1.0 Introduction
Infants born very preterm (≤32 weeks gestational age) spend weeks to months in neonatal intensive care units (NICU), undergoing on average 10 painful procedures per day [54] while their brains are rapidly developing. In rodents, early pain exposure has adverse effects on brain development [2,27,39] and social behaviors [1,11]. Clinical human prospective longitudinal cohort studies have demonstrated adverse effects of repetitive exposure to early pain on brain development and outcomes [16,25,48,49,67,70].
Effective pain management is crucial to help mitigate these negative consequences of early pain exposure. Currently, oral sucrose is administered routinely to reduce pain of minor procedures in infants and is recommended as standard care in international guidelines [4,5,43], which are based on evidence of safety and efficacy of a single dose of sucrose to reduce pain behaviors [62]. Importantly, long-term benefits or risks of repeated sucrose administration in the context of pain, in human or animal neonates, on brain development have not been studied. To date, one study in very preterm infants showed that more than 10 sucrose doses per day in the first week of life was associated with poorer attention and motor function, but the end-point was only at term-equivalent age [38]. Since oral sucrose is advocated as standard care worldwide [4,5,43], clinical equipoise no longer exists for a randomized controlled trial of sucrose in infants to evaluate effects on brain development.
Mechanisms of sucrose-induced analgesia are well established in rodent models. Oral administration of sweet substances, such as sucrose, inhibit pain by mediating endogenous opioid peptide and μ1-opioid receptor actions [21]. Others have suggested additional involvement of the 5-HT2A-serotonergic receptors in the antinociception effect of sweet solution administration[51]. Additionally, key brainstem sites critically involved in descending pain modulation (e.g. periaqueductal gray and rostroventromedial medullary region) have been shown to be activated by intraoral sucrose administration in rat pups [6]. One study has reported short-term memory impairment in rats repeadly exposed during the first 8 weeks of life to daily pain alone compared to pain combined with sucrose[45]. However, their pain model does not match that of the human infant exposure in the NICU.
Sugar consumption activates common reward pathways and repeated sugar exposure is known to cause excessive dopamine and acetylcholine release, as well as opioid stimulation [32,34,47,59]. In the developing brain, it is not known whether increases in dopamine and/or acetylcholine levels, as well as opioid stimulation would have positive or negative effect on related brain structures and functions [35].
Given that sucrose administration for procedural pain is a treatment that currently affects many thousands of preterm infants annually, it is crucial to determine the long-term benefits/risks of repetitive sucrose. In the present study we examined effects of neonatal repetitive sucrose treatment on brain development in a mouse pain model and amount of oral sucrose exposure that closely mimics NICU care.
2.0 Materials and methods
2.1. Animals
Mice make an excellent preclinical model to study conditions in the human preterm neonate because they are born neurologically immature, with the first postnatal week in the mouse corresponding to the development of the human limbic system, striatum and cerebellar development at 24 to 32 weeks gestation [12]. All procedures were approved by the University of British Columbia Animal Care Committee and conform to the guidelines of the Canadian Council on Animal Care. C57BL/6J mice used in this study were obtained from the Goldowitz mouse colony at the Center for Molecular Medicine and Therapeutics Animal Facility. Animals were maintained on a 14/10 hour light/dark cycle with food and water ad libitum. All mice were provided with nestlets and Plexiglas igloo-style houses as part of standard enrichment. A specific cellulose bedding was used during the entire protocol duration to minimize stimulation and discomfort of inflamed paws in the pups (¼ inch pelleted cellulose, Biofresh, USA). Multiparous female mice were individually mated with a male stud. Pregnant females were group housed until E17, upon which they were divided and housed with a nulliparous female CD-1 used as a “nanny” to improve survival and reduce cannibalism of mouse pups. This housing was maintained for the duration of pregnancy and lactation. The day of birth was considered P0. Pups were kept with their mother until wean age, postnatal day 21 (P21). After weaning, mice were housed together with appropriate nesting materials, enrichment and cellulose bedding (5 mice/cage, sex-matched cages) during aging until P85–P95.
2.2. Needle-prick model combined with oral sucrose
Newborn mouse pups on P0 were randomized to one of the six groups and tattooed on their paw using a single 30G needle prick to allow individual identification. Pups received either sterile water or sucrose (Vehicle; Treatment) given orally by a micropipette 2 minutes prior to one of the three interventions: paw needle-prick, light paw tactile pressure with a cotton-tipped swab, or only being handled in a similar manner to other intervention groups. Treatment/Intervention sequence duration was less than 5 minutes per interval. A solution of sucrose 24% w/v (Sucrose ≥99.5% (GC), Sigma, USA, cat#S7903) in sterile water, filtered with 0.22μm filter, or sterile water was used intraorally as analgesic treatment 2 minutes before the intervention. Using a micropipette and sterile tips, the treatment was administered either on the anterior part of the tongue or inner-cheek of the mouth. An equivalent dose of sucrose per weight comparable to the recommended dose for human neonates was given. The standard guideline recommends 0.5mL per dose for very preterm infants (24 to 32 weeks of gestation), which correspond to 0.08g–0.2g of sucrose per body weight (kg) [62]. Each pup received 0.1–0.2g of sucrose per kg of mouse body weight. Each dose was adjusted daily based on the mouse pup weight. Vehicle treatment group received orally an equivalent volume of sterile water. Treatment/Intervention was administered 10 times per day per mouse pup over a 10-hour period (from 8AM until 6PM) during the day (light) cycle from P1 to P6 inclusive. Each treatment/intervention was spaced by a minimum of 30 minutes to allow enough time for feeding and recovery from the interventions. Forepaws and hindpaws were alternated over each intervention and only one paw was touched or pricked during each interval. Needle-pricks were performed using a 30G sterile needle (0.3mm outer diameter) angled at 10–15 degrees from the skin to carefully pierce only the surface of the skin. Penetration of deeper layers, such as tendon or bone, was avoided. Mouse pups were returned to the dams between each hourly Treatment/Intervention sequences. During the procedure, home cages with nursing females were placed in a separate room to decrease their stress in response to mouse pup vocalizations related to the interventions. Each procedure was performed on a warmed heating pad to avoid any heat loss. At P7, mice pups remained in their home cage with their dam until weaning (P21). A total of 109 mouse pups were treated and survived to adulthood with an overall survival rate of 88%.
2.3. Perfusion and tissue collection
Adult brains were collected between P85–P95 for MRI scanning. Initially the mice were anesthetized and transcardially perfused with 10mL of 0.1M PBS containing 10U/mL heparin (PPC, CA, cat#C504805) and 2mM ProHance (a Gadolinium contrast agent, Bracco Diagnostics, USA, cat#111181) followed by 10mL of 4% paraformaldehyde (PFA) (Cedarlane, CA, cat#15710) in 0.1M PBS containing 2mM ProHance [60]. Perfusions were performed at a rate of approximately 60mL/hr. After perfusion, mice were decapitated. The brain and remaining skull structures were incubated in 4% PFA + 2mM ProHance overnight at 4°C then transferred to 0.1M PBS containing 2mM ProHance and 0.02% sodium azide for at least seven days prior to MRI scanning.
2.4. Magnetic resonance imaging, registration and analysis
A multi-channel 7.0 Tesla MRI scanner (Varian Inc., Palo Alto, CA) was used to image the brains within skulls. Sixteen custom-built solenoid coils were used to image the brains in parallel [15]. T2-weighted, three-dimensional fast spin echo sequence, with a cylindrical acquisition of k-space, and with repetition time TR = 350 ms, echo train length = 6, TEeff = 12 ms, field of view (FOV) 20mm×20mm×25mm3, and matrix size of 504 × 504 × 630, which provided an isotropic resolution of 0.04 mm were used to gather anatomical imaging and volume change parameters. Total imaging time was ~14 hours.
To visualize and compare any changes in the mouse brains, images were linearly (6 parameter followed by a 12 parameter) and non-linearly registered against a pre-existing atlas [26]. All scans were then re-sampled with the appropriate transformation and averaged to create a population atlas representing the average anatomy of the study sample. The resulting registration was to have all scans deformed into alignment with each other in an unbiased fashion. This strategy allowed for the analysis of the deformations needed to take each individual mouse’s anatomy into this final atlas space, the goal being to model how the deformation fields relate to treatment/intervention [42,44]. The Jacobian determinants of the deformation fields were calculated as measures of volume at each voxel. Significant volume changes were calculated by warping a pre-existing classified MRI atlas onto the population atlas, which allowed assessment of the volumes of 159 segmented structures encompassing cortical lobes, large white matter structures (i.e. corpus callosum), ventricles, cerebellum, brain stem, and olfactory bulbs in all brains [26]. The current atlas used is a combination of three atlas segmentations: A 62 region full brain segmentation [26], and additional segmentation of the cortex [66] and cerebellum [61,66]. This 159 region atlas was used to assess regional differences across the brain. Further, these measurements were examined on a voxel-wise basis to localize the differences found within regions or across the brain.
2.5. Statistical analysis
We first conducted multiple one-sided ANOVAs to determine whether there was any effect by intervention (Handle, Tactile, or Needle-prick), treatment (Vehicle or Sucrose), or an intervention by treatment interaction for each of the 159 regional brain volumes. Multiple comparisons were controlled for using the False Discovery Rate (FDR) [30].
Constrained principal component analysis (CPCA) was used to examine the group differences of effects of needle-prick and sucrose exposure upon all 159 regional brain volumes. CPCA is a 2-step process, referred to as the external and internal analysis. The external analysis consists of a multivariate least squares multiple regression of the dependent measures on the independent measures, producing predicted and residual scores for each dependent measure [48,49]. The internal analysis is a principal component analysis (PCA), which transforms aforementioned regression matrices into a few orthogonal components (or latency variables) which explain the maximum amount of variance.
CPCA captures the most prominent feature in a data matrix and projects it to a subspace of minimal dimensionality according to the external information [64]. The lowest dimension, which explains the greatest variation, depends on both the dataset and the external information. Since the aim of the study was to investigate the effects of sucrose on the interventions, three CPCA models were built to capture the contrasts between groups. The first CPCA was to compare regional brain volumes between Vehicle and Sucrose treatment groups. Second and third CPCA models were built to compare the effect of Treatment exposure within the Vehicle and Sucrose treatment group respectively.
In the first CPCA, the independent variables matrix of the external analysis included Vehicle treatment group and Sucrose treatment group, sex, and the adjusted total brain volume. The adjusted total brain volumes were computed as the residuals of regressing total brain volumes onto Vehicle/Sucrose groups and intervention groups. The purpose was to remove the possible association between sucrose and needle-prick exposure and the total brain volume. Two separate CPCAs were then performed by splitting the sample into Vehicle treated and Sucrose treated mice. The independent variable matrix of each CPCA included Intervention (i.e. Handle vs Tactile, Handle vs Needle-prick), sex, and adjusted total brain volume.
The internal analysis consists of PCAs on each of the aforementioned matrices. The resulting component solutions (overall, predicted, and residual solutions) are examined to determine which dimensions of the regional brain volumes can be explained by the independent variables. Details of CPCA have been described previously [19,48,49]. Computations for CPCA were done using MATLAB v 7.11 (The MathWorks, 2010, Natick, Massachusetts). Multiple comparisons were corrected by Bonferroni method. Data were graphically organized using GraphPad Prism (San Diego, CA).
3.0 Results
3.1. Repeated sucrose pain model details
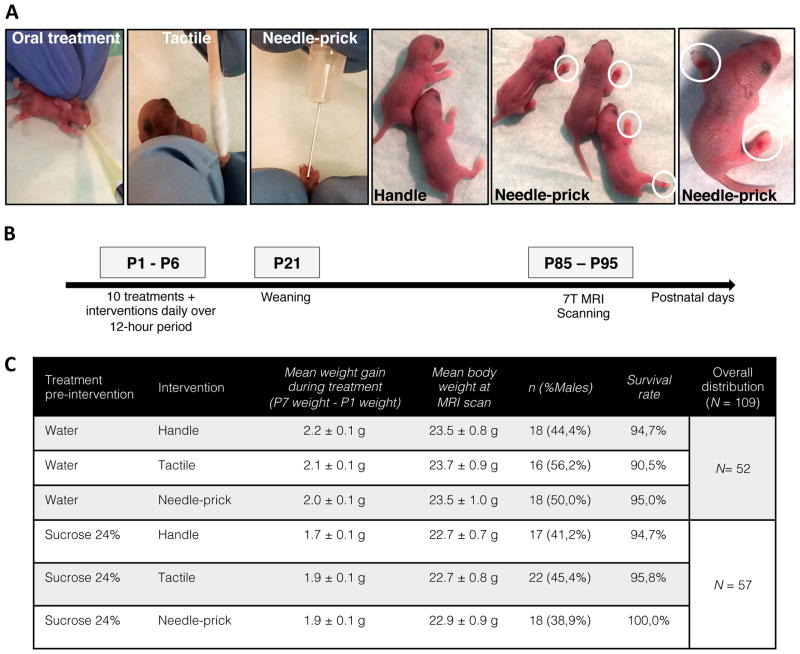
At P0, mouse pups (50 males; 59 females) were randomly allocated to one of six groups: Vehicle/Handle (n = 18), Vehicle/Tactile (n = 16), Vehicle/Needle-prick (n = 18), Sucrose/Handle (n = 17), Sucrose/Tactile (n = 22), or Sucrose/Needle-prick (n = 18) (Figure 1C). Each litter included at least two different groups and nearly equal distribution by sex per group. During the first days of life, from postnatal day 1 to 3 (P1 to P3), pups in the needle-prick groups showed visible paw inflammation at the site of skin-break as exposure to the intervention accumulated throughout the 10 hours (Figure 1A); in each case, local inflammation disappeared by the following morning. No signs of infection or other complications occurred. Timeline of the experimental design is shown in Figure 1B.
Figure 1. Neonatal mouse model of repetitive sucrose exposure given for procedural pain.
(A) Representative images showing C57Bl/6J mouse pups receiving oral sucrose treatment preceding 10 interventions daily: Handle, Tactile or Needle-prick. Ecchymosis and inflamed paws are highlighted by white circles showing focal needle-prick intervention effects. (B) Experimental protocol illustrating treatment/intervention timeline. Brain tissue was collected between P85–P95 for 7T MRI scanning. (C) Neonatal mice (n = 109) were assigned randomly across treatment/intervention groups (total of 6 groups). Distribution, mean weight gain over treatment period, mean body weight at MRI scan, sex ratio and survival amongst groups during the current study are provided.
Over the treatment period (P1–P7), suckling behavior was not affected by sucrose exposure reflected by non-significant changes in mean weight gain over the treatment period between groups (F5,103 = 2.050, P = 0.0778) (Figure 1C). Similar weight gain across groups persisted beyond the treatment period up to weaning (F5,102 = 1.639, P = 0.1564). Likewise, there was no significant difference in mean body weight between groups at scanning time (F5,103 = 0.350, P = 0.8815).
3.2. Early repetitive sucrose exposure leads to widespread smaller regional brain volumes
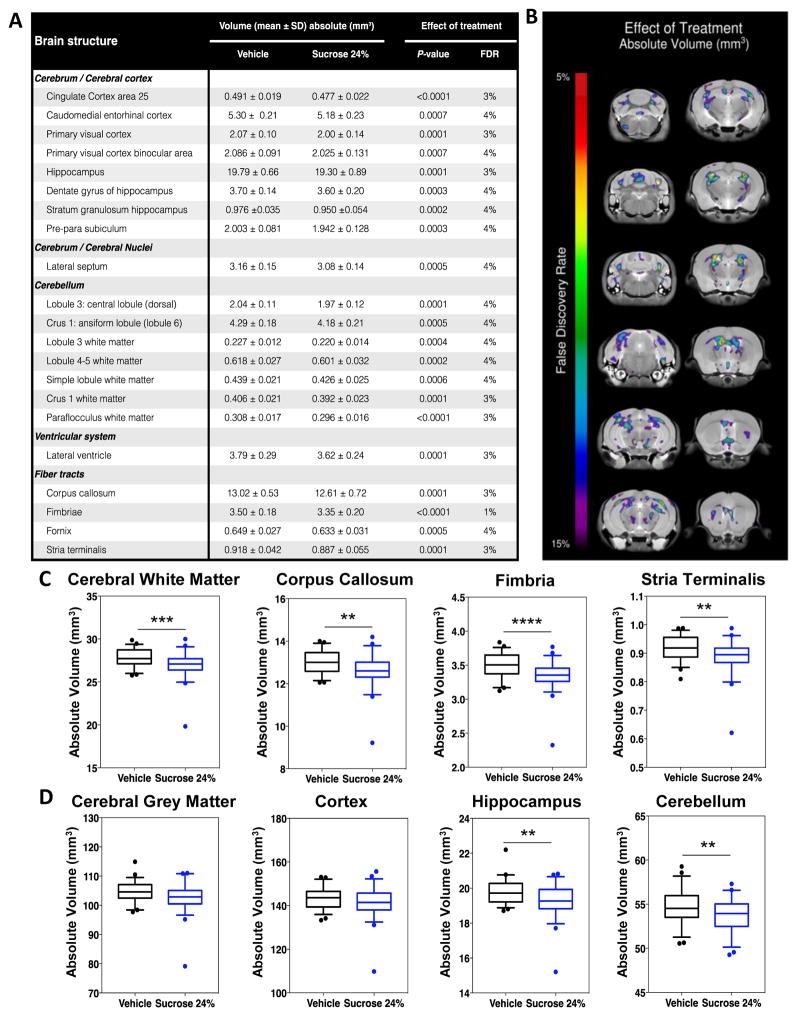
To explore the effects of P1 to P6 repeated exposure to sucrose on long-term brain development, we initially examined volumes of 159 independent brain regions in adult mice that either received Vehicle (n = 52) or Sucrose (n = 57) treatment before the intervention (either Handle, Tactile, or Needle-prick). Overall, we found significant differences in multiple regions with respect to treatment, with 21 out of 159 regions being significantly smaller in mice exposed to sucrose (P < 0.0001; false discovery rate [FDR] < 5%) (Figure 2A). White matter structures were particularly affected by sucrose treatment. For example, compared to the Vehicle groups, smaller volumes were found in the Sucrose groups for the corpus callosum (P < 0.0001; FDR = 3%) and fimbria (P < 0.0001; FDR = 1%). Only trends were seen when examining the effects of intervention only and treatment-intervention interaction; no significant sex differences were found. Figure 2A lists the results of the significant one-way ANOVAs (P < 0.0001, FDR < 5% to P < 0.03, FDR < 15%) for each brain region. We further explored the effect of sucrose exposure on brain volumes by grouping 159 brain regions into seven major divisions (cortex, cerebellum, ventricles, brainstem, olfactory region, grey and white matter) to limit the number of examined regions. Here, we found that adult mice exposed to sucrose as pups had significantly smaller volumes in the white matter, cerebellum and ventricles (P < 0.0001; FDR < 5%) (Figure 2B). Trends were observed in cerebral grey matter (P = 0.003; FDR = 6%). As before, we did not find any significant effects of intervention only, treatment-intervention interaction, or sex.
Figure 2. Widespread smaller volumes in 21 brain regions at adulthood in mice exposed to neonatal repetitive sucrose irrespective of intervention.
(A) List of the 21 brain regions, including cortical, subcortical, cerebellar structures and fiber tracts, that were significantly smaller (FDR < 5%) at adulthood. (B) Sample of coronal slices showing cross-regional voxel-wise differences of absolute brain regional volumes between mice exposed to repetitive Vehicle compared to Sucrose, irrespective of intervention. FDR 5–15% are shown. (C) Box-plot figures of brain volumes comparing mice in Vehicle versus Sucrose groups in white matter (FDR = 3%), corpus callosum (FDR = 3%), fimbria (FDR = 3%) and stria terminalis (FDR = 1%). (D) Box-plot figures of brain volumes comparing mice in Vehicle versus Sucrose groups in grey matter (FDR = 6%), cortex (FDR = 17%), hippocampus (FDR = 3%) and cerebellum (FDR = 4%). n = 52–57 per group. **P < 0.01, ***P < 0.001, ***P < 0.0001; FDR, false discovery rate.
Figures 2C and 2D provide box-plot examples of significant and non-significant volume differences found in specific brain regions. A complete list of brain mean volumes for the 159 regions measured is provided in supplementary material (Table S1 and Table S2) along with group comparisons for effect of Treatment (Vehicle vs Sucrose) or Intervention (Needle-prick [pain] vs all other interventions).
3.3. Constrained principal component analysis confirms Sucrose treatment damaging effect on brain volumes
To confirm and further explore the effect of exposure to treatment and intervention found using ANOVA, we used constrained principal component analysis (CPCA) [64] to examine the component structure of the variance in the brain volumes that is specifically predicted by a set of predictor variables: total brain volume, sex and study groups. CPCA allowed us to conduct a multiple regression analysis of the regional brain volumes in relation to the treatment group, sex and total brain volume, and then condense the results into components. Hence, in the present study, the matrix of predicted component scores reflected the variance in regional brain volumes that was accounted for by total brain volume, sex and study groups.
First we examined the 159 regional volumes in relation to treatment (i.e. Vehicle vs Sucrose), irrespective of intervention. The external analysis of CPCA (see Methods section for further details) showed that the three predictors included in our model (total brain volume, sex, and treatment) accounted for 49% of the overall variance in regional brain volume (Table 1). Two main principal components (PC) were extracted from the predicted solution, explaining respectively 26.2% and 22.7% of the variance in brain volumes of the 159 regions examined. Based on the distribution of predictors on each principal component, we interpreted PC1 as representing total brain volume and sex effects, and PC2 as representing treatment effects. Predictor variables with positive loadings were associated with larger regional brain volumes. Total brain volume positively loaded on both components, which indicated larger total brain volume was associated with larger regional volumes, whereas in contrast, the negative loading for treatment indicates smaller brain volumes was associated with sucrose exposure. Treatment (reflected in PC2) uniquely loaded on 22 out of the 159 brain regions largely comprising of cerebellar grey and white matter, showing smaller volumes in cortical amygdaloid, hippocampus, stria terminalis and hypothalamus; indicating that mice exposed to sucrose had smaller volumes in those areas compared to those exposed to vehicle (Table 2).
Table 1.
Component loadings for treatment in CPCA modeling
| Factors | PC1 | PC2 | ||
|---|---|---|---|---|
| Loading | P-value | Loading | P-value | |
|
| ||||
| Total brain volume | 0.746 | 0,0000 | 0.611 | 0.0000 |
| Sex | 0.623 | 0,0000 | −0.762 | 0.0000 |
| Vehicle vs Sucrose | −0.037 | 0.7005 | −0.360 | 0.0001 |
CPCA factor loadings for Vehicle versus Sucrose model; two principal components (PC)s were extracted from the predicted solution, where PC1 reflected total brain volume and sex and PC2 reflected treatment; explained variance for PC1 and PC2 are 26.2%, 22.7% respectively, n = 52–57 per group. Significant loadings (Bonferroni P < 0.013) particular to treatment shown in bold.
CPCA, constrained principal component analysis.
Table 2.
Regional brain volumes variance explained by treatment (Vehicle vs Sucrose) in CPCA modeling.
| Brain Structure | PC1 | PC2 | ||
|---|---|---|---|---|
| Loading | P-value | Loading | P-value | |
|
| ||||
| Cerebrum / Cerebral cortex | 0.279 | 0.0021 | 0.820 | 0.0000 |
| Amygdalo piriform transition area | 0.255 | 0.0004 | 0.685 | 0.0000 |
| Caudo medial entorhinal cortex | 0.297 | 0.0070 | 0.665 | 0.0000 |
| Postero lateral cortical amygdaloid area | 0.312 | 0.0013 | 0.554 | 0.0000 |
| Postero medial cortical amygdaloid area | 0.225 | 0.0017 | 0.748 | 0.0000 |
| Rostral amygdalo piriform area | 0.271 | 0.0092 | 0.525 | 0.0000 |
| Ventral tenia tecta | 0.191 | 0.0292 | 0.399 | 0.0000 |
| Cerebrum / Cerebral nuclei | ||||
| Bed nucleus of stria terminalis | 0.279 | 0.0021 | 0.820 | 0.0000 |
| Brainstem | ||||
| Medulla | 0.203 | 0.0094 | 0.351 | 0.0002 |
| Superior olivary complex | 0.061 | 0.4517 | 0.370 | 0.0001 |
| Cerebellum | ||||
| Lobules 4–5: culmen (ventral and dorsal) | 0.329 | 0.0004 | 0.536 | 0.0000 |
| Lobule 7: tuber (or folium) | 0.185 | 0.0129 | 0.418 | 0.0000 |
| Lobule 8: pyramis | 0.190 | 0.0233 | 0.439 | 0.0000 |
| Copula pyramis lobule 8 | 0.271 | 0.0020 | 0.427 | 0.0000 |
| Trunk of lobules 6–8 white matter | 0.265 | 0.0009 | 0.391 | 0.0000 |
| Lobule 8 white matter | 0.110 | 0.1854 | 0.394 | 0.0001 |
| Lobule 9: uvula | 0.266 | 0.0006 | 0.507 | 0.0000 |
| Lobule 9 white matter | 0.201 | 0.0106 | 0.484 | 0.0000 |
| Lobule 10: nodulus | 0.260 | 0.0015 | 0.433 | 0.0000 |
| Flocculus FL | 0.156 | 0.0549 | 0.509 | 0.0000 |
| Flocculus white matter | 0.099 | 0.2381 | 0.397 | 0.0000 |
| Paraflocculus PFL | 0.250 | 0.0019 | 0.329 | 0.0002 |
| Ventricular system | ||||
| Third ventricle | 0.410 | 0.0010 | 0.483 | 0.0001 |
CPCA factor loadings for analysis of treatment effect on regional brain volumes. Component loadings for PC1 and PC2 and their P-values for the 22 brain regions that passed the Bonferroni cut-off (P < 0.013) are listed. Treatment (represented in PC2) loaded uniquely on 22 brain regions, reflecting that mice exposed to Sucrose have significantly smaller volumes in these specific brain areas compared to Vehicle (P < 0.013), n per group 52–57.
CPCA, constrained principal component analysis; PC, principal component.
3.3.1 Needle-prick intervention has no effect on brain volumes
To examine the effects of treatment (Vehicle, Sucrose) within the three intervention groups (Handle, Tactile, and Needle-prick), two distinct CPCAs were conducted by splitting the sample by treatment group assignment, i.e. Vehicle versus Sucrose. To examine effects of Tactile and Needle-prick intervention, Handle was used as the reference intervention, i.e. Tactile vs Handle, Needle-prick vs Handle in each analysis.
When examining the effect of the interventions in mice exposed to vehicle, our CPCA showed that the four predictors included in our model (total brain volume, sex, Needle-prick intervention, and Tactile intervention) accounted for 44% of the overall variance in regional brain volumes. Three principal components (PC) were extracted from the predicted solution where PC1 reflected total brain volume and sex, PC2 reflected Tactile intervention, and PC3 reflected sex and Tactile intervention, explaining respectively 22%, 15.3%, and 6.3% of the variance in brain volumes of the 159 regions examined (Table 3). Predictor variables with positive loadings were associated with larger regional brain volumes. The Needle-prick intervention predictor did not load significantly on any PC, indicating that regional brain volumes were not different between the Vehicle/Needle-prick and Vehicle/Handle groups. Thus, mice repeatedly exposed to needle-pricks did not have significantly different regional brain volumes from those that were just handled. Tactile intervention positively loaded on PC2 and negatively on PC3, which indicated that Tactile intervention was not associated with those brain regions loaded on both components. Accordingly, Tactile intervention (reflected here in PC2) was solely related to volumes in 40 brain regions out of the 159 brain regions that were examined (Table 4; Bonferroni cut-off for significance [P < 0.013]). Therefore, mice in the Vehicle/Tactile group compared to Vehicle/Handle had bigger volumes in those structures, such as the hippocampus, midbrain, nucleus accumbens, and cerebellar subregions.
Table 3.
Component loadings for intervention split by treatment – Vehicle.
| Factors | PC1 | PC2 | PC3 | |||
|---|---|---|---|---|---|---|
| Loading | P-value | Loading | P-value | Loading | P-value | |
|
| ||||||
| Total brain volume | 0.678 | 0.0000 | 0.693 | 0.0000 | 0.242 | 0.0812 |
| Sex | 0.679 | 0.0000 | −0.602 | 0.0000 | −0.420 | 0.0026 |
| Needle-prick intervention | −0.035 | 0.8044 | −0.067 | 0.6315 | 0.079 | 0.5723 |
| Tactile intervention | −0.173 | 0.2238 | 0.469 | 0.0014 | −0.771 | 0.0000 |
Split analysis by treatment (Vehicle) CPCA factor loadings for Vehicle groups model; three PCs were extracted from the predicted solution, where PC1 reflected total brain volume and sex, PC2 Tactile intervention, and PC3 sex and Tactile intervention; explained variance for PC1, PC2, PC3 are 22%, 15.3%, and 6.3% respectively. Vehicle/Handle (n = 18), Vehicle/Tactile (n = 16), or Vehicle/Needle-prick (n = 18). Significant loadings (Bonferroni P < 0.013) particular to intervention shown in bold.
CPCA, constrained principal component analysis; PC, principal component.
Table 4.
Effects of exposure to interventions on adult mice regional brain volumes within the 3 groups: CPCA analysis split by treatment - Vehicle.
| Brain Structure | PC1 | PC2 | PC3 | |||
|---|---|---|---|---|---|---|
| Loading | P-value | Loading | P-value | Loading | P-value | |
|
| ||||||
| Cerebrum | ||||||
| Cerebral cortex/cortical plate | ||||||
| Cingulate cortex area 30 | −0.572 | 0.0000 | 0.398 | 0.0000 | −0.359 | 0.0004 |
| Secondary auditory cortex dorsal area | −0.594 | 0.0000 | 0.386 | 0.0002 | −0.331 | 0.0005 |
| Caudomedial entorhinal cortex | −0.096 | 0.3225 | 0.510 | 0.0001 | −0.376 | 0.0006 |
| Cingulum | −0.566 | 0.0000 | 0.358 | 0.0001 | −0.291 | 0.0010 |
| Ectorhinal cortex | −0.608 | 0.0000 | 0.446 | 0.0000 | −0.311 | 0.0147 |
| Postero lateral cortical amygdaloid area | −0.330 | 0.0050 | 0.488 | 0.0000 | −0.318 | 0.0216 |
| Perirhinal cortex | −0.525 | 0.0000 | 0.410 | 0.0000 | −0.319 | 0.0054 |
| Rostral amygdalo piriform area | −0.169 | 0.1585 | 0.548 | 0.0000 | −0.300 | 0.0072 |
| Temporal association area | −0.540 | 0.0000 | 0.400 | 0.0002 | −0.271 | 0.0361 |
| Primary visual cortex | −0.519 | 0.0000 | 0.490 | 0.0000 | −0.333 | 0.0010 |
| Secondary visual cortex lateral area | −0.625 | 0.0000 | 0.425 | 0.0000 | −0.332 | 0.0006 |
| Hippocampus | −0.433 | 0.0001 | 0.670 | 0.0000 | −0.351 | 0.0004 |
| Dentate gyrus of hippocampus | −0.241 | 0.0875 | 0.602 | 0.0000 | −0.264 | 0.0259 |
| Stratum granulosum of hippocampus | −0.318 | 0.0191 | 0.587 | 0.0000 | −0.241 | 0.0507 |
| Cerebral nuclei | ||||||
| Lateral septum | −0.671 | 0.0000 | 0.372 | 0.0000 | −0.287 | 0.0065 |
| Medial septum | −0.547 | 0.0000 | 0.487 | 0.0001 | −0.230 | 0.0481 |
| Nucleus accumbens | −0.669 | 0.0000 | 0.405 | 0.0000 | −0.252 | 0.0055 |
| Brainstem | ||||||
| Midbrain | −0.538 | 0.0000 | 0.518 | 0.0000 | −0.243 | 0.0286 |
| Cerebellum | ||||||
| Cerebellar cortex/vermis | ||||||
| Lobule 3 central lobule dorsal | −0.260 | 0.0048 | 0.540 | 0.0001 | −0.215 | 0.0987 |
| Lobule 3 white matter | −0.285 | 0.0027 | 0.483 | 0.0001 | −0.292 | 0.0308 |
| Lobules 4–5 culmen ventral and dorsal | −0.161 | 0.2575 | 0.493 | 0.0002 | −0.163 | 0.2286 |
| Lobule 7 tuberor folium | −0.083 | 0.4901 | 0.528 | 0.0000 | −0.043 | 0.7456 |
| Lobule 8 pyramis | −0.221 | 0.0430 | 0.517 | 0.0002 | −0.006 | 0.9641 |
| Lobule 9 uvula | −0.285 | 0.0065 | 0.566 | 0.0001 | −0.122 | 0.3305 |
| Cerebellar cortex/hemispheres | ||||||
| Simple lobule 6 | −0.533 | 0.0000 | 0.484 | 0.0000 | −0.121 | 0.3051 |
| Crus 1 white matter | −0.478 | 0.0000 | 0.525 | 0.0000 | −0.304 | 0.0005 |
| Crus 2 ansiform lobule 7 | −0.479 | 0.0000 | 0.530 | 0.0000 | −0.185 | 0.0301 |
| Crus 2 white matter | −0.541 | 0.0000 | 0.448 | 0.0000 | −0.267 | 0.0033 |
| Paramedian lobule 7 | −0.471 | 0.0000 | 0.503 | 0.0002 | −0.056 | 0.6141 |
| Trunk of crus 2 and paramedian white matter | −0.532 | 0.0000 | 0.404 | 0.0002 | −0.192 | 0.0482 |
| Flocculus FL | −0.120 | 0.2667 | 0.592 | 0.0000 | −0.077 | 0.5402 |
| Fiber Tracts | ||||||
| Cranial nerves | ||||||
| Lateral olfactory tract | −0.440 | 0.0000 | 0.385 | 0.0002 | −0.413 | 0.0047 |
| Medial lemniscus medial longitudinal fasciculus | −0.338 | 0.0006 | 0.536 | 0.0002 | −0.172 | 0.1684 |
| Lateral forebrain bundle system | ||||||
| Corticospinal tract pyramids | −0.197 | 0.0567 | 0.493 | 0.0002 | 0.038 | 0.7919 |
| Extrapyramidal fiber system | ||||||
| Ventral tegmental decussation | −0.506 | 0.0000 | 0.609 | 0.0000 | −0.184 | 0.0607 |
| Medial forebrain bundle system | ||||||
| Fimbria | −0.552 | 0.0000 | 0.360 | 0.0001 | −0.160 | 0.1743 |
| Fornix | −0.565 | 0.0000 | 0.454 | 0.0000 | −0.272 | 0.0133 |
| Fasciculus retroflexus | −0.645 | 0.0000 | 0.325 | 0.0000 | −0.152 | 0.1625 |
| Stria medullaris | −0.665 | 0.0000 | 0.338 | 0.0002 | −0.087 | 0.4835 |
| Other | ||||||
| Pre-para subiculum | −0.249 | 0.0171 | 0.495 | 0.0002 | −0.371 | 0.0077 |
Component loadings for PC1, PC2 and PC3 and their P-values for the 40 brain regions that passed the Bonferroni cut-off (P < 0.013; shown in bold font) for significance are listed; n = 16–18 per group. Brain regions are presented by anatomical distribution according to Allen Mouse Brain Atlas.
Significant P-values CPCA, constrained principal component analysis; PC, principal component.
3.3.2. Smallest brain volumes found in mice given sucrose treatment before needle-prick intervention
The CPCA for the Sucrose treatment showed that the four predictors included in our model (total brain volume, sex, Needle-prick intervention, and Tactile intervention) accounted for 55% of the overall variance in regional brain volumes. Three principal components (PC) were extracted from the predicted solution, where PC1 reflected Needle-prick and Tactile interventions, PC2 total brain volume and sex, and PC3 reflected all four predictors, explaining respectively 33.6%, 17.3%, and 4.3% of the variance in brain volumes of the 159 regions examined (Table 5). Predictor variables with positive loadings were associated with larger regional brain volumes. Dominant loadings on PC1 were widespread, with highly significant loadings in 95 brain regions (Table 6; Bonferroni cut-off for significance [P < 0.013]). Given that in PC1 the predictor loading for Needle-prick was negative and that the loading for Tactile was positive (loadings −0.469 and 0.428 respectively; see Table 5), this indicated that mice in the Sucrose/Needle-prick group compared to Sucrose/Handle group had smaller volumes in those structures with significant loadings on PC1, whereas mice in the Sucrose/Tactile group compared to Sucrose/Handle had bigger volumes in structures with significant loadings on PC1. These regions included the amygdala, cerebellum, primary and secondary somatosensory cortices, thalamus, hippocampus, cingulate cortex, and periaqueductal grey.
Table 5.
Component loadings for intervention split by treatment – Sucrose.
| Factors | PC1 | PC2 | PC3 | |||
|---|---|---|---|---|---|---|
| Loading | P-value | Loading | P-value | Loading | P-value | |
|
| ||||||
| Total brain volume | 0.723 | 0.0000 | 0.563 | 0.0000 | 0.392 | 0.0025 |
| Sex | 0.404 | 0.0015 | −0.833 | 0.0000 | 0.367 | 0.0067 |
| Needle-prick intervention | −0.469 | 0.0005 | 0.108 | 0.4286 | 0.787 | 0.0000 |
| Tactile intervention | 0.428 | 0.0014 | 0.020 | 0.8743 | −0.708 | 0.0000 |
CPCA, constrained principal component analysis; PC, principal component.
Table 6.
Effects of exposure to interventions on adult mice regional brain volumes within the 3 groups: CPCA analysis split by treatment - Sucrose.
| Brain Structure | PC1 | PC2 | PC3 | |||
|---|---|---|---|---|---|---|
| Loading | P-value | Loading | P-value | Loading | P-value | |
|
| ||||||
| Cerebrum | ||||||
| Cerebral cortex/cortical plate | ||||||
| Cingulate cortex area 24a | 0.736 | 0.0000 | 0.409 | 0.0005 | 0.187 | 0.0150 |
| Cingulate cortex area 24b | 0.636 | 0.0001 | 0.283 | 0.0232 | 0.187 | 0.0753 |
| Cingulate cortex area 24bapos | 0.685 | 0.0002 | 0.336 | 0.0143 | 0.115 | 0.2838 |
| Cingulate cortex area 25 | 0.717 | 0.0000 | 0.348 | 0.0002 | 0.053 | 0.5945 |
| Cingulate cortex area 29b | 0.707 | 0.0000 | 0.443 | 0.0008 | 0.163 | 0.0927 |
| Cingulate cortex area 30 | 0.811 | 0.0000 | 0.446 | 0.0017 | 0.161 | 0.0586 |
| Cingulate cortex area 32 | 0.742 | 0.0000 | 0.125 | 0.3233 | 0.246 | 0.0030 |
| Amygdalo piriform transition area | 0.398 | 0.0002 | 0.643 | 0.0000 | 0.157 | 0.1007 |
| Primary auditory cortex | 0.715 | 0.0000 | 0.483 | 0.0000 | 0.236 | 0.0016 |
| Secondary auditory cortex dorsal area | 0.707 | 0.0000 | 0.499 | 0.0000 | 0.261 | 0.0015 |
| Secondary auditory cortex ventral area | 0.683 | 0.0001 | 0.474 | 0.0002 | 0.151 | 0.0649 |
| Cingulum | 0.759 | 0.0000 | 0.417 | 0.0015 | 0.211 | 0.0059 |
| Dorsal intermediate entorhinal cortex | 0.550 | 0.0000 | 0.528 | 0.0000 | 0.220 | 0.0074 |
| Dorsolateral entorhinal cortex | 0.569 | 0.0001 | 0.426 | 0.0009 | 0.278 | 0.0035 |
| Dorsolateral orbital cortex | 0.585 | 0.0000 | 0.041 | 0.7359 | 0.264 | 0.0043 |
| Dorsal teniatecta | 0.651 | 0.0000 | 0.304 | 0.0030 | 0.032 | 0.7966 |
| Ectorhinal cortex | 0.762 | 0.0000 | 0.402 | 0.0002 | 0.250 | 0.0016 |
| Frontal cortex area 3 | 0.712 | 0.0002 | 0.259 | 0.0751 | 0.211 | 0.0078 |
| Insular region not subdivided | 0.691 | 0.0000 | 0.335 | 0.0002 | 0.105 | 0.2131 |
| Lateral orbital cortex | 0.761 | 0.0000 | 0.032 | 0.7989 | 0.219 | 0.0167 |
| Lateral parietal association cortex | 0.641 | 0.0000 | 0.452 | 0.0000 | 0.209 | 0.0453 |
| Primary motor cortex | 0.694 | 0.0001 | 0.371 | 0.0039 | 0.236 | 0.0053 |
| Secondary motor cortex | 0.695 | 0.0001 | 0.342 | 0.0060 | 0.178 | 0.0464 |
| Medial entorhinal cortex | 0.489 | 0.0001 | 0.466 | 0.0000 | 0.208 | 0.0330 |
| Medial orbital cortex | 0.731 | 0.0000 | −0.029 | 0.8023 | 0.254 | 0.0137 |
| Medial parietal association cortex | 0.679 | 0.0000 | 0.439 | 0.0001 | 0.216 | 0.0313 |
| Piriform cortex | 0.679 | 0.0000 | 0.467 | 0.0000 | 0.119 | 0.1831 |
| Perirhinal cortex | 0.704 | 0.0000 | 0.447 | 0.0001 | 0.284 | 0.0008 |
| Parietal cortex posterior area rostral part | 0.634 | 0.0000 | 0.432 | 0.0002 | 0.203 | 0.0658 |
| Primary somatosensory cortex | 0.761 | 0.0000 | 0.300 | 0.0274 | 0.234 | 0.0006 |
| Primary somatosensory cortex barrel field | 0.774 | 0.0000 | 0.412 | 0.0004 | 0.254 | 0.0007 |
| Primary somatosensory cortex dysgranular | 0.711 | 0.0000 | 0.389 | 0.0011 | 0.264 | 0.0006 |
| Primary somatosensory cortex forelimb | 0.711 | 0.0000 | 0.407 | 0.0005 | 0.256 | 0.0016 |
| Primary somatosensory cortex hindlimb | 0.660 | 0.0000 | 0.431 | 0.0000 | 0.254 | 0.0087 |
| Primary somatosensory cortex shoulder | 0.660 | 0.0000 | 0.438 | 0.0001 | 0.248 | 0.0142 |
| Primary somatosensory cortex trunk | 0.627 | 0.0000 | 0.458 | 0.0000 | 0.224 | 0.0354 |
| Primary somatosensory cortex upper lip | 0.762 | 0.0000 | 0.344 | 0.0046 | 0.214 | 0.0030 |
| Secondary somatosensory cortex | 0.738 | 0.0000 | 0.306 | 0.0015 | 0.140 | 0.1075 |
| Temporal association area | 0.736 | 0.0000 | 0.389 | 0.0008 | 0.205 | 0.0195 |
| Primary visual cortex | 0.675 | 0.0000 | 0.428 | 0.0000 | 0.216 | 0.0236 |
| Primary visual cortex binocular area | 0.719 | 0.0000 | 0.487 | 0.0000 | 0.233 | 0.0086 |
| Primary visual cortex monocular area | 0.710 | 0.0000 | 0.482 | 0.0001 | 0.203 | 0.0342 |
| Secondary visual cortex lateral area | 0.743 | 0.0000 | 0.419 | 0.0000 | 0.256 | 0.0015 |
| Secondary visual cortex mediolateral area | 0.688 | 0.0000 | 0.480 | 0.0001 | 0.220 | 0.0304 |
| Secondary visual cortex mediomedial area | 0.666 | 0.0000 | 0.541 | 0.0000 | 0.193 | 0.0343 |
| Claustrum ventral part | 0.689 | 0.0000 | 0.370 | 0.0003 | 0.084 | 0.3957 |
| Ventral intermediate entorhinal cortex | 0.462 | 0.0000 | 0.551 | 0.0000 | 0.192 | 0.0208 |
| Ventral orbital cortex | 0.802 | 0.0000 | 0.140 | 0.2341 | 0.187 | 0.0445 |
| Olfactory bulbs | 0.496 | 0.0000 | 0.677 | 0.0000 | −0.065 | 0.4716 |
| Hippocampus | 0.687 | 0.0001 | 0.667 | 0.0000 | 0.169 | 0.0056 |
| Dentate gyrus of hippocampus | 0.631 | 0.0002 | 0.648 | 0.0000 | 0.147 | 0.0876 |
| Stratum granulosum of hippocampus | 0.640 | 0.0001 | 0.626 | 0.0000 | 0.159 | 0.0738 |
| Cerebral cortex/subcortical plate | ||||||
| Claustrum | 0.737 | 0.0000 | 0.175 | 0.1660 | 0.307 | 0.0007 |
| Claustrum dorsal part | 0.683 | 0.0000 | 0.397 | 0.0000 | 0.139 | 0.1337 |
| Dorsal nucleus of the endopiriform | 0.762 | 0.0000 | 0.300 | 0.0092 | 0.130 | 0.1300 |
| Intermediate nucleus of the endopiriform claustrum | 0.736 | 0.0000 | 0.249 | 0.0356 | 0.187 | 0.0248 |
| Ventral nucleus endopiriform claustrum | 0.636 | 0.0001 | 0.490 | 0.0000 | 0.133 | 0.1561 |
| Amygdala | 0.613 | 0.0000 | 0.648 | 0.0000 | 0.087 | 0.3122 |
| Cerebral nuclei | ||||||
| Fundus of striatum | 0.737 | 0.0000 | 0.374 | 0.0030 | 0.102 | 0.2240 |
| Nucleus accumbens | 0.795 | 0.0000 | 0.343 | 0.0044 | 0.215 | 0.0020 |
| Globus pallidus | 0.785 | 0.0000 | 0.398 | 0.0027 | 0.166 | 0.0272 |
| Lateral septum | 0.682 | 0.0000 | 0.457 | 0.0000 | 0.225 | 0.0074 |
| Medial septum | 0.682 | 0.0000 | 0.478 | 0.0000 | 0.106 | 0.3023 |
| Olfactory tubercle | 0.572 | 0.0002 | 0.464 | 0.0001 | 0.019 | 0.8555 |
| Basal forebrain | 0.716 | 0.0000 | 0.565 | 0.0000 | 0.137 | 0.0676 |
| Brainstem | ||||||
| Interbrain | ||||||
| Thalamus | 0.845 | 0.0002 | 0.369 | 0.0188 | 0.216 | 0.0081 |
| Hypothalamus | 0.639 | 0.0001 | 0.671 | 0.0000 | 0.099 | 0.2161 |
| Midbrain | ||||||
| Midbrain | 0.788 | 0.0000 | 0.474 | 0.0000 | 0.195 | 0.0020 |
| Colliculus inferior | 0.687 | 0.0000 | 0.460 | 0.0000 | 0.171 | 0.0428 |
| Colliculus superior | 0.764 | 0.0000 | 0.507 | 0.0000 | 0.117 | 0.1248 |
| Periaqueductal grey | 0.757 | 0.0000 | 0.369 | 0.0001 | 0.245 | 0.0012 |
| Interpedunclar nucleus | 0.533 | 0.0000 | 0.396 | 0.0007 | 0.171 | 0.1388 |
| Hindbrain | ||||||
| Pons | 0.502 | 0.0000 | 0.409 | 0.0000 | 0.178 | 0.1237 |
| Cerebellum | ||||||
| Cerebellar cortex/vermis | ||||||
| Lobule 3 central lobule dorsal | 0.507 | 0.0001 | 0.297 | 0.0018 | 0.244 | 0.1156 |
| Lobules 4–5 culmen ventral and dorsal | 0.401 | 0.0001 | 0.443 | 0.0000 | 0.215 | 0.1028 |
| Lobule 3 white matter | 0.529 | 0.0001 | 0.242 | 0.0136 | 0.212 | 0.1491 |
| Lobules 4–5 white matter | 0.529 | 0.0000 | 0.319 | 0.0058 | 0.109 | 0.3825 |
| Cerebellar cortex/hemispheres | ||||||
| Crus 1 ansiform lobule 6 | 0.406 | 0.0000 | 0.535 | 0.0000 | 0.248 | 0.0021 |
| Simple lobule white matter | 0.554 | 0.0000 | 0.297 | 0.0012 | 0.350 | 0.0027 |
| Crus1 white matter | 0.515 | 0.0000 | 0.464 | 0.0000 | 0.287 | 0.0007 |
| Trunk of simple and crus1 white matter | 0.430 | 0.0000 | 0.407 | 0.0001 | 0.321 | 0.0021 |
| Simple lobule 6 | 0.557 | 0.0000 | 0.375 | 0.0000 | 0.378 | 0.0010 |
| Cerebellar nuclei | ||||||
| Dentate nucleus | 0.612 | 0.0000 | 0.277 | 0.0031 | 0.348 | 0.0063 |
| Nucleus interpositus | 0.541 | 0.0000 | 0.457 | 0.0000 | 0.300 | 0.0021 |
| Fastigial nucleus | 0.494 | 0.0000 | 0.346 | 0.0011 | 0.315 | 0.0031 |
| Fiber Tracts | ||||||
| Cranial nerves | ||||||
| Lateral olfactory tract | 0.573 | 0.0000 | 0.403 | 0.0001 | 0.074 | 0.4702 |
| Cerebellum related fiber tracts | ||||||
| Cerebellar peduncle superior | 0.716 | 0.0000 | 0.344 | 0.0000 | 0.229 | 0.0117 |
| Lateral forebrain bundle system | ||||||
| Internal capsule | 0.765 | 0.0001 | 0.511 | 0.0002 | 0.233 | 0.0005 |
| Extrapyramidal fiber system | ||||||
| Ventral tegmental decussation | 0.696 | 0.0000 | 0.462 | 0.0001 | 0.204 | 0.0039 |
| Medial forebrain bundle system | ||||||
| Anterior commissure pars anterior | 0.659 | 0.0002 | 0.613 | 0.0000 | 0.194 | 0.0273 |
| Anterior commissure pars posterior | 0.695 | 0.0000 | 0.446 | 0.0001 | 0.210 | 0.0196 |
| Fasciculus retroflexus | 0.778 | 0.0002 | 0.396 | 0.0058 | 0.244 | 0.0037 |
| Ventricular System | ||||||
| Ventricular System | ||||||
| Subependymale zone rhinocele | 0.458 | 0.0003 | 0.694 | 0.0000 | 0.067 | 0.4747 |
| Cerebral aqueduct | 0.644 | 0.0000 | 0.172 | 0.1101 | 0.130 | 0.2843 |
| Other | ||||||
| Pre-para subiculum | 0.663 | 0.0001 | 0.537 | 0.0000 | 0.162 | 0.0651 |
Component loadings for PC1, PC2 and PC3 and their P-values for the 95 brain regions that passed the Bonferroni cut-off (P < 0.013; shown in bold font) for significance are listed; n = 17–22 per group. Brain regions are presented by anatomical distribution according to Allen Mouse Brain Atlas.
CPCA, constrained principal component analysis; PC, principal component.
4.0 Discussion
Using a neonatal mouse model that closely mimics NICU procedural pain and treatment, we found widespread long-term alterations in adult white and grey matter brain volumes in mouse pups repeatedly exposed to sucrose in the first week of life, which approximates the preterm period in humans. Repetitive sucrose exposure induced smaller brain volumes mainly in white matter regions of the forebrain, cerebellum, and hippocampus. To our knowledge, this is the first animal or human study to examine long-term effects on brain of neonatal repetitive sucrose exposure for pain treatment relevant to NICU care. Whereas, Nuseir et al. [45] exposed newborn rats to daily repetitive pain and/or sucrose over a prolonged period of 8 weeks, which is exposure lasting far beyond the neonatal period, we used a pain model that matches that of the human infant exposure in the NICU. Importantly, we found deleterious effects of early sucrose treatment irrespective whether mouse pups were handled, touched or needle-pricked at the time sucrose was administered. Our findings raise serious questions relating to the current widespread use of sucrose for procedural pain management in NICUs worldwide. Of even greater concern, is that when analyzing the data by splitting the sample by intervention exposure (i.e. Vehicle versus Sucrose), we found that from all the pups that were exposed to sucrose prior to a treatment (i.e. handling, touch, needle-prick), it was the ones that received needle-pricks that had the worst outcome. This particular group of mice had smaller regional brain volumes predominately in areas involved in nociceptive processing (e.g. primary somatosensory cortices, thalamus, cingulate cortex, periaqueductal grey); whereas, this effect was not observed in mice that received water. This suggests that sucrose is not providing brain-protection and may even have an opposite effect on brain especially when given in combination with pain.
Sucrose is widely accepted as an effective nonpharmacological intervention for pain of minor procedures in preterm infants [29,62]. Exposure to single sucrose treatment is safe and spared of serious short-term side effects. However, one study has shown poorer neurological development around term equivalent age in preterm infants exposed to more than 10 doses of sucrose per 24h in the first week of life [38] and our results of reduced brain volume are consistent with these findings. There are increasing concerns regarding the limited evidence of long-term safety of repeated exposure to sucrose for procedural pain management in the preterm population [20].
The rewarding effects of ingesting sweet solutions, such as sucrose, a disaccharide combination of glucose and fructose, appear to be mediated by similar neuronal pathways and mechanisms implicated in drug abuse. Indeed, sucrose has opioid-like effects on dopamine receptors comparable to morphine [59]. Thus, the deleterious effect of sucrose on brain volumes after repeated administration seen in this study may be explained through the mechanism underlying sucrose’s mitigating effect on pain. Opioid-induced neurotoxicity has been largely explored in preclinical studies showing reduced neuronal density and dendritic length, as well as induced neuronal apoptosis in rodent pups exposed to morphine [8,33,53,57]. Early opioid exposure also induces apoptosis of fetal microglia and neurons in vitro [36] and compromises myelination [56]. Accordingly, these apoptotic and anti-proliferative effects described could also occur after sucrose exposure in the developing brain and could explain, at least partially, the widespread effect on brain regions we observed.
There are concerns currently about possible effects of pre-emptive morphine infusions in ventilated preterm infants, particularly at higher doses, based on findings from randomized control trials (RCTs) [3,50,58], leading to recommendations of judicious use in this population [10]. Follow-up studies of long-term neurodevelomental outcomes have reported mixed findings [22,23,28]. A single-site longitudinal cohort study of very preterm children showed an independent association between neonatal exposure to morphine and impaired cerebellar growth in the neonatal period (in a dose-dependent manner) [71]. Our current findings with early exposure to sucrose appear to be consistent with clinical and preclinical findings of opioid exposure, such that repetitive sucrose administration had adverse effects that were seen mainly in hippocampal areas and cerebellar lobules.
Importantly, Asmerom et al. [7] found that after a single dose of sucrose before a heel lance, preterm neonates had tachycardia, higher oxidative stress and adenosine triphosphate (ATP) breakdown. Given that multiple doses of sucrose are administered daily to preterm infants [18], the pathophysiological mechanisms involved in this recurrent sucrose-induced oxidative stress exposure may partially explain the widespread alterations of white matter tracts seen in our study. Indeed, excitotoxicity and oxidative stress are known as factors involved in maturation of pre-oligodendrocytes (pre-OLC) and oligodendrocytes (OLC) in premature brain white matter injury [9] leading to delayed or arrested OLC development [17] and delayed myelination [37]. In rodents, these cells proliferate, migrate and differentiate during the first two postnatal weeks, whereas in humans they develop predominantly in the second half of pregnancy [55]. Here, we found that repetitive sucrose during the first postnatal week in mice resulted in smaller cerebellar white matter volume and forebrain fiber tracts suggestive of a deleterious sucrose effect on OLC maturation and myelination.
Unique to our model is the administration of minute quantities of sucrose absorbed prominently through the oral mucosa, mimicking the standardized non-pharmacological pain treatment use of sucrose in NICU care worldwide. Absorption, metabolism and differing brain activation pathways must be taken into account when comparing our results to findings from models of sugar binge eating where sucrose solutions are being infused directly into the rodents’ stomach or animals feed ad libidum sucrose solutions/high caloric diets. Indeed, binge sugar intake has opioid-like effects and activates the reward system through the nucleus accumbens and the adjacent caudate-putamen [59], whereas repetitive exposure to small quantities of oral sucrose may activate different neurological pathways. Based on our findings that sucrose exposure induced altered regional brain volumes prominently in large afferent white matter tracts implicated in the hypothalamic–pituitary–adrenal (HPA) axis/stress system (e.g. fimbria, stria terminalis), and that we did not find significant volume alterations in the nucleus accumbens, we question which pathway is predominantly affected by early repetitive exposure to sucrose in this context. Evidence in rodents has shown the effects of sucrose/carbohydrates on regulation of the HPA axis [68]. Moreover, results of rodent studies have suggested that sweet solutions modulate pain through opioid mechanisms [13,40,52]. The evidence is less clear in human infants with few studies having examined potential mechanisms of action of sucrose [14,31,63] and results suggest that mechanisms other than those mediated by opioid pathways may be involved in the effects of sucrose [35]. Further studies are needed.
Surprisingly, we did not find a significant long-term effect of early repetitive pain exposure from needle-pricks on adult regional brain volumes, nor did we find a treatment-intervention interaction. According to previous studies in rodent pups, neonatal pain exposure (i.e. inflammatory pain from formalin injections or repetitive saline injections) was shown to modify both the structure and function of the developing brain [2,27,39]. Thus, we were expecting that mice exposed to needle-pricks during the first week of life would have altered brain development, which was not the case. Methodological differences may account for these findings, inflammatory pain or pain from injections may not have the same consequences as needle-pricks. We question the invasiveness of our needle-prick pain stimulus, given that we used very thin needles (30G) and did not penetrate deeper layers, such as tendon or bone, in contrast to the apparently more invasive procedures used in needle-prick pain in previous rat studies [1,27,41]. It should be noted that we undertook interventions 10 times daily for six days, based on the median exposure to painful procedures during NICU care [54].
Importantly, distinctive to our study was the addition of a CD-1 female mouse as a “nanny” to support the mother and improve survival of mouse pups, which could have added a buffering effect on pain through increased grooming and nurturing. Maternal behavior (grooming and licking) has been shown to modulate effects of early pain exposure in rats [24,69], where increased maternal behaviors reduced inflammation in response to neonatal formalin injections during the first two weeks of life of rat pups and thermal sensitivity once in adulthood. This “nanny plus mother” model emphasizes the robustness of our current findings of permanently smaller regional brain volumes in relation to early repetitive sucrose exposure.
Some limitations to our study exist. At this time, we do not have histological data to support our brain volume findings, thus complete interpretation cannot be determined (e.g. apoptosis or decrease neuronal proliferation or changes in cortical blood volume or water content) [46,65]. Consequences of repetitive sucrose exposure on the developing brain should be further studied at the functional level to assess if long-term effects on brain induce differences in learning and behavior.
Currently, it is not possible to conduct randomized controlled clinical trials of sucrose for pain management in preterm neonates in the NICU, since sucrose is the standard of care. Therefore, our findings from a neonatal mouse model are critical, suggesting cautious use of repetitive sucrose administration in very preterm infants is warranted. Our work reinforces recent guidelines from the American Academy of Pediatrics [20] highlighting the lack of evidence on mechanisms of action and long-term consequences of repetitive oral sucrose use for procedural pain.
Split analysis by treatment (Sucrose) CPCA factor loadings for Sucrose groups model; three PCs were extracted from the predicted solution, where PC1 reflected Needle-prick intervention and Tactile intervention, PC2 total brain volume and sex, and PC3 reflected all four predictors; explained variance for PC1, PC2 and PC3 are 33.6%, 17.3%, and 4.3% respectively. Sucrose/Handle (n = 17), Sucrose/Tactile (n = 22), Sucrose/Needle-prick (n = 18). Significant loadings (Bonferroni P < 0.013) particular to intervention shown in bold.
Supplementary Material
Acknowledgments
We would like to thank the help of Hannah McNeill (visiting undergraduate student from the University of Nottingham, UK) and Zahra Ezzat-Zadeh during the experiments. S. Tremblay and M. Ranger contributed equally to the work; R.E. Grunau and D. Goldowitz share senior authorship equally.
Funding: This work was supported in part by the Eunice Kennedy Shriver Institute of Child Health and Human Development (NICHD/NIH) grant RO1 HD039783 (to REG), funds from the Canadian Child Health Clinician Scientist Program (to ST and MR) and NeuroDevNet. Dr. Grunau holds a Senior Scientist salary award from the BC Children’s Hospital Research Institute; Fellowships support from the Canadian Institute of Health Research (CIHR) (to ST and MR), Pain In Child Health CIHR Strategic Training Initiative in Health (to MR), and Fonds Recherche Quebec-Santé (to ST and MR). We have no conflicts of interest to declare. Dr. Holsti is a lead inventor of a medical device for pain management for preterm infants. In partnership with the Provincial Health Services Association of British Columbia, Canada, she could, in the future, receive royalties as a result of licensing agreements made with private industry for commercialization of the device. She has not received any remuneration to date. In addition, she did not participate actively in the testing of the mice, or in the data analysis in this paper. None of the remaining authors have any affiliation, financial agreement, or other involvement that would place us in conflict of interest with this manuscript.
References
- 1.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ International Evidence-based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJS, Bergqvist L, Hall W, Carbajal R. Acute Pain Management in Newborn Infants. Pain (Clinical Updates) 2011;19:1–6. [Google Scholar]
- 6.Anseloni VC, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–243. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Asmerom Y, Slater L, Boskovic DS, Bahjri K, Holden MS, Phillips R, Deming D, Ashwal S, Fayard E, Angeles DM. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J Pediatr. 2013;163:29–35. e1. doi: 10.1016/j.jpeds.2012.12.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004;114:1001–1011. doi: 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- 9.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellu R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008;(1):CD004212. doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73:51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- 12.Biran V, Verney C, Ferriero DM. Perinatal cerebellar injury in human and animal models. Neurol Res Int. 2012;2012:858929. doi: 10.1155/2012/858929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blass E, Fitzgerald E, Kehoe P. Interactions between sucrose, pain and isolation distress. Pharmacol Biochem Behav. 1987;26:483–489. doi: 10.1016/0091-3057(87)90153-5. [DOI] [PubMed] [Google Scholar]
- 14.Blass EM, Ciaramitaro V. A new look at some old mechanisms in human newborns: taste and tactile determinants of state, affect, and action. Monogr Soc Res Child Dev. 1994;59:I–V. 1–81. [PubMed] [Google Scholar]
- 15.Bock NA, Nieman BJ, Bishop JB, Mark Henkelman R. In vivo multiple-mouse MRI at 7 Tesla. Magn Reson Med. 2005;54:1311–1316. doi: 10.1002/mrm.20683. [DOI] [PubMed] [Google Scholar]
- 16.Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boelle PY, Annequin D, Cimerman P, Anand KJ, Breart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 19.Chau CMY, Ranger M, Sulistyoningrum D, Devlin AM, Oberlander TF, Grunau RE. Neonatal pain and COMT Val158Met genotype in relation to serotonin transporter (SLC6A4) promoter methylation in very preterm children at school age. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and Management of Procedural Pain in the Neonate: An Update. Pediatrics. 2016;137:e20154271–4271. doi: 10.1542/peds.2015-4271. Epub 2016 Jan 25. [DOI] [PubMed] [Google Scholar]
- 21.de Freitas RL, Kubler JM, Elias-Filho DH, Coimbra NC. Antinociception induced by acute oral administration of sweet substance in young and adult rodents: the role of endogenous opioid peptides chemical mediators and mu(1)-opioid receptors. Pharmacol Biochem Behav. 2012;101:265–270. doi: 10.1016/j.pbb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 22.de Graaf J, van Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, Roofthooft DW, Groot Jebbink LJ, Veenstra RR, Tibboel D, van Dijk M. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152:1391–1397. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 23.de Graaf J, van Lingen RA, Valkenburg AJ, Weisglas-Kuperus N, Groot Jebbink L, Wijnberg-Williams B, Anand KJ, Tibboel D, van Dijk M. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain. 2013;154:449–458. doi: 10.1016/j.pain.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 24.de Medeiros CB, Fleming AS, Johnston CC, Walker CD. Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr Res. 2009;66:272–277. doi: 10.1203/PDR.0b013e3181b1be06. [DOI] [PubMed] [Google Scholar]
- 25.Doesburg SM, Chau CM, Cheung TP, Moiseev A, Ribary U, Herdman AT, Miller SP, Cepeda IL, Synnes A, Grunau RE. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain. 2013;154:1946–1952. doi: 10.1016/j.pain.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage. 2008;42:60–69. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 27.Duhrsen L, Simons SH, Dzietko M, Genz K, Bendix I, Boos V, Sifringer M, Tibboel D, Felderhoff-Mueser U. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology. 2013;103:35–43. doi: 10.1159/000341769. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012;34:47–55. doi: 10.1016/j.ntt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Gao H, Gao H, Xu G, Li M, Du S, Li F, Zhang H, Wang D. Efficacy and safety of repeated oral sucrose for repeated procedural pain in neonates: A systematic review. Int J Nurs Stud. 2016;62:118–125. doi: 10.1016/j.ijnurstu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 31.Gradin M, Schollin J. The role of endogenous opioids in mediating pain reduction by orally administered glucose among newborns. Pediatrics. 2005;115:1004–1007. doi: 10.1542/peds.2004-1189. [DOI] [PubMed] [Google Scholar]
- 32.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 33.Hammer RP, Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology. 1989;10:475–483. [PubMed] [Google Scholar]
- 34.Hoebel BG, Avena NM, Bocarsly ME, Rada P. Natural addiction: a behavioral and circuit model based on sugar addiction in rats. J Addict Med. 2009;3:33–41. doi: 10.1097/ADM.0b013e31819aa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics. 2010;125:1042–1047. doi: 10.1542/peds.2009-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 37.Jablonska B, Scafidi J, Aguirre A, Vaccarino F, Nguyen V, Borok E, Horvath TL, Rowitch DH, Gallo V. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston CC, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker CD, Veilleux A, Pelausa E, Cake H, Stone S, Sherrard A, Boyer K. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics. 2002;110:523–528. doi: 10.1542/peds.110.3.523. [DOI] [PubMed] [Google Scholar]
- 39.Juul SE, Beyer RP, Bammler TK, Farin FM, Gleason CA. Effects of neonatal stress and morphine on murine hippocampal gene expression. Pediatr Res. 2011;69:285–292. doi: 10.1203/PDR.0b013e31820bd165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kehoe P, Blass EM. Behaviorally functional opioid systems in infant rats: II. Evidence for pharmacological, physiological, and psychological mediation of pain and stress. Behav Neurosci. 1986;100:624–630. doi: 10.1037//0735-7044.100.5.624. [DOI] [PubMed] [Google Scholar]
- 41.Knaepen L, Patijn J, van Kleef M, Mulder M, Tibboel D, Joosten EA. Neonatal repetitive needle pricking: plasticity of the spinal nociceptive circuit and extended postoperative pain in later life. Dev Neurobiol. 2013;73:85–97. doi: 10.1002/dneu.22047. [DOI] [PubMed] [Google Scholar]
- 42.Lerch JP, Carroll JB, Spring S, Bertram LN, Schwab C, Hayden MR, Henkelman RM. Automated deformation analysis in the YAC128 Huntington disease mouse model. Neuroimage. 2008;39:32–39. doi: 10.1016/j.neuroimage.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 43.McKechnie L, Levene M. Procedural pain guidelines for the newborn in the United Kingdom. J Perinatol. 2008;28:107–111. doi: 10.1038/sj.jp.7211822. [DOI] [PubMed] [Google Scholar]
- 44.Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics. 2006;24:154–162. doi: 10.1152/physiolgenomics.00217.2005. [DOI] [PubMed] [Google Scholar]
- 45.Nuseir KQ, Alzoubi KH, Alabwaini J, Khabour OF, Kassab MI. Sucrose-induced analgesia during early life modulates adulthood learning and memory formation. Physiol Behav. 2015;145:84–90. doi: 10.1016/j.physbeh.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Pomares FB, Funck T, Feier NA, Roy S, Daigle-Martel A, Ceko M, Narayanan S, Araujo D, Thiel A, Stikov N, Fitzcharles MA, Schweinhardt P. Histological Underpinnings of Grey Matter Changes in Fibromyalgia Investigated Using Multimodal Brain Imaging. J Neurosci. 2017;37:1090–1101. doi: 10.1523/JNEUROSCI.2619-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 48.Ranger M, Chau CM, Garg A, Woodward TS, Beg MF, Bjornson B, Poskitt K, Fitzpatrick K, Synnes AR, Miller SP, Grunau RE. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013;8:e76702. doi: 10.1371/journal.pone.0076702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranger M, Zwicker JG, Chau CM, Park MT, Chakravarthy MM, Poskitt K, Miller SP, Bjornson BH, Tam EW, Chau V, Synnes AR, Grunau RE. Neonatal Pain and Infection Relate to Smaller Cerebellum in Very Preterm Children at School Age. J Pediatr. 2015;167:292–298. e1. doi: 10.1016/j.jpeds.2015.04.055. [DOI] [PubMed] [Google Scholar]
- 50.Rao R, Sampers JS, Kronsberg SS, Brown JV, Desai NS, Anand KJ. Neurobehavior of preterm infants at 36 weeks postconception as a function of morphine analgesia. Am J Perinatol. 2007;24:511–517. doi: 10.1055/s-2007-986675. [DOI] [PubMed] [Google Scholar]
- 51.Reboucas EC, Segato EN, Kishi R, Freitas RL, Savoldi M, Morato S, Coimbra NC. Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology (Berl) 2005;179:349–355. doi: 10.1007/s00213-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 52.Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proc Natl Acad Sci U S A. 1997;94:1471–1475. doi: 10.1073/pnas.94.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricalde AA, Hammer RP., Jr Perinatal opiate treatment delays growth of cortical dendrites. Neurosci Lett. 1990;115:137–143. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- 54.Roofthooft DW, Simons SH, Anand KJ, Tibboel D, van Dijk M. Eight Years Later, Are We Still Hurting Newborn Infants? Neonatology. 2014;105:218–226. doi: 10.1159/000357207. [DOI] [PubMed] [Google Scholar]
- 55.Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia. 2008;56:1017–1027. doi: 10.1002/glia.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seatriz JV, Hammer RP., Jr Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30:523–527. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 58.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 59.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Spring S, Lerch JP, Henkelman RM. Sexual dimorphism revealed in the structure of the mouse brain using three-dimensional magnetic resonance imaging. Neuroimage. 2007;35:1424–1433. doi: 10.1016/j.neuroimage.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Steadman PE, Ellegood J, Szulc KU, Turnbull DH, Joyner AL, Henkelman RM, Lerch JP. Genetic effects on cerebellar structure across mouse models of autism using a magnetic resonance imaging atlas. Autism Res. 2014;7:124–137. doi: 10.1002/aur.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. 2016;7:CD001069. doi: 10.1002/14651858.CD001069.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taddio A, Shah V, Shah P, Katz J. Beta-endorphin concentration after administration of sucrose in preterm infants. Arch Pediatr Adolesc Med. 2003;157:1071–1074. doi: 10.1001/archpedi.157.11.1071. [DOI] [PubMed] [Google Scholar]
- 64.Takane Y. Constrained principal component analysis and related techniques. Boca Raton, FL: Chapman and Hall/CRC Press; 2014. [Google Scholar]
- 65.Tardif CL, Steele CJ, Lampe L, Bazin PL, Ragert P, Villringer A, Gauthier CJ. Investigation of the confounding effects of vasculature and metabolism on computational anatomy studies. Neuroimage. 2017;149:233–243. doi: 10.1016/j.neuroimage.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 66.Ullmann JF, Watson C, Janke AL, Kurniawan ND, Reutens DC. A segmentation protocol and MRI atlas of the C57BL/6J mouse neocortex. Neuroimage. 2013;78:196–203. doi: 10.1016/j.neuroimage.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Vinall J, Miller S, Bjornson B, Fitzpatrick K, Poskitt K, Brant R, Synnes A, Cepeda I, Grunau R. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics. 2014;133:412–421. doi: 10.1542/peds.2013-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker CD. Nutritional aspects modulating brain development and the responses to stress in early neonatal life. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1249–1263. doi: 10.1016/j.pnpbp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Walker CD, Xu Z, Rochford J, Johnston CC. Naturally occurring variations in maternal care modulate the effects of repeated neonatal pain on behavioral sensitivity to thermal pain in the adult offspring. Pain. 2008;140:167–176. doi: 10.1016/j.pain.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Zwicker JG, Grunau RE, Adams E, Chau V, Brant R, Poskitt KJ, Synnes A, Miller SP. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol. 2013;48:123–129. e1. doi: 10.1016/j.pediatrneurol.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, Liu M, Synnes A, Poskitt KJ, Stiver ML, Tam EW. Smaller Cerebellar Growth and Poorer Neurodevelopmental Outcomes in Very Preterm Infants Exposed to Neonatal Morphine. J Pediatr. 2016;172:81–87. e2. doi: 10.1016/j.jpeds.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.