Abstract
In asthma elevated rates of exhaled breath temperature changes (Δe°T) and bronchial blood flow (Qaw) may be due to increased vascularity of the airway mucosa as a result of inflammation.
We investigated the relationship of Δe°T with Qaw and airway inflammation as assessed by exhaled nitric oxide (NO). We also studied the anti-inflammatory and vasoactive effects of inhaled corticosteroid and β2-agonist.
Δe°T was confirmed to be elevated (7.27 ± 0.6 Δ°C/s) in 19 asthmatic subjects (mean age ± SEM, 40 ± 6 yr; 6 male, FEV1 74 ± 6 % predicted) compared to 16 normal volunteers (4.23 ± 0.41 Δ°C/s, p < 0.01) (30 ± 2 yr) and was significantly increased after salbutamol inhalation in normal subjects (7.8 ± 0.6 Δ°C/ s, p < 0.05) but not in asthmatic patients. Qaw, measured using an acetylene dilution method was also elevated in patients with asthma compared to normal subjects (49.47 ± 2.06 and 31.56 ± 1.6 μl/ml/min p < 0.01) and correlated with exhaled NO (r = 0.57, p < 0.05) and Δe°T (r = 0.525, p < 0.05). In asthma patients, Qaw was reduced 30 minutes after the inhalation of budesonide 400 μg (21.0 ± 2.3 μl/ml/min, p < 0.05) but was not affected by salbutamol.
Δe°T correlates with Qaw and exhaled NO in asthmatic patients and therefore may reflect airway inflammation, as confirmed by the rapid response to steroids.
Keywords: asthma, nitric oxide, temperature, bronchial blood flow, inflammation.
Asthma is an inflammatory disease of the airways. Vasodilatation is a critical feature of inflammation, and angiogenesis and vascular remodelling are features of chronic inflammatory diseases, such as asthma [1]. The increased vascularity of the airways in asthma [2] is partly due to the elevated number of vessels associated with angiogenesis and partly due to vasodilation caused by the release of vasodilator mediators, such as, histamine, bradykinin [3], and nitric oxide (NO) [4]. In a recent study we have found that patients with asthma have higher increases of exhaled breath temperature (Δe°T) compared with normal subjects and that this is correlated to the concentration of exhaled nitric oxide (NO) [5]. Therefore, we suggested that patients with asthma have high Δe°T. and that this may be due to airway inflammation and elevated levels of NO. In the present study we hypothesise that elevated Δe°T may result from increased heat exchange in the airways due to elevated bronchial blood flow (Qaw) caused by inflammation and airway remodelling. To test this, we investigate the relationship between airway inflammation as assessed by exhaled NO, with Qaw and Δe°T measured non-invasively. Qaw is an expression of bronchial blood flow, whereas Δe°T reflects the rate of temperature increase in the exhaled breath. We hypothesised that Qaw changes may contribute to the levels of Δe°T and we studied their relationship considering that, potentially, minor changes of bronchial blood flow may not affect exhaled breath temperature.
Elevated levels of exhaled NO in asthma [6,7] are likely to be due to the activation of the inducible form of NO synthase (iNOS) by inflammatory cytokines [8] and therefore, reflect airway inflammation. Because NO also regulates bronchial vascular tone [9] and may increase bronchial blood flow [10,11] we measured its concentration in the exhaled breath as a marker of inflammation and we analysed its relationship with Qaw and Δe°T.
The measurement of Qaw is non-invasive and was standardised and adapted from a previously validated technique [12,13]. Δe°T and bronchial blood flow (Qaw) were also measured non-invasively allowing us to make repeated measurements and to study the interactions of these two markers and exhaled NO.
The inhalation of corticosteroids has been shown to have an acute vasocostrictive effect on the bronchial circulation [14] and the measurement of Qaw has been advocated to assess airway steroid sensitivity. In order to evaluate further the correlation of Qaw and Δe°T with inflammation, we studied the acute effect of inhaled budesonide on these parameters and their reciprocal changes. Furthermore, to validate our methods, we also evaluated the vasodilating effect of the short-acting β2-agonist salbutamol.
Methods
Patients
Nineteen asthmatic patients were studied (7 male, age 40 ± 5 yr, FEV1 74 ± 6 %, 9 patients were on inhaled steroid treatment and 10 patients had mild persistent asthma and were on β2-agonist inhalers only). These two groups of patients were chosen because they are representative of the larger majority of asthmatic patients; in addition this allowed us to verify cross sectionally the effect of inhaled steroids on bronchial blood flow and exhaled NO.
We also examined 16 control subjects (age 30 ± 2 yr, 8 male) recruited from our outpatient clinic and from volunteers (Table 1). Most of these subjects had previously taken part in other published studies. The diagnosis of asthma was established in each patient according to American Thoracic Society criteria [15]. Patients with acute chest infection or disease exacerbation during the month before enrolment were excluded. Patients with history of diabetes, liver disease, heart failure, lung cancer, or alcohol/drug abuse were not eligible for the study. All subjects were life-long non-smokers. All asthmatic patients refrained from using β2 agonists and corticosteroids for at least 12 h prior to the study. The tests were carried out at ambient temperature between 23 and 25C° and humidity 60% and 65%.
Table 1.
PATIENT CHARACTERISTICS
| Asthma not steroid treated (n = 10) | Steroid treated asthma (n = 9) | Normals (n = 16) | |
| Age (years) | 37 ± 7 | 45 ± 7 | 30 ± 2 |
| Sex (M/F) | 4/6 | 3/6 | 8/8 |
| FEV1 (% predicted) | 93 ± 10 | 75 ± 11 | 97 ± 9 |
| Smokers | 0 | 0 | 0 |
| Ex smokers | 0 | 0 | 0 |
| Therapy: | |||
| Inhaled β-drenergics | 10 | 10 | 0 |
| Theophylline | 0 | 0 | 0 |
| Inhaled steroids | 0 | 10 | 0 |
| Oral steroids | 0 | 0 | 0 |
Definition of abbreviations:; FEV1 = forced expiratory volume in one second. Values are means ± SEM.
Study design
The study was approved by the Brompton and Harefield NHS trust Ethics Committee. After a clinical examination was carried out, Δe°T, Qaw and exhaled NO were measured at least after one hour of rest in the laboratory. This was followed by spirometry.
Eight asthmatic subjects and eight normal volunteers agreed to have Δe°T and Qaw measured after the inhalation of budesonide 400 μg and salbutamol 200 μg or placebo, which were administered in three different visits. The measurements were repeated every 15 minutes for 1 h after budesonide and placebo inhalation and at baseline and every 10 minutes for half an hour after the inhalation of salbutamol.
Exhaled breath temperature measurement
During a flow and pressure controlled single breath exhalation exhaled breath temperature gradients were measured as previously described [5]. As previously shown [5], during a flow and pressure controlled exhalation from total lung capacity through a 2.77 mm mouthpiece[16] exhaled breath temperature was measured by a fast response (1 ms) high accuracy (0.015 ± 0.027°C), thermometer (Picotech Ltd) interfaced with a computer by a single channel Picotech Oscilloscope (model ADC 42, resolution 12 bits) allowing online recording of exhaled breath temperature.
In a preliminary study exhaled breath temperature tracings were analyzed mathematically. The tracings proved to have an exponential rise and the point at 63% of the total temperature increase was chosen to study the slope of the curves because it represents two time constants of the maximal °T change and therefore allows a better mathematical characterization of the tracings before plateau.
The time constant of the thermometer response was found by measuring the time for the temperature to reach 63% of the final reading. This avoided large errors in estimating when the asymptotic final reading had been reached.
The exhaled breath temperature changes exponentially with time. The shape of the curve depends upon the time constant (T). In one time constant the response reaches 63% of its final change.
The response is of the form
![]()
Where V2 is the final value, V1 the initial value, and t is the time after exhalation was started. As t > infinity, the exponential term > 0. Hence the response rises asymptotically to its final value. When performing experiments to determine the time response of a system, it is difficult to tell at which time the final value is obtained, since there will be a small change in the response for a relatively long time as the asymptote is approached. Jitter and noise in the signal will also add to this problem. It is easier to estimate the asymptotic value and find the time at which a certain percentage of this is reached. Various percentages are possible, the most commonly used in physics and biology is the time constant, although in some applications (particularly electronic engineering) the rise time to 95% of the final value is used.
The 63% arises because when t = T, the expression above becomes
(V2-V1)(1-1/e) = (V2-V1)(1-0.37) = 0.63(V2-V1)
In n time constants the percentage reached is 100 × (1-1/en)
which, approaches 98% after about 5 time constants.
The rate of temperature increase (Δe°T) calculated between the beginning of exhalation and 63 % of the total temperature increase (a/b, where "a" is 63% of Δ°T and "b" the time to reach "a") proved to be the more reproducible parameter to characterize the curves.
We evaluated the effect of different exhalation flow rates, distance of the thermocouple from the edge of the mouthpiece and ambient temperature on Δe°T and end-expiratory plateau temperatures. We found that Δe°T but not plateau temperatures were elevated at low (2–3 L/min) compared to higher (5–6 L/min) exhalation flow rates (6.25 ± 0.4°C/s and 4.45 ± 0.8°C/s, p < 0.01) in 8 normal subjects. When the thermocouple was inserted close to the edge of the mouthpiece (1 cm) the Δe°T was significantly higher (7.15 ± 0.2°C/s) compared to when it was located farther (2 cm) (4.45 ± 0.8°C/s, p < 0.01). There was a tendency for faster Δe°T when the subjects were starting exhaling from higher baseline ambient temperatures but this was not significant for temperature changes within ± 3°C (5.05 ± 0.8°C/s at 28°C and 4.45 ± 0.8°C/s at 22°C p > 0.05). The volume ventilation did not influence the Δe°T value. The difference in exhaled breath Δe°T and plateau temperatures measured during two successive collections at five minutes intervals (single session variability) was 4.4%, while between sessions variability (n = 6, one day interval) was 6.8%. The reproducibility of the test was confirmed by the Bland and Altman test [17].
Exhaled NO measurements
Exhaled NO was measured using a modified chemiluminescence analyzer (model LR2000; Logan Research, Rochester, Kent) as previously described [18].
Bronchial blood flow
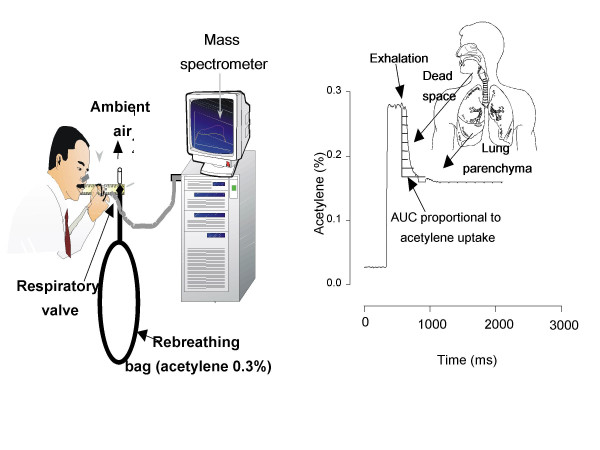
We modified a previously validated soluble inert gas uptake method to measure Qaw, using acetylene rather than the potentially explosive dimethylether [12,13]. The subjects were sitting in front of a valve system inhaling through a mouthpiece (with nose clips on) initially room air and then a gas mixture from a Teflon bag containing 35% O2, 0,3% acetylene, 5% sulphur hexafluoride, CO 3% and a balance of nitrogen. During the exhalation the concentration of acetylene was measured directly online by a mass spectrometer, and Qaw was calculated from the Fick principle (dilution of acetylene concentration) (Figure 1), the area under the curve (AUC) being inversely proportional to the bronchial blood flow. Qaw was expressed as μl/ml/min representing the volume of blood per volume of dead space per time.
Figure 1.
Method for the measurement of bronchial blood flow (Qaw). Subjects inhale 60% of their vital capacity from a reservoir containing acetylene 0.3% and then exhale into a mass spectrometer (Panel A). Panel B shows a tracing of acetylene, the area under the curve, corresponding to the conducting airways, is proportional to airway blood flow.
Previous studies have used dimethyl ether instead of acetylene for the measurement of bronchial blood flow. In the current study we used acetylene because of the higher explosive potential of dimethylether when in contact with O2. This method was previously validated in the sheep where the invasive inoculation of radioactive micro spheres directly into the bronchial circulation provided similar results [19]. The use of micro spheres is considered the Gold Standard for the measurement of blood flow, however, this method is invasive and presents limitations such as the recirculation of the radioactive spheres.
Acetylene and diethyl ether present similar blood solubility and affinity for haemoglobin when measured at the same temperature [20]. Gas exchange efficiency is largely dependent on solubility. Because these two gases have similar physicochemical characteristics we assume that they can be used interchangeably. This is further confirmed by the finding of a similar range of bronchial blood flow and similar response to corticosteroids (vasoconstriction) and beta 2 agonists (vasodilatation) in this study compared to previously published studies which used the dimethyl ether method.
Statistics
Comparisons between groups were made by one-way analysis of variance (ANOVA).. Data were expressed as means ± standard error of mean. The relationship between the exhaled breath temperature, Qaw, NO and FEV1 were tested with the linear correlation coefficient.
Results
Bronchial blood flow (Qaw)
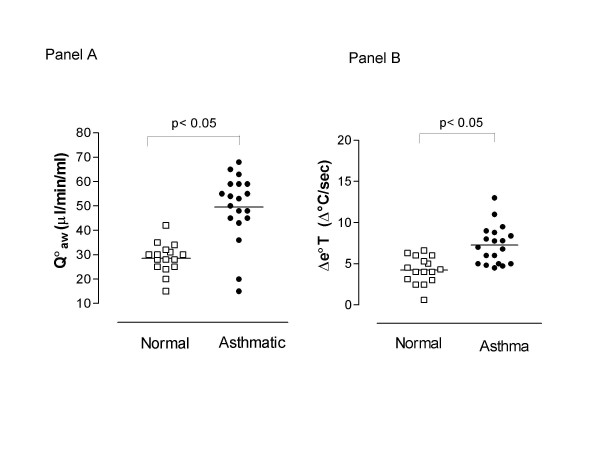
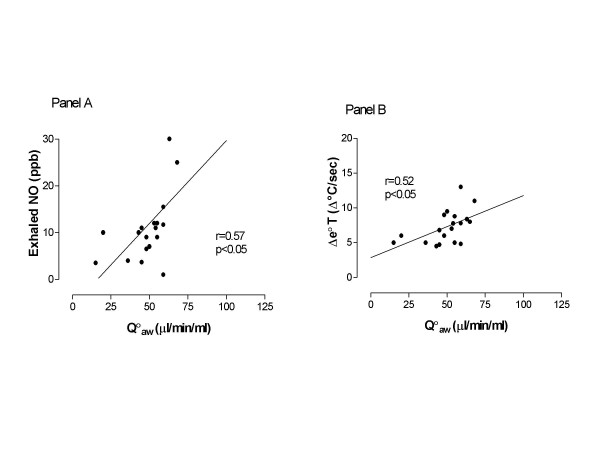
Qaw was elevated to a similar extent in patients with mild persistent and moderate asthma (on regular inhaled corticosteroids and β2-agonists) (46.0 ± 51 μl/ml/min) and patients with mild intermittent asthma (on β2-agonists as needed only) (52.64 ± 3.0 μl/ml/min, p > 0.05) compared to normal subjects (31.56 ± 1.6 μl/ml/min, p < 0.01, Figure 2, Panel A). Qaw was correlated with exhaled NO (r = 0.57, p < 0.05, Figure 3, Panel A) and Δe°T (r = 0.52, p < 0.05, Figure 3, Panel B).
Figure 2.
Levels of bronchial blood flow (Qaw) (Panel A) and exhaled breath temperature gradients (Δe°T) (Panel B) in normal subjects (□) and patients with asthma (•).
Figure 3.
Correlation of bronchial blood flow (Qaw) with exhaled nitric oxide (NO) (Panel A) and exhaled breath temperature gradients (Δe°T) (Panel B) in patients with asthma.
Exhaled air temperature
Δe°T was higher in asthmatic patients (7.27 ± 0.6 Δ°C/ sec) compared to normal subjects (4.23 ± 0.41 Δ°C/ sec, p < 0.01, Figure 2, Panel B) and was not statistically different in steroid treated (7.56 ± 0.99 Δ°C/ sec) compared to untreated patients (6.83 ± 0.78 Δ°C/ sec, p > 0.05).
Exhaled NO
NO levels were elevated in asthmatic subjects not on steroid treatment (15.6 ± 2.8 ppb) compared to steroid treated patients (7.5.6 ± 2.3 ppb, p < 0.05) and normal subjects (4.7 ± 0.3 ppb, p < 0.05).
Effect of budesonide and salbutamol inhalation
Bronchial blood flow
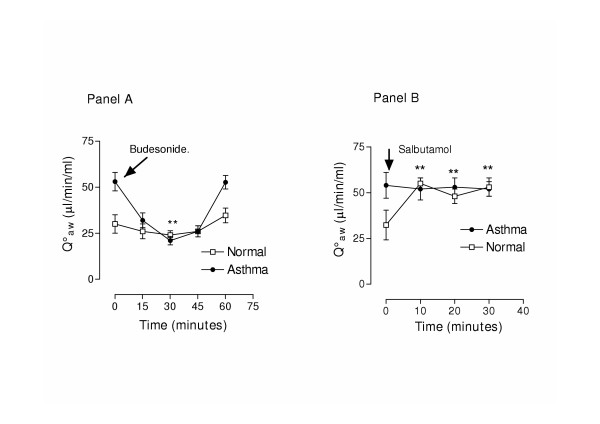
In asthmatic patients Qaw was significantly reduced 30 minutes after the inhalation of budesonide compared to baseline (53.0 ± 5.0 μl/ml/min and 21.3 ± 2.32 μl/ml/min respectively, p < 0.05 Figure 4 Panel A) and returned to baseline levels at 60 minutes (52.6 ± 4.0 μl/ml/min). In normal subjects there was a tendency for lower Qaw after the inhalation of budesonide but such changes were not significant (30.3 ± 5.0 μl/ml/min and 26.3 ± 3.0 μl/ml/min at baseline and at 30 minutes respectively, p > 0.05, n = 5, Figure 4 Panel A).
Figure 4.
Acute effect of budesonide inhalation (400 μg) (Panel A) and salbutamol (200 μg) (Panel B) on bronchial blood flow (Qaw).
In 8 normal volunteers Qaw was increased after salbutamol inhalation (32.3 ± 8.1 μl/ml/min and 55.0 ± 3.0 μl/ml/min at baseline and at 10 minutes respectively, p < 0.05), while this effect was not present in asthmatic patients (54.0 ± 7.0 μl/ml/min and 52.0 ± 6.0 μl/ml/min, p > 0.05, n = 5, Figure 4 Panel B).
Placebo had no effect on Qaw in subjects with asthma (54.0 ± 3.0, 56.1 ± 4.5 and 53.1 ± 8.1 μl/min/ml/ at baseline, 30 and 60 minutes respectively, p > 0.05) nor in normal subjects (34.0 ± 3.0,38.1 ± 4.5 and 35.1 ± 8.1 μl/min/ ml/ p > 0.05)
Exhaled breath temperature
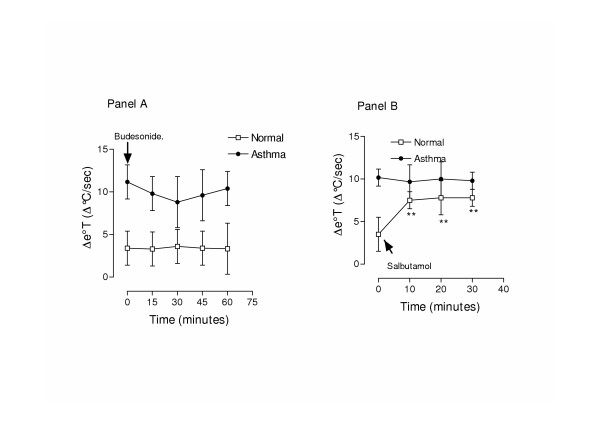
The inhalation of budesonide was associated with a tendency for a decrease of Δe°T in asthmatic patients (from 10.17 ± 2 Δ°C/ sec at baseline to 8.6 ± 3 Δ°C/sec 30 minutes after inhalation Figure 5 Panel A), even though this decrease was not significant; the changes in Qaw and Δe°T were correlated in asthmatic patients (r = 0.78, p < 0.05). In normal subjects, no significant changes of Δe°T were found at any of the time points after budesonide inhalation (3.5 ± 2 Δ°C/sc at baseline, 3.5 ± 1 Δ°C/sc at 30 minutes, 3.53 ± 2 Δ°C/sc at 60 minutes, p > 0.05).
Figure 5.
Acute effect of budesonide inhalation (400 μg) (Panel A) and salbutamol (200 μg) (Panel B) on exhaled breath temperature gradients (Δe°T).
In 5 normal volunteers Δe°T was increased after the inhalation of 200 μg of salbutamol (3.50 ± 0.29 Δ°C/ sec and 7.8 ± 0.6 Δ°C/ sec, p < 0.01), while this effect was not present in asthmatic patients (10.1 ± 0.44 Δ°C/ sec and 9.67 ± 0.51 Δ°C/ sec, p > 0.05, n = 5, Figure 5 Panel B)
Δe°T was unchanged in subjects with asthma (9.17 ± 2 and 10.23 ± 3.5 Δ°C/ sec at baseline and 10 minutes respectively, p > 0.05) and in normal subjects (3.50 ± 0.29 Δ°C/ sec and 4.20 ± 0.32 Δ°C/ sec p > 0.05) after the inhalation of placebo.
Discussion
This is the first study to show that elevated levels of Qaw and Δe°T are correlated with one another and with airway inflammation as assessed by exhaled NO. We propose that high levels of NO generated in asthmatic patients as a result of airway inflammation may cause vasodilatation of the bronchial circulation contributing to increased heat exchange. This is supported by the demonstration that lower Δe°T levels, after the inhalation of corticosteroids, are correlated with reduced levels of bronchial blood flow. We propose that these non-invasive measurements may be useful to evaluate airway inflammation and may provide a tool to assess steroid sensitivity.
Angiogenesis and microvascular remodelling are features of chronic inflammatory diseases, such as asthma [21]. As inflammatory diseases evolve, the microvasculature undergoes progressive changes in structure and function. Blood vessels enlarge and proliferate supplying inflammatory cells in chronically inflamed tissues. Because of these changes, asthmatic patients have increased vascularity of the airway mucosa which is related to the severity of the disease [2]. Airway vascular remodelling and inflammation maybe responsible for increased bronchial blood flow [22] and exhaled breath temperature gradients in asthmatic patients [5].
In a previous study [23] we have proved that patients with asthma have elevated Δe°T compared to normal subjects and because we found a significant correlation with exhaled NO we suggested that this was due to airway inflammation. In the present study we hypothesised that Δe°T is elevated in asthma as a result of increased bronchial blood flow and we studied the relationship between Qaw and Δe°T and airway inflammation as assessed by exhaled NO. Even though the patients enrolled in this study were significantly older than the control group, our previous studies [5,24] indicate that that age does not affect Δe°T. We studied airway inflammation measuring exhaled NO and we investigates its relationship with Qaw which has also been suggested as a marker of inflammation. We also studied the interaction of these two parameters after inhaled corticosteroids β2 agonists.
We confirmed previous data showing elevated levels of Qaw in asthmatic subjects compared to normal volunteers, using a modified method developed by Onorato et al [25]. In the current study we preferred the use of acetylene over dimethyl ether because the latter is highly explosive when in contact with oxygen. Gas exchange efficiency is largely dependent on solubility, because these two gases have similar physicochemical characteristics we assume that they can be used interchangeably. Furthermore, the measurement of Qaw in this study presented the same response pattern and timing to the inahaltion of steroids [12] and beta agonists [26] as previously showed using the dimethyl ether method confirming that the method presented in our study is an acceptable measurement of Qaw.
The method used in this study for the measurement of Qaw produces results which include the contribution of the dead space and trachea blood flow to the total bronchial blood flow. We acknowledge that the trachea may not be the main site of inflammation in asthma, but inflammation in asthma extends from the larynx to the terminal bronchioles and the tracheal mucosa certainly appears inflamed in many asthmatic patients. When tracheal inflammation occurs there will be a good separation between normal subjects and patients with asthma because the measurements of bronchial blood flow and exhaled breath temperature will particularly reflect the contribution of this part of the respiratory tract
For the first time we have shown, using non-invasive methods, that changes in bronchial blood flow can alter exhaled breath temperature indicating that the bronchial circulation may control airstream temperature. Furthermore, in this study not only we have shown a correlation between Qaw and Δe°T but we have also shown that these measurements respond similarly to steroids and beta 2 agonists. Therefore, patients with asthma have a significantly faster rise of breath temperature and Qaw and these are correlated. We presume that this is due to the increased vascularity of the bronchial vessels [27] and elevated blood supply and therefore heat transfer across the bronchial wall. Hyperaemia and hyperperfusion are consistent features of tissue inflammation, therefore, the finding of increased exhaled air temperature and bronchial blood flow in asthmatic patients may be due to the elevated levels of exhaled NO which is a marker of inflammation and a potent bronchial vasodilator. Even though the correlation between Qaw and Δe°T may appear to be weak, it is noteworthy that the acute changes of these variable was significantly correlated, reinforcing the hypothesis that elevated breath temperature gradients in asthmatic patients may reflect increased bronchial blood flows.
We were able to show a positive correlation between exhaled NO and Δe°T. NO is a gas produced by several types of pulmonary cells, including inflammatory, endothelial and airway epithelial cells. Elevated levels of exhaled NO in asthma [6], and interstitial lung disease [28] are likely to be due to the activation of the inducible form of NO synthase (iNOS) and therefore may reflect airway inflammation, alternatively NO maybe produced by the bronchial epithelium. In addition the activity of iNOS, the inducible enzyme responsible for the synthesis of NO, is temperature-dependent [29], therefore elevated airway temperatures in patients with asthma [5] may induce further synthesis of NO. NO is a potent vasodilator and may play a role in the regulation of bronchial vasomotor tone [10], so that elevated levels of NO may lead to vasodilatation and increased bronchial blood flow as shown by the correlation between exhaled NO and Δe°T. Unfortunately, in the current study, we were unable to establish the contribution of NO produced by the bronchial vasculature compared to the pulmonary circulation.
In this cross-sectional study we could not show any differences of Δe°T or Qaw in corticosteroid-treated compared to untreated asthmatic patients, despite the efficacy of steroids in reducing bronchial blood flow [12]. This is consistent with previous studies published by our group and others showing that steroid-treated asthmatic patients have similar Δe°T [5] and Qaw [22] compared to untreated patients. One hypothesis is that the vasoconstrictive action of inhaled corticosteroids may have been balanced by β2 induced vasodilatation resulting in minimal changes in bronchial artery diameter and blood flow and therefore no changes of Qaw and Δe°T. However, these results must be confirmed by placebo controlled studies.
In contrast to the effect of chronic treatment with corticosteroids, the acute inhalation of budesonide caused a significant temporary reduction of Qaw which returned to baseline one hour after inhalation. This is also consistent with a previous publication [22], notably, in the current study Qaw and Δe°T and their interaction were studied simultaneously in the same group of patients for the first time. Corticosteroids may cause vasoconstriction by numerous mechanisms. They can potentiate the vasoconstrictor actions of noradrenalin and angiotensin II by upregulating their vascular receptors [30]. Furthermore, corticosteroids may inhibit the synthesis of NO [31], thus causing vasoconstriction. In addition to these mechanisms of action, corticosteroids may also have a very rapid action (less than 5 minutes) by inhibiting noradrenalin uptake in bronchial blood vessels [32]. The glucocorticoid-induced vasoconstriction in asthmatics seems to be accompanied by a greater α1-adrenergic vasoconstrictor response [26]. This adds further support to a α1-adrenergic steroid interplay in the regulation of vascular tone.
Further studies are required, investigating the dose response relationship for inhaled steroids would provide valuable information.
The cardinal signs of inflammation are rubor (redness), calor (heat), tumor (swelling), dolor (pain), and impaired function (functio laesa). Exhaled breath temperature and bronchial blood flow may reflect rubor and calor in the airways and therefore may be markers of tissue inflammation and remodelling as confirmed by the positive correlation between Δe°T, Qaw and exhaled NO which was shown for the first time in this study. Measurement of exhaled breath temperature and bronchial blood flow may provide means of detecting airway inflammation and vascular remodelling in a non-invasive way.
Acknowledgments
Acknowledgments
This study was supported by the National Heart and Lung Institute, London UK No Part of the research presented was founded by tobacco industry sources
Contributor Information
Paolo Paredi, Email: p.paredi@imperial.ac.uk.
Sergei A Kharitonov, Email: s.kharitonov@imperial.ac.uk.
Peter J Barnes, Email: p.j.barnes@ic.ac.uk.
References
- Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123:417S–422S. doi: 10.1378/chest.123.3_suppl.417S-a. [DOI] [PubMed] [Google Scholar]
- Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons GH, Nichol GM, Barnes PJ, Chung KF. Peptide mediator effects on bronchial blood velocity and lung resistance in conscious sheep. J Appl Physiol. 1992;72:1118–22. doi: 10.1152/jappl.1992.72.3.1118. [DOI] [PubMed] [Google Scholar]
- Charan NB, Johnson SR, Lakshminarayan S, Thompson WH, Carvalho P. Nitric oxide and beta-adrenergic agonist-induced bronchial arterial vasodilation. J Appl Physiol. 1997;82:686–692. doi: 10.1152/jappl.1997.82.2.686. [DOI] [PubMed] [Google Scholar]
- Paredi P, Kharitonov SA, Barnes PJ. Faster rise of exhaled breath temperature in asthma: a novel marker of airway inflammation? Am J Respir Crit Care Med. 2002;165:181–184. doi: 10.1164/ajrccm.165.2.2103053. [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/S0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- Lanz MJ, Leung DY, McCormick DR, Harbeck R, Szefler SJ, White CW. Comparison of exhaled nitric oxide, serum eosinophilic cationic protein, and soluble interleukin-2 receptor in exacerbations of pediatric asthma. Pediatr Pulmonol. 1997;24:305–311. doi: 10.1002/(sici)1099-0496(199711)24:5<305::aid-ppul1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Robbins RA, Springall DR, Warren JB, et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem Biophys Res Commun. 1994;203:209–18. doi: 10.1006/bbrc.1994.2169. [DOI] [PubMed] [Google Scholar]
- Cremona G, Wood AM, Hall LW, Bower EA, Higenbottam T. Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol (Lond) 1994;481:185–195. doi: 10.1113/jphysiol.1994.sp020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KGJ, Huang PL, McMurtry IF, Rodman DM. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–L478. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- Degnim AC, Nakayama DK. Nitric oxide and the pulmonary artery smooth muscle cell. Semin Pediatr Surg. 1996;5:160–164. [PubMed] [Google Scholar]
- Brieva JL, Danta I, Wanner A. Effect of an Inhaled Glucocorticosteroid on Airway Mucosal Blood Flow in Mild Asthma. Am J Respir Crit Care Med. 2000;161:293–296. doi: 10.1164/ajrccm.161.1.9905068. [DOI] [PubMed] [Google Scholar]
- Chediak AD, Elsasser S, Csete ME, Gazeroglu H, Wanner A. Effect of histamine on tracheal mucosal perfusion, water content and airway smooth muscle in sheep. Respir Physiol. 1991;84:231–243. doi: 10.1016/0034-5687(91)90120-8. [DOI] [PubMed] [Google Scholar]
- Mendes ES, Pereira A, Danta I, Duncan RC, Wanner A. Comparative bronchial vasoconstrictive efficacy of inhaled glucocorticosteroids. Eur Respir J. 2003;21:989–993. doi: 10.1183/09031936.03.00072402. [DOI] [PubMed] [Google Scholar]
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- Paredi P, Loukides S, Ward S, et al. Exhalation flow and pressure-controlled reservoir collection of exhaled nitric oxide for remote and delayed analysis. Thorax. 1998;53:775–9. doi: 10.1136/thx.53.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Kharitonov S, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur Respir J. 1997;10:1683–1693. doi: 10.1183/09031936.97.10071683. [DOI] [PubMed] [Google Scholar]
- Scuri M, McCaskill V, Chediak AD, Abraham WM, Wanner A. Measurement of airway mucosal blood flow with dimethylether: validation with microspheres. J Appl Physiol. 1995;79:1386–1390. doi: 10.1152/jappl.1995.79.4.1386. [DOI] [PubMed] [Google Scholar]
- Jibelian G, Mitchell RR, Overland ES. Influence of hematocrit and temperature on solubility of acetylene and dimethyl ether. J Appl Physiol. 1981;51:1357–1361. doi: 10.1152/jappl.1981.51.5.1357. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and remodelling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med JID. 2001;164(10 Pt 2):S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- Kumar SD, Emery MJ, Atkins ND, Danta I, Wanner A. Airway mucosal blood flow in bronchial asthma. Am J Respir Crit Care Med. 1998;158:153–156. doi: 10.1164/ajrccm.158.1.9712141. [DOI] [PubMed] [Google Scholar]
- Laitinen LA, Laitinen MA, Widdicombe JG. Dose-related effects of pharmacological mediators on tracheal vascular resistance in dogs. Br J Pharmacol. 1987;92:703–709. doi: 10.1111/j.1476-5381.1987.tb11374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredi P, Caramori G, Cramer D, Ward S, Kharitonov SA, Barnes PJ. Slower rise of exhaled breath temperature in COPDSlower rise of exhaled breath temperature in COPD. Eur Respir J. 2003;21:439–443. doi: 10.1183/09031936.03.00067103. [DOI] [PubMed] [Google Scholar]
- Onorato DJ, Demirozu MC, Breitenbucher A, Atkins ND, Chediak AD, Wanner A. Airway mucosal blood flow in humans. Response to adrenergic agonists. Am J Respir Crit Care Med. 1994;149:1132–1137. doi: 10.1164/ajrccm.149.5.8173752. [DOI] [PubMed] [Google Scholar]
- Brieva J, Wanner A. Adrenergic airway vascular smooth muscle responsiveness in healthy and asthmatic subjects. J Appl Physiol. 2001;90:665–669. doi: 10.1152/jappl.2001.90.2.665. [DOI] [PubMed] [Google Scholar]
- Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- Paredi P, Kharitonov SA, Loukides S, Pantelidis P, du BR, Barnes PJ. Exhaled nitric oxide is increased in active fibrosing alveolitis. Chest. 1999;115:1352–1356. doi: 10.1378/chest.115.5.1352. [DOI] [PubMed] [Google Scholar]
- Venturini G, Colasanti M, Fioravanti E, Bianchini A, Ascenzi P. Direct effect of temperature on the catalytic activity of nitric oxide synthases types I, II, and III. Nitric Oxide. 1999;3:375–382. doi: 10.1006/niox.1999.0250. [DOI] [PubMed] [Google Scholar]
- Ullian ME. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/S0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- Redington AE, Meng QH, Springall DR, Evans TJ, Creminon C, Maclouf J, Holgate ST, Howarth PH, Polak JM. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax. 2001;56:351–357. doi: 10.1136/thorax.56.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Lieb T, Conner GE, Salathe M, Wanner A. Steroid sensitivity of norepinephrine uptake by human bronchial arterial and rabbit aortic smooth muscle cells. Am J Respir Cell Mol Biol. 2001;25:500–506. doi: 10.1165/ajrcmb.25.4.4559. [DOI] [PubMed] [Google Scholar]