Abstract
BACKGROUND
Evaluate long-term outcomes after initial definitive or adjuvant radiotherapy for T3-larynx cancers.
METHODS
We reviewed 412 patients treated for T3 laryngeal squamous cell cancer 1985–2011.
RESULTS
10-year OS was 35%; disease-specific-survival (DSS), 61%; locoregional control (LRC), 76%; freedom from distant metastasis, 83%. Chemotherapy, age, performance status <2, node-negative status, and glottic sub-site were associated with improved survival (all P<0.03). Larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT) had better laryngectomy-free survival (LFS) than radiotherapy alone (LP-RT) (HR, 0.62; 95% CI, 0.47–0.81; P=0.0005); 10-year LFS rates of the LP-CRT cohort (37%) were higher than those of the LP-RT cohort (18%). 5-year DSS and OS rates of the LP-CRT cohort (79% and 67%) were better laryngectomy with post-operative radiotherapy (TL-PORT) (61% and 50%) and LP-RT (64% and 46%; P<0.006 for all).
CONCLUSIONS
In patients with T3-larynx cancers, LP-CRT provides better functional, oncologic, and survival outcomes than historical TL-PORT or LP-RT does.
Keywords: T3, radiotherapy, total laryngectomy, outcomes, survival
INTRODUCTION
Following the publication of the landmark Veterans Affairs (VA) larynx cancer study and the Radiation Therapy Oncology Group (RTOG) 91-11 randomized trials,(1, 2) which demonstrated the viability of non-surgical larynx preservation approaches, strategies using sequential or concurrent chemoradiotherapy have increasingly been used for the definitive treatment of locally advanced larynx cancers. However, several studies’ findings have implied that non-surgical larynx preservation approaches may be associated with survival decrement.(3–7) Specifically, Hoffman et al., using large-scale series from the National Cancer Data Base (NCDB), reported that the increased use of organ preservation approaches was associated with a decrement in relative survival in patients with T3 larynx cancer.(3) Gourin et al., in a retrospective series of locally advanced disease demonstrated that non-surgical larynx preservation was associated with poorer survival particularly for patients with T4 disease.(4) In a retrospective series from the Alberta Cancer Registry, Dziegielewski et al. showed that T3 as well as T4a larynx cancer patients had inferior survival for both radiation alone and concurrent chemoradiation cohorts compared to surgical cohort.(5) Another NCDB analysis reported by Chen et al., showed increased mortality risk among T3 patients treated with non-surgical approaches particularly in patients treated by radiation alone.(6) More recently, Megwalu et al., analyzing grouped survival data from the Surveillance, Epidemiology, and End Results (SEER) 18 registry, reported that patients with some T3 larynx cancers who were treated with surgery had better survival outcomes than those who were treated with non-surgical management.(7) These findings have led some researchers to question the utility of chemoradiation as first-line therapy for T3 larynx cancer.(3–7)
This is not an invalid concern, as the VA and RTOG trials took place in an era before the introduction of modern radiotherapy techniques. The VA(1) and RTOG(2) studies, designed and conducted several decades ago, enrolled patients who were treated with non-volumetric imaging–based conventional 2- or 3-dimensional radiotherapy (2D/3DCRT). Since then, technical gains in radiotherapy techniques via computed tomography (CT)-guided intensity-modulated radiotherapy (IMRT) have revolutionized the capacity for normal tissue sparing to such a degree that assessing data from the pre-IMRT era would be considered anachronistic, given published data demonstrating definitively the reduced toxicity and improved quality of life outcomes achieved with IMRT.(8–13) Similarly, the improved survival outcomes provided by IMRT(14) suggest that grossly grouping 2D/3DCRT and IMRT in studying oncologic and functional outcomes provides a limited, if not specious, risk estimation in modern-day patients.
Given these considerations, the purpose of the present study was to use a robust dataset to investigate survival, disease control, and functional outcomes with modern larynx preservation approaches. We used our institutional historic radiotherapy-alone and total laryngectomy with post-operative radiotherapy (TL-PORT) cohorts as comparators. We evaluated oncologic and functional outcomes for T3 larynx cancer patients treated at our institution in the previous three decades and compared outcomes of chemoradiotherapy larynx preservation cohorts with those of historical surgical and non-surgical cohorts.
MATERIALS AND METHODS
This retrospective chart review was approved by The University of Texas MD Anderson Cancer Center’s (MDACC) Institutional Review Board. Sequential patients with locally advanced squamous cell carcinoma of the larynx who received radiotherapy with curative intent at MDACC between January 1, 1985, and December 31, 2011, were identified from an institutional registry. The paper and electronic records of all patients whose disease met the criteria for T3N0-3M0 squamous cell cancer of the larynx (per the 7th [2010] edition of the American Joint Committee on Cancer [AJCC] staging manual) were reviewed. Patients with distant metastasis at initial presentation and patients treated for salvage or palliative intents were excluded. Patient demographics, Eastern Cooperative Oncology Group (ECOG) performance status scores, tumor data (pathologic grade and subsite of origin), and clinical TNM staging data were extracted from patient records. TNM staging was manually reviewed and revised as necessary according to the 7th edition of the AJCC staging manual (e.g., after the AJCC 6th edition partial cartilage involvement is considered T3 disease whereas previous editions considered it as T4). Baseline pretreatment comorbidity data were collected and comorbidity score index was calculated for each case as described by Charlson et al.(15) All extracted information was recorded in a database (SPSS, IBM Inc., Chicago, IL). Staging findings from computed tomography (CT) and/or positron emission tomography (PET)-CT were recorded, as were pathologic data from patients who had had surgery as primary treatment; chemotherapy regimen(s) and their sequence with radiotherapy (induction, concurrent, or both); and radiotherapy dose, fractionation, technique, beam energy, and delivery technique. Biologically equivalent dose (BED) was calculated using the simple BED equation without correction for repopulation.(16) Disease recurrence was coded as local recurrence (i.e., occurring in the treated primary site), locoregional recurrence (i.e., occurring in the treated primary site or treated lymph nodes), or distant metastases (i.e., squamous carcinomas occurring outside the treated head and neck). Because records of distant metastases and second primary squamous carcinomas could not be reliably separated, these pathologies were grouped together as metastatic events in the current analysis. Cause of death was manually coded; patients with active cancer at the time of death were coded as having “cancer-related death,” whereas patients without active disease at last follow-up were coded as having “non-cancer death.”
Survival Endpoints
The Kaplan-Meier product limit method was used to calculate, from the date of diagnosis, rates of 5- and 10-year overall survival (OS; defined as death from any cause as an event, all others censored), local control (LC; defined as time without local recurrence, with local recurrence or cancer in the larynx coded as an event and all others censored), locoregional control (LRC; defined as time without locoregional disease, with local recurrence or cancer in the treated fields coded as an event and all others censored), freedom from distant disease (FDD; defined as time without disease outside the therapy field, with any cancer occurring outside the treated fields coded as an event and all others censored), recurrence-free survival (RFS; defined as time without any recurrence, censoring all others, including deaths), cancer event–free survival (EFS; recurrence and death coded as events, all others censored), disease–specific survival (DSS, death from disease coded as an event and censoring all others). Three composite functional/mortality endpoints were calculated for patients who had larynx preservation: actuarial freedom from laryngectomy (FFL, in which salvage laryngectomy was coded as an event, censoring all others; laryngectomy-free survival (LFS, in which any death or salvage total laryngectomy were coded as events, censoring all others; and laryngoesophageal dysfunction (LED)-free survival (LEDFS, in which any death, local relapse, salvage total laryngectomy, tracheotomy and/or feeding tube placement/persistence recorded after 2 years was coded as an event, censoring all others. Non–cancer cause–specific survival (NCCSS, in which all deaths recorded in patients without active cancer at last follow-up were coded as events, and all others censored) was used as a crude estimator of non-cancer mortality events.
Statistical Analysis
Competing risk survival and hazard calculation were performed with Weibull parametric fitting using cause of death as a competing risk variable for uncensored data. For actuarial comparisons, the log-rank test was used. Univariate and multivariate Cox proportional hazards assessments were performed to determine whether the following variables were correlated with disease control and/or survival endpoints: ECOG performance status score at treatment (0–4), age (as a continuous variable), nodal positivity (as a binary variable, N0 vs. N+), sex (male or female), histologic grade (low, intermediate, or high), Charlson comorbidity index (as a continuous variable), chemotherapy cohort (radiotherapy alone, induction/concurrent chemoradiotherapy), surgery cohort (larynx preservation vs. post-laryngectomy radiotherapy), and radiotherapy dose (as a continuous variable). After initial assessment, a second simplified multivariate model specifying dichotomized binary parameters was constructed for the following variables, using the second listed cohort as a comparator: ECOG performance status score (0 or 1 vs. 2–4), age (<60 vs. ≥60 years), nodal positivity (N0 vs. N+), surgery cohort (larynx preservation vs. post-laryngectomy radiotherapy), sex (female vs. male), and chemotherapy cohort (induction/concurrent chemoradiotherapy vs. radiotherapy alone). The simplified multivariate model was used to investigate all actuarial and survival endpoints using a 2-degree full factorial proportional hazards calculation, and the resultant calculated hazard ratio (HR) confidence intervals (CIs) were visually plotted as compared to the reference cohort.
Furthermore, product limit survival differences were interrogated for listed outcomes as a function of binary N category (i.e., N0 vs. N+). At our facility, for T3 larynx cancer patients who were candidates for organ preservation, chemoradiotherapy and IMRT were consistently implemented together, as the use of IMRT occurred solely in the era in which larynx preservation strategies involved chemoradiotherapy. Owing to changing institutional treatment paradigms, patients were categorized into three broad therapy cohorts for Kaplan-Meier analysis: Total laryngectomy followed by post-operative radiotherapy (TL-PORT), larynx preservation with definitive radiotherapy alone (LP-RT), and larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT). Univariate proportional hazards assessment was performed for all calculated endpoints stratified by therapy cohort.
A post hoc assessment using univariate Cox proportional hazards for all calculated endpoints stratified by radiotherapy technique, chemotherapy use for larynx preservation patients, and by treatment decade (80’s, 90’s, and 2000’s) was performed to assess the impact of IMRT or conventional radiotherapy, different chemotherapy–radiotherapy sequences used in larynx preservation, and different era of treatment on disease control and survival. Data were analyzed using JMP 11.2 Pro statistical software (SAS Institute, Cary, NC), and statistical significance was determined using a specified non–Bonferroni-corrected α of 0.05.
RESULTS
Patient and Treatment Characteristics
A total of 417 sequential patients with previously untreated T3 larynx squamous cell carcinoma presented to our facility during the study period. Five patients had incomplete records and were excluded, leaving 412 patients for analysis. The patients’ median age at diagnosis was 61 years (range, 30–88 years), and 287 patients (70%) were men. The overwhelming majority of tumors were of supraglottic origin (73%). Of the 287 patients (70%) who were treated using a larynx preservation approach, 166 (58%) received induction (n=53), concurrent (n=87), or both induction and concurrent (n=26) chemotherapy. Of the 125 patients (30%) who underwent TL-PORT, 113 (90%) received radiotherapy alone following total laryngectomy, and 12 (10%) received induction (n=7) or concurrent (n=5) chemotherapy. Patients’ demographic data and treatment characteristics are given in Table 1.
TABLE 1.
Patient, Disease, and Treatment Characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Female | 125 (30) |
| Male | 287 (70) |
| Ethnicity | |
| Asian/Pacific Islander | 52 (13) |
| Black/African-American | 3 (1) |
| Hispanic/Latino | 43 (10) |
| Other/unspecified | 9 (2) |
| White | 305 (74) |
| Initial disease site | |
| Supraglottic | 299 (73) |
| Glottic | 63 (15) |
| Subglottic | 1 (<1) |
| Transglottic | 49 (12) |
| Nodal classification | |
| N0 | 198 (48) |
| Nx | 2 (<1) |
| N1 | 58 (14) |
| N2a | 16 (4) |
| N2b | 52 (13) |
| N2c | 60 (15) |
| N3 | 26 (6) |
| Pathologic grade | |
| Well differentiated | 31 (8) |
| Moderately differentiated | 213 (52) |
| Poorly differentiated | 106 (26) |
| Unknown/unspecified | 62 (15) |
| Smoking history at diagnosis | |
| None | 16 (4) |
| Unknown/unspecified | 7 (2) |
| Positive | 389 (94) |
| Radiotherapy technique | |
| 2D/3DCRT | 343 (83) |
| IMRT | 69 (17) |
| Radiation beam energy | |
| 6 MV | 181 (43) |
| 60Co | 231 (57) |
| Mean total radiation dose ± SD, Gy | 68.7 ± 6.5 |
| Mean no. of fractions received | 42 ± 14 |
| Fractionation schedule | |
| Conventional | 262 (64) |
| Altered | 107 (26) |
| Twice daily | 43 (10) |
| Concomitant boost | |
| Radiation aim | |
| Definitive | 287 (70) |
| Postoperative | 125 (30) |
| Chemotherapy | |
| Induction + concurrent | 26 (6) |
| Concurrent | 92 (22) |
| Induction | 60 (15) |
| None | 234 (57) |
Note: All data are no. of patients (%) unless otherwise indicated.
Survival Endpoint Analysis
The median follow-up duration was 57 months (range, 4–321 months) for all patients and 80 months (range, 6–307 months) for patients alive at last contact. The median OS duration was 73 months (95% CI, 61–88 months). The 5- and 10-year OS rates were 56% and 35%, respectively; the 5- and 10-year DSS rates were 69% and 61%, respectively; the actuarial 5- and 10-year LC probabilities were 84% and 82%, respectively; the 5- and 10-year LRC rates were 78% and 76%, respectively; and the 5- and 10-year FDD rates were 84% and 83%, respectively (Figure 1).
Figure 1.
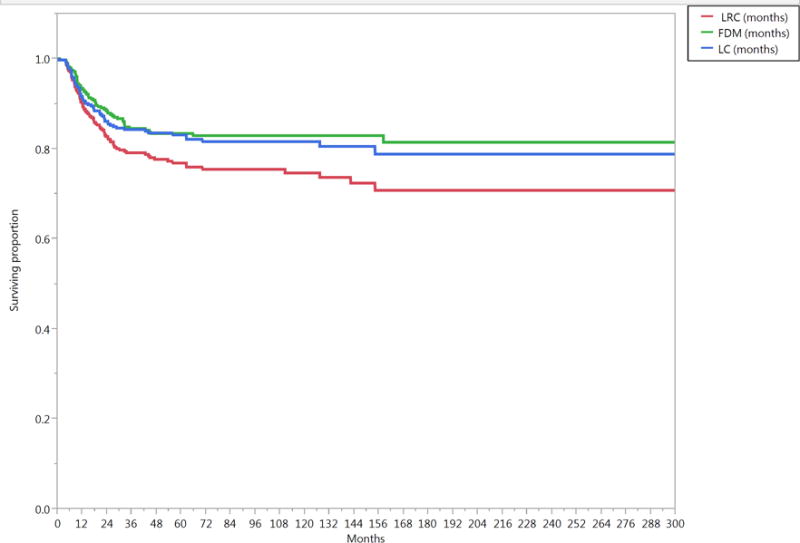
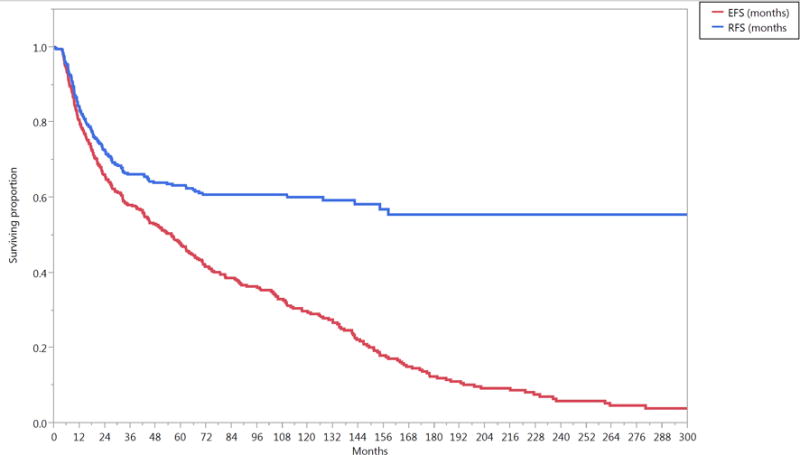
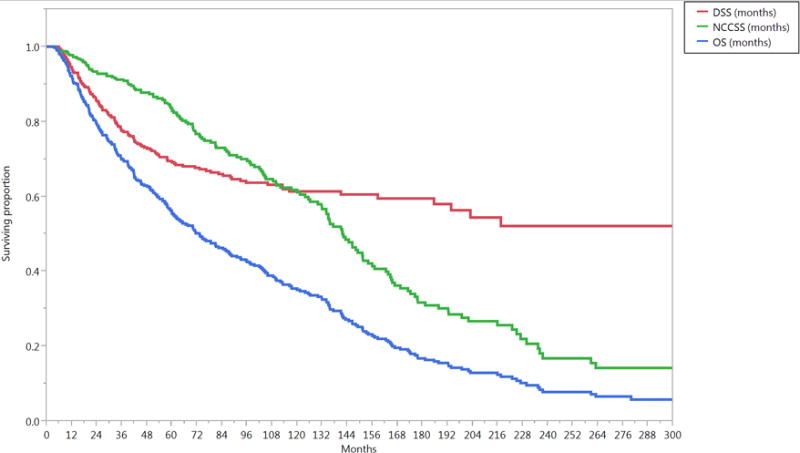
Kaplan-Meier curves calculated for all patients (n=412) showing (A) local control (LC), locoregional control (LRC), freedom from distant disease (FDD), (B) relapse free survival (RFS), cancer-event free survival (EFS), and (C) disease specific survival (DSS), non-cancer cause specific survival (NCCSS), and Overall survival (OS).
Assessment of competing causes of survival for all patients revealed the late predominance of non-cancer mortality, with non-cancer-related death predominating approximately 8 years after diagnosis (Supplementary Figure S1).
Correlates of Survival
Univariate analysis revealed that chemotherapy use (HR, 0.72; P=0.006), younger age (P=0.0001), better ECOG performance status (P=0.03), lower comorbidity index (P=0.03), node-negative status (HR, 0.75; P=0.01), and glottic sub-site (HR, 0.54; P=0.0002) were associated with improved OS. Surgery, sex, pathologic grade, and radiotherapy dose were not associated with OS. Multivariate analysis revealed that chemotherapy use (HR, 0.66; P=0.003), age <60 years (HR, 0.48; P=0.0001), ECOG performance status <2 (HR, 0.57; P=0.03), node-negative status (HR, 0.69; P=0.002), and glottic sub-site (HR, 0.54; P=0.0003) were independently associated with better OS. Simplified cohort full-factorial multivariate Cox proportional hazards model results, including intra-variable risk ratio significance and magnitude for all endpoints listed, are given in Figure 2.
Figure 2.
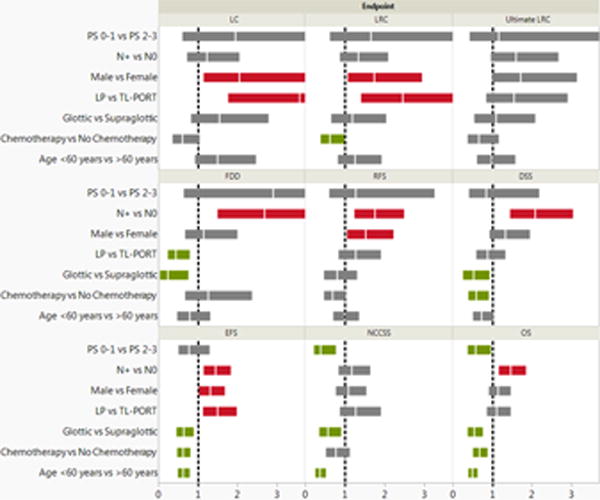
Results of a multivariate analysis for all survival endpoints. The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines); red boxplots represent increased risk of endpoint occurrence, green boxplots indicate increased reduced risk and grey boxplots indicate no statistically significant difference. Abbreviations: Local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant disease (FDD), relapse-free survival (RFS), disease-specific survival (DSS), cancer event–free survival (EFS), non–cancer cause–specific survival (NCCSS), and overall survival (OS).
Therapy Cohorts
The median follow-up times of the LP-CRT cohort (n=166; 60 months), LP-RT cohort (n=121; 53 months), and the TL-PORT cohort (n=125; 56 months) did not differ significantly (P=0.7). Charlson’s comorbidity index was slightly higher in LP-RT cohort (1±1.4) compared to LP-CRT (0.7±1) and TL-PORT (0.7±1) cohorts. The vast majority of patients in the three cohorts completed the prescribed radiation course within the planned treatment duration. Only 3 patients in LP-RT, 6 in LP-CRT, and 9 in TL-PORT cohort had more than 3 days of delay during radiation course. The details of demographics and disease characteristics in each therapy cohort is provided in supplementary table 1.
Log-rank assessment of product limit survival revealed that the median survival duration of the LP-CRT cohort (102 months [95% CI, 79–126 months]) was significantly higher than those of the TL-PORT cohort (61 months [95% CI, 42–82 months]) and LP-RT cohort (53 months [95% CI, 43–73 months]; P=0.006). The 5- and 10-year OS rates of the LP-CRT cohort (67% and 43%, respectively) were higher than those of the TL-PORT cohort (50% and 35%, respectively), which were higher than those of the LP-RT cohort (46% and 25%, respectively).
Pairwise comparison of the 10-year LRC showed that TL-PORT cohort was statistically better than the LP-RT cohort (83% vs 64%; P=0.003) but only numerically better than the LP-CRT cohort (83% vs 77%; P=0.2). Ultimately, 66% (n=23) and 58% (n=22) of patients with local and/or regional failure in the LP-CRT and LP-RT cohorts, respectively, underwent a subsequent salvage surgery following the initial recurrence, that maintained the ultimate LRC (i.e., no second locoregional relapse) in 48% (n=11) and 50% (n=11) of salvaged patients in the LP-CRT and LP-RT cohorts, respectively. Consequently, the 10-year ultimate LRC rates of the TL-PORT, LP-CRT, and LP-RT cohorts (83%, 84%, and 74%, respectively) were not significantly different.
The 10-year FDD rate of the TL-PORT cohort (71%) was significantly lower than those of the LP-RT cohort (90%) and LP-CRT cohort (87%; P=0.0005). DSS was superior in LP-CRT cohort (79% at 5-year and 73% at 10-year) compared to both TL-PORT (61% at 5-year and 53% at 10-year) and LP-RT (64% at 5-year and 56% at 10-year) cohorts (p=0.002). All disease control and survival endpoints for the three therapy cohorts are presented in Figure 3 while hazard ratios and confidence intervals are presented in Figure 4.
Figure 3.
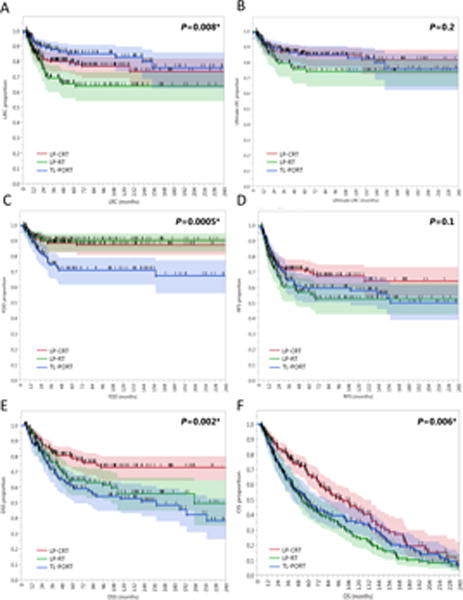
Kaplan-Meier curves calculated for all patients (n=412) showing (A) locoregional control (LRC), (B) ultimate LRC, (C) freedom from distant disease (FDD), (D) relapse-free survival (RFS), (E) disease-specific survival (DSS), and (F) overall survival (OS) in patients who underwent larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT) versus larynx preservation with definitive radiotherapy alone (LP-RT) versus total laryngectomy and post-operative radiotherapy (TL-PORT). Shaded colors represent 95% confidence intervals, short vertical lines represent censored data, and asterisk indicates significant log-rank p values.
Figure 4.
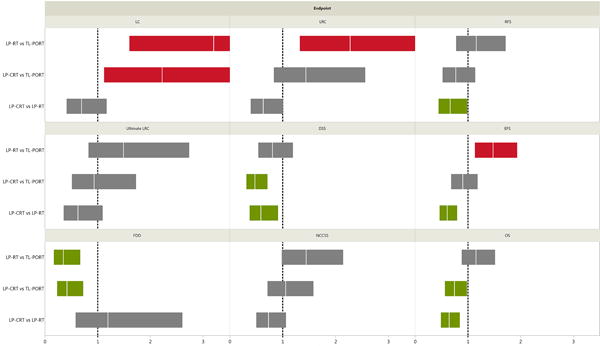
Results of a multivariate Cox model for survival endpoints for cohorts of patients with T3 larynx cancer who underwent total laryngectomy followed by post-operative radiotherapy (TL-PORT), larynx preservation with definitive radiotherapy alone (LP-RT), or larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT). The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines); red boxplots represent increased probability of endpoint occurrence, whereas green boxplots indicate reduced risk of endpoint occurrence. Overall survival (OS), local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant disease (FDD), recurrence-free survival (RFS), cancer event–free survival (EFS), disease-specific survival (DSS), and non–cancer cause–specific survival (NCCSS) HRs were assessed for all patients (n=412).
Assessment of competing risks of failure and Weibull competing causes of failure revealed that the primary pattern of failure in the TL-PORT cohort was distant failure; the Weibull-estimated 10-year cumulative incidences of distant failure and locoregional failure were 32% and 18%, respectively. However, the primary pattern of failure in the LP-RT cohort was locoregional failure, which had a 10-year cumulative incidence of 40%, compared to a 20% for distant failure at the same time. In the LP-CRT cohort, the risks of locoregional and distant failure were similar, with a 10-year cumulative incidence of 23% for locoregional failure and 19% for distant failure (Figure 5a–c). Assessment of competing risks of survival revealed a relatively prolonged cancer-specific cause of death in the TL-PORT cohort for approximately 14 years post-diagnosis compared with the LP-CRT (~6 years) and LP-RT cohorts (~5.5 years, Figure 5d–f).
Figure 5.
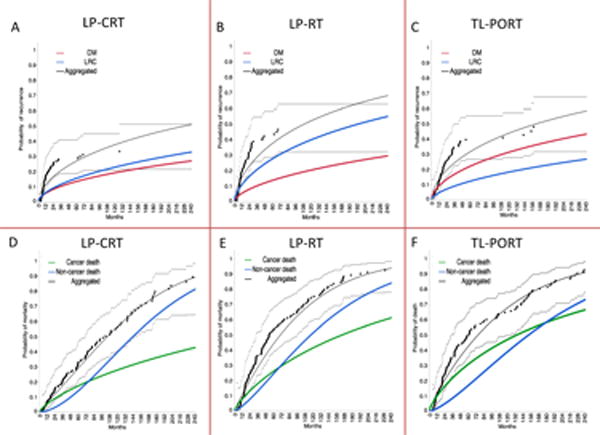
Competing risk analysis for patients with T3 larynx cancer who underwent (a,d) larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT), (b,e) larynx preservation with definitive radiotherapy alone (LP-RT), or (c,f) total laryngectomy followed by post-operative radiotherapy (TL-PORT).Patterns of failure are presented in the upper panels (a, b, and c); patterns of competing risk of demise by cause are presented in the lower panels (d, e, and f). Black dots represent aggregated data points and dashed lines represent confidence intervals.
Composite Endpoints and Functional Outcomes
Compared with patients treated with an LP-RT approach (n=121), patients treated with an LP-CRT approach (n=166) had better LFS (HR, 0.62; 95% CI, 0.47–0.81; P=0.0005) and LEDFS (HR, 0.63; 95% CI, 0.49–0.82; P=0.0006). The 5- and 10-year LFS rates of the LP-CRT cohort (62% [95% CI, 54–69%] and 37% [95% CI, 28–46%], respectively) were higher than those of the LP-RT cohort (38% [95% CI, 29–47%] and 18% [95% CI, 12–25%], respectively). Similarly, the 5- and 10-year LEDFS rates of the LP-CRT cohort (59% [95% CI, 51–66%] and 35% [95%, CI 26–44%], respectively) were higher than those of the LP-RT cohort (37% [95% CI, 29–46%] and 18% [95% CI, 12–25%], respectively). The FFL of the LP-CRT cohort was higher than that of the LP-RT cohort, but this difference was not statistically significant (HR, 0.71; 95% CI, 0.4–1.3; P=0.3). All composite endpoints are presented in Figure 6.
Figure 6.
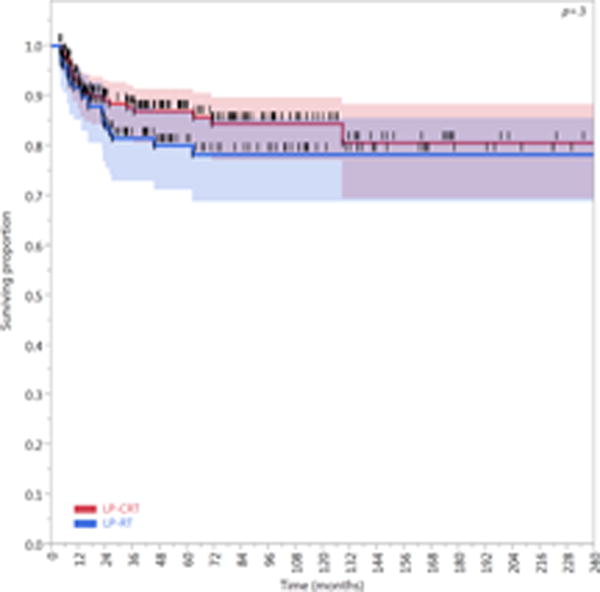
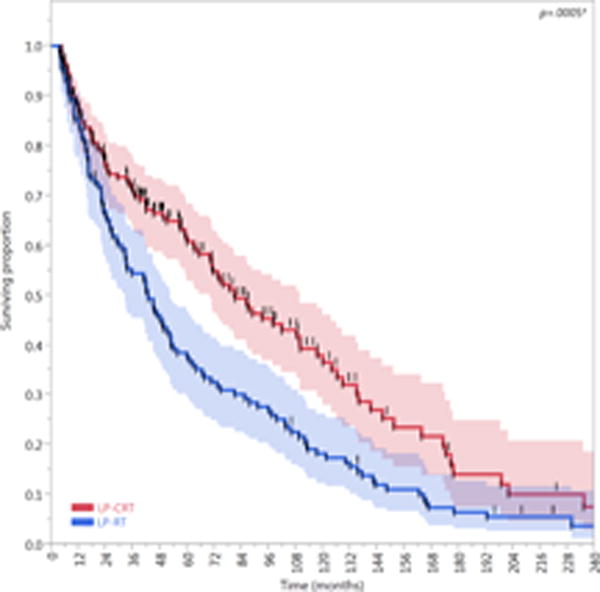
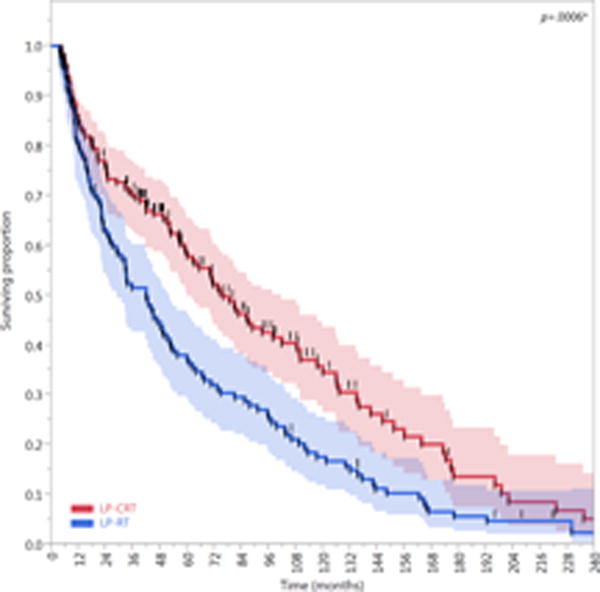
Kaplan-Meier curves calculated for patients treated with larynx preservation approaches (n=287) showing (A) Freedom from laryngectomy (FFL), (B) laryngectomy-free survival (LFS), and (C) laryngoesophageal dysfunction–free survival (LEDFS) in patients who underwent larynx preservation with induction and/or concurrent chemoradiotherapy (LP-CRT) versus larynx preservation with definitive radiotherapy alone (LP-RT). Shaded colors represent 95% confidence intervals, short vertical lines represent censored data, and asterisk indicates significant log-rank p values.
At last follow up, the LP-CRT cohort had a tracheostomy rate of 20%, any-event aspiration rate of 22%, and a feeding tube rate of 15%, and the LP-RT cohort had a tracheostomy rate of 23%, any-event aspiration rate of 15%, and a feeding tube rate of 12%. Although the TL-PORT cohort had a permanent tracheostomy rate of 100%, it also had few aspiration events in 9% of patients caused by leakage around the voice prosthesis. Feeding tube rate was 10% in the TL-PORT cohort which was not significantly different from those of the larynx preservation cohorts (P=0.48). The long-term functional outcomes for the three therapy cohorts are shown in Table 2.
TABLE 2.
Late Functional Outcomes by Therapy Cohort
| Functional Outcome | Therapy Cohort
|
||
|---|---|---|---|
| LP-CRT (n=166) No. of event/No. at risk (%) |
LP-RT (n=121) No. of event/No. at risk (%) |
TL-PORT (n=125) No. of event/No. at risk (%) |
|
| PEG/DHT Tube | |||
| At 6 months | |||
| No | 124/164 (76) | 100/119 (84) | 110/123 (90) |
| Yes | 29/164 (18) | 11/119 (9) | 10/123 (8) |
| Unspecified | 11/164 (6) | 8/119 (7) | 3/123 (2) |
| At 12 months | |||
| No | 125/154 (81) | 103/113 (91) | 107/113 (95) |
| Yes | 13/154 (9) | 8/113 (7) | 6/113 (5) |
| Unspecified | 16/154 (10) | 2/113 (2) | 0/113 (0) |
| At last contact | |||
| No | 133/166 (80) | 99/121 (82) | 103/125 (82) |
| Yes | 25/166 (15) | 15/121 (12) | 13/125 (10) |
| Unspecified | 8/166 (5) | 7/121 (6) | 9/125 (8) |
| Aspiration* | |||
| No | 104/166 (63) | 87/121 (72) | 102/125 (82) |
| Yes | 37/166 (22) | 18/121 (15) | 11/125 (9)£ |
| Unspecified | 25/166 (15) | 16/121 (13) | 12/125 (9) |
| Tracheostomy | |||
| At baseline | |||
| No | 138/166 (83) | 95/121 (79) | 6/125 (5) |
| Yes | 28/166 (17) | 26/121 (21) | 97/125 (78) |
| Unspecified | 0/166 (0) | 0/121 (0) | 22/125 (17) |
| At 6 months | |||
| No | 138/164 (84) | 95/119 (80) | 0/123 (0) |
| Yes | 18/164 (11) | 20/119 (17) | 123/123 (100) |
| Unspecified | 8/164 (5) | 4/119 (3) | 0/123 (0) |
| At 12 months | |||
| No | 92/154 (60) | 31/113 (27) | 0/113 (0) |
| Yes | 19/154 (12) | 15/113 (13) | 113/113 (100) |
| Unspecified | 43/154 (28) | 67/113 (60) | 0/113 (0) |
| At last contact | |||
| No | 109/166 (66) | 42/121 (35) | 0/125 (0) |
| Yes | 33/166 (20) | 28/121 (23) | 125/125 (100) |
| Unspecified | 24/166 (14) | 51/121 (42) | 0/125 (0) |
Note: All data are no. of event/no. at risk (%).
Aspiration is recorded as “any-event” aspiration during the post-treatment follow-up, £ Aspiration in TL-PORT is caused by leak around the voice prosthesis.
Abbreviations: DHT, Dobhoff tube; PEG, percutaneous endoscopic gastrostomy tube.
IMRT vs 2D/3DCRT
Patients treated with larynx preservation approaches (n=287) were classified by radiotherapy technique to an IMRT cohort (n=61) or a 2D/3DCRT cohort (n=226). Compared with those in the 2D/3DCRT cohort, patients in the IMRT cohort had better LRC, LFS, RFS, and EFS. Nonetheless, the median OS duration of the IMRT cohort (102 months [95% CI, 64–102 months]) was not significantly different from that of the 2D/3DCRT cohort (73 months [95% CI, 59–93 months]; P=0.18; Figure 7). The HRs for all endpoints for the IMRT cohort vs. the 2D/3DCRT cohort are shown in Supplementary Figure S2.
Figure 7.
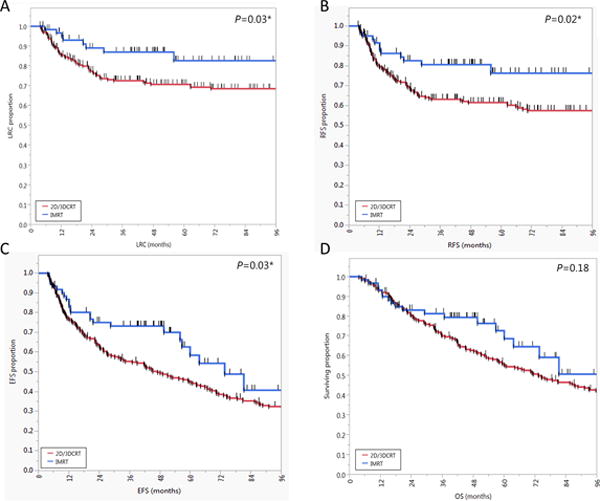
Kaplan-Meier plots of actuarial survival endpoints, including (a) locoregional control (LRC), (b) relapse-free survival (RFS), (c) event-free survival (EFS), and (d) overall survival (OS), for patients who underwent 2- or 3-dimensional conventional radiotherapy (2D/3DCRT) or intensity-modulated radiotherapy (IMRT) for larynx preservation. Short vertical lines represent censored data, and asterisk indicates significant log-rank p values.
Chemotherapy
Patients who received concurrent chemoradiotherapy either after induction chemotherapy (n=26) or upfront (n=87) had better LRC and RFS than did patients who received induction chemotherapy followed by radiotherapy alone (n=53) or no chemotherapy (n=121; P=0.02 for both LRC and RFS; Figure 8a,b). The three cohorts that received chemotherapy had better OS than the cohort that didn’t receive chemotherapy (P=0.0006; Figure 8c). The results of a univariate Cox proportional hazards model for chemotherapy use in larynx preservation patients are shown in Supplementary Figure S3.
Figure 8.
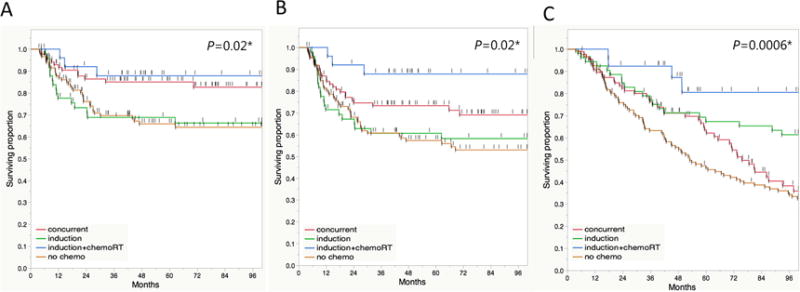
Kaplan-Meier plots of (a) Locoregional control (LRC), (b) recurrence-free survival (RFS), and (c) overall survival (OS) in different chemotherapy cohorts treated using larynx preservation approaches (n=287). Short vertical lines represent censored data, and asterisk indicates significant log-rank p values.
Treatment decade
Changes in the selection of alternative treatment options for T3 larynx cancer patients in different decades elucidate the shifts in the preferred strategy over time where TL-PORT was the most common in 80’s (61%, n=53), LP-RT was the most common in 90’s (43%, n=83), while LP-CRT was the most common in the new millennium (76%, n=101); Supplementary table 1 shows the distribution of treatment strategies over different decades. The 5-year OS rates were 46%, 54%, and 65% for patients treated in 80’s, 90’s, and 2000’s, respectively (P=0.1, Supplementary Figure S4). Patients treated in 2000’s had a non-significant trend of decreased mortality hazard compared to 80’s (HR, 0.75; P=0.09), and 90’s (HR, 0.9; P=0.6).
DISCUSSION
In this large-scale retrospective study we found that age <60yrs, better PS, lower comorbidity index, node negative category, glottic sub-site and patients who received chemotherapy were all associated with improved overall survival for T3 larynx cancer. The ultimate LRC for patients treated using LP-CRT was equivalent to those treated using TL-PORT, and better than those treated using LP-RT. In addition, LP-CRT patients had better long-term survival outcomes with acceptable functional outcomes represented as 59% rate of laryngoesophageal dysfunction free survival (i.e. alive with fully functional larynx) at 5-year follow up. IMRT achieved better tumor control and DSS in patients who had larynx preservation than conventional radiotherapy did. Furthermore, patients who received induction and/or concurrent chemotherapy for larynx preservation had better tumor control and long-term survival than patients who received no chemotherapy. Intriguingly, the outcomes of patients who had induction chemotherapy followed by concurrent chemoradiotherapy appeared to be better than those of patients who had upfront concurrent chemoradiotherapy or induction chemotherapy followed by radiotherapy alone.
Larynx preservation with chemoradiotherapy became an alternative to total laryngectomy following the landmark VA and RTOG trials(1, 2) in the 1980’s and 1990’s but only came of age with the dissemination of these trials’ data in the mid 1990’s and early 2000’s.(17) Simultaneously, advances in radiotherapy were made, and IMRT, with its unmatched conformality and lower toxicity, demonstrably improved survival outcomes in head and neck cancer patients.(14) Consequently, the current standard of care for patients with T3 larynx cancers who have good laryngeal and swallowing function is laryngeal preservation surgery or chemoradiotherapy with IMRT.
Several reports have implied that non-surgical therapy conveys a decrement in survival and tumor control outcomes.(3–7) For example, Hoffman et al., using a robust National Cancer Data Base, reported that increased use of non-surgical approaches for larynx preservation was concurrent with decreased relative survival in patients with T3 larynx cancers.(3) However, because Hoffman et al.(3) culled data from between 1985 and 2001, before the implementation of IMRT and the publication of RTOG 9001 and the more widespread use of induction and concurrent chemotherapy, their findings, although timely when published, do not accurately reflect the use of radiotherapy and combined modality techniques in the current era. Our findings agree with the notion that radiation without chemotherapy is no panacea. Although IMRT can reduce the radiation dose to swallowing structures,(18, 19) conceivably leading to improved functional outcomes, optimal larynx preservation appears to also have a formal chemotherapy component. In both the present study and the study by Hoffman et al.(3), outcomes achieved with radiotherapy alone were worse than those achieved with other therapy regimens. In contrary to the results previously reported by Dziegielewski et al. and Chen et al.(5, 6), our results showed that LP-CRT cohort was associated with lower mortality risk compared to TL-PORT (HR, 0.74; P=0.03, Fig. 5).
The outcomes of treatment of the subset of T3 larynx cancer with N0 disease reported by Megwalu et al.,(7) showed inferior 5-year OS (48% vs. 59%) and DSS (63% vs. 69%) for larynx preservation compared to surgical cohort. However, the discrimination between IMRT and conventional radiotherapy is not possible, and dichotmomizing radiotherapy alone and chemoradiotherapy for larynx preservation cannot be performed in this SEER database. For this reason, the differences between surgical and non-surgical cohorts potentially obscure radical shifts in therapy that occurred during the long period sampled (1973–2009). For instance, a patient whose disease was staged using physical examination alone and who was treated with opposed lateral fields on a Cobalt-60 unit in 1973 and a patient whose disease was staged using thin-slice angled-gantry CT for local staging and PET-CT for nodal assessment and who was treated with induction chemotherapy followed by concurrent chemoradiotherapy with daily image-guided IMRT in 2009 could both be included in the same “non-surgical” cohort, but this type of classification would be unlikely to provide insight into the specifics of modern management. In our series, T3N0M0 subset treated with “non-surgical” approaches did no worse in terms of overall survival, with no observed statistical difference between radiotherapy alone or chemoradiation and surgical patients. However, post hoc assessment showed a demonstrable difference (P=.03, Supplementary figure S5) between radiotherapy alone and chemoradiation cohorts favoring chemoradiation.
In the present study, we attempted to broadly ascertain whether the chemotherapy component of treatment (induction chemotherapy followed by radiotherapy, concurrent chemoradiotherapy, or induction chemotherapy followed by concurrent chemoradiotherapy) had distinct outcome profiles. Our findings suggest that the outcome profiles are substantially different, with induction chemotherapy followed by chemoradiotherapy eliciting better survival outcomes. Our long-term data suggest that the different chemotherapy approaches are associated with distinct patterns of failure, with induction chemotherapy primarily reducing distant failure and concurrent chemoradiotherapy improving locoregional control. Interestingly, our analysis of non–cancer-related death, using NCCSS as an endpoint, revealed that concurrent chemoradiotherapy resulted in a decrease in non-cancer survival, although a similar decrease was not observed in patients receiving induction chemotherapy followed by concurrent chemoradiotherapy (Supplementary Figure S3).
Data from our facility, as well as those from the VA and RTOG 91-11 trials, suggest that larynx preservation approaches elicit exceedingly poor functional and comparatively worse oncologic outcomes than TL-PORT does in patients with T4 cancers with demonstrable cartilage penetration.(20) On the basis of our experience, we believe that in general, patients with T3 larynx cancer who are candidates for larynx preservation are more likely to benefit from chemotherapy and IMRT, whereas most T4 patients require upfront TL-PORT. In addition, we eschew larynx preservation in both T3 and T4 larynx cancer patients who, on pretreatment barium swallow, have poor baseline airway functions such as demonstrable aspiration to a degree that suggests that airway protection following therapy is not possible. For this reason, careful multidisciplinary evaluation, including direct pre-therapy assessment by medical oncology, head and neck surgery, radiation oncology, and experienced speech pathology personnel, is the cornerstone of our process for selecting patient-specific therapy.
The present study is not without limitations. The typical caveats of any retrospective analysis apply (e.g. selection bias for induction/concurrent chemotherapy, unequal distribution of N-stage in the three cohorts, or increased disposition for surgery in previously tracheostomized patients). While performance status, age, and sex were well-balanced across cohorts, the nature of our patient-specific multi-disciplinary conferences to drive longitudinal care practices is potentially an occult confounder, as our informal consensus has shifted over the decades with regard to therapeutic selection. On the basis of early work from Maccomb et al.(21), Jesse(22), and Terhaard et al.(23), surgery alone has not been a routinely used therapy for T3 larynx cancer at our institution since the mid-1970s. Therefore, because the earliest records we reviewed in the present study dated to only the mid-1980s, we could not investigate this surgery-alone cohort to determine how its outcomes compared with those of the archaic radiotherapy-alone cohorts. Other unidentified confounders, such as technical progress in CT-based imaging, refined surgical approaches, improved speech pathology practices, and demographic and staging shifts likely inflated the role of modern chemotherapy/IMRT in improving survival outcomes. Finally, we couldn’t investigate the role of larynx conservation surgery (LCS) versus the non-surgical approaches because LCS is not considered the standard surgical approach for T3 disease in our institution.
Despite its potential limitations, our study represents, to our knowledge, the largest single-site retrospective series of T3 larynx cancer patients in the modern era. Furthermore, because our study analyzed multiple relevant oncologic, functional, and survival endpoints simultaneously and was sufficiently numerically robust to detect significant between-cohort differences, our study’s findings can be used to guide future prospective studies, such as those evaluating the use of induction or concurrent chemoradiotherapy for larynx preservation in patients with T3 larynx cancer. We aim to implement this data for advanced risk-stratification in future studies investigating novel chemotherapy agents or radiotherapy techniques. To conclude, in patients with T3 larynx cancers, modern larynx preservation regimens employing both chemotherapy and radiotherapy, in addition to providing satisfactory long-term functional outcomes, resulted in comparatively better oncologic outcomes than historical approaches utilizing laryngectomy plus adjuvant radiotherapy or radiotherapy alone. Such modern approaches should be considered the preferred standard of care for both therapy selection and future outcome assessment.
Supplementary Material
Supplementary Figure S1. Weibull plot of competing risks of all-cause mortality, cancer-associated mortality, and non-cancer-associated mortality over time. It shows the late predominance of non-cancer-associated mortality approximately 8 years after diagnosis.
Supplementary Figure S2. Results of simplified univariate Cox model for radiotherapy technique (2- or 3-dimensional radiotherapy [2D/3DCRT] or intensity-modulated radiotherapy [IMRT]) in patients who had larynx preservation. The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines; the green boxplots indicate statistically significant risk reduction). Overall survival (OS), local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant disease (FDD), recurrence-free survival (RFS), cancer event–free survival (EFS), disease-specific survival (DSS), laryngoesophageal dysfunction–free survival (LEDFS), laryngectomy-free survival (LFS), actuarial freedom from laryngectomy (FFL), and non–cancer cause–specific survival (NCCSS) HRs were assessed for all patients who had larynx preservation (n=287). Multivariate analysis of revealed that collinearity between IMRT and chemotherapy cohorts precluded the differentiation of distinct effects on outcomes (i.e., because almost all IMRT patients received chemotherapy, no distinction between the effects of chemotherapy and those of IMRT on outcome were identifiable).
Supplementary Figure S3. Results of simplified univariate Cox model of chemotherapy use in patients who underwent larynx preservation. The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if the a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines); the green boxplots indicate risk reduction. Overall survival (OS), local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant metastasis (FDM), recurrence-free survival (RFS), cancer event–free survival (EFS), disease-specific survival (DSS), and non–cancer cause–specific survival (NCCSS) HRs were assessed for all patients (n=287).
Supplementary Figure S4. Kaplan-Meier plots of OS by treatment decade. Short vertical lines represent censored data.
Supplementary Figure S5. Kaplan-Meier plots of OS for T3N0M0 subset. Short vertical lines represent censored data, and asterisk indicates significant log-rank p values.
Acknowledgments
We thank Nebil Ark, MD, and Cidney Hulett, MD, MPH, for their assistance with data collection.
Funding sources and financial disclosures: This work was supported in part by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. Dr. Fuller receives grant support from the National Institutes of Health/National Cancer Institute’s Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant: The Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. Dr. Mohamed receives salary support from the Union for International Cancer Control /American Cancer Society International Fellowships for Beginning Investigators (UICC/ACSBI) mechanism. Dr. Heukelom receives support from the Koningin Wilhemina Fonds/René Vogels Foundation grant. These entities played no role in designing the study; collecting, analyzing, or interpreting its data; writing the manuscript; or making the decision to submit the report for publication.
References
- 1.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–90. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 Pt 2 Suppl 111):1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 4.Gourin CG, Conger BT, Sheils WC, Bilodeau PA, Coleman TA, Porubsky ES. The effect of treatment on survival in patients with advanced laryngeal carcinoma. Laryngoscope. 2009;119(7):1312–7. doi: 10.1002/lary.20477. [DOI] [PubMed] [Google Scholar]
- 5.Dziegielewski PT, O’Connell DA, Klein M, et al. Primary total laryngectomy versus organ preservation for T3/T4a laryngeal cancer: a population-based analysis of survival. J Otolaryngol Head Neck Surg. 2012;41(Suppl 1):S56–64. [PubMed] [Google Scholar]
- 6.Chen AY, Halpern M. Factors predictive of survival in advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1270–6. doi: 10.1001/archotol.133.12.1270. [DOI] [PubMed] [Google Scholar]
- 7.Megwalu UC, Sikora AG. Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):855–60. doi: 10.1001/jamaoto.2014.1671. [DOI] [PubMed] [Google Scholar]
- 8.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–91. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nutting CM. Intensity Modulated Radiotherapy (IMRT) in head and neck cancers - an overview. Gulf J Oncolog. 2012;(12):17–26. [PubMed] [Google Scholar]
- 11.Al-Mamgani A, Van Rooij P, Tans L, Teguh DN, Levendag PC. Toxicity and outcome of intensity-modulated radiotherapy versus 3-dimensional conformal radiotherapy for oropharyngeal cancer: a matched-pair analysis. Technol Cancer Res Treat. 2013;12(2):123–30. doi: 10.7785/tcrt.2012.500305. [DOI] [PubMed] [Google Scholar]
- 12.Rathod S, Gupta T, Ghosh-Laskar S, Murthy V, Budrukkar A, Agarwal J. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): evidence from a prospective randomized study. Oral Oncol. 2013;49(6):634–42. doi: 10.1016/j.oraloncology.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–9. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 14.Beadle BM, Liao KP, Elting LS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer. 2014;120(5):702–10. doi: 10.1002/cncr.28372. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 83(991):554–68. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen AY, Schrag N, Hao Y, et al. Changes in treatment of advanced laryngeal cancer 1985-2001. Otolaryngol Head Neck Surg. 2006;135(6):831–7. doi: 10.1016/j.otohns.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? International Journal of Radiation Oncology Biology Physics. 2004;60(5):1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NP, Chi A, Betz M, et al. Feasibility of Intensity-Modulated and Image-Guided Radiotherapy for Functional Organ Preservation in Locally Advanced Laryngeal Cancer. Plos One. 2012;7(8) doi: 10.1371/journal.pone.0042729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal DI, Mohamed AS, Weber R, et al. Long-term Outcomes after Surgical or Nonsurgical Initial Therapy for T4 Squamous Cell Carcinoma of the Larynx: A Three Decades Survey. Cancer. 2014 doi: 10.1002/cncr.29241. Inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccomb WS, Fletcher GH. Planned combination of surgery and radiation in treatment of advanced primary head and neck cancers. Am J Roentgenol Radium Ther Nucl Med. 1957;77(3):397–414. [PubMed] [Google Scholar]
- 22.Jesse RH. The evaluation of treatment of patients with extensive squamous cancer of the vocal cords. Laryngoscope. 1975;85(9):1424–9. doi: 10.1288/00005537-197509000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Terhaard CH, Hordijk GJ, van den Broek P, et al. T3 laryngeal cancer: a retrospective study of the Dutch Head and Neck Oncology Cooperative Group: study design and general results. Clin Otolaryngol Allied Sci. 1992;17(5):393–402. doi: 10.1111/j.1365-2273.1992.tb01681.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Weibull plot of competing risks of all-cause mortality, cancer-associated mortality, and non-cancer-associated mortality over time. It shows the late predominance of non-cancer-associated mortality approximately 8 years after diagnosis.
Supplementary Figure S2. Results of simplified univariate Cox model for radiotherapy technique (2- or 3-dimensional radiotherapy [2D/3DCRT] or intensity-modulated radiotherapy [IMRT]) in patients who had larynx preservation. The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines; the green boxplots indicate statistically significant risk reduction). Overall survival (OS), local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant disease (FDD), recurrence-free survival (RFS), cancer event–free survival (EFS), disease-specific survival (DSS), laryngoesophageal dysfunction–free survival (LEDFS), laryngectomy-free survival (LFS), actuarial freedom from laryngectomy (FFL), and non–cancer cause–specific survival (NCCSS) HRs were assessed for all patients who had larynx preservation (n=287). Multivariate analysis of revealed that collinearity between IMRT and chemotherapy cohorts precluded the differentiation of distinct effects on outcomes (i.e., because almost all IMRT patients received chemotherapy, no distinction between the effects of chemotherapy and those of IMRT on outcome were identifiable).
Supplementary Figure S3. Results of simplified univariate Cox model of chemotherapy use in patients who underwent larynx preservation. The vertical white lines on the colored boxplots represent hazard ratios (HRs); the colored boxplots themselves represent 95% confidence intervals (CIs). Statistical significance is indicated if the a boxplot does not encroach upon a risk ratio of 1.0 (indicated by vertical dashed lines); the green boxplots indicate risk reduction. Overall survival (OS), local control (LC), locoregional control (LRC), ultimate LRC, freedom from distant metastasis (FDM), recurrence-free survival (RFS), cancer event–free survival (EFS), disease-specific survival (DSS), and non–cancer cause–specific survival (NCCSS) HRs were assessed for all patients (n=287).
Supplementary Figure S4. Kaplan-Meier plots of OS by treatment decade. Short vertical lines represent censored data.
Supplementary Figure S5. Kaplan-Meier plots of OS for T3N0M0 subset. Short vertical lines represent censored data, and asterisk indicates significant log-rank p values.


