with age-associated B cells, their exact role (e.g., whether they are involved in early or late stages of the disease) remains unknown. Additionally, little is known about newly identified cell types, such as the innate lymphoid cells, a family of innate lymphocytes enriched at mucosal epithelial barrier surfaces, where they have been implicated in both homeostatic immunity and immune pathology. The family comprises three different subsets known as Groups 1, 2, and 3, which mirror the functional characteristics of T helper cell type 1 (Th1), Th2, and Th17 cells, respectively (9). Interleukin-17-expressing innate lymphoid cells have been identified in the human and mouse periodontium (54, 187), although their exact role awaits elucidation.
Another open issue is the dichotomy between homeostatic protective and destructive host responses. The components of these responses and mechanisms that regulate them have not yet been adequately illuminated. If the microbial challenge is controlled by innate and – if necessary – adaptive immune mechanisms, then the inflammation is resolved and tissue healing ensues. In such cases, some initial tissue damage would be a small price to pay. If the inflammatory response, however, does not control the dysbiotic insult, then the stage is set for persistent chronic inflammation and overt periodontitis, owing to collateral release of inflammatory mediators, tissue-destructive enzymes, and cytotoxic substances from the activated innate and adaptive leukocytes, as well as the generation of cleavage products of complement activation that have chemoattractant and cell-activating properties.
In the last 40 years, we came to know more about the ‘bad’ and ‘good’ players in periodontitis but we are still experiencing a journey of unknowns. Indeed, the periodontal research field has generated more questions than provided answers in the past four decades, but this is an inevitable consequence of the progress made: the more we understand, the more we know to ask. Nevertheless, the increased understanding of the cytokine and complement networks for innate-adaptive immune crosstalk, the dissection to some extent of the molecular mechanisms governing the recruitment, activation, and regulation of leukocytes, and insights into the immune regulation of osteoclastogenesis, have produced a number of promising therapeutic targets for human periodontitis on the basis of successful preclinical studies (73, 78, 95, 99, 131, 169, 179, 254, 275).
Acknowledgments
We thank Debbie Maizels (Zoobotanica Scientific Illustration) for redrawing the figures in this paper. The authors’ research is supported by NIH grants; AI068730, DE015254, DE021685, DE024153, DE024716, and DE026152.
References
- 1.Abe T, AlSarhan M, Benakanakere MR, Maekawa T, Kinane DF, Cancro MP, Korostoff JM, Hajishengallis G. The B cell-stimulatory cytokines BLyS and APRIL are elevated in human periodontitis and are required for B cell-dependent bone loss in experimental murine periodontitis. J Immunol. 2015;195:1427–1435. doi: 10.4049/jimmunol.1500496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe T, Hosur KB, Hajishengallis E, Reis ES, Ricklin D, Lambris JD, Hajishengallis G. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189:5442–5448. doi: 10.4049/jimmunol.1202339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allam JP, Duan Y, Heinemann F, Winter J, Gotz W, Deschner J, Wenghoefer M, Bieber T, Jepsen S, Novak N. IL-23-producing CD68(+) macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J Clin Periodontol. 2011;38:879–886. doi: 10.1111/j.1600-051X.2011.01752.x. [DOI] [PubMed] [Google Scholar]
- 6.Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22:775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 7.Arizon M, Nudel I, Segev H, Mizraji G, Elnekave M, Furmanov K, Eli-Berchoer L, Clausen BE, Shapira L, Wilensky A, Hovav AH. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc Natl Acad Sci U S A. 2012;109:7043–7048. doi: 10.1073/pnas.1116770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 9.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 10.Attstrom R, Laurel AB, Lahsson U, Sjoholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J Periodont Res. 1975;10:19–27. doi: 10.1111/j.1600-0765.1975.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 11.Baeza M, Garrido M, Hernandez-Rios P, Dezerega A, Garcia-Sesnich J, Strauss F, Aitken JP, Lesaffre E, Vanbelle S, Gamonal J, Brignardello-Petersen R, Tervahartiala T, Sorsa T, Hernandez M. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: An exploratory study. J Clin Periodontol. 2015 doi: 10.1111/jcpe.12479. [DOI] [PubMed] [Google Scholar]
- 12.Baker PJ, Boutaugh NR, Tiffany M, Roopenian DC. B Cell IgD deletion prevents alveolar bone loss following murine oral infection. Interdiscip Perspect Infect Dis. 2009;2009:864359. doi: 10.1155/2009/864359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 15.Beklen A, Ainola M, Hukkanen M, Gurgan C, Sorsa T, Konttinen YT. MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res. 2007;86:347–351. doi: 10.1177/154405910708600409. [DOI] [PubMed] [Google Scholar]
- 16.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 17.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32(Suppl 6):87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 18.Berglundh T, Donati M, Zitzmann N. B cells in periodontitis: friends or enemies? Periodontol 2000. 2007;45:51–66. doi: 10.1111/j.1600-0757.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 19.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittner-Eddy PD, Fischer LA, Kaplan DH, Thieu K, Costalonga M. Mucosal langerhans cells promote differentiation of Th17 cells in a murine model of periodontitis but are not required for Porphyromonas gingivalis-driven alveolar bone destruction. J Immunol. 2016 doi: 10.4049/jimmunol.1502693. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bluestone JA, Mackay CR, O'Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 23.Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 24.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nat Rev Immunol. 2009;9:657–661. doi: 10.1038/nri2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso CR, Garlet GP, Moreira AP, Junior WM, Rossi MA, Silva JS. Characterization of CD4+CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leukoc Biol. 2008;84:311–318. doi: 10.1189/jlb.0108014. [DOI] [PubMed] [Google Scholar]
- 29.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 32.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen-Kiang S. Regulation of terminal differentiation of human B-cells by IL-6. Curr Top Microbiol Immunol. 1995;194:189–198. doi: 10.1007/978-3-642-79275-5_23. [DOI] [PubMed] [Google Scholar]
- 34.Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;41:541–549. doi: 10.1111/jcpe.12238. [DOI] [PubMed] [Google Scholar]
- 35.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi EY, Lim JH, Neuwirth A, Economopoulou M, Chatzigeorgiou A, Chung KJ, Bittner S, Lee SH, Langer H, Samus M, Kim H, Cho GS, Ziemssen T, Bdeir K, Chavakis E, Koh JY, Boon L, Hosur K, Bornstein SR, Meuth SG, Hajishengallis G, Chavakis T. Developmental endothelial locus-1 is a homeostatic factor in the central nervous system limiting neuroinflammation and demyelination. Mol Psychiatry. 2015;20:880–888. doi: 10.1038/mp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coat J, Demoersman J, Beuzit S, Cornec D, Devauchelle-Pensec V, Saraux A, Pers JO. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. J Clin Periodontol. 2015;42:817–823. doi: 10.1111/jcpe.12433. [DOI] [PubMed] [Google Scholar]
- 40.Cooper PR, Palmer LJ, Chapple IL. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol 2000. 2013;63:165–197. doi: 10.1111/prd.12025. [DOI] [PubMed] [Google Scholar]
- 41.Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977;56:327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- 42.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 43.Cutler CW, Teng YT. Oral mucosal dendritic cells and periodontitis: many sides of the same coin with new twists. Periodontol 2000. 2007;45:35–50. doi: 10.1111/j.1600-0757.2007.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dababneh R, Al-Wahadneh AM, Hamadneh S, Khouri A, Bissada NF. Periodontal manifestation of leukocyte adhesion deficiency type I. J Periodontol. 2008;79:764–768. doi: 10.1902/jop.2008.070323. [DOI] [PubMed] [Google Scholar]
- 45.Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol 2000. 2003;32:82–104. doi: 10.1046/j.0906-6713.2003.03207.x. [DOI] [PubMed] [Google Scholar]
- 46.Deban L, Russo RC, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, Garlanda C, Danese S, Salvatori G, Sassano M, Evangelista V, Rossi B, Zenaro E, Constantin G, Laudanna C, Bottazzi B, Mantovani A. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 47.Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, Graves DT. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol. 2001;28:233–240. doi: 10.1034/j.1600-051x.2001.028003233.x. [DOI] [PubMed] [Google Scholar]
- 48.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- 49.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donati M, Liljenberg B, Zitzmann NU, Berglundh T. B-1a cells and plasma cells in periodontitis lesions. J Periodontal Res. 2009;44:683–688. doi: 10.1111/j.1600-0765.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 51.Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 52.Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. ISME J. 2014;8:1659–1672. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutzan N, Gamonal J, Silva A, Sanz M, Vernal R. Over-expression of forkhead box P3 and its association with receptor activator of nuclear factor-kappa B ligand, interleukin (IL) -17, IL-10 and transforming growth factor-beta during the progression of chronic periodontitis. J Clin Periodontol. 2009;36:396–403. doi: 10.1111/j.1600-051X.2009.01390.x. [DOI] [PubMed] [Google Scholar]
- 54.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016 doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutzan N, Vernal R, Vaque JP, Garcia-Sesnich J, Hernandez M, Abusleme L, Dezerega A, Gutkind JS, Gamonal J. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol. 2012;83:948–954. doi: 10.1902/jop.2011.110482. [DOI] [PubMed] [Google Scholar]
- 56.Ebersole JL, Kirakodu S, Novak MJ, Stromberg AJ, Shen S, Orraca L, Gonzalez-Martinez J, Burgos A, Gonzalez OA. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J Clin Periodontol. 2014;41:853–861. doi: 10.1111/jcpe.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, Akira S, Miyasaka M. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer's patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003;171:1642–1646. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 58.El-Awady AR, Arce RM, Cutler CW. Dendritic cells: microbial clearance via autophagy and potential immunobiological consequences for periodontal disease. Periodontol 2000. 2015;69:160–180. doi: 10.1111/prd.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung KJ, Hashim A, Curtis MA, Chavakis T, Hajishengallis G. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13:465–473. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 61.Fine N, Hassanpour S, Borenstein A, Sima C, Oveisi M, Scholey J, Cherney D, Glogauer M. Distinct oral neutrophil subsets define health and periodontal disease states. J Dental Res. 2016 doi: 10.1177/0022034516645564. [DOI] [PubMed] [Google Scholar]
- 62.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 63.Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujihashi K, Kono Y, Beagley KW, Yamamoto M, McGhee JR, Mestecky J, Kiyono H. Cytokines and periodontal disease: immunopathological role of interleukins for B cell responses in chronic inflamed gingival tissues. J Periodontol. 1993;64:400–406. [PubMed] [Google Scholar]
- 66.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garlet GP. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 69.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 70.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13:17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 71.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 72.Gibson FC, 3rd, Genco CA. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infect Immun. 2001;69:7959–7963. doi: 10.1128/IAI.69.12.7959-7963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci U S A. 2013;110:18525–18530. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzales JR. T- and B-cell subsets in periodontitis. Periodontol 2000. 2015;69:181–200. doi: 10.1111/prd.12090. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez OA, Novak MJ, Kirakodu S, Stromberg A, Nagarajan R, Huang CB, Chen KC, Orraca L, Martinez-Gonzalez J, Ebersole JL. Differential gene expression profiles reflecting macrophage polarization in aging and periodontitis gingival tissues. Immunol Invest. 2015;44:643–664. doi: 10.3109/08820139.2015.1070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 77.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graves DT, Li J, Cochran DL. Inflammation and uncoupling as mechanisms of periodontal bone loss. J Dent Res. 2011;90:143–153. doi: 10.1177/0022034510385236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greer A, Irie K, Hashim A, Leroux BG, Chang AM, Curtis MA, Darveau RP. Site-specific neutrophil migration and CXCL2 expression in periodontal tissue. J Dent Res. 2016 doi: 10.1177/0022034516641036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res. 2014;93:231–237. doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35:3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajishengallis G, Chavakis T. Endogenous modulators of inflammatory cell recruitment. Trends Immunol. 2013;34:1–6. doi: 10.1016/j.it.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 2015;98:539–548. doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hajishengallis G, Lamont RJ. Dancing with the Stars: How choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24:477–89. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hajishengallis G, Lamont RJ, Graves DT. The enduring importance of animal modelsin understanding periodontal disease. Virulence. 2015;6:229–235. doi: 10.4161/21505594.2014.990806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hajishengallis G, Moutsopoulos NM. Etiology of leukocyte adhesion deficiency-associated periodontitis revisited: not a raging infection but a raging inflammatory response. Expert Rev Clin Immunol. 2014;10:973–975. doi: 10.1586/1744666X.2014.929944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hajishengallis G, Moutsopoulos NM. Role of bacteria in leukocyte adhesion deficiency-associated periodontitis. Microb Pathog. 2016;94:21–26. doi: 10.1016/j.micpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hajishengallis G, Moutsopoulos NM, Hajishengallis E, Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. 2016;28:146–158. doi: 10.1016/j.smim.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 95.Han X, Kawai T, Taubman MA. Interference with immune-cell-mediated bone resorption in periodontal disease. Periodontol 2000. 2007;45:76–94. doi: 10.1111/j.1600-0757.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 96.Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. 2012;1250:50–55. doi: 10.1111/j.1749-6632.2011.06389.x. [DOI] [PubMed] [Google Scholar]
- 97.Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harada Y, Han X, Yamashita K, Kawai T, Eastcott JW, Smith DJ, Taubman MA. Effect of adoptive transfer of antigen-specific B cells on periodontal bone resorption. J Periodontal Res. 2006;41:101–107. doi: 10.1111/j.1600-0765.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 99.Hasturk H, Kantarci A, Van Dyke TE. Paradigm shift in the pharmacological management of periodontal diseases. Front Oral Biol. 2012;15:160–176. doi: 10.1159/000329678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 101.Hernandez M, Gamonal J, Tervahartiala T, Mantyla P, Rivera O, Dezerega A, Dutzan N, Sorsa T. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. J Periodontol. 2010;81:1644–1652. doi: 10.1902/jop.2010.100196. [DOI] [PubMed] [Google Scholar]
- 102.Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun. 2011;36:65–75. doi: 10.1016/j.jaut.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL. Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem. 1997;272:22053–22058. doi: 10.1074/jbc.272.35.22053. [DOI] [PubMed] [Google Scholar]
- 104.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10:103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huntington ND, Carpentier S, Vivier E, Belz GT. Innate lymphoid cells: parallel checkpoints and coordinate interactions with T cells. Curr Opin Immunol. 2016;38:86–93. doi: 10.1016/j.coi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 108.Ito H, Honda T, Domon H, Oda T, Okui T, Amanuma R, Nakajima T, Yamazaki K. Gene expression analysis of the CD4+ T-cell clones derived from gingival tissues of periodontitis patients. Oral Microbiol Immunol. 2005;20:382–386. doi: 10.1111/j.1399-302X.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 109.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 110.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J Clin ;nvest. 120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jarry CR, Martinez EF, Peruzzo DC, Carregaro V, Sacramento LA, Araujo VC, Weitzmann MN, Napimoga MH. Expression of SOFAT by T- and B-lineage cells may contribute to bone loss. Mol Med Rep. 2016;13:4252–4258. doi: 10.3892/mmr.2016.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joachim F, Barber P, Newman HN, Osborn J. The plasma cell at the advancing front of the lesion in chronic periodontitis. J Periodontal Res. 1990;25:49–59. doi: 10.1111/j.1600-0765.1990.tb01206.x. [DOI] [PubMed] [Google Scholar]
- 114.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 115.Jonsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011;46:153–157. doi: 10.1111/j.1600-0765.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 116.Jusko M, Potempa J, Karim AY, Ksiazek M, Riesbeck K, Garred P, Eick S, Blom AM. A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J Immunol. 2012;188:2338–2349. doi: 10.4049/jimmunol.1101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kajiya M, Giro G, Taubman MA, Han X, Mayer MPA, Kawai T. Role of periodontal pathogenic bacteria in RANKL-mediated bone destruction in periodontal disease. J Oral Microbiol. 2010;2:5532. doi: 10.3402/jom.v2i0.5532. doi:5510.3402/jom.v5532i5530.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kalyan S, Kabelitz D. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol. 2014;44:627–633. doi: 10.1002/eji.201344195. [DOI] [PubMed] [Google Scholar]
- 119.Kanzaki H, Chiba M, Shimizu Y, Mitani H. Dual regulation of osteoclast differentiation by periodontal ligament cells through RANKL stimulation and OPG inhibition. J Dent Res. 2001;80:887–891. doi: 10.1177/00220345010800030801. [DOI] [PubMed] [Google Scholar]
- 120.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katz J, Black KP, Michalek SM. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect Immun. 1999;67:4352–4359. doi: 10.1128/iai.67.9.4352-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I, Mizukami S, Maiya N, Yasuda H, Kumanogoh A, Kikuchi K, Germain RN, Ishii M. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest. 2013;123:866–873. doi: 10.1172/JCI65054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim PD, Xia-Juan X, Crump KE, Abe T, Hajishengallis G, Sahingur SE. Toll-like receptor 9-mediated inflammation triggers alveolar bone loss in experimental murine periodontitis. Infect Immun. 2015;83:2992–3002. doi: 10.1128/IAI.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim YC, Ko Y, Hong SD, Kim KY, Lee YH, Chae C, Choi Y. Presence of Porphyromonas gingivalis and plasma cell dominance in gingival tissues with periodontitis. Oral Dis. 2010;16:375–381. doi: 10.1111/j.1601-0825.2009.01649.x. [DOI] [PubMed] [Google Scholar]
- 128.Kinane DF, Demuth DR, Gorr SU, Hajishengallis GN, Martin MH. Human variability in innate immunity. Periodontol 2000. 2007;45:14–34. doi: 10.1111/j.1600-0757.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 129.Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430–449. doi: 10.1177/154411130301400605. [DOI] [PubMed] [Google Scholar]
- 130.Kinane DF, Mooney J, Ebersole JL. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 131.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 133.Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, Fujihashi K, Ruby J, Kataoka K, Wada M, Yamamoto M, Fujihashi K. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res. 2011;90:653–658. doi: 10.1177/0022034510397838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 135.Kometani K, Kurosaki T. Differentiation and maintenance of long-lived plasma cells. Curr Opin Immunol. 2015;33:64–69. doi: 10.1016/j.coi.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 136.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 137.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 138.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kotake S, Yago T, Kawamoto M, Nanke Y. Role of osteoclasts and interleukin-17 in the pathogenesis of rheumatoid arthritis: crucial 'human osteoclastology'. J Bone Miner Metab. 2012;30:125–135. doi: 10.1007/s00774-011-0321-5. [DOI] [PubMed] [Google Scholar]
- 140.Lakschevitz FS, Aboodi GM, Glogauer M. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS One. 2013;8:e68983. doi: 10.1371/journal.pone.0068983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lally ET, McArthur WP, Baehni PC. Biosynthesis of complement components in chronically inflamed gingiva. J Periodontal Res. 1982;17:257–262. doi: 10.1111/j.1600-0765.1982.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 142.Lam RS, O'Brien-Simpson NM, Lenzo JC, Holden JA, Brammar GC, Walsh KA, McNaughtan JE, Rowler DK, Van Rooijen N, Reynolds EC. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. J Immunol. 2014;193:2349–2362. doi: 10.4049/jimmunol.1400853. [DOI] [PubMed] [Google Scholar]
- 143.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M. Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J Periodontal Res. 2014 doi: 10.1111/jre.12211. [DOI] [PubMed] [Google Scholar]
- 145.Larjava H, Koivisto L, Heino J, Hakkinen L. Integrins in periodontal disease. Exp Cell Res. 2014;325:104–110. doi: 10.1016/j.yexcr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 146.Larsson L, Castilho RM, Giannobile WV. Epigenetics and its role in periodontal diseases: a state-of-the-art review. J Periodontol. 2015;86:556–568. doi: 10.1902/jop.2014.140559. [DOI] [PubMed] [Google Scholar]
- 147.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 148.Lester SR, Bain JL, Johnson RB, Serio FG. Gingival concentrations of interleukin-23 and -17 at healthy sites and at sites of clinical attachment loss. J Periodontol. 2007;78:1545–1550. doi: 10.1902/jop.2007.060458. [DOI] [PubMed] [Google Scholar]
- 149.Lewkowicz N, Mycko MP, Przygodzka P, Cwiklinska H, Cichalewska M, Matysiak M, Selmaj K, Lewkowicz P. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.66. [DOI] [PubMed] [Google Scholar]
- 150.Li KL, Vogel R, Jeffcoat MK, Alfano MC, Smith MA, Collins JG, Offenbacher S. The effect of ketoprofen creams on periodontal disease in rhesus monkeys. J Periodontal Res. 1996;31:525–532. doi: 10.1111/j.1600-0765.1996.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 151.Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin D, Li L, Sun Y, Wang W, Wang X, Ye Y, Chen X, Xu Y. IL-17 regulates the expressions of RANKL and OPG in human periodontal ligament cells via TRAF6/TBK1-JNK/NF-kappaB pathways. Immunology. 2014 doi: 10.1111/imm.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infect Immun. 2011;79:911–917. doi: 10.1128/IAI.00944-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ling MR, Chapple IL, Matthews JB. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015 doi: 10.1177/1753425915589387. [DOI] [PubMed] [Google Scholar]
- 156.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 157.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 158.Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maekawa T, Briones RA, Resuello RR, Tuplano JV, Hajishengallis E, Kajikawa T, Koutsogiannaki S, Garcia CA, Ricklin D, Lambris JD, Hajishengallis G. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. J Clin Periodontol. 2016;43:238–249. doi: 10.1111/jcpe.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maekawa T, Hajishengallis G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J Periodontal Res. 2014 doi: 10.1111/jre.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maekawa T, Hosur K, Abe T, Kantarci A, Ziogas A, Wang B, Van Dyke TE, Chavakis T, Hajishengallis G. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nat Commun. 2015;6:8272. doi: 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, Lambris JD, Hajishengallis G. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maffia PC, Zittermann SE, Scimone ML, Tateosian N, Amiano N, Guerrieri D, Lutzky V, Rosso D, Romeo HE, Garcia VE, Issekutz AC, Chuluyan HE. Neutrophil elastase converts human immature dendritic cells into transforming growth factor-beta1-secreting cells and reduces allostimulatory ability. Am J Pathol. 2007;171:928–937. doi: 10.2353/ajpath.2007.061043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mahanonda R, Champaiboon C, Subbalekha K, Sa-Ard-Iam N, Rattanathammatada W, Thawanaphong S, Rerkyen P, Yoshimura F, Nagano K, Lang NP, Pichyangkul S. Human Memory B Cells in Healthy Gingiva, Gingivitis, and Periodontitis. J Immunol. 2016 doi: 10.4049/jimmunol.1600540. [DOI] [PubMed] [Google Scholar]
- 165.Malcolm J, Millington O, Millhouse E, Campbell L, Adrados Planell A, Butcher JP, Lawrence C, Ross K, Ramage G, McInnes IB, Culshaw S. Mast Cells contribute to Porphyromonas gingivalis-induced bone loss. J Dent Res. 2016 doi: 10.1177/0022034516634630. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 166.Malm S, Jusko M, Eick S, Potempa J, Riesbeck K, Blom AM. Acquisition of complement inhibitor serine protease factor I and its cofactors C4b-binding protein and factor H by Prevotella intermedia. PLoS One. 2012;7:e34852. doi: 10.1371/journal.pone.0034852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 168.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 169.Mastellos DC, Ricklin D, Hajishengallis E, Hajishengallis G, Lambris JD. Complement therapeutics in inflammatory diseases: promising drug candidates for C3-targeted intervention. Mol Oral Microbiol. 2016;31:3–17. doi: 10.1111/omi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Matsuda T, Yamasaki K, Taga T, Hirano T, Kishimoto T. Current concepts of B cell modulation. Int Rev Immunol. 1989;5:97–109. doi: 10.3109/08830188909061976. [DOI] [PubMed] [Google Scholar]
- 171.Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple IL. Neutrophil hyper-responsiveness in periodontitis. J Dent Res. 2007;86:718–722. doi: 10.1177/154405910708600806. [DOI] [PubMed] [Google Scholar]
- 172.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 173.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 175.Meyle J, Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 2015;69:7–17. doi: 10.1111/prd.12104. [DOI] [PubMed] [Google Scholar]
- 176.Miller DP, Bell JK, McDowell JV, Conrad DH, Burgner JW, Heroux A, Marconi RT. Structure of factor H-binding protein B (FhbB) of the periopathogen, Treponema denticola: insights into progression of periodontal disease. J Biol Chem. 2012;287:12715–12722. doi: 10.1074/jbc.M112.339721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 178.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 179.Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mizutani Y, Tsuge S, Takeda H, Hasegawa Y, Shiogama K, Onouchi T, Inada K, Sawasaki T, Tsutsumi Y. In situ visualization of plasma cells producing antibodies reactive to Porphyromonas gingivalis in periodontitis: the application of the enzyme-labeled antigen method. Mol Oral Microbiol. 2014;29:156–173. doi: 10.1111/omi.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mohamed SG, Sugiyama E, Shinoda K, Taki H, Hounoki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Ogawa H, Miyahara T. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW264. 7 cells and mouse bone marrow cells. Bone. 2007;41:592–602. doi: 10.1016/j.bone.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 184.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moutsopoulos NM, Chalmers NI, Barb JJ, Abusleme L, Greenwell-Wild T, Dutzan N, Paster BJ, Munson PJ, Fine DH, Uzel G, Holland SM. Subgingival microbial communities in leukocyte adhesion deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11:e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39:294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Sci Transl Med. 2014;6:229ra240. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Moutsopoulos NM, Lionakis MS, Hajishengallis G. Inborn errors in immunity: unique natural models to dissect oral immunity. J Dent Res. 2015;94:753–758. doi: 10.1177/0022034515583533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, Kropf P, Mueller I, Kolb A, Modolell M, Ho AD. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 190.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakajima T, Amanuma R, Ueki-Maruyama K, Oda T, Honda T, Ito H, Yamazaki K. CXCL13 expression and follicular dendritic cells in relation to B-cell infiltration in periodontal disease tissues. J Periodontal Res. 2008;43:635–641. doi: 10.1111/j.1600-0765.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 193.Nakajima T, Ueki-Maruyama K, Oda T, Ohsawa Y, Ito H, Seymour GJ, Yamazaki K. Regulatory T-cells infiltrate periodontal disease tissues. J Dent Res. 2005;84:639–643. doi: 10.1177/154405910508400711. [DOI] [PubMed] [Google Scholar]
- 194.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23:582–590. doi: 10.1016/j.tem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 195.Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 196.Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016;269:118–129. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- 197.Niederkorn JY, Stein-Streilein J. History and physiology of immune privilege. Ocul Immunol Inflamm. 2010;18:19–23. doi: 10.3109/09273940903564766. [DOI] [PubMed] [Google Scholar]
- 198.Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J Periodontal Res. 1985;20:268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 199.Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- 200.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–677. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 201.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 202.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013 doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 203.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Odobasic D, Kitching AR, Yang Y, O'Sullivan KM, Muljadi RC, Edgtton KL, Tan DS, Summers SA, Morand EF, Holdsworth SR. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood. 2013;121:4195–4204. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 205.Offenbacher S, Braswell LD, Loos AS, Johnson HG, Hall CM, McClure H, Orkin JL, Strobert EA, Green MD, Odle BM. Effects of flurbiprofen on the progression of periodontitis in Macaca mulatta. J Periodontal Res. 1987;22:473–481. doi: 10.1111/j.1600-0765.1987.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 206.Offenbacher S, Divaris K, Barros SP, Moss KL, Marchesan JT, Morelli T, Zhang S, Kim S, Sun L, Beck JD, Laudes M, Munz M, Schaefer AS, North KE. Genome-wide association study of biologically-informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, Yamada N, Hata M, Yamane J, Terada N. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res. 2009;88:633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 208.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–579. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 209.Oliver-Bell J, Butcher JP, Malcolm J, MacLeod MK, Adrados Planell A, Campbell L, Nibbs RJ, Garside P, McInnes IB, Culshaw S. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol Oral Microbiol. 2015;30:160–169. doi: 10.1111/omi.12082. [DOI] [PubMed] [Google Scholar]
- 210.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Page RC, Lantz MS, Darveau R, Jeffcoat M, Mancl L, Houston L, Braham P, Persson GR. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral Microbiol Immunol. 2007;22:162–168. doi: 10.1111/j.1399-302X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 212.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 213.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- 214.Page RC, Schroeder HE. Current status of the host response in chronic marginal periodontitis. J Periodontol. 1981;52:477–491. doi: 10.1902/jop.1981.52.9.477. [DOI] [PubMed] [Google Scholar]
- 215.Papadopoulos G, Weinberg EO, Massari P, Gibson FC, 3rd, Wetzler LM, Morgan EF, Genco CA. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol. 2013;190:1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Parachuru VP, Coates DE, Milne TJ, Hussaini HM, Rich AM, Seymour GJ. Forkhead box P3-positive regulatory T-cells and interleukin 17-positive T-helper 17 cells in chronic inflammatory periodontal disease. J Periodontal Res. 2014;49:817–826. doi: 10.1111/jre.12169. [DOI] [PubMed] [Google Scholar]
- 217.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J Clin Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- 219.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2009;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 220.Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page RC. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–1031. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. γ δ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 225.Potempa M, Potempa J, Kantyka T, Nguyen KA, Wawrzonek K, Manandhar SP, Popadiak K, Riesbeck K, Eick S, Blom AM. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009;5:e1000316. doi: 10.1371/journal.ppat.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rajapakse PS, O'Brien-Simpson NM, Slakeski N, Hoffmann B, Reynolds EC. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infect Immun. 2002;70:2480–2486. doi: 10.1128/IAI.70.5.2480-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 228.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rifas L, Weitzmann MN. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009;60:3324–3335. doi: 10.1002/art.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Roberts HM, Ling MR, Insall R, Kalna G, Spengler J, Grant MM, Chapple IL. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J Clin Periodontol. 2015;42:1–11. doi: 10.1111/jcpe.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Roes J, Rajewsky K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J Exp Med. 1993;177:45–55. doi: 10.1084/jem.177.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 234.Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Ann N Y Acad Sci. 2013;1285:97–114. doi: 10.1111/nyas.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rotondo R, Bertolotto M, Barisione G, Astigiano S, Mandruzzato S, Ottonello L, Dallegri F, Bronte V, Ferrini S, Barbieri O. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–727. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 236.Ryder MI. Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000. 2010;53:124–137. doi: 10.1111/j.1600-0757.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- 237.Sadallah S, Eken C, Schifferli JA. Ectosomes as immunomodulators. Semin Immunopathol. 2011;33:487–495. doi: 10.1007/s00281-010-0232-x. [DOI] [PubMed] [Google Scholar]
- 238.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Scapini P, Bazzoni F, Cassatella MA. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol Lett. 2008;116:1–6. doi: 10.1016/j.imlet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 240.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, Tamassia N, Pieropan S, Biasi D, Sbarbati A, Sozzani S, Bambara L, Cassatella MA. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 241.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 242.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Schenkein HA. Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000. 2006;40:77–93. doi: 10.1111/j.1600-0757.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 244.Schenkein HA, Fletcher HM, Bodnar M, Macrina FL. Increased opsonization of a prtH-defective mutant of Porphyromonas gingivalis W83 is caused by reduced degradation of complement-derived opsonins. J Immunol. 1995;154:5331–5337. [PubMed] [Google Scholar]
- 245.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. I. Quantitative study of immunoglobulins, complement components, and other plasma proteins. J Periodontol. 1977;48:772–777. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- 246.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- 247.Schenkein HA, Genco RJ. Complement cleavage products in inflammatory exudates from patients with periodontal diseases. J Immunol. 1978;120:1796. [Google Scholar]
- 248.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2012;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 250.Seguier S, Godeau G, Brousse N. Collagen fibers and inflammatory cells in healthy and diseased human gingival tissues: a comparative and quantitative study by immunohistochemistry and automated image analysis. J Periodontol. 2000;71:1079–1085. doi: 10.1902/jop.2000.71.7.1079. [DOI] [PubMed] [Google Scholar]
- 251.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shaker OG, Ghallab NA. IL-17 and IL-11 GCF levels in aggressive and chronic periodontitis patients: relation to PCR bacterial detection. Mediators Inflamm. 2012;2012:174764. doi: 10.1155/2012/174764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shin J, Hosur KB, Pyaram K, Jotwani R, Liang S, Chavakis T, Hajishengallis G. Expression and function of the homeostatic molecule Del-1 in endothelial cells and the periodontal tissue. Clin Dev Immunol. 2013 doi: 10.1155/2013/617809. Article ID 617809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shin J, Maekawa T, Abe T, Hajishengallis E, Hosur K, Pyaram K, Mitroulis I, Chavakis T, Hajishengallis G. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Sci Transl Med. 2015;7:307ra155. doi: 10.1126/scitranslmed.aac5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernandez M, Gamonal J. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23:329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sima C, Glogauer M. Neutrophil dysfunction and host susceptibility to periodontal inflammation: Current state of knowledge. Curr Oral Health Rep. 2014;1:95–103. [Google Scholar]
- 258.Sollecito TP, Sullivan KE, Pinto A, Stewart J, Korostoff J. Systemic conditions associated with periodontitis in childhood and adolescence. A review of diagnostic possibilities. Med Oral Patol Oral Cir Bucal. 2005;10:142–150. [PubMed] [Google Scholar]
- 259.Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 261.Steinmetz O, Hoch S, Avniel-Polak S, Gavish K, Eli-Berchoer L, Wilensky A, Nussbaum G. CX3CR1hi monocytes support bacterial survival and experimental infection-driven bone resorption. J Infect Dis. 2015 doi: 10.1093/infdis/jiv763. [DOI] [PubMed] [Google Scholar]
- 262.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86:1105–1109. doi: 10.1189/jlb.0209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 265.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 266.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–374. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 269.Tamassia N, Zimmermann M, Castellucci M, Ostuni R, Bruderek K, Schilling B, Brandau S, Bazzoni F, Natoli G, Cassatella MA. Cutting edge: An inactive chromatin configuration at the IL-10 locus in human neutrophils. J Immunol. 2013;190:1921–1925. doi: 10.4049/jimmunol.1203022. [DOI] [PubMed] [Google Scholar]
- 270.Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, Zheng SG, Sun B, Ryffel B. RORγt+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 272.Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Teng YTA. Protective and destructive immunity in the periodontium: Part 2--T-cell-mediated immunity in the periodontium. J Dent Res. 2006;85:209–219. doi: 10.1177/154405910608500302. [DOI] [PubMed] [Google Scholar]
- 274.Thorbert-Mros S, Larsson L, Berglundh T. Cellular composition of long-standing gingivitis and periodontitis lesions. J Periodontal Res. 2015;50:535–543. doi: 10.1111/jre.12236. [DOI] [PubMed] [Google Scholar]
- 275.Tonetti MS, Chapple IL. Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(Suppl 11):114–118. doi: 10.1111/j.1600-051X.2010.01675.x. [DOI] [PubMed] [Google Scholar]
- 276.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 277.Toto PD, Lin L, Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J Dent Res. 1978;57:696. doi: 10.1177/00220345780570050501. [DOI] [PubMed] [Google Scholar]
- 278.Toto PD, Lin LM, Gargiulo AW. Immunoglobulins and complement in human periodontitis. J Periodontol. 1978;49:631–634. doi: 10.1902/jop.1978.49.12.631. [DOI] [PubMed] [Google Scholar]
- 279.Tseng CW, Liu GY. Expanding roles of neutrophils in aging hosts. Curr Opin Immunol. 2014;29:43–48. doi: 10.1016/j.coi.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 280.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 281.Van Dyke TE. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 2011;38(Suppl 11):119–125. doi: 10.1111/j.1600-051X.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 282.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 283.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 284.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Waldrop TC, Anderson DC, Hallmon WW, Schmalstieg FC, Jacobs RL. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J Periodontol. 1987;58:400–416. doi: 10.1902/jop.1987.58.6.400. [DOI] [PubMed] [Google Scholar]
- 286.Wang J, Shiratori I, Uehori J, Ikawa M, Arase H. Neutrophil infiltration during inflammation is regulated by PILRalpha via modulation of integrin activation. Nat Immunol. 2013;14:34–40. doi: 10.1038/ni.2456. [DOI] [PubMed] [Google Scholar]
- 287.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Whitney C, Ant J, Moncla B, Johnson B, Page RC, Engel D. Serum immunoglobulin G antibody to Porphyromonas gingivalis in rapidly progressive periodontitis: titer, avidity, and subclass distribution. Infect Immun. 1992;60:2194–2200. doi: 10.1128/iai.60.6.2194-2200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wilensky A, Chaushu S, Shapira L. The role of natural killer cells in periodontitis. Periodontol 2000. 2015;69:128–141. doi: 10.1111/prd.12092. [DOI] [PubMed] [Google Scholar]
- 291.Wilensky A, Segev H, Mizraji G, Shaul Y, Capucha T, Shacham M, Hovav AH. Dendritic cells and their role in periodontal disease. Oral Dis. 2014;20:119–126. doi: 10.1111/odi.12122. [DOI] [PubMed] [Google Scholar]
- 292.Xiao W, Dong G, Pacios S, Alnammary M, Barger LA, Wang Y, Wu Y, Graves DT. FOXO1 deletion reduces dendritic cell function and enhances susceptibility to periodontitis. Am J Pathol. 2015;185:1085–1093. doi: 10.1016/j.ajpath.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yamashita K, Eastcott JW, Taubman MA, Smith DJ, Cox DS. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect Immun. 1991;59:1529–1534. doi: 10.1128/iai.59.4.1529-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Younes R, Ghorra C, Khalife S, Igondjo-Tchen-Changotade S, Yousfi M, Willig C, Senni K, Godeau G, Naaman N. Pertinent cell population to characterize periodontal disease. Tissue Cell. 2009;41:141–150. doi: 10.1016/j.tice.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 296.Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol. 2016:1–21. doi: 10.1902/jop.2016.160081. [DOI] [PubMed] [Google Scholar]
- 297.Zappa U, Reinking-Zappa M, Graf H, Espeland M. Cell populations and episodic periodontal attachment loss in humans. J Clin Periodontol. 1991;18:508–515. doi: 10.1111/j.1600-051x.1991.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 298.Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zenobia C, Luo XL, Hashim A, Abe T, Jin L, Chang Y, Jin ZC, Sun JX, Hajishengallis G, Curtis MA, Darveau RP. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15:1419–1426. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhan Y, Zhang R, Lv H, Song X, Xu X, Chai L, Lv W, Shang Z, Jiang Y, Zhang R. Prioritization of candidate genes for periodontitis using multiple computational tools. J Periodontol. 2014;85:1059–1069. doi: 10.1902/jop.2014.130523. [DOI] [PubMed] [Google Scholar]
- 301.Zhao L, Zhou Y, Xu Y, Sun Y, Li L, Chen W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. J Clin Periodontol. 2011;38:509–516. doi: 10.1111/j.1600-051X.2011.01712.x. [DOI] [PubMed] [Google Scholar]
- 302.Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, Gyurko R, Nikolajczyk BS. B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J Leukoc Biol. 2014;96:349–357. doi: 10.1189/jlb.4A0214-095R. [DOI] [PMC free article] [PubMed] [Google Scholar]