Abstract
Polysialic acid (PSA) is a large, negatively charged, linear homopolymer of alpha2-8-linked sialic acid residues. It is generated by two polysialyltransferases and attached to N- and/or O-linked glycans, and its main carrier is the neural cell adhesion molecule NCAM. PSA controls the development and regeneration of the nervous system by enhancing cell migration, axon path finding, synaptic targeting, synaptic plasticity, by regulating the differentiation of progenitor cells and by modulating cell-cell and cell-matrix adhesions. In the adult, PSA plays a role in the immune system, and PSA mimetics promote functional recovery after nervous system injury. In search for novel small molecule mimetics of PSA that are applicable for therapy, we identified idarubicin, an antineoplastic anthracycline, and irinotecan, an antineoplastic agent of the topoisomerase I inhibitor class, as PSA mimetics using a competition enzyme-linked immunosorbent assay. Idarubicin and irinotecan compete with the PSA-mimicking peptide and colominic acid, the bacterial analogue of PSA, for binding to the PSA-specific monoclonal antibody 735. Idarubicin and irinotecan stimulate neurite outgrowth and survival of cultured cerebellar neurons after oxidative stress via protein kinase C and Erk1/2 in a similar manner as colominic acid, whereas Fyn, casein kinase II and the phosphatase and tensin homolog PTEN are only involved in idarubicin and irinotecan-stimulated neurite outgrowth. These novel results show that the structure and function of PSA can be mimicked by the small organic compounds irinotecan and idarubicin which trigger the same signaling cascades as PSA, thus introducing the possibility of retargeting these drugs to treat nervous system injuries.
Keywords: irinotecan, idarubicin, polysialic acid, neurite outgrowth, cell survival
Introduction
Polysialylation is a posttranslational modification executed by two polysialyltransferases (Rutishauser 2008). The polysialyltransferases generate a long homopolymer of α2,8-linked N-acetylneuraminic acid units, which is mostly attached to the neural cell adhesion molecule NCAM. Less prominent carriers of PSA are SynCAM-1, the polysialyltransferase ST8SiaII, neuropilin-2, the chemokine receptor CCR7, and the scavenger receptor CD36 (Mühlenhoffet al. 2013; Hildebrandt and Dityatev 2015; Kiermaier et al. 2016). Polysialylation mainly controls the developmental plasticity of the vertebrate nervous system by modulating neural cell migration, axon path finding and synaptic targeting (Schnaar et al. 2014). In the adult nervous system, the expression of PSA becomes restricted to regions of neuronal and glial plasticity, such as the dentate gyrus of the hippocampus, where it enables synaptic plasticity (Rutishauser 2008; Bonfanti and Theodosis 2009; Senkov et al. 2012). PSA attached to a transmembrane proteolytic NCAM fragment was shown to enter the cell nucleus of cultured cerebellar granule neurons and of neurons in different brain regions of adult mice where PSA-carrying NCAM contributed to the regulation of clock-related gene expression and of the circadian rhythm (Westphal et al. 2016). Recent evidence also suggests an involvement of PSA during immune responses (Curelli et al. 2007; Drake et al., 2008; Rey-Gallardo et al., 2010; Kiermaier et al. 2016). Furthermore, PSA is aberrantly re-expressed on many tumors of neuroendocrine origin and promotes cancer growth and metastasis by a mechanism that has yet to be described (Suzuki et al. 2005; Falconer et al. 2012). A transient re-expression of PSA was also detected in neurons and glial cells in different lesion models using adult animals (Brezun and Daszuta 2000; Bonfanti 2006) and levels and localization of PSA were found to be critical for early peripheral nerve regeneration (Jungnickel et al. 2009). Re-introduction of PSA into the adult nervous system by transducing the tissue with PSA, by injecting lentiviral vectors, or by implanting PSA-overexpressing Schwann cells into the spinal cord after injury, enhanced regeneration of sensory axons. Furthermore, PSA-overexpressing Schwann cells showed improved migration and promoted axonal regeneration, remyelination and functional recovery (Lavdas et al. 2006; Zhang et al. 2007; Luo et al. 2011; Ghosh et al. 2012). Similarly, application of PSA mimicking peptides or small molecules improved functional recovery after spinal cord and femoral nerve injury (Marino et al. 2009; Mehanna et al. 2009, 2010; Bushman et al. 2014; Pan et al. 2014; Saini et al. 2016). In addition, it is assumed that the increased expression of PSA in the hippocampus and entorhinal cortex of patients suffering from temporal lobe epilepsy, as well as in the molecular layer of the dentate gyrus in patients with Alzheimer's disease indicates attempts of repair and disease-related plasticity (Mikkonen et al. 1998, 1999). These favorable effects of PSA overproduction and appliction of PSA mimetics make this carbohydrate a promising candidate for the development of novel therapeutic strategies to treat nervous system injuries and diseases.
Treatment with PSA transducing viruses or application of PSA expressing cells as a therapeutic strategy has the disadvantage that PSA can be cleaved in vivo by neuraminidases and sialidases, such as sialidase NEU4, which is highly expressed in the central nervous system (Takahashi et al. 2012). Furthermore, PSA is difficult to isolate, purify or produce from biological sources, especially when a defined PSA with a certain number of sialic acid residues is needed. Peptide mimetics of PSA discovered by screening of phage display libraries with PSA monoclonal antibodies (Torregrossa et al. 2004; Mehanna et al. 2009) promoted functional recovery and plasticity after injury of the murine peripheral and central nervous systems (Marino et al. 2009; Mehanna et al. 2009, 2010). However, peptides can be more instable in vivo and display a short half-life due to enzymatic degradation by proteases and fast renal clearance (Sato et al. 2006; Li et al. 2015; Penchala et al. 2015). Therefore, we searched for novel, small organic compounds that mimic PSA structurally and functionally and are approved pharmaceuticals. These compounds are assumed to provide a longer half-life, stability and safety and can be easier and cheaper to synthesize. Their toxicology is known for certain indications and re-purposing of these drugs should allow a facilitated procedure for therapeutic acceptance. We assumed that these compounds will trigger the same beneficial functions as PSA in vitro and in vivo and that they will signal via the same pathways as PSA. We identified idarubicin, a clinically effective synthetic anthracycline analog used in the treatment of several human neoplasms, and irinotecan, an antineoplastic agent of the topoisomerase I inhibitor class used for treatment of small cell lung cancer and advanced colorectal cancer, as novel PSA mimetics and tested their function and signaling pathways in vitro using cultures of murine and rat primary neurons of central nervous system origin. Our results show that idarubicin and irinotecan bind to the PSA-specific monoclonal antibody 735, modulate in vitro outgrowth and survival of cerebellar granule neurons in a manner similar to colominic acid, the bacterial analogue of PSA, and signal via protein kinase C and extracellular regulated kinase 1/2 to stimulate neuronal survival and neurite outgrowth. Additionally, Scr family kinases, casein kinase II and the phosphatase and tensin homolog PTEN are involved in the induction of neurite outgrowth. These novel results show that the structure and function of PSA can be mimicked by the small organic compounds irinotecan and idarubicin and that these compounds trigger the same intracellular signaling cascades as PSA to promote neurite outgrowth and neuronal survival.
Materials and Methods
Antibodies and reagents
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) if not indicated otherwise. (7S,9S)-9-acetyl-7-(4-amino-5-hydroxy-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione hydrochloride (idarubicin hydrochloride; idarubicin), (S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3, 14-dioxo-1 H-pyrano [3′,4′:6,7] indolizino [1,2-b] quinolin-9-yl-[1,4′-bipiperidine]-1′-carboxylate monohydrochloride trihydrate (irinotecan hydrochloride; irinotecan), (7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione hydrochloride (epirubicin hydrochloride; epirubicin), Scr and Abl inhibitor 1-cyclopentyl-3-(1H-pyrrolo[2,3-b]pyridin-5-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP121), v-Scr and c-Fyn inhibitor 1-(1,1-dimethylethyl)-3-(1-naphthalenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1-naphthyl PP1) and PKA inhibitor (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT 5720) were obtained from Tocris Bioscience (Bristol, UK). The PSA mimicking peptide (NTHTDPYIYPID; Mehanna et al. 2009) and the scrambled control peptide were obtained from Schafer-N (Copenhagen, Denmark); ortho-phenylenediamine dihydrochloride (OPD), calcein-AM and propidium iodide were from ThermoFisher Scientific (Waltham, MA). Protein kinase C inhibitor 2,2′,3,3′,4,4′-hexahydroxy-1,1′-biphenyl-6,6′-dimethanol-dimethyl ether (HBDDE), casein kinase II inhibitor (E)-3-(2,3,4,5-tetrabromophenyl)acrylic acid (TBCA), Erk inhibitor 1-nitro-2-[(Z)-[5-(3-nitrophenyl)furan-2-yl]methylideneamino]guanidine (Erk inhibitor III), phosphatase and tensin homolog (PTEN) and protein phosphotyrosine phosphatase (PTP) inhibitor dipotassium bisperoxo (5-hydroxypyridine-2-carboxyl) oxovanadate (bpv(HOpic)) and were purchased from Santa Cruz Biotechnology (Dallas, TX). The NIH Clinical Collection 1 library was obtained from the National Institutes of Health (Bethesda, MD). PSA-specific monoclonal antibody 735 (RRID:AB_2619682) was a kind gift of Rita Gerardy-Schahn (Department of Biochemistry, Institute for Cellular Chemistry, Hannover Medical School, Hannover, Germany) and secondary anti-mouse antibodies coupled to horse radish peroxidase or Alexa488 (cat# 715-035-150, RRID:AB_2340770; cat# 715-545-150, RRID:AB_2340846) were obtained from Jackson ImmunoResearch (West Grove, PA). Goat anti-NCAM antibody (SAB2501672) was from Sigma-Aldrich (St. Louis, MO), rabbit anti-GAPDH antibody (cat# sc-25778; RRID:AB_10167668) was from Santa Cruz Biotechnology (Heidelberg, Germany) and anti-goat antibody coupled to Cy3 (cat# 705-165-147; RRID:AB_2307351), and anti-rabbit and anti-goat antibodies coupled to HRP (cat# 711-035-152, RRID:AB_10015282; cat# 705-035-147, RRID:AB_2313587) were from Jackson-ImmunoResearch. The Annexin V-FITC Apoptosis detection kit was from Miltenyi Biotec (San Diego, CA).
Animals and cell lines
Postnatal day 5-7 (P5-P7) old Sprague-Dawley (SD) rats (RRID:RGD_7246927) were ordered from Taconic Farms Inc. (Germantown, NY). Six-week-old CB6F1/J mice (RRID:IMSR_JAX:100007) were ordered from Jackson Laboratory (Bar Harbor, ME). C57BL/6J mice (RRID:IMSR_JAX:000664) were obtained from the central breeding facility of the University Medical Center Hamburg-Eppendorf. Mice were maintained for breeding P6-P7 old offspring with ad libitum access to food and water and a 12 hour light and 12 hour dark cycle in the animal facility of the Division of Life Sciences at the Nelson Biology Laboratories of Rutgers University or at the University Medical Center Hamburg-Eppendorf. Rats and mice of either sex were used for primary cerebellar granule cell culture. All animal experiments were approved by the Institutional Animal Care and Use Committee of Rutgers University (protocol # 09-051) or by the responsible committee of the State of Hamburg (permission number ORG 679), and all experiments were conducted in compliance with the ARRIVE guidelines for reports on animal research.
Human IMR-32 neuroblastoma cells (cat# 300148/p666_IMR-32, RRID:CVCL_0346) were obtained from the National Center for Cell Science (Pune, India) and maintained in DMEM (Sigma-Aldrich) supplemented with 1× penicillin/streptomycin/neomycin (GIBCO) and 10% fetal bovine serum at 37°C and 5% CO2.
ELISA screening of a small organic compound library for PSA mimetics
The NIH Clinical Collection 1 Library containing 446 small organic compounds was screened for molecules structurally mimicking PSA using competitive enzyme-linked immunosorbent assay (ELISA) as described (Loers et al. 2014). In brief, catalase-coupled PSA-mimicking peptides were immobilized on the surface of 384-well flat-bottom microtiter well plates (3 μg/ml; 25 μl/well), which was then washed with PBS and blocked with 1% bovine serum albumin (BSA) for 1 hour at room temperature. PSA-specific antibody 735 (0.1 μg/ml; 25 μl/well) incubated with either phosphate-buffered saline solution, pH 7.4 (PBS) serving as a negative control, PBS and 1% dimethyl sulfoxide (DMSO) serving as a solvent control, colominic acid (CA; 10 μM), the bacterial analogue of PSA, serving as positive control or 10 μM of the NIH library compounds in DMSO were then added to the PSA-mimicking peptide-containing wells. Following 5 subsequent washes with 0.5% Tween-20 diluted in PBS (PBST), addition of horseradish peroxidase (HRP)-coupled secondary antibody (1:5,000 in PBS), visualization with 5 minutes of incubation with 25 μl of 0.5 mg/mL OPD and termination of the resultant reaction with 25 μl 2.5 M H2SO4, absorbance was quantified at 490 nm using an ELISA reader (EnVision Plateworks software, Perkin Elmer, Waltham, MA). Compounds were considered to be PSA mimetics, when they competed with a PSA mimicking peptide for binding to the PSA-specific antibody (reduction in signal intensity by more than 30%). Confirmation that molecules producing hits in the screening were true structural PSA mimetics was obtained by testing their ability to bind to antibody 735 in both a specific and concentration-dependent manner. Competition ELISA was carried out, as described, using antibody 735 pre-incubated with increasing concentrations of the compounds or a control peptide, nitrendipine (Loers et al. 2014).
In vitro neurite outgrowth assay and cell migration
Primary cerebellar granule cells were prepared from wild-type mice or rats as described (Loers et al. 2005). For neurite outgrowth studies, cells were seeded at a density of 125,000 cells/ml (250 μl each well) in 0.01% poly-D-lysine (PDL)-coated 48-well Falcon tissue culture plates (ThermoFisher Scientific). Cells were allowed to settle down for 1 hour and then incubated with the test compounds, solvent or positive controls for 23 hours at 37°C with 5% CO2 and 90% humidity, fixed with 2.5% glutaraldehyde for 30 minutes and stained with 1% toluidine blue and 0.1% methylene blue in 1% Na-tetraborate. Neurites were imaged and quantified using an AxioObserver.A1 microscope (Carl Zeiss, Oberkochen, Germany) with a 20× objective and AxioVision 4.6 software. The longest neurite lengths were measured from the edge of the cell body to the end of the process, taking into account only neurites with a length equal to or greater than the diameter of the soma from which they originated and those that showed no contact with other neurites or cell bodies. Measurements were taken from 50 cells in each of two wells per condition carried out in three independent experiments. All measurements were conducted using ImageJ software (National Institutes of Health, Bethesda, MD). Cerebellar explants were prepared as described and migration of neurons was determined from at least 12 explants per treatment and experiment (Jakovcevski et al. 2009).
IMR-32 cells were sub-cultured by trypsinization (0.01% in PBS) and maintained in 12- or 24-well plates according to the requirement of the experiment. Migration of IMR-32 cells was assessed using a scratch injury assay performed according to Etienne-Manneville (2006) with slight modifications (Loers et al. 2014). Confluent IMR-32 cell monolayers were wounded by scratching with a sterile 22 gauge needle, resulting in a cell-free cleft ∼800μm wide. Directly after scratching, compounds were added and the cell free areas were determined microscopically with a 10× objective. Time point zero indicates the maximal scratch size determined directly after the injury (100% gap size). To analyze the migration of cells into the cell free/scratched area, pictures of the gap were taken 24 hours after scratch injury using a phase contrast microscope (Nikon TE2000). From these images, the size of the remaining cell-free area, as well as the distances the cells migrated into the cell-free area, were measured using the Image-Pro Plus software (Media Cybernetics, Silver Spring, USA). The cell-free area was determined from six wells per treatment group.
Cell survival
A cell survival assay was conducted as described (Loers et al. 2005) with slight modifications. Briefly, cerebellar granule cells cultured from P6-P7 mice were seeded at a density of 1×106 cells/ml (250 μl each well) in 0.01% PDL-coated 48-well flat-bottom tissue culture plates (ThermoFisher Scientific) and maintained at 37°C with 5% CO2 and 90% humidity overnight. Subsequently, cells were treated with inhibitors, solvent control or left untreated. Twenty minutes after inhibitor addition mimetics at different concentrations and controls (DMSO; CA) were added to the neurons, which were incubated for an additional 30 minutes. Oxidative stress was then induced by the addition of 10 μM H2O2 for 24 hours. Afterwards, live and apoptotic cells were stained for one hour at 37°C using 1 μg/ml calcein-AM and 1 μg/ml propidium iodide, and then fixed with 4% formaldehyde for 30 minutes at room temperature. The cells were washed three times with PBS and imaged using a Zeiss Axiovert 200M inverted transmitted-light microscope (Carl Zeiss). A count of living cells and apoptotic cells was taken from 4 images for each of 3 wells per condition using ImageJ. Experiments were carried out three independent times and for each experiment all images were processed the same way. First, the contrast/brightness threshold was adjusted manually until there was a clear shape of the cell somas visible. Subsequently, the background noise was eliminated using the despeckle and erode functions. To return the remaining cells back to their original shape, the dilate function was used. Finally, the watershed function was implemented to improve accuracy before the cells were counted using the analyze particles function. Cell numbers of living and apoptotic cells were summarized for each image and the percentage of living cells was calculated.
To determine IMR-32 cell survival, cells were seeded at a density of 15×103 cells/ml in 96-well plates, and cultured for 2 hours to allow for cell adhesion and treated with the test compounds at different concentrations (0.5 nM-5 μM). Cells were cultured for additional 72 hours and then stained with MTT and DAPI to determine the number of live cells (Loers et al. 2014). The cell number was determined from three wells for each treatment group.
Cell signaling experiments
Cell signaling that is essential for enhanced neurite outgrowth and neuronal survival after CA and PSA mimetic-induced stimulation was analyzed using cerebellar granule cells of P6-P7 mice. Neurons were pre-treated for 20 minutes with different signal transducer molecule inhibitors (120 nM KT5720, PKA inhibitor; 100 μM HBDDE, PKC inhibitor; 220 nM TBCA, casein kinase II inhibitor; 4 μM ERK inhibitor III; 40 nM PP121, Src family inhibitor; 1.2 μM 1-Naphthyl PP1, Src family inhibitor; 28 nM bpV(HOpic), PTEN inhibitor) before the mimetics or CA were added. Three independent neurite outgrowth and cell survival experiments were then performed as described to determine which inhibitors alter the effects of CA and/or the mimetics on these cellular processes.
Immunocytofluorescence staining
Cells were fixed with 4% formaldehyde and permeabilized with 0.3% Triton X-100 in PBS (PBST). For dual immunostaining, cells were incubated together with antibodies against PSA and NCAM in blocking solution (PBS with 2% BSA; 1:250) at 4°C overnight. After three washes with PBS, cells were incubated with anti-mouse IgM 488 and anti-mouse IgG 546 antibodies in blocking solution for 2 hours at room temperature and counterstained with DAPI to visualize nuclei. Images were captured using a confocal microscope (Nikon A1R, Nikon Corporation, Tokyo, Japan) and relative immunofluorescence intensities were determined using NIS elements AR analysis software version 4.11.00 (Nikon Corporation, Tokyo, Japan).
Cell stimulation and Western blot analysis
Cerebellar neurons were seeded at a density of 2×106 cells/well into PLL-coated 6-well culture plates and maintained for 16-24 hours in defined serum-free medium. Afterwards, cells were treated with vehicle control (0.001% DMSO), 1 nM irinotecan, 1 nMidarubicin, 30 μg/ml colominic acid or 1 nM nitrendipine (control compound) for 24 hours, lysed with ice cold lysis buffer [20 mM Tris/HCl pH 7.4, 140 mM NaCl, 1% NP-40, 1 mM EDTA and protease inhibitor cocktail (Roche)] and centrifuged at 1,000 g and 4°C for 15 minutes. Protein concentrations in the supernatants were determined with the BCA test (ThermoFisher Scientific) and probes were mixed with SDS sample buffer (60 mM Tris/HCl, pH 6.8, 2% SDS, 1%β-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) and incubated at 95°C for 5 minutes. Twenty microgram protein were loaded in each lane. Western blot analysis was performed as described (Makhina et al. 2009) and membranes were incubated with primary antibodies [anti-PSA 735 (1:2,000), anti-NCAM (1:1,000), or/and anti-GAPDH (1:1,000)] followed by incubation with HRP-conjugated secondary antibodies (1:20,000) for 1 hour at room temperature. Immunoreactive bands were visualized using the advanced chemiluminescent substrate (GE Healthcare) and a gel imaging system (ImageQuant LAS 4000; GE Healthcare).
Statistics
An independent scientist encrypted all chemicals which were used for the different treatments, and experiments were performed and analyzed in a blinded manner. Average values and standard error of the mean (SEM) were calculated from a pool of at least three independent experiments and statistical comparisons were conducted by one-way analysis of variance (ANOVA) followed by Holm-Sidak or by Fisher's protected least significant difference (PLSD) post hoc tests using StatView and Microsoft Excel.
Results
Idarubicin and irinotecan compete with a PSA-mimicking peptide for binding to anti-PSA antibody 735
To identify novel PSA mimicking compounds, the NIH Clinical Collection 1 Library was screened for compounds that interfere with binding of the PSA receptor site of antibody 735 to the PSA-mimicking peptide coupled to catalase. Two of these compounds that were able to bind to antibody 735 were idarubicin and irinotecan. To verify the results of the initial screen, a competition ELISA was performed using different molar concentrations (1-100 μM) of idarubicin and irinotecan and nitrendipine as a control compound. Idarubicin and irinotecan reduced binding of antibody 735 to PSA-mimicking peptide coupled to catalase as well as colominic acid, the bacterial analog of PSA, coupled to catalase in a concentration-dependent manner (Fig. 1). The maximal effect was observed at a 60 to 100 μM compound concentrations. At a 100 μM concentration, idarubicin reduced binding of the PSA-mimicking peptide to antibody 735 by 37% and irinotecan by 45%, respectively. Nitrendipine, which served as a control compound, did not significantly impede antibody binding to colominic acid. The reduction in the observed signal intensity is probably due to the presence of the solvent DMSO which reduced binding of the antibody 735 to the PSA-mimicking peptide in a similar manner as seen for nitrendipine. Free colominic acid maximally reduced binding of the PSA-mimicking peptide to antibody 735 by 48-51% at 20 - 100 μM concentrations. The results show that irinotecan is as potent as colominic acid in binding to the PSA-specific antibody 735, while idarubicin is slightly less potent.
Fig. 1.
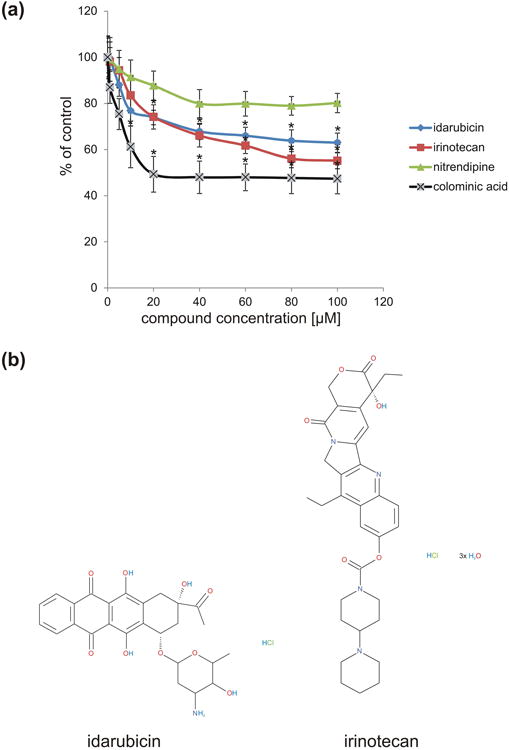
Idarubicin and irinotecan compete with the PSA-mimicking peptide for binding to the PSA-specific antibody 735. (a) PSA-mimicking peptide-coupled catalase was immobilized and incubated with anti-PSA antibody 735 in the presence of idarubicin (blue), irinotecan (red) or control compound nitrendipine (green) at 1 - 100 μM concentration or colominic acid (black). The signal from 735 antibody binding to PSA-mimicking peptide coupled catalase was set to 100%. Idarubicin and irinotecan compete with PSA-mimicking peptide for binding to 735 antibody in a concentration dependent manner reaching a maximal effect at 60 to 100 μM concentrations. *p< 0.01 (3 wells from three independent experiments; n = 3); data were compared by one-way analysis of variance (ANOVA). (b) Chemical structure of idarubicin and irinotecan.
Idarubicin and irinotecan induce neurite outgrowth and neuronal survival
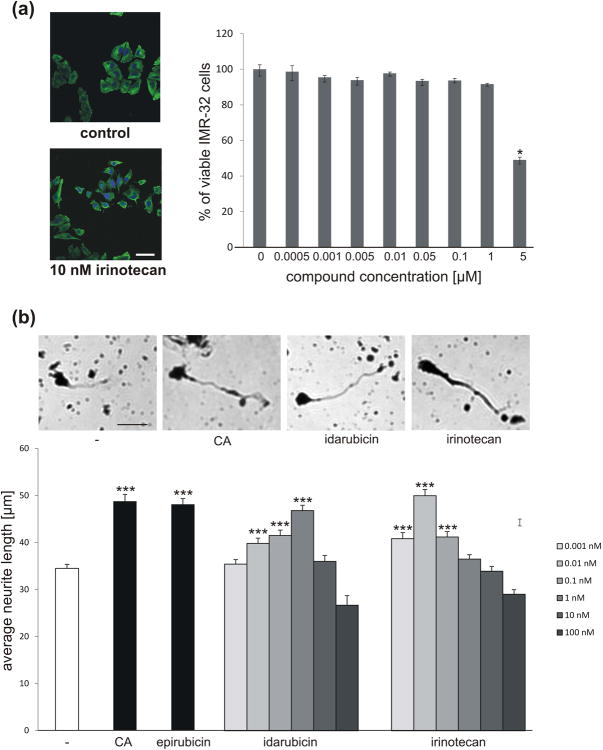
To analyze whether idarubicin and irinotecan also function in a similar manner as PSA, we determined neurite outgrowth and neuronal survival of PSA-responsive cerebellar granule neurons. Since both compounds are topoisomerase inhibitors that generate DNA strand breaks and induce apoptosis, IMR-32 neuroblastoma cells were used to determine the compound concentrations that can be applied to proliferating cells without inducing cell death. Application of 0.5 nM to 1 μMirinotecan did not alter cell survival, but higher concentrations induced cell death (Fig. 2a). This result shows that treatment of postmitotic and proliferating cells is not deleterious at concentrations in the nanomolar range or at even lower concentrations. Therefore, 1 pM to 100 nM inrinotecan and idarubicin were applied to cultured cerebellar granule neurons to test their effect on neurite outgrowth. Inrinotecan and idarubicin stimulated neurite outgrowth in a concentration-dependent manner, with the highest stimulatory effects seen at a 1 nM concentration of idarubicin and 10 pM irinotecan, which reached similar values as colominic acid treatment and treatment with the previously identified PSA-mimicking compound epirubicin (Fig. 2b). At concentrations higher than 1 nM irinotecan and idarubicin showed a reduced effect on neurite outgrowth leading to a bell-shaped response curve as it was previously observed for the PSA mimetics epirubicin and vinorelbine (Loers et al. 2016).
Fig. 2.
Idarubicin and irinotecan are not toxic to IMR-32 neuroblastoma cells at sub-micromolar concentration and enhance neurite outgrowth of cerebellar granule cells in a concentration-dependent manner. (a) Representative images of irinotecan-treated IMR-32 cells and control cells and quantification of cell survival after treatment of cells with different concentrations of irinotecan. Three wells per treatment group were used to determine the number of live cells (mean ± SEM) and the experiment was repeated thrice (n = 3). Asterisks signify statistically significant differences as compared to untreated control (p < 0.05, one-way ANOVA with Holm Sidak post-hoc test). (b) Representative images of mouse cerebellar granule cells left untreated (-), treated with 1 nM idarubicin or epirubicin, 0.01 nM irinotecan or 30 μg/ml colominic acid (CA). Bar diagram shows the average neurite length (mean + SEM) from the longest neurite of 300 cells (n = 300 cells from 6 wells out of 3 independent experiments) treated with different concentrations of idarubicin and irinotecan, or treated with epirubicin and CA as positive controls. Experiments were performed 3 independent times. Asterisks signify statistically significant differences versus untreated neurons (-) as determined by one-way ANOVA with Fisher's PLSD test (F=27.744, p<0.0001; PLSD *p<0.05). Scale bar, 25 μm.
PSA has been described to beneficially influence the survival of glial and neuronal cells. PSA-NCAM was reported to support survival of injured retinal ganglion cells (Lobanovskaya et al. 2015). PSA-expressing Schwann cells survived better after transplantation into the lesioned spinal cord than control cells (Luo et al. 2011). Colominic acid, a PSA-mimicking peptide and the PSA-mimicking compound tegaserod were shown to increase the survival of stressed motor neurons and cerebellar granule neurons (Bushman et al. 2014). Therefore, we investigated whether application of irinotecan and idarubicin to cultured granule cells can protect the cells from oxidative stress. Idarubicin and irinotecan enhanced neuronal survival at concentrations of 0.1 pM to 100 nM with strongest protection of cells seen at 1 nM concentration (Fig. 3). Higher compound concentrations were less effective in supporting cell survival, an effect also observed in cell survival assays with the PSA mimetics epirubicin and vinorelbine (Loers et al. 2016). The pro-survival effect of idarubicin was comparable to the protective effects achieved by treatment of neurons with colominic acid and epirubicin, whereas irinotecan was slightly less effective.
Fig. 3.
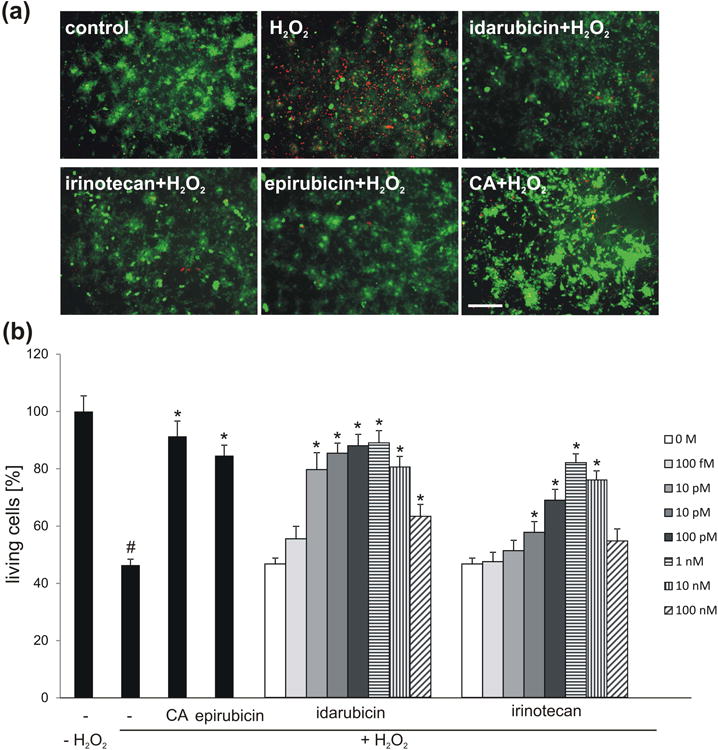
Idarubicin and irinotecan enhance survival of cerebellar granule cells in a concentration-dependent manner. (a) Representative images of cerebellar granule cells treated with 1 nM idarubicin, irinotecan or epirubicin or 30 μg/ml colominic acid (CA) and subsequently stressed using hydrogen peroxide (H2O2), and stained with calcein-AM (green) and propidium iodide (red). (b) Bar diagram shows the relative number of live neurons (mean + SEM) treated with different concentrations of irdarubicin and irinotecan, or treated with 1 nM epirubicin and 30 μg/ml CA as positive controls and stressed with H2O2. The experiment was performed independently five times with three biological replicates in each experiment (n = 5). The hash sign shows significant difference between the unstressed group (-; - H2O2) and stressed group (-; + H2O2). Asterisks signify statistically significant differences between the stressed group (-; + H2O2) and stressed and compound treated groups (epirubicin/CA/idarubicin/irinotecan; + H2O2) as determined by one-way ANOVA with Holm-Sidak post hoc test#p< 0.0001; *p< 0.005). Scale bar, 50 μm.
Irinotecan and idarubicin do not alter cell migration
Since PSA-expressing Schwann cells and stem cells were shown to migrate farther in lesioned tissue than control cells, and since treatment of IMR-32 cells and cerebellar neurons with colominic acid and the PSA mimetic 5-nonyloxytryptamine enhanced migration of these cells (Loers et al. 2014), we were interested in determining if the novel PSA mimetics might also influence cell migration. A scratch injury assay was performed using IMR-32 cells. The cells were treated with and without irinotecan and cell migration into the cell-free wound area was monitored. Results showed that in contrast to PSA, colominic acid and 5-nonyloxytryptamine, irinotecan did not alter cell migration of IMR-32 cells (Fig. 4a). Furthermore, irinotecan and idarubicin did not enhance the migration of cerebellar neurons out of cerebellar explants at femto- or picomolar concentrations (Fig. 4b) but reduced migration at nanomolar concentrations (data not shown). It is thus likely that irinotecan and idarubicin act more as cell cycle inhibitors than as PSA mimetics under these experimental conditions.
Fig. 4.
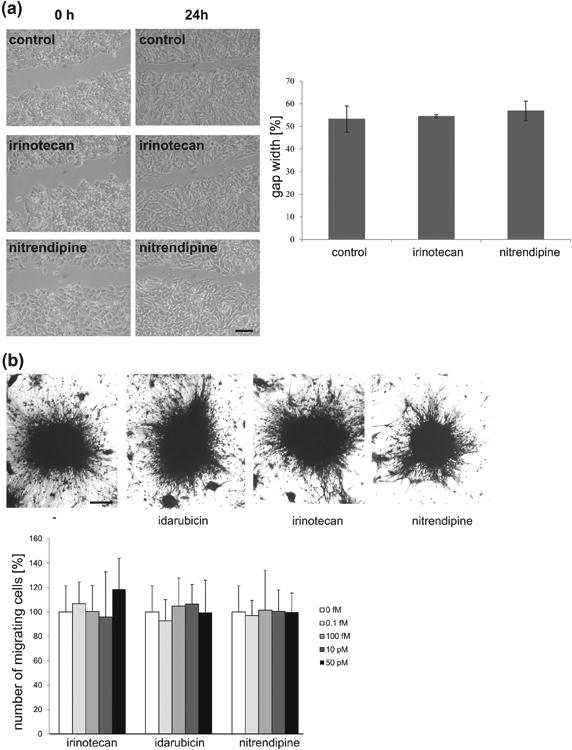
Irinotecan and idarubicin do not alter migration of IMR-32 cells after scratch injury or of neurons from cerebellar explants. (a) Representative phase contrast images of IMR-32 cells, 0 and 24 hours after scratch injury depicting gap size in untreated control, irinotecan (10 nM) and nitrendipine (negative control; 10 nM) treated cells. Scale bar = 200 μm. Histogram represents % gap size (mean ± SEM) in control, irinotecan and nitrendipine treated cells after 24 hours of treatment (initial gap width was taken as 100%). The experiment was performed thrice with two wells per condition (n = 3). No statistically significant differences between control and treated cells were seen (p > 0.05, one-way ANOVA with Holm-Sidak post hoc test). (b) Representative phase contrast images of untreated explants (-) and explants treated with idarubicin, irinotecan or nitrendipine (negative control) (all 100 fM) 24 hours after treatment. Histogram shows the number of migrating cells out of cerebellar explants (mean + SD) from control, irinotecan, idarubicin or nitrendipine treated explants at indicated concentrations 24 hours after treatment. The experiment was performed independently four times and at least 12 explants were analyzed in each experiment (n = 4). No statistically significant differences between control and treated explants were seen (p > 0.05, one-way ANOVA with Holm-Sidak post hoc test). Scale bar, 50 μm.
Idarubicin and irinotecan stimulate neurite outgrowth and survival of cerebellar neurons via protein kinase C and Erk1/2
As a next step, we analyzed whether idarubicin and irinotecan treatment activates the same signaling pathways as PSA and PSA-NCAM. NCAM was shown to activate cAMP-dependent protein kinase (PKA) as well as protein kinase C (PKC), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), Ca2+/calmodulin-dependent protein kinase II (CaMKII), Fyn and extracellular regulated kinases (Erk) 1/2 (Loers and Schachner 2007; Maness and Schachner 2007). Additionally, NCAM interacts with the fibroblast growth factor receptor, which leads to the activation of phospholipase C and PKC, while PSA was shown to interact with myristoylated alanine-rich C kinase substrate through the plasma membrane (Theis et al. 2013). It was therefore conceivable that idarubicin and irinotecan would activate the same signaling molecules, if they are true mimetics of PSA. Indeed, treatment of cerebellar neurons with inhibitors of PKC, CK2, Src/Fyn and PTEN reduced the neurite outgrowth effect of idarubicin and irinotecan (Fig. 5). Interestingly, only the PKC inhibitor and Erk inhibitor significantly reduced the pro-survival effect of idarubicin and irinotecan as well as of colominic acid, whereas PKA, CK2, Src/Fyn and PTEN inhibitors did not reduce the pro-survival effect of the compounds or colominic acid (Fig. 6).
Fig. 5.
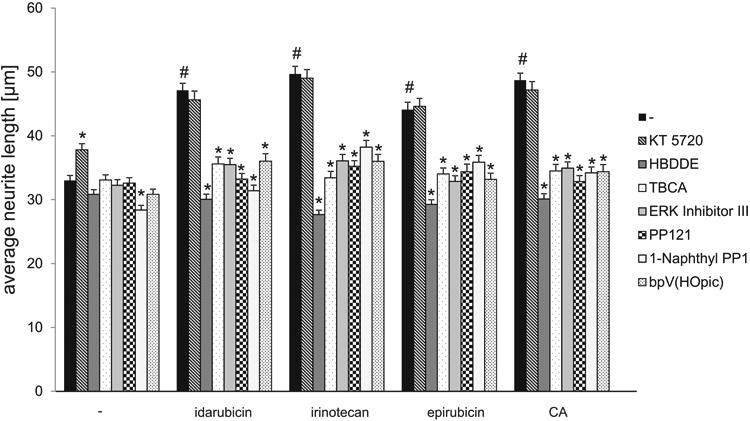
Cell signaling essential for neurite outgrowth that is triggered by idarubicin, irinotecan, epirubicin and CA.Bar diagram displays the average longest neurite length (mean + SEM) of rat cerebellar granule cells pre-treated with different signal transducer molecule inhibitors (KT 5720 (PKA), HBDDE (PKC), TBCA (CKII), Erk inhibitor III (Erk), PP121 (Src), 1-Naphthyl PP1 (Fyn), bpV(HOpic) (PTEN)) and untreated cells or cells treated with 1 nM idarubicin or epirubicin, 0.01 nM irinotecan or 30 μg/ml CA. The hash sign shows a significant difference between the untreated group (control; -) and the groups treated only with PSA mimetics or CA (#p< 0.001). Asterisks signify statistically significant differences within the groups as determined by one-way ANOVA with Fisher's PLSD test (n = 300 cells from 6 wells out of 3 independent experiments; F=36.575 p < 0.0001; PLSD *p< 0.05).
Fig. 6.
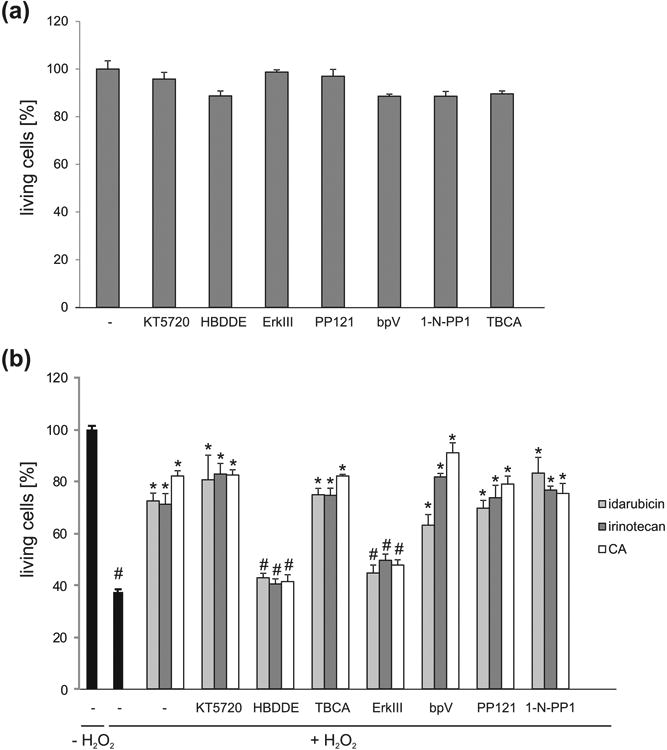
Cell signaling triggered by idarubicin, irinotecan, epirubicin and CA which is essential for neuronal survival. (a) Inhibitors do not influence basal cell survival. Bar diagram displays the average number of living murine cerebellar granule cells (mean + SEM) treated with different signal transducer molecule inhibitors (KT 5720 (PKA), HBDDE (PKC), TBCA (CKII), ERK inhibitor III (Erk), PP121 (Src), 1-Naphthyl PP1 (1-N-PP1; Fyn), bpV(HOpic) (bpV; PTEN)). (b) Bar diagram displays the average number of living murine cerebellar granule cells (mean + SEM) pre-treated with different signal transducer molecule inhibitors and treated with 1 nM idarubicin, irinotecan or epirubicin or 30 μg/ml CA followed by cell death induction with H2O2. For each treatment and experiment 6 wells were used and the experiment was repeated four times (n = 4). The pound sign shows significant difference between the untreated group (-; - H2O2) and the stressed group (-; + H2O2) or the stressed groups treated with compounds (-; + H2O2; idarubicin/irinotecan/CA) and the stressed groups treated with inhibitors and compounds (inhibitor; + H2O2; idarubicin/irinotecan/CA) (#p< 0.001). Asterisks signify statistically significant difference compared to the hydrogen peroxide treated group as determined by one-way ANOVA with test Holm-Sidak post-hoc test (*p< 0.005).
Idarubicin and irinotecan enhance expression of PSA and NCAM
In addition to activating signaling pathways, PSA mimetics were shown to enhance the endogenous expression of PSA and NCAM (Loers et al. 2014, 2016). To determine whether idarubicin and irinotecan are also able to upregulate PSA and NCAM expression, IMR-32 cells were treated with compounds for 24 hours and then stained with antibodies against PSA and NCAM. Alternatively, cerebellar neurons were treated with compounds for 24 hours, and cell lysates were then probed with PSA and NCAM antibodies. Results showed that irinotecan, idarubicin and colominic acid enhanced the expression of PSA and NCAM in a similar manner as the previously described PSA mimetics, 5-nonyloxytryptamine and vinorelbine (Fig. 7).
Fig. 7.
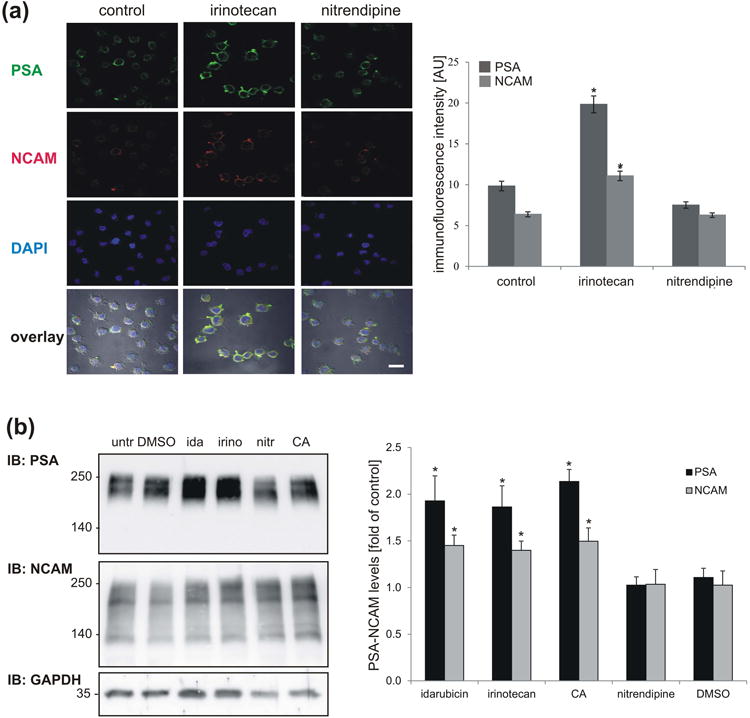
Expression of PSA and NCAM is enhanced by irinotecan and idarubicin treatment. (a) IMR-32 cells were treated with 10 nM irinotecan, 10 nM nitrendipine (control compound) and DMSO (formulation control) for 72 hours, fixed and stained with antibodies against PSA (green) and NCAM (red). Nuclei are visualized with DAPI (blue). Scale bar: 50 μm. Histogram shows data representing mean values ± SEM from three independent experiments (100 cells were counted per experiment; n = 300) representing the intensity of PSA and NCAM staining in irinotecan (10 nM) and nitrendipine (10 nM) treated cells compared to control cells. * p < 0.05, one-way ANOVA with Holm-Sidak post-hoc test. (b) Cerebellar neurons were left untreated (untr) or treated with 1 nM irinotecan (irino), 1 nM idarubicin (ida), 1 nM nitrendipine (nitr; control compound), 30 μg/ml colominic acid (CA) or DMSO (formulation control) for 24 h, lysed and subjected to Western blot analysis (IB) with antibodies against PSA and NCAM. GAPDH was analyzed to control for loading. PSA and NCAM levels were quantified by densitometry and normalized to the GAPDH levels. A representative immunoblot out of six experiments is shown. Histogram shows data representing mean values + SD from six independent experiments (n = 6) representing the levels of PSA and NCAM levels in compound treated cells normalized to GAPDH levels and compared to PSA and NCAM levels in control cells. * p < 0.05, one-way ANOVA with Holm-Sidak post hoc test.
The combined results indicate that idarubicin and irinotecan act as PSA mimetics and stimulate neurite outgrowth and neuronal survival via PKC and Erk1/2.
Discussion
With recent advances in the identification of glycan receptors, discovery of glycomimetics, construction of synthetic glycans for drug delivery and drug design, glycans and glycomimetics have become increasingly attractive for therapeutic applications (Irintchev et al. 2011; Masand et al. 2012; Mehanna et al. 2010; Prost et al. 2012; Rowlands et al. 2015; Cunha and Grenha 2016; Hockl et al. 2016). One important drug target and glycan for drug delivery is PSA. PSA was shown to be involved in invasive meningococcal diseases, influenza virus infections (Rameix-Welti et al. 2009), cancer progression (Falconer et al. 2012), schizophrenia and depression (Senkov et al. 2012), synaptic plasticity (Bonfanti 2006; Bonfanti and Theodosis 2009; Senkov et al. 2012), development of the nervous system (Bonfanti 2006; Franceschini et al. 2011; Rutishauser 2008) and neuroregeneration (Ghosh et al. 2012; Masand et al. 2012; Mehanna et al. 2010). PSA-carboxymethyl chitosan hydrogel, amphiphilic PSA derivatives and PSA grafted with polycaprolactone are currently being explored as promising drug-delivery systems (Deepagan et al. 2013; Wilson et al. 2014; Wu et al. 2015). Thus, manipulation of PSA functions and application of PSA mimetics is likely to be of important therapeutic value for treatment of nervous system injuries and neurological disorders.
In our search for PSA mimetics with known toxicology and pharmacological profiles, we identified idarubicin and irinotecan as novel PSA-mimicking compounds. Irinotecan (drug name Onivyde®) is the hydrochloride salt of a semisynthetic derivative of camptothecin, a cytotoxic, quinoline-based alkaloid extracted from the Asian tree Camptotheca acuminata. Irinotecan is a pro-drug that is converted to a biologically active metabolite, 7-ethyl-10-hydroxy-camptothecin (SN-28), by a carboxylesterase-converting enzyme. SN-38 inhibits topoisomerase I activity by stabilizing the cleavable complex between topoisomerase I and DNA, resulting in DNA breaks that inhibit DNA replication and trigger apoptotic cell death (National Cancer Institute; http://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C1381). Idarubicin is an anthracycline antineoplastic antibiotic. Idarubicin (drug names idamycin PFS and idarubicin hydrochloride) intercalates into DNA and inhibits topoisomerase II, thereby inhibiting DNA replication and, ultimately, interfering with RNA and protein synthesis (National Cancer Institute; http://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C1587). We could show that in addition to their published action as topoisomerase inhibitors, both compounds bind to PSA-specific antibody 735 and stimulate neurite outgrowth and neuronal survival in a concentration-dependent manner. The observed bell-shaped response curves were also seen when using the previously described PSA mimetics epirubicin and vinorelbine (Loers et al. 2016) suggesting that these results are probably due to the cell cycle inhibiting effect of the compounds at higher concentrations. Furthermore, idarubicin and irinotecan enhanced the expression of PSA and NCAM. This treatment most likely increased PSA and NCAM levels by contributing to the effect of idarubicin and irinotecan on neurite outgrowth and neuronal survival by enhancing the stimulation of PSA- and NCAM-mediated signaling pathways.
These functions of idarubicin and irinotecan are likely mediated by PKC, a known down-stream signaling component of NCAM signaling pathways shown to be important for NCAM-mediated neurite outgrowth (Kolkova et al. 2005). In addition, PKC regulates polysialyltransferase activity and the NCAM polysialylation state (Gallagher et al. 2001). Therefore, activation of PKC by idarubicin and irinotecan could both stimulate signaling pathways and provide a feedback mechanism to regulate PSA expression. Altered PSA levels could then further influence NCAM signaling, as shown in recent in vitro studies (Eggers et al. 2011; Röckleet al. 2008). Here, we also show that the neurite outgrowth stimulatory effect of irinotecan and idarubicin depends on the activation of the Src non-receptor tyrosine kinase family members, Erk1/2 and CKII. This is in agreement with previous studies demonstrating that NCAM-mediated neurite outgrowth was abolished by pharmacological inhibition of Src-family kinases (Kolkova et al. 2000) and NCAM clustering on the cell surface by means of NCAM antibodies resulted in transient Fyn phosphorylation (Beggs et al. 1997). The activation of NCAM-mediated down-stream signaling led to phosphorylation and stimulation of Erk1 and Erk2 (Schmid et al. 1999; Kolkova et al. 2000). Similar pathways were shown to be activated in primary neurons by application of the PSA mimetics 5-nonyloxytryptamine, vinorelbine and epirubicin (Loers et al. 2014, 2016). It is noteworthy in this context that EndoN-mediated removal of PSA from human neuroblastoma cells initiated NCAM interactions at cell-cell contacts and resulted in reduced cell proliferation and, in parallel, activation of the Erk1/2 pathway (Cavallaro et al. 2001; Francavilla et al. 2009). Phosphorylation of growth-associated protein GAP-43 by PKC, as well as by CKII, was shown to be important for NCAM-induced neurite outgrowth (Korshunova et al. 2007), while CKI was identified as a protein kinase able to phosphorylate NCAM (Mackie et al. 1989). Thus, it is conceivable that irinotecan and idarubicin as PSA mimetics also signal via CKII, Erk1/2 and Scr family kinases to induce neurite outgrowth. Interestingly, idarubicin and irinotecan also activated PTEN to induce neurite outgrowth, but an involvement of PTEN in NCAM or PSA signaling or an association of PTEN with NCAM has not yet been reported. From the protein family of cell adhesion molecules, only L1 and CHL1 have been shown to influence PTEN expression. L1 was shown to bind to CKIIα via its intracellular domain and to trigger neuroprotection via inhibition of PTEN and p53. Application of a CKII inhibitor or transfection with CKIIαsiRNA increased levels of PTEN and p53 in primary neurons (Wang and Schachner 2015). CHL1 was shown to regulate phosphorylation of the serotonin 2c receptor and its association with PTEN and β-arrestin 2 (Kleene et al. 2015). Therefore, NCAM, with its attached glycan PSA, might also influence PTEN functions, which might be reflected in our findings with irinotecan and idarubicin.
It is at the moment difficult to compare or even compatibilize the effects of the two compounds in their impact on their functions in cancer biology and neurobiology. These remain to be studied in the future by detailed experiments aiming at the elucidation of underlying molecular mechanisms in regard to the two cellular systems. In conclusion, irinotecan and idarubicin activate neurite outgrowth and survival by similar mechanisms as PSA and NCAM, suggesting that they are indeed mimetics of PSA, but with different signaling mechanisms: Erk and PKC signal transduction for neuroprotection and all signal transducers acting on neurite outgrowth, thus dissecting the actions of PSA (see graphical abstract).
Acknowledgments
The authors thank Eva Kronberg for excellent animal care, Markus Wolf for technical assistance and Noelle Messina for help with image acquisition. We are grateful to the National Institute of Health for funding this project as part of the R01 grant 4R01NS078385-05 and the Bundesministerium fuer Bildung und Forschung (BMBF) and Indian Council of Medical Research (ICMR) (Indo-German Research Project 10/050) for support. V.S. thanks the Council of Scientific and Industrial Research (CSIR) India for a Senior Research Fellowship. UGC, India grant under UPE and CPEPA schemes is acknowledged for providing infrastructure in Guru Nanak Dev University. The authors declare no conflict of interest.
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CA
colominic acid
- CKII
casein kinase II
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco's Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- ELISA
enzyme-linked immunosorbent assay
- epirubicin
(7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione hydrochloride
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- idarubicin
(7S,9S)-9-acetyl-7-(4-amino-5-hydroxy-6-methyloxan-2-yl)oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione hydrochloride
- irinotecan
(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3, 14-dioxo-1 H-pyrano [3′,4′6,7] indolizino [1,2-b] quinolin-9-yl-[1,4′-bipiperidine]-1′-carboxylate monohydrochloride trihydrate
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NCAM
neural cell adhesion molecule
- OPD
ortho-phenylenediamine dihydrochloride
- PBS
phosphate buffered saline solution
- PBST
phosphate buffered saline solution containing Tween-20
- PDL
poly-D-lysine
- PLL
poly-L-lysine
- PLSD
protected least significant difference
- PSA
polysialic acid
- PPT
protein phosphotyrosine phosphatase
- PTEN
phosphatase and tensin homolog
- SD
standard deviation
- SEM
standard error of the mean
- SN-28
7-ethyl-10-hydroxy-camptothecin
Footnotes
ARRIVE guidelines have been followed: Yes, => if No or if it is a Review or Editorial, skip complete sentence => if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.” unless it is a Review or Editorial
References
- Bax M, van Vliet SJ, Litjens M, García-Vallejo JJ, van Kooyk Y. Interaction of polysialic acid with CCL21 regulates the migratory capacity of human dendritic cells. PLOS ONE. 2009;4:e6987. doi: 10.1371/journal.pone.0006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT. Polysialic acid and activity-dependent synapse remodeling. Cell Adh Migr. 2009;3:43–50. doi: 10.4161/cam.3.1.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus. 2000;10:37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Bushman J, Mishra B, Ezra M, Gul S, Schulze C, Chaudhury S, Ripoll D, Wallqvist A, Kohn J, Schachner M, Loers G. Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacology. 2014;79:456–466. doi: 10.1016/j.neuropharm.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- Cunha L, Grenha A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar Drugs. 2016;14:E42. doi: 10.3390/md14030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreli S, Arany Z, Gerardy-Schahn R, Mann D, Stamatos NN. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- Deepagan VG, Thambi T, Ko H, Kang YM, Park JH. Amphiphilic polysialic acid derivatives: synthesis, characterization, and in-vitro cytotoxicity. J Nanosci Nanotechnol. 2013;13:7312–7318. doi: 10.1166/jnn.2013.8089. [DOI] [PubMed] [Google Scholar]
- Eggers K, Werneburg S, Schertzinger A, Abeln M, Schiff M, Scharenberg MA, Burkhardt H, Mühlenhoff M, Hildebrandt H. Polysialic acid controls NCAM signals at cell-cell contacts to regulate focal adhesion independent from FGF receptor activity. J Cell Sci. 2011;124:3279–3291. doi: 10.1242/jcs.084863. [DOI] [PubMed] [Google Scholar]
- Falconer RA, Errington RJ, Shnyder SD, Smith PJ, Patterson LH. Polysialyltransferase: a new target in metastatic cancer. Curr Cancer Drug Targets. 2012;12:925–939. doi: 10.2174/156800912803251225. [DOI] [PubMed] [Google Scholar]
- Francavilla C, Cattaneo P, Berezin V, Bock E, Ami D, de Marco A, Christofori G, Cavallaro U. The binding of NCAM to FGFR1 induces a specific cellular response mediated by receptor trafficking. J Cell Biol. 2009;187:1101–1116. doi: 10.1083/jcb.200903030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Desroziers E, Caraty A, Duittoz A. The intimate relationship of gonadotropin-releasing hormone neurons with the polysialylated neural cell adhesion molecule revisited across development and adult plasticity. Eur J Neurosci. 2010;32:2031–2041. doi: 10.1111/j.1460-9568.2010.07517.x. [DOI] [PubMed] [Google Scholar]
- Gallagher HC, Murphy KJ, Foley AG, Regan CM. Protein kinase C delta regulates neural cell adhesion molecule polysialylation state in the rat brain. J Neurochem. 2001;77:425–434. doi: 10.1046/j.1471-4159.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Tuesta LM, Puentes R, Patel S, Melendez K, El Maarouf A, Rutishauser U, Pearse DD. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. 2012;60:979–992. doi: 10.1002/glia.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Dityatev A. Polysialic acid in brain development and synaptic plasticity. Top Curr Chem. 2015;366:55–96. doi: 10.1007/128_2013_446. [DOI] [PubMed] [Google Scholar]
- Hockl PF, Wolosiuk A, Pérez-Sáez JM, Bordoni AV, Croci DO, Toum-Terrones Y, Soler-Illia GJ, Rabinovich GA. Glyco-nano-oncology: Novel therapeutic opportunities by combining small and sweet. Pharmacol. 2016;109:45–54. doi: 10.1016/j.phrs.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Wu MM, Lee HJ, Zhu H, Feng YP, Liu YS, Bernreuther C, Loers G, You SW, Schachner M. Glycomimetic improves recovery after femoral injury in a non-human primate. J Neurotrauma. 2011;28:1295–1306. doi: 10.1089/neu.2011.1775. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Siering J, Hargus G, Karl N, Hoelters L, Djogo N, Yin S, Zecevic N, Schachner M, Irintchev A. Close homologue of adhesion molecule L1 promotes survival of Purkinje and granule cells and granule cell migration during murine cerebellar development. J Comp Neurol. 2009;513:496–510. doi: 10.1002/cne.21981. [DOI] [PubMed] [Google Scholar]
- Jungnickel J, Brämer C, Bronzlik P, Lipokatic-Takacs E, Weinhold B, Gerardy-Schahn R, Grothe C. Level and localization of polysialic acid is critical for early peripheral nerve regeneration. Mol Cell Neurosci. 2009;40:374–381. doi: 10.1016/j.mcn.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Kiermaier E, Moussion C, Veldkamp CT, Gerardy-Schahn R, de Vries I, Williams LG, Chaffee GR, Phillips AJ, Freiberger F, Imre R, Taleski D, Payne RJ, Braun A, Förster R, Mechtler K, Mühlenhoff M, Volkman BF, Sixt M. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science. 2016;351:186–190. doi: 10.1126/science.aad0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Chaudhary H, Karl N, Katic J, Kotarska A, Guitart K, Loers G, Schachner M. Interaction between CHL1 and serotonin receptor 2c regulates signal transduction and behavior in mice. J Cell Sci. 2015;128:4642–4652. doi: 10.1242/jcs.176941. [DOI] [PubMed] [Google Scholar]
- Kolkova K, Novitskaya V, Pedersen N, Berezin V, Bock E. Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway. J Neurosci. 2000;20:2238–2246. doi: 10.1523/JNEUROSCI.20-06-02238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolkova K, Stensman H, Berezin V, Bock E, Larsson C. Distinct roles of PKC isoforms in NCAM-mediated neurite outgrowth. J Neurochem. 2005;92:886–894. doi: 10.1111/j.1471-4159.2004.02919.x. [DOI] [PubMed] [Google Scholar]
- Korshunova I, Novitskaya V, Kiryushko D, Pedersen N, Kolkova K, Kropotova E, Mosevitsky M, Rayko M, Morrow JS, Ginzburg I, Berezin V, Bock E. GAP-43 regulates NCAM-180-mediated neurite outgrowth. J Neurochem. 2007;100:1599–1612. doi: 10.1111/j.1471-4159.2006.04316.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Parkash J, Kataria H, Kaur G. Enzymatic removal of polysialic acid from neural cell adhesion molecule interrupts gonadotropin releasing hormone (GnRH) neuron-glial remodeling. Mol Cell Endocrinol. 2012;348:95–103. doi: 10.1016/j.mce.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Franceschini I, Dubois-Dalcq M, Matsas R. Schwann cells genetically engineered to express PSA show enhanced migratory potential without impairment of their myelinating ability in vitro. Glia. 2006;53:868–878. doi: 10.1002/glia.20340. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Wie Q, Zheng X, Tang L, Kong D, Min Gong M. Variant fatty acid-like molecules Conjugation, novel approaches for extending the stability of therapeutic peptides. Sci Rep. 2015;5:18039. doi: 10.1038/srep18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobanovskaya N, Zharkovsky T, Jaako K, Jürgenson M, Aonurm-Helm A, Zharkovsky A. PSA modification of NCAM supports the survival of injured retinal ganglion cells in adulthood. Brain Res. 2015;1625:9–17. doi: 10.1016/j.brainres.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Loers G, Chen S, Grumet M, Schachner M. Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J Neurochem. 2005;92:1463–1476. doi: 10.1111/j.1471-4159.2004.02983.x. [DOI] [PubMed] [Google Scholar]
- Loers G, Schachner M. Recognition molecules and neural repair. J Neurochem. 2007;101:865–882. doi: 10.1111/j.1471-4159.2006.04409.x. [DOI] [PubMed] [Google Scholar]
- Loers G, Saini V, Mishra B, Papastefanaki F, Lutz D, Chaudhury S, Ripoll DR, Wallqvist A, Gul S, Schachner M, Kaur G. Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture. J Neurochem. 2014;128:88–100. doi: 10.1111/jnc.12408. [DOI] [PubMed] [Google Scholar]
- Loers G, Saini V, Mishra B, Gul S, Chaudhury S, Wallqvist A, Kaur G, Schachner M. Vinorelbine and epirubicin share common features with polysialic acid and modulate neuronal and glial functions. J Neurochem. 2016;136:48–62. doi: 10.1111/jnc.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Bo X, Wu D, Yeh J, Richardson PM, Zhang Y. Promoting survival, migration, and integration of transplanted Schwann cells by over-expressing polysialic acid. Glia. 2011;59:424–434. doi: 10.1002/glia.21111. [DOI] [PubMed] [Google Scholar]
- Mackie K, Sorkin BC, Nairn AC, Greengard P, Edelman GM, Cunningham BA. Identification of two protein kinases that phosphorylate the neural cell-adhesion molecule, N-CAM. J Neurosci. 1989;9:1883–1896. doi: 10.1523/JNEUROSCI.09-06-01883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhina T, Loers G, Schulze C, Ueberle B, Schachner M, Kleene R. Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol Cell Neurosci. 2009;41:206–218. doi: 10.1016/j.mcn.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Maness P, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Marino P, Norreel JC, Schachner M, Rougon G, Amoureux MC. A polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Exp Neurol. 2009;219:163–174. doi: 10.1016/j.expneurol.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Masand SN, Chen J, Perron IJ, Hammerling BC, Loers G, Schachner M, Shreiber DI. The effect of glycomimetic functionalized collagen on peripheral nerve repair. Biomaterials. 2012;33:8353–8362. doi: 10.1016/j.biomaterials.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna A, Mishra B, Kurschat N, Schulze C, Bian S, Loers G, Irintchev A, Schachner M. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain. 2009;132:1449–1462. doi: 10.1093/brain/awp128. [DOI] [PubMed] [Google Scholar]
- Mehanna A, Jakovcevski I, Acar A, Xiao M, Loers G, Rougon G, Irintchev A, Schachner M. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol Ther. 2010;18:34–43. doi: 10.1038/mt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Kälviänen R, Tapiola T, Ylinen A, Vapalahti M, Paljärvi L, Pitkänen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44:923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- Mikkonen M, Soininen H, Tapiola T, Alafuzoff I, Miettinen R. Hippocampal plasticity in Alzheimer's disease: changes in highly polysialylated NCAM immunoreactivity in the hippocampal formation. Eur J Neurosci. 1999;11:1754–1764. doi: 10.1046/j.1460-9568.1999.00593.x. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Rollenhagen M, Werneburg S, Gerardy-Schahn RH, Hildebrandt H. Polysialic acid: Versatile modification of NCAM, SynCAM 1 and neuropilin-2. Neurochem Res. 2013;38:1134–1143. doi: 10.1007/s11064-013-0979-2. [DOI] [PubMed] [Google Scholar]
- Pan HC, Shen YQ, Loers G, Jakovcevski I, Schachner M. Tegaserod, a small compound mimetic of polysialic acid, promotes functional recovery after spinal cord injury in mice. Neuroscience. 2014;277:356–366. doi: 10.1016/j.neuroscience.2014.06.069. [DOI] [PubMed] [Google Scholar]
- Parkash J, Kaur G. Neuronal-glial plasticity in gonadotrophin releasing hormone release in adult female rats: role of the polysialylated form of neural cell adhesion molecule. J Endocrinol. 2005;186:397–409. doi: 10.1677/joe.1.06156. [DOI] [PubMed] [Google Scholar]
- Penchala SC, Miller MR, Pal A, Dong J, Madadi NR, Xie J, Joo H, Tsai J, Batoon P, Samoshin V, Franz A, Cox T, Miles J, Chan WK, Park MS, Alhamadsheh MM. Nat Chem Biol. 2015;11:793–798. doi: 10.1038/nchembio.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost LR, Grim JC, Tonelli M, Kiessling LL. Noncarbohydrate glycomimetics and glycoprotein surrogates as DC-SIGN antagonists and agonists. ACS Chem Biol. 2012;7:1603–1608. doi: 10.1021/cb300260p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameix-Welti MA, Zarantonelli ML, Giorgini D, Ruckly C, Marasescu M, van der Werf S, Alonso JM, Naffakh N, Taha MK. Influenza A virus neuraminidase enhances meningococcal adhesion to epithelial cells through interaction with sialic acid-containing meningococcal capsules. Infect Immun. 2009;77:3588–3595. doi: 10.1128/IAI.00155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Gallardo A, Escribano C, Delgado-Martín C, Rodriguez-Fernández JL, Gerardy-Schahn R, Rutishauser U, Corbi AL, Vega MA. Polysialylated neuropilin-2 enhances human dendritic cell migration through the basic C-terminal region of CCL21. Glycobiology. 2010;20:1139–1146. doi: 10.1093/glycob/cwq078. [DOI] [PubMed] [Google Scholar]
- Röckle I, Seidenfaden R, Weinhold B, Mühlenhoff M, Gerardy-Schahn R, Hildebrandt H. Polysialic acid controls NCAM-induced differentiation of neuronal precursors into calretinin-positive olfactory bulb interneurons. Dev Neurobiol. 2008;68:1170–1184. doi: 10.1002/dneu.20649. [DOI] [PubMed] [Google Scholar]
- Rowlands D, Sugahara K, Kwok JC. Glycosaminoglycans and glycomimetics in the central nervous system. Molecules. 2015;20:3527–3548. doi: 10.3390/molecules20033527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Saini V, Lutz D, Kataria H, Kaur G, Schachner M, Loers G. The polysialic acid mimetics 5-nonyloxytryptamine and vinorelbine facilitate nervous system repair. Sci Rep. 2016;6:26927. doi: 10.1038/srep26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato AK, Viswanathan M, Kent RB, Wood CR. Therapeutic peptides: technological advances driving peptides into development. Curr Opin Biotechnol. 2006;17:638–642. doi: 10.1016/j.copbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Graff RD, Schaller MD, Chen S, Schachner M, Hemperly JJ, Maness PF. NCAM stimulates the Ras-MAPK pathway and CREB phosphorylation in neuronal cells. J Neurobiol. 1999;38:542–558. [PubMed] [Google Scholar]
- Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkov O, Tikhobrazova O, Dityatev A. PSA-NCAM: synaptic functions mediated by its interactions with proteoglycans and glutamate receptors. Int J Biochem Cell Biol. 2012;44:591–595. doi: 10.1016/j.biocel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nakayama J, Suzuki A, Angata K, Chen S, Sakai K, Hagihara K, Yamaguchi Y, Fukuda M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15:887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- Theis T, Mishra B, von der Ohe M, Loers G, Prondzynski M, Pless O, Blackshear PJ, Schachner M, Kleene R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. J Biol Chem. 2013;288:6726–6742. doi: 10.1074/jbc.M112.444034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Schachner M. The intracellular domain of L1CAM binds to casein kinase 2α and is neuroprotective via inhibition of the tumor suppressors PTEN and p53. J Neurochem. 2015;133:828–843. doi: 10.1111/jnc.13083. [DOI] [PubMed] [Google Scholar]
- Westphal N, Kleene R, Lutz D, Theis T, Schachner M. Polysialic acid enters the cell nucleus attached to a fragment of the neural cell adhesion molecule NCAM to regulate the circadian rhythm in mouse brain. Mol Cell Neurosci. 2016;74:114–127. doi: 10.1016/j.mcn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Wilson DR, Zhang N, Silvers AL, Forstner MB, Bader RA. Synthesis and evaluation of cyclosporine A-loaded polysialic acid-polycaprolactone micelles for rheumatoid arthritis. Eur J Pharm Sci. 2014;51:146–156. doi: 10.1016/j.ejps.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Wu JR, Zhan XB, Zheng ZY, Zhang HT. Synthesis and characterization of polysialic acid/carboxymethyl chitosan hydrogel with potential for drug delivery. Bioorg Khim. 2015;41:627–632. doi: 10.7868/s0132342315040132. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ghadiri-Sani M, Zhang X, Richardson PM, Yeh J, Bo X. Induced expression of polysialic acid in the spinal cord promotes regeneration of sensory axons. Mol Cell Neurosci. 2007;35:109–119. doi: 10.1016/j.mcn.2007.02.011. [DOI] [PubMed] [Google Scholar]