Abstract
Purpose
This study evaluates the feasibility and acceptability of a Self-Management Survivorship Care Planning (SM-SCP) intervention in colorectal and lung cancer survivors.
Methods
This is a single-group, pre- and post-mixed methods study of an advance practice nurse-driven survivorship care intervention that integrates a survivorship care plan with self-management skills coaching. Colorectal and lung cancer survivors with stage I–III disease were enrolled at 3–6 months after completing treatments, and the intervention was administered in one in-person or telephone session. Survivor outcome measures included depression, anxiety, self-efficacy, QOL, and satisfaction. Paired t-tests were used for exploratory evaluations of pre- to post-intervention score changes. Content analysis was conducted to analyze the qualitative data to describe survivors’ experience with the intervention.
Results
Thirty participants (15 colorectal, 15 lung) enrolled and completed the study (73% retention). It took an average of 40 minutes to complete the TS/CP and 34.2 minutes to deliver the intervention. Exploratory analysis revealed significant differences from baseline to post-intervention in depression, anxiety, self-efficacy, physical functioning, role limitations-physical, pain, general health, health transition, physical health summary, and total QOL. Three qualitative themes emerged: 1) Feeling empowered about having a plan; 2) Struggling with psychosocial concerns; and 3) Suggestions for intervention content and delivery.
Conclusions
The SM-SCP intervention was feasible and acceptable for colorectal and lung cancer survivors after treatment completion. Survivorship care interventions have potential to fulfill the unmet needs of colorectal and lung cancer survivors. Their effectiveness might be greater by integrating conceptually-based models of care, such as self-management skills building.
Keywords: Self-Management, Care Plans, Colorectal Cancer, Lung Cancer, Self-Efficacy, Mixed-methods
Introduction
The need for evidence-based models of comprehensive survivorship care that address long-term survivorship issues has been the subject of numerous Institute of Medicine (IOM) reports (Hewitt et al., 2006). Survivorship care planning is considered an essential component of high-quality cancer care (Levit et al., 2013). The American Society of Clinical Oncology (ASCO) has developed and endorsed the use of treatment summary/care plans (TS/CPs) as a vehicle to guide personalized survivorship care planning (McCabe et al., 2013). The American College of Surgeon’s Commission on Cancer (CoC) has included requirements related to the provision of TS/CPs into their Cancer Program Standards (American College of Surgeons, 2012).
Despite the endorsements, guidelines, and standards, there remains a critical paucity in empirical evidence for the value of survivorship care planning on outcomes. This gap in evidence and various barriers associated with delivering TS/CPs has resulted in the slow integration of survivorship care into routine oncology care. Current evidence suggests that the development and preparation of TS/CPs is resource-intensive, lacks evidence-informed integration with technology platforms, and lacks clear reimbursement mechanisms (Parry et al., 2013). The slow uptake of survivorship care planning can be attributed, in part, to the challenges associated with their development and implementation into diverse contexts and settings (Mayer et al., 2015b; Parry et al., 2013; Selove et al., 2016).
Colorectal and lung cancer survivors are underrepresented in the survivorship literature. Colorectal cancer survivors experience bowel dysfunction, pain, fatigue, and sexual dysfunction that negatively affects their quality of life (QOL) (Bours et al., 2016; Gosselin et al., 2016; Sun et al., 2015; Sun et al., 2016; Walling et al., 2015). Among rectal cancer survivors, permanent colostomies represent a major life adjustment (McMullen et al., 2016; Sun et al., 2013; Wright et al., 2015). Colorectal cancer survivors are also less likely to receive appropriate preventive and co-morbid condition care (Hardcastle et al., 2017; Lafata et al., 2015; Sun et al., 2014). Lung cancer survivors have impaired pulmonary functions, and symptoms such as chronic pain, psychological distress, cough, fatigue persisting following treatment (Kenzik et al., 2015; Kim et al., 2016; Oksholm et al., 2015). Large population-based studies have estimated that the rate of smoking among survivors to be at least 20% (Shiels et al., 2014; Yang et al., 2015). Only 45% of survivors were meeting 5-a-day recommendations for fruit and vegetable consumption (Zhang et al., 2015). Survivor adherence to physical activity recommendations ranges from 30% to 52% (Kohler et al., 2016).
A primary goal of comprehensive survivorship care is to inform survivors about the types of treatments received, anticipate late and long-term effects of treatment, and how to maintain health and well-being after treatment (Jacobsen et al., 2016). Although intended to reduce care fragmentation and facilitate care coordination, survivorship care should also enable and empower survivors to participate in their own care. Providing information about ongoing care as part of survivorship care planning could potentially foster confidence in self-management by helping survivors monitor for late and long-term effects, successfully adopt healthy living behaviors, and undergo appropriate surveillance and screening. The primary purpose of this mixed-methods, single group, pre- and post-intervention pilot study was to describe the feasibility and acceptability of an advanced practice nurse (APN)-driven Self-Management Survivorship Care Planning (SM-SCP) intervention in colorectal and lung cancer. Qualitative data were collected to provide contextual information about feasibility and survivors’ experience with the intervention. A convergent-parallel mixed methods design was used to gain a better understanding of the intervention so it can be refined for a larger randomized study. Exploratory aims were to examine trends in survivor-specific outcome scores pre- and post-intervention.
Methods and Materials
Study Design
The study design and outcomes selection were guided by a modified version of Parry et al.’s Conceptual Framework for Survivorship Care Planning Research (Parry et al., 2013). The concentric circles represent key components of the SM-SCP (Figure 1). At the center of the circle is the TS/CP, which serves as the document that guides survivorship care and transitions from active treatment to surveillance. The delivery and implementation of TS/CPs involve three key constructs: the process of care, the model of care, and the use of technology platforms. The process of care refers to how key stakeholders (i.e. survivors and providers) interact within a given care system. The quality of these interactions is thought to affect survivor and system level outcomes through the intermediary constructs of care coordination and the quality of patient/provider communication (Parry et al., 2013). For the SM-SCP, APNs served as a vital link for care coordination and communication between survivor and oncologists. The model of care defines the target population, setting, and way the care is organized. Our intervention is disease-specific and follows the principles of integrated survivorship care where post-treatment care is provided in the same clinical setting and location where survivors received their treatments. Preliminary evidence suggests that this model of care is preferred by oncologists and survivors (Klemanski et al., 2016) and facilitates communication and efficient care transitions. The incorporation of technology can facilitate the preparation of TS/CPs, which can be resource-intensive. We used a simple and basic approach for electronic TS/CP development through Adobe PDF templates completed using tablets. Implementation, system and process-specific outcomes include recruitment, retention, time to complete TS/CPs, time to administer the intervention, and measures of resource utilization. Survivor outcome measures include intervention acceptability, depression, anxiety, self-efficacy, and QOL.
Figure 1.
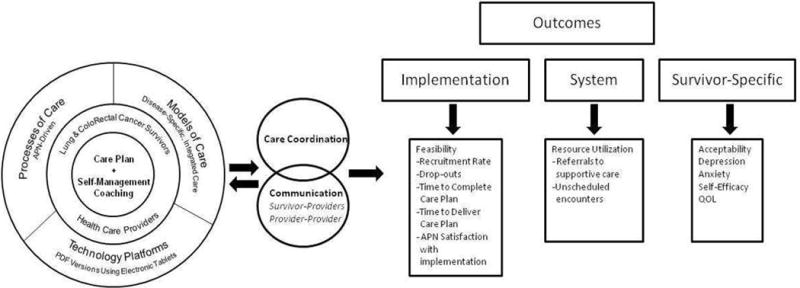
Adapted Conceptual Framework for Survivorship Care Planning Research
Intervention Design
The SM-SCP is based on the Chronic Care Self-Management Model (CCM) in which providers form partnerships with survivors to enable them to assume a more active role in managing their symptoms and achieving their goals of care (McCorkle et al., 2011). Self-management can empower cancer survivors to report and manage potential late and long-term effects of treatment, understand when to seek support, and make lifestyle changes to promote healthy living after treatment (Foster and Fenlon, 2011). The CCM model addresses survivors’ goals/preferences and fosters confidence in their ability to perform care activities (Rosenberg et al., 2015). It coaches survivors on key self-management skills such as problem-solving, decision-making, taking action or goal-setting, communication with providers, regular assessment of progress, and resource utilization (Klabunde et al., 2017). Improvements in self-efficacy are likely necessary for improvements to occur in other key outcomes such as QOL and health care resource utilization. The current evidence on CCM models of care supports this concept (McCorkle et al., 2011). Evidence for this mechanism is found in studies with chronic illnesses such as COPD, diabetes, and more recently, cancer patients (Risendal et al., 2014a; Risendal et al., 2014b; Salvatore et al., 2015).
The SM-SCP intervention is a multi-component model of survivorship care. It includes the following: 1) preparation of a personalized TS/CP, and 2) provision of the document through one post-treatment review session using self-management principles to support survivor self-efficacy. The APNs reviewed the personalized TS/CP with patients, but also incorporated self-management approaches to engage survivors. Behavioral approaches such as goal setting, problem-solving skills building and self-monitoring skills for late and long-term effects were used. The TS/CP was developed based on IOM recommendations (Hewitt et al., 2006) and existing ASCO templates. Two versions were developed: 1) a shorter TS/CP for providers in response to their preference for a brief, treatment-focused version; and 2) a TS/CP for survivors that includes additional content to support self-management skills building (i.e. strategies to cope with long-term symptoms and promote healthy lifestyle behaviors).
Setting/Sample
The study was conducted at an NCI designated comprehensive cancer center in Southern California and was approved by the Institutional Review Board. Informed consent was obtained from all individual participants included in this study. Survivors were recruited from the surgical and medical oncology ambulatory clinics. Eligibility criteria included: 1) history of stage I–III colorectal or non-small cell lung cancer (NSCLC); 2) within 3 to 6 months since completing treatment; and 3) ability to read and understand English.
Outcome Measures
Implementation Outcomes
Table 1 presents detailed information on the outcome measures for this pilot study. To assess feasibility of the SM-SCP, the following outcomes were obtained: 1) recruitment/retention rates, 2) attrition rates, 3) time spent completing the TS/CPs (both clinician and survivor versions), and 4) length of intervention sessions. We defined recruitment rate as 1) the number of eligible patients who were approached during the study period; 2) the number who declined; 3) the number enrolled per month; and 4) the total number enrolled. APN perception of intervention implementation was assessed using an investigator-developed debriefing form. The debriefing form includes questions about the APN’s overall impression and satisfaction with the session; perception of survivor’s responsiveness and knowledge about follow-up care; and the presence of any distractions or interruptions. It includes 2 open-ended questions addressing any other comments or issues that influenced the session.
Table 1.
Outcome Measures
| CONSTRUCT | INSTRUMENT | DESCRIPTION/PSYCHOMETRICS | Format | Scoring |
|---|---|---|---|---|
| Social Support | Medical Outcomes Study Social Support Survey Subscales | Two subscales used: a) Emotional/informational (expression of positive affect and empathetic understanding, and b) Tangible (access to material or behavioral assistance). | Six items rated on 5 point Likert scale ranging from “none of the time” to “all of the time. | Scores were transformed to a 1–100 scale with higher scores indicating greater support. |
| Instrumental Activities of Daily Living | OARS Multidimensional Functional Assessment Questionnaire | Assesses level of functioning and degree to which the activity can be performed independently. | Seven questions rated on a 3-point likert scale. | Score range 0–14 with higher score indicating more independence. |
| Physical Activity | The Rapid Assessment of Physical Activity (RAPA) | Brief assessment tool for level of physical activity in adults. Scoring is categorized to reflect one of five levels of activity ranging from underactive/sedentary to regular active (vigorous). | Seven items assessing level of physical activity and 2 items assessing strength and flexibility. | Total score ranges from 1–7. Any score less than 6 is suboptimal. Strength training and flexibility are scored separately. |
| Diet | Starting the Conversation (adapted version) | 8-item dietary assessment tool designed for non-dieticians in clinical practice for assessment and counseling. | Item scores range 0–2 and are added to create a summary score (0–16). | Lower summary scores indicate a healthier diet while higher scores indicate need for improvement. |
| Depression | Center for Epidemiological Studies-Depression Scale (CES-D) | Assesses the number, types, and duration of depressive symptoms. | 20 items, 4 point likert scale ranging from 0 to 3. | Scores range 0–60 with higher scores indicating more depressive symptoms. |
| Anxiety | State Trait Anxiety Inventory (STAI) | Measure of anxiety that can be used in clinical settings to diagnose anxiety and to distinguish it from depressive disorders. | 20 items, 4 point Likert scale (1–4). | Higher scores indicate greater anxiety; Range 20–80. |
| Quality of Life | Medical Outcomes Study- Short form-36 (SF-36) | 8 scales that measure physical functioning, role-physical, bodily pain, general health, vitality, mental health, role-emotional, and social functioning. Yields 2 summary scores: physical component summary (PCS) and mental component summary (MCS). | 36 items | High score indicates better QOL. Subscale scores range from 0–100. Score of <50 indicates worse physical or mental health. |
| Self-Efficacy | Self-Efficacy to Perform Self-Management Behaviors | 8 items that assess survivor’s confidence in performing self-management activities. | 8 items rated on 4-point Likert scale (1–4); total scores range from 8–32. | Higher scores represent greater confidence |
| Satisfaction (Survivor) | Survivor Satisfaction Tool | 14 fixed response items; 3 open-ended questions assess overall experience with the timing and content of the intervention. | 5 point likert scale; item scores range from 0–4; two fixed response items rated on a 3 point likert scale. | Higher scores for fixed response items indicate greater satisfaction |
| APN Perception of the Intervention | Debriefing Form | Assess APN overall impression and satisfaction with the intervention. | Likert scale format. Items range from 0–10. | Higher scores indicate greater satisfaction |
System Outcomes
Health care resource utilization measures were used to assess resource and support service needs associated with comprehensive survivorship care. These included: 1) supportive care referrals, 2) unscheduled outpatient encounters, and 3) unscheduled hospital admissions.
Survivor-Specific Outcomes
Survivors completed an investigator-developed satisfaction tool at 2-months post-intervention to assess acceptability. The tool included both fixed response items and open-ended questions assessing a) acceptability and content of the SM-SCP; b) helpfulness and understanding of information about long-term effects, lifestyle measures; preventive screening; and follow-up care; and c) the format and timing of the SM-SCP. The 3 open-ended questions assessed the content (“Looking back at your experience in the study, were there any additional information you would have liked to receive? Was there anything in the intervention that shouldn’t be included?”), timing of delivery of the intervention (“Your survivorship care plan and intervention was given to you a few months after you completed your primary treatment. Was this a good timing?”), and their overall experience (“Is there anything else that you feel is important for us to know about your overall experience?”).
The following validated measures were used to assess survivor-specific outcomes: 1) Rapid Assessment of Physical Activity (RAPA) (Topolski et al., 2006); 2) Starting the Conversation (STC) for diet behaviors (Frick et al., 2017); 3) Center for Epidemiological Studies-Depression Scale (CES-D) (Knight et al., 1997); 4) State Trait Anxiety Inventory (STAI) (Meyerhardt et al., 2016); 5) Self-Efficacy to Perform Self-Management Behaviors Scale (Hershman et al., 2014); and 6) Medical Outcomes Study – Short Form-36 (SF-36) for QOL (McHorney et al., 1993). Demographics data was obtained using a self-report tool and included Instrumental Activities of Daily Living (IADL) (Fillenbaum and Smyer, 1981; Siegel et al., 2014) and perceived social support (Medical Outcomes Study Social Support Survey) (Edwards et al., 2014). Co-morbidities were assessed using the Physical Health subscale of the Older Americans Resources and Services Questionnaire (OARS) which lists concurrent illnesses and the degree that they impair daily activities, rated on a 3-point scale ranging from “not at all” to “a great deal” (Fillenbaum and Smyer, 1981). Clinical characteristics were obtained through an audit of the EHR. Survivors completed all outcome measures at baseline and 2-months, except for the RAPA and STC (baseline only).
Study Procedures
All eligible survivors were referred by the multidisciplinary GI and lung disease teams. Two APN interventionists were utilized in the study, and each was assigned to work with one disease group only. The APNs were masters-prepared with experience caring for patients with colorectal and lung cancer. They approached all eligible survivors during a regularly scheduled clinic visit to explain the study and ascertain interest. Following informed consent, survivors completed baseline measures within 3–5 days prior to the intervention. APNs completed and prepared TS/CPs for all survivors. The SM-SCP intervention session was administered within one month following baseline assessment. To decrease participant burden, survivors were given the option to have the session administered by phone or during a regular clinic visit. During the session, the APNs reviewed self-management tips and strategies and tailored the recommendations based on the survivor’s goals and preferences. The TS/CPs were either emailed, mailed, or given to the survivors in-person based on selected mode of intervention delivery. Survivors were instructed to contact the APNs with questions regarding the TS/CPs. If any symptoms or support service needs were identified throughout the study, the APNs notified the treating oncologists and appropriate referrals were made.
Statistical Analysis
Quantitative analysis
Data were entered, audited, programmed, and then analyzed in SAS®. All multi-item instruments were scored according to manuals or other formal scoring rules available. Survivors’ sociodemographic and clinical characteristics were summarized. Outcome measure scores were examined for normality of distribution. Normalizing transformations were considered when necessary to meet the assumptions of the statistical methods utilized. Descriptive statistics were determined for feasibility of the SM-SCP. Quantitative data on acceptability of the SM-SCP were analyzed using descriptive statistics to determine survivor satisfaction with the timing, content, and delivery of the SM-SCP. Descriptive analyses were computed to assess intervention delivery, implementation issues, and barriers to intervention delivery from the APN perspective. Change in survivor-specific outcomes (depression, anxiety, self-efficacy, QOL) were examined between baseline and 2-month assessments, using paired t-tests. Standard t-tests were used to explore baseline differences in various metrics by diagnosis.
Qualitative analysis
Content analysis with an inductive approach (Elo and Kyngas, 2008) was used to analyze qualitative data. We included data from 3 open-ended questions on the satisfaction tool, survivor’s hand-written comments on fixed response items in the satisfaction tool, and the APN’s comments from the Debriefing Form. The qualitative data were analyzed independently by two team members (Dr. Reb and Dr. Sun). The responses to the open-ended questions were transcribed into a table; these were broadly categorized as “general feedback” or “specific suggestions.” The APN’s comments were entered into tables organized by participant study ID numbers. The data was reviewed several times and preliminary codes or key phrases were generated using open-coding. Preliminary subcategories were created and reviewed for each survivor across all data sources. The number of survivors in each response category were tabulated for frequency and response patterns to aid in identifying themes and categories (Sandelowski, 2000). Data that were discordantly coded were discussed by team members for refinement and consensus.
Data Integration
The quantitative and qualitative data were analyzed separately and then compared and integrated in the discussion section. A narrative approach was used to compare and expand on the findings.
Results
Sociodemographic and Clinical Characteristics
A total of 30 survivors completed the study with evaluable data (15 colorectal, 15 lung). Survivors had a mean age of 65 (range 40–90), were primarily Caucasian and retired (Table 2). The majority had at least a college education and self-reported household income was high overall. The majority of survivors had a diagnosis of stage I or II disease (57%). The mean number of co-morbidities was 2 (range 0–7). The most common categories of co-morbidities included cardiovascular (73%), musculoskeletal (60%), and respiratory (23%). For physical activity at baseline (n=29), 50% were classified as “regular active” and 47% as “underactive.” The majority of lung cancer survivors were underactive (54%). Only 36.7% of survivors reported engaging in both strength and flexibility activities. Dietary patterns at baseline were generally healthy; 76.7% consumed 3 or more servings of beans, chicken, or fish per week and 60% consumed no sweet beverages (soda or tea). However, only 43% consumed 3 or more servings of fruits/vegetables per day while 50% consumed 3 or more servings of desserts per week.
Table 2.
Sociodemographics and Clinical Characteristics
| Variable | Total (N=30) N (%) |
CRC (N=15) N (%) |
NSCLC (N=15) N (%) |
|---|---|---|---|
| Age Mean (Range) | |||
| 65 (40–90) | 60 (40–79) | 70 (49–90) | |
| Gender | |||
| Male | 12 (40) | 6 (40) | 6 (40) |
| Female | 18 (60) | 9 (60) | 9 (60) |
| Race/Ethnicity | |||
| White, Non-Hispanic | 26 (87) | 13 (86) | 14 (93) |
| Hispanic | 2 (7) | 1 (7) | 1 (7) |
| Asian | 1 (3) | 1 (7) | – |
| Black | 1 (3) | – | – |
| Education | |||
| No high school | 1 (3) | – | 1 (7) |
| Secondary/High School | 2 (7) | – | 2 (13) |
| College | 17 (57) | 10 (66) | 7 (47) |
| Graduate School | 10 (33) | 5 (34) | 5 (33) |
| Marital Status | |||
| Single | 3 (10) | 2 (13) | 1 (7) |
| Married | 15 (50) | 8 (53) | 7 (46) |
| Divorced/Separated | 6 (20) | 3 (20) | 3 (20) |
| Widowed | 6 (20) | 2 (13) | 4 (27) |
| Current Employment Status | |||
| Employed | 8 (27) | 6 (40) | 2 (13) |
| Retired | 16 (52) | 6 (40) | 10 (67) |
| Homemaker | 2 (7) | 2 (13) | – |
| On disability | 4 (14) | 1 (7) | 3 (20) |
| Annual Household Income | |||
| $15–30K | 4 (13) | 1 (7) | 3 (20) |
| $30–50K | 7 (23) | 3 (20) | 4 (27) |
| $50–75K | 4 (13) | 3 (20) | 1 (6) |
| $75–100K | 5 (17) | 2 (13) | 3 (20) |
| >$100K | 10 (34) | 6 (40) | 4 (27) |
| Perceived Level of Social Support | |||
| (Median) Range=1–100; higher score=higher perceived social support | |||
| Physical/Functional Support | 71.8 | 62.5 | 75.0 |
| Emotional Support | 81.2 | 87.5 | 78.1 |
| Composite Score | 81.2 | 83.3 | 79.1 |
| Stage of Disease | |||
| I | 9 (30) | 1 (6) | 8 (54) |
| II | 8 (27) | 6 (40) | 3 (20) |
| III | 13 (43) | 8 (54) | 4 (26) |
| *Co-Morbidities | |||
| Cardiovascular | 22 (73) | 7 (46) | 15 (100) |
| Musculoskeletal | 18 (60) | 7 (46) | 11 (73) |
| Respiratory | 7 (23) | 1 (7) | 6 (40) |
| Mental Disorders | 6 (22) | 3 (25) | 3 (20) |
| Digestive | 4 (13) | 1 (7) | 3 (20) |
| Soft Tissue/Sensory | 3 (10) | 1 (7) | 2 (13) |
| Endocrine | 2 (7) | 1 (7) | 1 (7) |
Subjects can select more than one answer
Feasibility and Implementation Outcomes
A total of 54 patients were eligible for the study and were invited to participate over a 6-month period. Of these, 13 declined participation (24%). Reasons for declining included being overwhelmed (1); no time (1); unable to reach patient (5); and no reason provided (6). Forty-one survivors (23 colorectal, 18 lung) provided consent and were enrolled (average of four patients per month). Attrition rate for the 41 consented and enrolled survivors was 26.8%. Reasons for drop-out included experiencing a cancer recurrence (3); too busy (2), no longer interested (1) and unknown (5). After accounting for attrition, a total of 30 survivors completed the study with evaluable data (73%).
On average, it took the APNs approximately 40 minutes (range 12–60) to complete the Clinician TS/CP (colorectal 32.8 minutes; lung 47.3 minutes). The average time to complete the Survivor TS/CP was 20.6 minutes (range 10–60; colorectal 26.6 minutes, lung 14.6 minutes). Overall, the average time for completing the SM-SCP session was 34.2 minutes (range 15–75). By diagnosis, the average time for session delivery was 20.6 minutes (range 15–25) for colorectal cancer survivors and 42.3 minutes (range 30–75) for lung cancer survivors. Overall, the APNs were very satisfied with the session (7.0/10.0) and rated their overall impression of the session as very effective (7.1/10.0).
System Outcomes
There were two unscheduled outpatient encounters during the study; one to an oncologist office and another to Urgent Care. There was one unscheduled hospital admission for symptom management. There were two supportive care referrals; one for nutrition and one to the Rehabilitation service (PT/OT Incontinence Program) and these 2 survivors followed up with the respective service.
Survivor-Specific Outcomes
We explored trends in score changes pre- and post-intervention for survivor outcomes (Table 3). Baseline scores for depression and anxiety were low overall and below the cut-off levels for clinically meaningful differences. Survivors reported high levels of self-efficacy at baseline. For QOL, lung cancer survivors scored significantly lower on the physical functioning subscale (58.0 vs. 85.3, p<.01) and the physical health summary score (54.0 vs. 72.3, p=.02) compared to the colorectal cancer survivors at baseline. We observed a positive trend for scores on QOL subscales at 2 months including vitality, mental health, social functioning, and mental health summary score. Significant differences were found from baseline to post-intervention in depression, anxiety, self-efficacy, QOL subscales including physical functioning, role-physical, bodily pain, general health, health transition, physical health summary, and total QOL.
Table 3.
Trends in Score Changes for Survivor-Specific Outcomes Pre- and Post-Intervention
| Total (N=30) |
Colorectal (N=15) |
Lung (N=15) |
|||||
|---|---|---|---|---|---|---|---|
| Outcome measure | Variable | Mean (SD) | P | Mean (SD) | P | Mean (SD) | P |
|
Depression (CES-D)– Higher score=more depressive symptoms |
Baseline | 9.9 (6.1) | 0.001* | 8.1 (5.0) | 0.11 | 11.3 (6.8) | 0.001* |
| 2-Months | 6.4 (5.2) | 5.7 (4.5) | 7.1 (5.7) | ||||
|
Anxiety (STAI)– Higher score=more anxiety |
Baseline | 33.5 (10.9) | 0.02* | 33.3 (11.5) | 0.09 | 32.3 (10.6) | 0.16 |
| 2-Months | 28.8 (9.1) | 29.6 (8.7) | 28.1 (9.6) | ||||
|
Self-Efficacy– Higher score=more confidence in self-management |
Baseline | 3.5 (0.54) | 0.05* | 3.5 (0.6) | 0.18 | 3.5 (0.4) | 0.14 |
| 2-Months | 3.7 (0.35) | 3.7 (0.3) | 3.7 (0.3) | ||||
| QOL: SF-36 Subscales | |||||||
|
Physical Functioning Range = 15–100; higher score=better QOL |
Baseline | 70.6 (22.6) | 0.03* | 85.3 (15.2) | 0.33 | 58.0 (21.3) | 0.05* |
| 2-Months | 77.5 (21.2) | 87.8 (12.9) | 68.0 (23.3) | ||||
|
Role Limitations due to Physical Health Problems - Range = 0–100; higher score=better QOL |
Baseline | 44.2 (40.8) | 0.01* | 58.8 (40.3) | 0.06 | 33.3 (39.7) | 0.10 |
| 2-Months | 61.7 (38.6) | 75.0 (35.3) | 49.4 (38.6) | ||||
|
Role Limitations due to Emotional Problems– Range = 0–100; higher score=better QOL |
Baseline | 80.4 (31.5) | 0.86 | 80.0 (30.3) | 0.58 | 80.0 (27.6) | 0.84 |
| 2-Months | 81.6 (28.9) | 83.3 (31.3) | 82.2 (33.0) | ||||
|
Vitality– Range = 0–100; higher score=better QOL |
Baseline | 54.2 (23.1) | 0.12 | 61.2 (23.3) | 0.43 | 49.3 (22.6) | 0.13 |
| 2-Months | 59.3 (21.3) | 64.1 (22.0) | 54.8 (20.4) | ||||
|
Mental Health– Range = 52–100; higher score=better QOL |
Baseline | 76.8 (13.1) | 0.12 | 75.2 (12.0) | 0.11 | 78.4 (14.3) | 0.08 |
| 2-Months | 82.9 (12.2) | 80.8 (12.8) | 84.9 (11.7) | ||||
|
Social Functioning– Range = 25–100; higher score=better QOL |
Baseline | 72.8 (23.4) | 0.30 | 76.6 (18.8) | 0.53 | 70.0 (27.0) | 0.42 |
| 2-Months | 78.0 (24.9) | 80.3 (21.7) | 75.8 (28.1) | ||||
|
Bodily Pain– Range = 10–100; higher score=better QOL |
Baseline | 69.8 (25.1) | 0.009* | 76.5 (18.3) | 0.05* | 63.6 (29.1) | 0.08 |
| 2-Months | 77.5 (22.3) | 83.5 (17.8) | 71.8 (25.0) | ||||
|
General Health– Range = 20–100; higher score=better QOL |
Baseline | 64.8 (20.1) | 0.01* | 68.6 (20.1) | 0.12 | 61.0 (20.0) | 0.05* |
| 2-Months | 71.6 (20.2) | 75.0 (14.5) | 68.2 (24.7) | ||||
|
Health Transition– Range = 0–100; higher score=better QOL |
Baseline | 64.6 (30.2) | 0.05* | 80.0 (27.0) | 0.27 | 51.6 (27.4) | 0.11 |
| 2-Months | 75.8 (27.1) | 83.9 (23.2) | 68.3 (29.0) | ||||
|
Physical Health Summary Range = 27.5–100; higher score=better QOL |
Baseline | 63.1 (22.0) | 0.0005* | 72.3 (19.9) | 0.05* | 54.0 (20.7) | 0.002* |
| 2-Months | 72.3 (18.7) | 80.3 (13.5) | 64.3 (20.2) | ||||
|
Mental Health Summary Range = 28–100; higher score=better QOL |
Baseline | 71.1 (18.1) | 0.18 | 73.2 (16.0) | 0.35 | 69.9 (20.1) | 0.36 |
| 2-Months | 75.5 (15.3) | 77.1 (16.3) | 73.9 (14.7) | ||||
|
Total Score– Range = 28–100; higher score=better QOL |
Baseline | 67.4 (18.4) | 0.006* | 72.8 (16.7) | 0.11 | 61.9 (19.0) | 0.01* |
| 2-Months | 74.0 (14.9) | 78.8 (12.1) | 69.1 (16.2) | ||||
Overall, survivors found that the intervention was very helpful for information related to treatment summary and follow-up surveillance tests (3.5/4.0). They rated information on physical activity, diet and overall resources as moderately helpful (2.7/4.0 and 2.6/4.0, respectively). Survivors’ ratings regarding their understanding of follow-up care and late/long-term effects of treatment were high overall (3.6/4.0 and 3.3/4.0, respectively). They rated the format of the TS/CP as very user-friendly (3.4/4.0) and the majority rated the amount of information and time spent to deliver the intervention as “the right amount.”
Qualitative Findings on Survivor’s Experience
Analysis of the survivor’s experience with the SM-SCP intervention was based on data from 29 survivors. This analysis included responses from 25 participants on the open-ended questions on the satisfaction survey; comments from 4 participants on the fixed response items of this survey; and comments entered by the APN’s on the debriefing forms for 18 participants. Three themes emerged from the analysis, and these included: 1) feeling empowered about having a plan; 2) struggling with psychosocial concerns; and 3) suggestions regarding the content and delivery of the intervention.
Feeling empowered about having a plan
The majority of survivors (n=16) felt that the TS/CP was organized and comprehensive. They felt reassured to have a plan to help them understand symptoms that may occur and what they need to do in the future:
“I just think it’s important for this follow-up paperwork… I think it is important for people to know there is more to it once you think you are done. The more you can do the better it is for the survivors.”
Although three survivors commented that they were still dealing with recovery issues, they felt that the intervention helped them think about their health plan going forward and reinforced what they were already doing. Another survivor wanted to focus on living life and not dwell on the cancer but appreciated having a TS/CP to refer to when needed. Alternatively, one colorectal cancer survivor commented that since the doctors said “cancer free” she did not think she needed to do anything further.
Struggling with psychosocial concerns
Some survivors were overwhelmed and stressed about their cancer journey. One survivor commented that she was still in a daze dealing with recovery from treatment and did not remember a lot of the information that was reviewed.
Another survivor received conflicting information during treatment:
“This whole cancer from diagnosis to today has kind of been a blur… I would have to say a lot of these things have not been explained to me in depth… I already had radiation prior to surgery, the surgeon (said), oh we don’t do that, the radiation prior to surgery can cause healing issues; plus it never works. And I’m like; I wish I knew that ahead of time…. That kind of stuff was never discussed to me as a patient or given the option, it was like, here is what we are going to do and we are going to do it. I would say it is important for the patient needs to understand what is going on.”
Another survivor felt that her family did not understand what she was going through:
“I think maybe a little bit more on how to express or tell or deal with family members, when you are told you have cancer and what is going to happen… How do you express it to your family and for them not to worry and what steps to take to prepare your family. It took a year and a half to find out. This was a little stressful as you are on this rollercoaster… and you still don’t have the answer and it is difficult because your emotions are going crazy…. I didn’t tell my family until after the surgery, if there is a way of letting someone know it’s okay not to tell people right away. I don’t know I kind of got lost there. They don’t see me as ill or struggling because I didn’t do radiation or chemo.”
One survivor cried during her intervention session explaining that her friend who had lung cancer recently died and had been a support to her. Two other survivors expressed needing more support services including assistance with transportation and socialization. Another lived 2 hours away and was uncertain about where he would receive pulmonary rehabilitation. One survivor wanted to connect with others going through the same experience. A survivor with lung cancer wanted information on spiritual concerns in the TS/CP.
“I didn’t really see a whole lot of spiritual needs or concerns as to what one’s faith is and how much you really need to put your faith in there. I understand as doctors you are not supposed to impose faith, but I feel that spiritual faith can do a lot of healing.”
Suggestions for intervention content and delivery
Survivors offered specific suggestions for additional information in the TS/CP including more information on nutrition, other supportive care providers, and cancer screening information for family members. Some survivors wanted more information on symptom management such as dealing with incontinence, bowel habits, and managing pain at home. Another wanted the TS/CP to include information on reasons for surveillance blood work. One survivor commented that it would be helpful to put the TS/CP on an iPad or card stock so she could carry it in her purse. Other suggestions included a medications list for the TS/CP. Alternatively, three survivors felt that certain information on the TS/CP did not apply to them. Some commented that they were already active and eating healthy and that some information was not helpful:
“When I looked at it, the things that weren’t important to me were in one ear and out the other but I can see that it can be helpful for others who that information is important for.”
“Much of it did not pertain to me; things on there were for really sick people. I didn’t have any troubles.”
Suggestions regarding intervention delivery
Most survivors (n=16) felt that the timing of the intervention (3–6 months after treatment completion) was appropriate and felt it was helpful during the recovery process.
The timing of it was great because it helped me to be aware of everything going on. If it was given to (me) earlier I would have put it aside and forgotten about it but if it was too late, then I would have missed my opportunity of when I needed to do things.
Alternatively, some survivors (n=9) felt that the intervention should have been delivered sooner; e.g. shortly after surgery or treatments. Two commented that having the TS/CP sooner would have helped them with managing doctors’ appointments:
“Timing-between surgery and care plan was too long, biggest stressor after surgery was trying to manage doctors’ appointments… Didn’t have doctors’ numbers, schedules…I tried to make my own grid and had a notebook but I was always shuffling through my purse trying to find things.”
Another survivor felt they needed more time to go over intervention content and their TS/CP.
Discussion
The aim of this study was to pilot-test the SM-SCP intervention, an APN-driven model of survivorship care that focuses on empowering colorectal and lung cancer survivors after treatment completion. The intervention, which incorporated a personalized TS/CP and self-management skills building, was feasible to implement and acceptable for colorectal and lung cancer survivors. Although enrollment was about 76% for eligible survivors within a 6 month timeframe, the retention rate was 73% over the 2-month study. The intervention session was completed within 1-month following enrollment for all survivors. Importantly, survivors in the current study generally derived benefits from the SM-SCP intervention as indicated by their scores on the satisfaction tool. Overall, the intervention scored highly for content, usefulness, and timing of delivery. Others have reported similar findings, where survivors found that TS/CPs and the information provided to be useful (Salz et al., 2014; Weaver et al., 2014). The qualitative data support and expand on these findings. Survivors felt empowered to have a plan and found the SM-SCP intervention and content to be organized and comprehensive in understanding risks, symptoms, and the surveillance plan going forward. Studies suggest that many survivors experience uncertainty, anxiety, and confusion regarding follow-up care (Jefford et al., 2011; Klemanski et al., 2016; Marbach and Griffie, 2011). Integrating self-management coaching and its principles to communicate TS/CP content may help to alleviate uncertainty and promote a sense of control (Baravelli et al., 2009). Findings from the Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) study of long-term colorectal and lung cancer survivors suggest that survivors who received care planning were more likely to have positive self-efficacy and to have seen a physician for follow-up care (Chrischilles et al., 2015).
The qualitative findings also revealed specific psychosocial and informational needs and preferences for CRC and lung cancer survivors. Survivors wanted more content to address grief, symptoms, nutrition, family issues, and spiritual concerns. Current models of survivorship care do not address the significant psychosocial needs that survivors experience after treatment (Blanch-Hartigan et al., 2015; Keesing et al., 2015). Some survivors were overwhelmed and commented that they did not remember much information from the intervention. Other survivors suggested a more tailored or individualized TS/CP to better meet their needs. Survivors may need an initial “personalized transition” approach, depending on the degree of symptom severity, needs, and preferences at the end of treatment. The TS/CP can be viewed as a “living document” that can be reviewed and revised over time based on the survivor’s goals and readiness for information. Although most survivors felt that the timing of intervention delivery was appropriate, several commented that the intervention should have been delivered immediately following surgery or treatment completion. Studies suggest that survivors prefer a wide range of timing for receiving TS/CP, which ranges from immediately after treatment completion to within 3 to 6 months after (Dulko et al., 2013; Mayer et al., 2014).
The preparation of a TS/CP for each survivor as part of the intervention shows that the process can be relatively time-consuming and labor-intensive. Although our institution uses EHRs, most information was documented in separate locations. This resulted in increased preparation time because of the need to access multiple electronic sites. Similar challenges are reported in other studies; average time taken to complete a TS/CP ranges from 49 to 90 minutes in the current literature (Mayer et al., 2014; McCollum et al., 2014). More research is needed to identify strategies to leverage technological advances and streamline TS/CP preparation.
The exploratory analysis of pre-and post-intervention outcomes observed significant changes in depression, anxiety, self-efficacy, physical function domains subscales of QOL, general health, health transition, and total QOL. Baseline anxiety and depression were low overall in this study, similar to findings in a recent RCT of a nurse-led supportive care intervention in colorectal cancer survivors (Jefford et al., 2016). However, other studies found that CRC survivors experience significant levels of anxiety and distress (D’Souza et al., 2016). Studies show that SCP may lead to improved follow-up care, better long-term physical well-being, positive self-efficacy, improved survivor knowledge on survivorship care, and positive lifestyle changes (Chrischilles et al., 2015; Klemanski et al., 2016; Nissen et al., 2013). The qualitative data also provided support for improved self-efficacy and confidence in understanding their health plan going forward.
The current evidence on best survivorship care models is mixed at best with most published studies showing low to moderate benefits of care planning on outcomes (Grunfeld et al., 2011; Nicolaije et al., 2015; Partridge et al., 2013). This may be partially attributed to limitations in the current evidence including: 1) heterogeneity in the models of survivorship care which makes it difficult to compare results across studies; 2) a near exclusive focus on breast cancer; 3) a lack of conceptually-based interventions; and 4) a focus on survivor- reported outcomes with limited focus on provider and systems outcomes such as care coordination and health care utilization (Halpern et al., 2014; Jacobsen et al., 2016; Mayer et al., 2015a). The SM-SCP intervention is an evidence-informed, conceptually-based model of post-treatment survivorship care that integrates a personalized TS/CP with self-management skills building to enable and empower survivors. It targets two understudied cancer survivor populations (colorectal and lung) in an effort to expand the scope of diseases studied in the current literature.
This pilot study has several important limitations that warrant further discussion including the small sample size. We did not perform power calculations to statistically derive the sample size. The sample size was derived to be realistic and practical for the six month study timeframe, and our primary intent was to determine whether the components of the SM-SCP intervention can be feasibly administered in our target populations. Since our analysis was limited to feasibility and acceptability, the pre-and post-intervention comparisons were exploratory in nature. Since there was no control group and survivor-specific outcomes likely improve over time after treatment completion, non-specific factors may have contributed to the beneficial effects reported. A future larger, randomized trial is needed to verify the benefits of the intervention. Although the qualitative data were robust, a limitation is that the satisfaction survey data did not allow for probing or clarification as it was self-administered.
In conclusion, the SMP-SCP intervention was feasible to implement in an ambulatory care setting and acceptable for colorectal and lung cancer survivors. The study findings support the benefits of an APN-administered self-management intervention in this setting. Advance practice nurses can incorporate self-management skills in providing survivorship care including coaching patients about problem-solving, symptom management, communication with health care providers, and goal setting to increase survivor engagement. Further research on creative models of survivorship care delivery is needed to address the significant psychosocial and information needs of colorectal and lung cancer survivors in a cost-effective manner. Future research should focus on the optimal dose and frequency of follow-up care based on the needs and preferences of survivors during the early transition period. Studies are needed to address the impact of survivorship care planning on cancer care delivery outcomes such as care coordination and healthcare utilization as these factors may influence the effectiveness of interventions and patient reported outcomes (Mayer et al., 2015b; Parry et al., 2013). Findings from this pilot study will set the stage for a randomized trial to deliver and sustain the SM-SCP intervention in real-world care settings.
Table 4.
Intervention Acceptability
| Total (N=30) |
Colorectal (N=15) |
Lung (N=15) |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| How helpful was the SM-SCP for the following: | |||
| (Range=0–4; 0=not helpful at all, 4=very helpful) | |||
| Contact Information for your doctors | 3.33 (1.02) | 3.07 (1.22) | 3.60 (.74) |
| Information about the types of treatments that you received | 3.50 (.82) | 3.33 (.90) | 3.67 (.72) |
| When to see your cancer doctor for follow-up and what types of scans/blood work you need | 3.50 (.90) | 3.13 (1.13) | 3.87 (.35) |
| Information on how to manage possible late/long-term effects from treatments | 3.28 (1.16) | 2.80 (1.37) | 3.79 (.58) |
| Information about how to stay physically active and eat a better diet | 2.77 (1.43) | 2.47 (1.41) | 3.07 (1.44) |
| Information about screening for other cancers | 2.83 (1.34) | 2.33 (1.29) | 3.33 (1.23) |
| Information about resources that you can use on the internet or through telephone | 2.60 (1.45) | 2.47 (1.13) | 2.73 (1.75) |
| When to see your cancer doctor for follow-up and what types of scans/blood work you need | 3.62 (.86) | 3.50 (1.09) | 3.73 (.59) |
| Possible late/long-term effects from treatments | 3.30 (1.06) | 3.13 (1.06) | 3.47 (1.06) |
| How to stay physically active and eat a better diet | 3.37 (1.16) | 3.07 (1.22) | 3.67 (1.05) |
| Screening for other cancers | 3.17 (1.07) | 2.93 (1.03) | 3.43 (1.09) |
| How user-friendly was the format of TS/CP? (Range=0–4; 0=not user-friendly at all, 4=very user-friendly) | 3.43 (.69) | 3.40 (.63) | 3.47 (.74) |
| *Was the amount of information in the TS/CP: | 2.10 (.48) | 2.07 (.59) | 2.13 (.35) |
| *Was the time spent going over the care plan: | 2.00 (.46) | 1.93 (.46) | 2.07 (.46) |
1=too little; 2=the right amount; 3=too much
Highlights.
We pilot-tested a self-management survivorship care planning intervention in colorectal and lung cancer.
Survivorship care interventions have potential to fulfill the unmet needs of cancer survivors.
Interventions may be more effective by integrating conceptually-based models of care, such as self-management skills building.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 7th Biennial American Cancer Society/National Cancer Institute Cancer Survivorship Research Conference, June 18–20, 2014, Atlanta, GA
References
- American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient-Centered Care 2012 [Google Scholar]
- Baravelli C, Krishnasamy M, Pezaro C, Schofield P, Lotfi-Jam K, Rogers M, Milne D, Aranda S, King D, Shaw B, Grogan S, Jefford M. The views of bowel cancer survivors and health care professionals regarding survivorship care plans and post treatment follow up. Journal of cancer survivorship: research and practice. 2009;3:99–108. doi: 10.1007/s11764-009-0086-1. [DOI] [PubMed] [Google Scholar]
- Blanch-Hartigan D, Chawla N, Beckjord EI, Forsythe LP, de Moor JS, Hesse BW, Arora NK. Cancer survivors’ receipt of treatment summaries and implications for patient-centered communication and quality of care. Patient Education and Counseling. 2015;98:1274–1279. doi: 10.1016/j.pec.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, van der Linden BW, Winkels RM, van Duijnhoven FJ, Mols F, van Roekel EH, Kampman E, Beijer S, Weijenberg MP. Candidate Predictors of Health-Related Quality of Life of Colorectal Cancer Survivors: A Systematic Review. The oncologist. 2016;21:433–452. doi: 10.1634/theoncologist.2015-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrischilles EA, McDowell BD, Rubenstein L, Charlton M, Pendergast J, Juarez GY, Arora NK. Survivorship care planning and its influence on long-term patient-reported outcomes among colorectal and lung cancer survivors: the CanCORS disease-free survivor follow-up study. Journal of cancer survivorship: research and practice. 2015;9:269–278. doi: 10.1007/s11764-014-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza V, Daudt H, Kazanjian A. Survivorship care plans for people with colorectal cancer: do they reflect the research evidence? Current oncology. 2016;23:e488–e498. doi: 10.3747/co.23.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulko D, Pace CM, Dittus KL, Sprague BL, Pollack LA, Hawkins NA, Geller BM. Barriers and facilitators to implementing cancer survivorship care plans. Oncology nursing forum. 2013;40:575–580. doi: 10.1188/13.ONF.575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo S, Kyngas H. The qualitative content analysis process. Journal of advanced nursing. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of gerontology. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- Foster C, Fenlon D. Recovery and self-management support following primary cancer treatment. Br J Cancer. 2011;105:S21–S28. doi: 10.1038/bjc.2011.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick MA, Vachani CC, Hampshire MK, Bach C, Arnold-Korzeniowski K, Metz JM, Hill-Kayser CE. Survivorship after lower gastrointestinal cancer: Patient-reported outcomes and planning for care. Cancer. 2017 doi: 10.1002/cncr.30527. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Gosselin TK, Beck S, Abbott DH, Grambow SC, Provenzale D, Berry P, Kahn KL, Malin JL. The Symptom Experience in Rectal Cancer Survivors. Journal of pain and symptom management. 2016;52:709–718. doi: 10.1016/j.jpainsymman.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, Joy AA, Provencher L, Rayson D, Rheaume DE, Porter GA, Paszat LF, Pritchard KI, Robidoux A, Smith S, Sussman J, Dent S, Sisler J, Wiernikowski J, Levine MN. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:4755–4762. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of Cancer Survivorship Care: Overview and Summary of Current Evidence. Journal of oncology practice/American Society of Clinical Oncology. 2014;11:e19–e27. doi: 10.1200/JOP.2014.001403. [DOI] [PubMed] [Google Scholar]
- Hardcastle SJ, Maxwell-Smith C, Zeps N, Platell C, O’Connor M, Hagger MS. A qualitative study exploring health perceptions and factors influencing participation in health behaviors in colorectal cancer survivors. Psycho-oncology. 2017;26:199–205. doi: 10.1002/pon.4111. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical, O. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- Hewitt ME, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivors: Lost in Transition. National Academies Press; Washington, DC: 2006. [Google Scholar]
- Jacobsen PB, Rowland JH, Paskett ED, Van Leeuwen F, Moskowitz C, Katta S, Wollins D, Robison LL. Identification of Key Gaps in Cancer Survivorship Research: Findings From the American Society of Clinical Oncology Survey. Journal of oncology practice/American Society of Clinical Oncology. 2016;12:190–193. doi: 10.1200/JOP.2015.009258. [DOI] [PubMed] [Google Scholar]
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow P, Phipps-Nelson J, Young J, Krishnasamy M, Ugalde A, King D, Strickland A, Franco M, Blum R, Johnson C, Ganju V, Shapiro J, Chong G, Charlton J, Haydon A, Schofield P. A Randomized Controlled Trial of a Nurse-Led Supportive Care Package (SurvivorCare) for Survivors of Colorectal Cancer. The oncologist. 2016;21:1014–1023. doi: 10.1634/theoncologist.2015-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefford M, Lotfi-Jam K, Baravelli C, Grogan S, Rogers M, Krishnasamy M, Pezaro C, Milne D, Aranda S, King D, Shaw B, Schofield P. Development and pilot testing of a nurse-led posttreatment support package for bowel cancer survivors. Cancer nursing. 2011;34:E1–10. doi: 10.1097/NCC.0b013e3181f22f02. [DOI] [PubMed] [Google Scholar]
- Keesing S, McNamara B, Rosenwax L. Cancer survivors’ experiences of using survivorship care plans: a systematic review of qualitative studies. Journal of cancer survivorship: research and practice. 2015;9:260–268. doi: 10.1007/s11764-014-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzik KM, Ganz PA, Martin MY, Petersen L, Hays RD, Arora N, Pisu M. How much do cancer-related symptoms contribute to health-related quality of life in lung and colorectal cancer patients? A report from the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium. Cancer. 2015;121:2831–2839. doi: 10.1002/cncr.29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Sun V, Raz DJ, Williams AC, Fujinami R, Reckamp K, Koczywas M, Cristea M, Hurria A, Ferrell B. The impact of lung cancer surgery on quality of life trajectories in patients and family caregivers. Lung Cancer. 2016;101:35–39. doi: 10.1016/j.lungcan.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Haggstrom D, Kahn KL, Gray SW, Kim B, Liu B, Eisenstein J, Keating L. Oncologists’ perspectives on post-cancer treatment communication and care coordination with primary care physicians. European journal of cancer care. 2017 doi: 10.1111/ecc.12628. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemanski DL, Browning KK, Kue J. Survivorship care plan preferences of cancer survivors and health care providers: a systematic review and quality appraisal of the evidence. Journal of cancer survivorship: research and practice. 2016;10:71–86. doi: 10.1007/s11764-015-0452-0. [DOI] [PubMed] [Google Scholar]
- Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behaviour research and therapy. 1997;35:373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25:1018–1028. doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafata JE, Salloum RG, Fishman PA, Ritzwoller DP, O’Keeffe-Rosetti MC, Hornbrook MC. Preventive care receipt and office visit use among breast and colorectal cancer survivors relative to age- and gender-matched cancer-free controls. Journal of cancer survivorship: research and practice. 2015;9:201–207. doi: 10.1007/s11764-014-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit L, Balogh E, Nass S, Ganz P. Delivering high-quality cancer care: charting a new course for a system in crisis. The National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- Marbach TJ, Griffie J. Patient preferences concerning treatment plans, survivorship care plans, education, and support services. Oncology nursing forum. 2011;38:335–342. doi: 10.1188/11.ONF.335-342. [DOI] [PubMed] [Google Scholar]
- Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: An integrative review of studies of cancer survivorship care plans (2006–2013) Cancer. 2015a;121:978–996. doi: 10.1002/cncr.28884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DK, Birken SA, Chen RC. Avoiding Implementation Errors in Cancer Survivorship Care Plan Effectiveness Studies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015b;33:3528–3530. doi: 10.1200/JCO.2015.62.6937. [DOI] [PubMed] [Google Scholar]
- Mayer DK, Gerstel A, Walton AL, Triglianos T, Sadiq TE, Hawkins NA, Davies JM. Implementing survivorship care plans for colon cancer survivors. Oncology nursing forum. 2014;41:266–273. doi: 10.1188/14.ONF.266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS, Hudson MM. American Society of Clinical Oncology Statement: Achieving High-Quality Cancer Survivorship Care. Journal of Clinical Oncology. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum KH, Wood FG, Auriemma K. Evaluation of a breast and colon cancer survivorship program. Clinical journal of oncology nursing. 2014;18:231–236. doi: 10.1188/14.CJON.231-236. [DOI] [PubMed] [Google Scholar]
- McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling LS, Lorig K, Wagner EH. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: a cancer journal for clinicians. 2011;61:50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- McMullen CK, Bulkley JE, Altschuler A, Wendel CS, Grant M, Hornbrook MC, Sun V, Krouse RS. Greatest Challenges of Rectal Cancer Survivors: Results of a Population-Based Survey. Diseases of the colon and rectum. 2016;59:1019–1027. doi: 10.1097/DCR.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, Cespedes EM, Xiao J, Caan BJ. Association of Weight Change after Colorectal Cancer Diagnosis and Outcomes in the Kaiser Permanente Northern California Population. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;26:30–37. doi: 10.1158/1055-9965.EPI-16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaije KA, Ezendam NP, Vos MC, Pijnenborg JM, Boll D, Boss EA, Hermans RH, Engelhart KC, Haartsen JE, Pijlman BM, van Loon-Baelemans IE, Mertens HJ, Nolting WE, van Beek JJ, Roukema JA, Zijlstra WP, Kruitwagen RF, van de Poll-Franse LV. Impact of an Automatically Generated Cancer Survivorship Care Plan on Patient-Reported Outcomes in Routine Clinical Practice: Longitudinal Outcomes of a Pragmatic, Cluster Randomized Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:3550–3559. doi: 10.1200/JCO.2014.60.3399. [DOI] [PubMed] [Google Scholar]
- Nissen M, Tsai M, Blaes A, Swenson K, Koering S. Effectiveness of treatment summaries in increasing breast and colorectal cancer survivors’ knowledge about their diagnosis and treatment. Journal of Cancer Survivorship. 2013;7:211–218. doi: 10.1007/s11764-012-0261-7. [DOI] [PubMed] [Google Scholar]
- Oksholm T, Rustoen T, Cooper B, Paul SM, Solberg S, Henriksen K, Kongerud JS, Miaskowski C. Trajectories of Symptom Occurrence and Severity From Before Through Five Months After Lung Cancer Surgery. Journal of pain and symptom management. 2015;49:995–1015. doi: 10.1016/j.jpainsymman.2014.11.297. [DOI] [PubMed] [Google Scholar]
- Parry C, Kent EE, Forsythe LP, Alfano CM, Rowland JH. Can’t see the forest for the care plan: a call to revisit the context of care planning. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:2651–2653. doi: 10.1200/JCO.2012.48.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge AH, Norris VW, Blinder VS, Cutter BA, Halpern MT, Malin J, Neuss MN, Wolff AC, on behalf of the, A.B.C.R.P.S.G. Implementing a breast cancer registry and treatment plan/summary program in clinical practice: A pilot program. Cancer. 2013;119:158–163. doi: 10.1002/cncr.27625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risendal B, Dwyer A, Seidel R, Lorig K, Katzenmeyer C, Coombs L, Kellar-Guenther Y, Warren L, Franco A, Ory M. Adaptation of the chronic disease self-management program for cancer survivors: feasibility, acceptability, and lessons for implementation. Journal of cancer education: the official journal of the American Association for Cancer Education. 2014a;29:762–771. doi: 10.1007/s13187-014-0652-8. [DOI] [PubMed] [Google Scholar]
- Risendal BC, Dwyer A, Seidel RW, Lorig K, Coombs L, Ory MG. Meeting the challenge of cancer survivorship in public health: results from the evaluation of the chronic disease self-management program for cancer survivors. Frontiers in public health. 2014b;2:214. doi: 10.3389/fpubh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg CA, Flanagan C, Brockstein B, Obel JC, Dragon LH, Merkel DE, Wade EL, Law TM, Khandekar JD, Hensing TA. Promotion of self-management for post treatment cancer survivors: evaluation of a risk-adapted visit. Journal of Cancer Survivorship. 2015;10:206–219. doi: 10.1007/s11764-015-0467-6. [DOI] [PubMed] [Google Scholar]
- Salvatore AL, Ahn S, Jiang L, Lorig K, Ory MG. National study of chronic disease self-management: 6-month and 12-month findings among cancer survivors and non-cancer survivors. Psycho-oncology. 2015;24:1714–1722. doi: 10.1002/pon.3783. [DOI] [PubMed] [Google Scholar]
- Salz T, Baxi SS, Blinder VS, Elkin EB, Kemeny MM, McCabe MS, Moskowitz CS, Onstad EE, Saltz LB, Temple LK, Oeffinger KC. Colorectal cancer survivors’ needs and preferences for survivorship information. Journal of oncology practice/American Society of Clinical Oncology. 2014;10:e277–282. doi: 10.1200/JOP.2013.001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski M. Whatever happened to qualitative description? Research in nursing & health. 2000;23:334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Selove R, Birken SA, Skolarus TA, Hahn EE, Sales A, Proctor EK. Using Implementation Science to Examine the Impact of Cancer Survivorship Care Plans. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016 doi: 10.1200/JCO.2016.67.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Gibson T, Sampson J, Albanes D, Andreotti G, Beane Freeman L, Berrington de Gonzalez A, Caporaso N, Curtis RE, Elena J, Freedman ND, Robien K, Black A, Morton LM. Cigarette smoking prior to first cancer and risk of second smoking-associated cancers among survivors of bladder, kidney, head and neck, and stage I lung cancers. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:3989–3995. doi: 10.1200/JCO.2014.56.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- Sun V, Grant M, McMullen CK, Altschuler A, Mohler MJ, Hornbrook MC, Herrinton LJ, Baldwin CM, Krouse RS. Surviving colorectal cancer: long-term, persistent ostomy-specific concerns and adaptations. Journal of wound, ostomy, and continence nursing: official publication of The Wound, Ostomy and Continence Nurses Society/WOCN. 2013;40:61–72. doi: 10.1097/WON.0b013e3182750143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun V, Grant M, McMullen CK, Altschuler A, Mohler MJ, Hornbrook MC, Herrinton LJ, Krouse RS. From diagnosis through survivorship: health-care experiences of colorectal cancer survivors with ostomies. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2014;22:1563–1570. doi: 10.1007/s00520-014-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun V, Grant M, Wendel CS, McMullen CK, Bulkley JE, Altschuler A, Ramirez M, Baldwin CM, Herrinton LJ, Hornbrook MC, Krouse RS. Dietary and Behavioral Adjustments to Manage Bowel Dysfunction After Surgery in Long-Term Colorectal Cancer Survivors. Annals of surgical oncology. 2015;22:4317–4324. doi: 10.1245/s10434-015-4731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun V, Grant M, Wendel CS, McMullen CK, Bulkley JE, Herrinton LJ, Hornbrook MC, Krouse RS. Sexual Function and Health-Related Quality of Life in Long-Term Rectal Cancer Survivors. The journal of sexual medicine. 2016;13:1071–1079. doi: 10.1016/j.jsxm.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Preventing chronic disease. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- Walling AM, Weeks JC, Kahn KL, Tisnado D, Keating NL, Dy SM, Arora NK, Mack JW, Pantoja PM, Malin JL. Symptom prevalence in lung and colorectal cancer patients. Journal of pain and symptom management. 2015;49:192–202. doi: 10.1016/j.jpainsymman.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Aziz NM, Arora NK, Forsythe LP, Hamilton AS, Oakley-Girvan I, Keel G, Bellizzi KM, Rowland JH. Follow-up care experiences and perceived quality of care among long-term survivors of breast, prostate, colorectal, and gynecologic cancers. Journal of oncology practice/American Society of Clinical Oncology. 2014;10:e231–239. doi: 10.1200/JOP.2013.001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Downing A, Morris EJ, Corner JL, Richards MA, Sebag-Montefiore D, Finan P, Glaser AW. Identifying Social Distress: A Cross-Sectional Survey of Social Outcomes 12 to 36 Months After Colorectal Cancer Diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:3423–3430. doi: 10.1200/JCO.2014.60.6129. [DOI] [PubMed] [Google Scholar]
- Yang B, Jacobs EJ, Gapstur SM, Stevens V, Campbell PT. Active Smoking and Mortality Among Colorectal Cancer Survivors: The Cancer Prevention Study II Nutrition Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:885–893. doi: 10.1200/JCO.2014.58.3831. [DOI] [PubMed] [Google Scholar]
- Zhang FF, Liu S, John EM, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer. 2015;121:4212–4221. doi: 10.1002/cncr.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]


