Abstract
High level of homocysteine (hyperhomocysteinemia, HHcy) is associated with increased risk for vascular disease. Evidence for this emerges from epidemiological studies which show that HHcy is associated with premature peripheral, coronary artery and cerebrovascular disease independent of other risk factors. Possible mechanisms by which homocysteine causes vascular injury include endothelial injury, DNA dysfunction, proliferation of smooth muscle cells, increased oxidative stress, reduced activity of glutathione peroxidase and promoting inflammation. HHcy has been shown to cause direct damage to endothelial cells both in vitro and in vivo. Clinically, this manifests as impaired flow-mediated vasodilation and is mainly due to a reduction in nitric oxide synthesis and bioavailability. The effect of impaired nitric oxide release can in turn trigger and potentiate atherothrombogenesis and oxidative stress. Endothelial damage is a crucial aspect of atherosclerosis and precedes overt manifestation of disease. In addition, endothelial dysfunction is also associated with hypertension, diabetes, ischemia reperfusion injury and neurodegenerative diseases. Homocysteine is a precursor of hydrogen sulfide (H2S) which is formed by transulfuration process catalyzed by the enzymes, cystathionine β-synthase and cystathionine γ-lyase. H2S is a gasotransmitter that has emerged recently as a novel mediator in cardiovascular homeostasis. As a potent vasodilator, it plays several roles which include regulation of vessel diameter, protection of endothelium from redox stress, ischemia reperfusion injury and chronic inflammation. However, the precise mechanism by which it mediates these beneficial effects is complex and still remains unclear. Current evidence indicates H2S modulates cellular functions by a variety of intracellular signaling processes. In this review, we summarize the mechanisms of HHcy-induced endothelial dysfunction and the metabolism and physiological functions of H2S as a protective agent.
Keywords: Dysfunction, Endothelium, homocysteine, hydrogen sulfide
INTRODUCTION
The vascular endothelium is a single layer of dynamic cells which through a variety of stimuli produces vasoactive substances to maintain vascular tone and regulates blood flow to the tissues. Early work by Furchgott and Zawadzki showed that the presence of endothelium was vital to acetylcholine induced vasorelaxation in isolated artery preparations and this effect was attributed to a substance(s) released by the endothelium [1] which was subsequently identified as nitric oxide (NO) [2]. The endothelium also mediates smooth muscle relaxation by endothelium-derived hyperpolarizing factor [3] and prostacyclin [4, 5] and vasoconstriction by endothelin [6], superoxide and thromboxane [7]. In addition to its role as a vasoregulator, the release and control of these bioactive molecules has been shown to counteract thrombosis [8], inflammation [9] and vascular smooth muscle cell migration and proliferation [10]. A balance in the above interacting factors is crucial for maintaining homeostasis.
Endothelial dysfunction results from a disruption in the cellular integrity leading to impaired endothelium-dependent relaxation mainly due to a reduction in the NO bioavailability. NO is produced from its precursor L-arginine by endothelial nitric oxide synthase (eNOS). Under physiological conditions, following production, NO diffuses across the endothelial cell membrane into the vascular smooth muscle cells to activate guanylate cyclase leading to subsequent cyclic guanosine-3′,5-monophosphate (cGMP) mediated vasodilation. Several molecules such as acetylcholine, bradykinin, serotonin and substance P can induce eNOS. Another important stimulus is the shear stress exerted by the flowing blood which can cause ion channel activation for a rapid response or through a process of phosphorylation induce sustained release of NO to maintain vasodilation [11].
Current evidence implicates endothelial dysfunction in several diseases including hypertension, diabetes, atherosclerosis, renal and cardiac failure and neurodegeneration [12-15]. An early event in this process is endothelial activation wherein cells are primed for a series of events which include leucocyte recruitment and degranulation, expression of adhesion molecules, release of cytokines, and change in the phenotype to an invasive and proliferative type [16]. Under pathophysiological conditions the activation of endothelium entails suppression of NO signaling and an increase in redox signaling by reactive oxygen species [17]. Normally, a system of antioxidant mechanisms control the production of reactive oxygen species (ROS), however, during adverse and chronic conditions, an imbalance in the system causes disruption of NO and ROS generation leading to endothelial dysfunction. Elevated level of homocysteine increases the risk for developing hypertension, coronary artery disease, myocardial infarction and strokes [18, 19]. The overall mortality for patients with homocysteine level greater than 15 (μM/L in the plasma is reported to be 24.7% compared to 3.8% in patients with values less than 9 (μM/L [20]. Oxidative stress, thiolactone formation and protein homocysteinylation are directly related to endothelial toxicity [21-23].
Hydrogen sulfide (H2S) is a pungent gas synthesized in mammalian tissues from L-cysteine by enzymatic and non-enzymatic reactions [24]. In the body, H2S is produced in (μM concentrations and is known to exert a variety of physiological functions by influencing several intracellular signaling mechanisms. A decrease in the production of H2S has been implicated in several diseases such as hypertension, atherosclerosis, diabetes, cardiac and renal failure [25-27]. In the recent years, H2S has gained importance for its multiple effects on individual body systems such as cardioprotection and prevention of ischemia/reperfusion injury.
In this review, we summarize the mechanism of endothelial dysfunction caused by hyperhomocysteinemia (HHcy). In addition, we also discuss the synthesis, metabolism and potential role of hydrogen sulfide (H2S) as a therapeutic agent to ameliorate HHcy-induced endothelial injury.
HOMOCYSTEINE METABOLISM AND PATHOGENESIS OF HYPERHOMOCYSTEINEMIA
Homocysteine is a thiol-containing amino acid derived from the metabolism of methionine in dietary protein and is situated at the junction of remethylation and transulfuration pathways (Fig. 1). Under physiological conditions, the remethylation pathway aims to conserve methionine and recycle methyltetrahydrofolate, a process dependent on vitamin B12 as a cofactor or betaine as a substrate [28]. Methionine is then converted to S-adenosylmethionine (SAM) which serves as a methyl donor for several reactions involving DNA, RNA, and proteins. The hydroxylation of SAM regenerates homocysteine.
Fig. (1).
Schematic representation of homocysteine metabolism pathway. B12, vitamin B12; CβS, Cystationine β-synthase; CSE, Cystathionine γ-lyase; PLP, pyridoxal-5′-phosphate; 5-Methyl TH4-Folate, 5-Methyl tetrahydrofolate.
The transulfuration process is catalyzed by two vitamin B6-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). In the first reaction involving CBS, homocysteine condenses with serine to form cystathionine, in the next step, this is converted to cysteine and α-ketobutyrate by the enzyme, CSE.
There are three main causes of HHcy, 1) genetic defects of Hcy metabolism enzymes, 2) malnutrition and 3) impaired renal clearance. Several genetic variants responsible for HHcy have been described, one of the most common mutation involves 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C→T. This defect causes a decrease in the conversion of homocysteine to methionine causing HHcy and is reported to be a risk factor for the development of essential hypertension [29]. A combination of T133C mutation in the CBS gene and C677T mutation in the methyle-netetrahydrofolate reductase (MTHFR) gene has been reported to result in HHcy in certain populations [30]. Other gene variants associated with hypercysteinuria include p.I278T and p.T191M mutations in CBS gene [31].
Nutritional causes of HHcy include a deficiency of folate or vitamin B12 in the diet. Folate is a precursor of 5-methyl tetrahydrofolate which is necessary for remethylation of homocysteine by methionine synthase (Fig. 1). Folate transfers 1-carbon groups for purine biosynthesis essential for cellular process such as growth and differentiation [32]. Folate deficiency can occur due to malabsorption syndromes, alcoholism and liver diseases. Since the source of vitamin B12 is meat and dairy products, vegans are increasingly susceptible to B12 deficiency. Like folate, B12 is required for methionine synthase activity. At low levels of B12, the formation of MTHFR is impaired resulting in buildup of homocysteine [33]. Additionally, low levels of pyridoxal phosphate, a cofactor for CBS enzyme are inversely related to homocysteine levels and has been shown to be an independent risk factor for coronary artery disease [34].
HHcy is a common finding in patients suffering from chronic kidney disease and end-stage renal failure and is a major risk factor for mortality from cardiovascular events in this population [35]. Although the exact mechanism of homocysteine accumulation in patients with renal disease is incompletely understood, impaired clearance and/or reduction in the extrarenal Hcy metabolism has been suggested as probable causes [36, 37]. Studies conducted using an isotope for sulfur containing amino acid metabolism in end-stage renal disease (ESRD) patients revealed a reduction in the rate of remethylation and transmethylation whereas transulfuration remained normal [38]. Several clinical trials have used vitamin B6 or B12 to study the effect of lowering Hcy levels on vascular events in ESRD patients. In patients undergoing dialysis, a combination of B6 and B12 was more effective in reducing plasma Hcy than B6 alone [39]. Despite a moderate reduction in Hcy levels by these treatments, no improvement was seen in endothelial dysfunction [40] or mortality rates from cardiovascular events [41]. In a separate study, high dose of folic acid and B vitamins did not reduce the incidence of myocardial infarction, strokes, or vascular complications in ESRD patients [42] leading to the speculation that the possible causes for this failure in improvement could be due to advanced stage of the disease and the presence of other associated risk factors [42].
Homocysteine-Induced Oxidative Stress and Endothelial Dysfunction
Elevated plasma homocysteine (Hcy) known as HHcy is well recognized as a risk factor for cardiovascular [43, 44], renal [45] and cerebrovascular diseases [46]. HHcy-induced endothelial damage is a crucial initiating event in the above diseases followed by inflammation. Early studies in our laboratory showed impaired endothelial function in the endocardium and coronary arteries in patients and animal models of HHcy [47, 48]. In the coronary arteries, HHcy caused inhibition of collagenase activity leading to collagen accumulation and fibrosclerosis [48].
Nicotinamide adenine dinucleotide phosphate oxidases (NADPH oxidases) are a group of proteins which generate superoxide during the process of transferring electrons across cell membranes [49]. The NOX family as they are known, comprise seven members sharing homology with gp91phox, the catalytic component of NADPH oxidase [49, 50]. Within the vascular compartment, endothelial cells express NOX4, NOX1, NOX2 and NOX5 isoforms and are the main source of ROS [51, 52]. The other sources include mitochondrial respiration, uncoupled eNOS and the xanthine oxidase pathway. The activation of Nox generates hydrogen peroxide causing sequestration of NO and tyrosine phosphorylation of key proteins thereby altering cellular function [53]. Several studies have shown that homocysteine causes endothelial damage by increasing oxidative stress [54-56]. HHcy enhances ROS production by autoxidation process catalyzed by metal cation such as copper and the resulting superoxide anion reacts with NO to form peroxynitrate adducts which reduces the bioavailability of NO [57]. Furthermore, HHcy-induced oxidative stress is known to activate matrix metallo-proteinases (MMPs) causing disruption of extracellular matrix (ECM) metabolism and increases collagen deposition leading to vascular fibrosis [58]. In an earlier study, our lab demonstrated that HHcy causes dose dependent injury to cardiac microvascular endothelial cells by a mechanism involving activation of protease-activated receptor-4 which upregulates nicotinamide adenine dinucleotide phosphate (NADPH) for ROS production [59]. In the same study, HHcy was also associated with a reduction in thioredoxin expression and NO bioavailability [59]. Both in vitro and in vivo experiments have shown that HHcy can suppress intracellular glutathione peroxidase activity and heme oxygenase 1 leading to oxidative stress and endothelial dysfunction [60, 61]. HHcy has also been shown to impair NO synthesis by a separate mechanism involving inhibition of dimethylargininedimethylaminohydrolase (DDAH), an enzyme involved in the metabolism of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide synthase [62].
In the mammalian cells, mitochondria are a major source of ROS production during electron transport. Although ROS was originally thought as molecules causing cellular damage, in the last decade, a variety of ROS signaling mechanisms have also been described contributing to cellular homeostasis [63]. In response to stimuli such as inflammatory cytokines, chemical compounds, transition metals, radiation etc., the generation of ROS from mitochondria exceeds its scavenging capacity resulting in mitochondrial damage. A synergistic effect of Hcy and hydrogen peroxide (H2O2) was shown to cause mitochondrial damage by decreasing the expression of mitochondrial RNA levels of cytochrome c oxidase III/ATPase 6, 8 and heat shock protein 60 [64]. Similarly, other reports also indicate increased sensitivity of mitrochodrial DNA to superoxide, H2O2, and peroxynitrite causing damage and development of atherosclerosis [65, 66]. In a recent study, folate deficiency and consequent increase in plasma Hcy was shown to cause mitochondrial DNA mutations along with a significant reduction in their content in the heart, brain and liver [67]. These changes were associated with elevated oxidative DNA damage and mitochondrial biogenesis [67]. Furthermore, Hcy has been reported to increase mitochondrial biogenesis in human endothelial cells, an activity dependent on ROS mediation and occurs by increasing the expression of nuclear respiratory factor-1 and mitochondrial transcription factor A [68]. A consequence of oxidative stress-induced increase in mitochondria content could further increase ROS production worsening cell damage [69].
Homocysteine Promotes Endothelial Inflammation and Coagulation
The proinflammatory effect of HHcy is related to ROS generation and involves the activation of nuclear transcription factor κB (NF-κB) which controls the genes for the expression of adhesion molecules, cytokines and chemokines [70]. Under normal conditions, NF-κB is present in an inactive form in the cytosol. Stimuli such as homocysteine and ROS cause phosphorylation of the inhibitory protein IκB alpha for degradation and NF-κB is translocated into the nucleus where it activates the target genes. HHcy-induced activation of NF-κB has been shown to increase the expression of intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), vascular adhesion molecule-1 (VCAM-1) and E-selectin which promote the interaction of inflammatory cells with the endothelium leading to the development of atherosclerosis [71-73]. In response to cell activation, the translocation of NF-κB into the nucleus is accompanied by translocation of subunits p47phox, p67phox and G protein Rac from the cytosol to complex with two other subunits, gp91phox and p22phox located in the cell membrane [74]. The assembled oxidase produces superoxide by one-electron reduction of oxygen by using NADPH as electron donor [75, 76]. Furthermore, HHcy induces redox factor-1 (Ref-1) expression which in turn upregulates monocyte chemoattractant protein-1 (MCP-1) expression via activation of NF-κB [77].
The endothelium achieves hemostasis by maintaining a balance in the production of pro- and anti-coagulant factors. In in vitro studies, HHcy has been shown to promote activation of factor V, inhibit thrombomodulin-dependent protein C activation, impair secretion of vonWillebrand factor (VWF) and induce tissue factor all of which propagate coagulation [78]. Activation of coagulation cascade has also been described in HHcy patients with a history of venous thrombosis and acute coronary artery disease [79, 80].
In the quiescent state, the endothelium maintains an antithrombotic surface for blood flow by regulating several molecules which include thrombomodulin, heparan sulfate and glycosaminoglycans. HHcy has been shown to affect the expression and activity of these molecules. When porcine aortic endothelial cells were treated with various concentrations of Hcy, there was a reduction in the antithrombin III binding activity in a dose and time dependent manner [81]. In a primate study involving diet-induced HHcy, Lentz et al. reported a significant decrease in endothelium-dependent vasodilation along with reduction in the thrombomodulin anticoagulant activity [82]. Furthermore, homocysteinuria patients were found to have low levels of antithrombin III [83]. Similarly, other studies involving HHcy have reported a reduction in activated protein C and irreversible inhibition of thrombomodulin which promotes procoagulant state [84, 85]. HHcy has also been shown to enhance the binding of lipoprotein (a) to fibrin suggesting a link between thrombosis and the development of atherosclerosis [86]. Additionally, HHcy has been shown to reduce the binding of tissue-plasminogen activator to cultured endothelial cells in a dose and time dependent manner thereby decreasing the fibrinolytic activity [87].
Interaction of Endothelium, Leukocytes and Platelets
Incubation of human umbilical vein endothelial cells (HUVECs) with L-Hcy has been reported to increase ROS production along with upregulation of intercellular adhesion molecule-1 (ICAM-1) expression via activation of NF-κB leading to adhesion of monocytes [71]. Similarly, increased expression of P-selectin has been observed in the aorta of mildly hyperhomocysteinemic CBS+/- mice [88] and increased vascular cell adhesion molecule (VCAM) and E-selectin in HHcy rats [73]. When CBS+- mice were treated with L-2-oxo-4-thiazolidine carboxylate (OTC), a compound which increases reduced glutathione and total thiols, the expression of P-selectin was reduced suggesting that increased P-selectin was the result of Hcy-induced oxidative stress [88]. HHcy has also been shown to promote the expression of chemokines, MCP-1 and IL-8 in monocytes along with increased expression of chemokine receptor type 2 (CCR2) [89, 90]. The abolishment of this effect by the addition of superoxide dismutase suggested that it was mediated mainly by Hcy-induced ROS production [91]. The production of the above molecules and the interaction of endothelium and inflammatory cells can stimulate the development of atherosclerosis.
Hcy-induced vascular inflammation involves migration of leukocytes from the vascular compartment into the tissue in a multistep process. In the initial step, neutrophils are recruited by margination along the vessel wall followed by rolling. Under appropriate stimuli, both neutrophils and endothelial cells express adhesion molecules and their ligands which enables adhesion and eventual migration into the tissues [92]. Hcy induces the expression of CD11B/CD18 proteins which form a docking complex enabling interaction between inflammatory cells and the endothelium [93]. The sum effect of these processes cause damage and loss of endothelial cells. HHcy increases ROS generation in neutrophils by activation of Nox family as described above. Recent studies have suggested that this involves mitogen-activated phosphokinases (MAPKs) activation in neutrophils and c-Jun NH(2)-terminal kinase in the vascular endothelial cells [94, 95].
Platelet activation during HHcy leads to increased secretion of growth factors, chemokines and production of coagulation factors [96]. In patients with peripheral vascular disease, mild HHcy was shown to increase the sensitivity of platelets to adenosine diphosphate (ADP) and thrombin leading to platelet activation [97]. In addition, the sensitivity to ADP significantly increased the expression of P-selectin [97]. In rats treated with high methionine diet, moderate HHcy increased the aggregation of platelets and enhanced thromboxane synthesis creating pro-thrombotic state [98]. Several pathways have been shown to be involved in the activation of platelets by HHcy which include phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK), cytosolic phospholipase A2 (cPLA2) and more recently phospholipase C gamma 2 (PLCγ2) [99, 100].
HYDROGEN SULFIDE SYNTHESIS AND METABOLISM
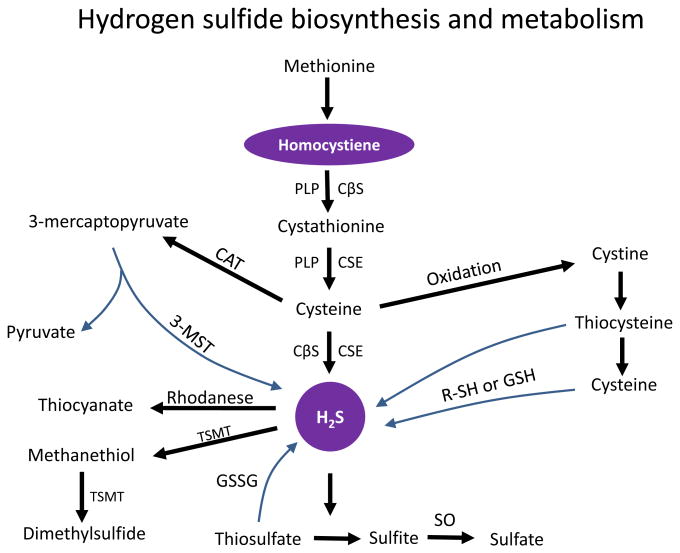
Hydrogen sulfide (H2S) is a colorless gas with a rotten egg odour. For a long time, H2S was known only for its neurotoxicity and as an environmental hazard. In the recent years however, H2S has been named as the third endogenous gasotransmitter along with nitric oxide and carbon monoxide. It has a very short half-life (minutes) and is easily diffusible through the plasma membrane because of its high lipid solubility. The majority of H2S in the mammalian tissues is produced by pyridoxal-5′-phosphate dependent enzymes in four ways - a) cystathionine β synthetase (CBS, EC 4.2.1.22) acts on L-cysteine to give H2S, b) cystathionine γ lyase (CSE, EC 4.4.1.1) forms thiocysteine from cystine and then yields H2S, c) cysteine aminotransferase (CAT, EC 2.6.1.3) first forms 3-mercaptopyruvate from L-cysteine and then by a process of desulfuration by 3-mercaptopyruvate sulfurtransferase (3-MST, EC 2.8.1.2) yields H2S, and finally d) cysteine lyase (CL, EC 4.4.1.10) converts L-cysteate to H2S (Fig. 2) [24].
Fig. (2).
Schematic representation of hydrogen sulfide synthesis and degradation. CβS, Cystationine β-synthase; CSE, Cystathionine γ-lyase; 3-MST, 3-mercaptopyruvate sulfurtransferase; CAT, cysteine aminotransferase; PLP, pyridoxal-5′-phosphate, GSSG, oxidized glutathione, GSH, reduced glutathione, R-SH, thiol-bearing intermediate; SO, sulfite oxidase; and TSMT, thiol S-methyltransferase.
The distribution of CBS, CSE and 3-MST enzymes are-tissue specific but both CBS and CSE have been identified in several mammalian tissues including liver, kidney and brain [101]. The expression of CSE has been demonstrated in thoracic aorta, portal vein and mesenteric artery [102, 103] including other vascular beds and has been shown in both smooth muscle cells and the endothelium [103, 104]. Within the cells, CBS and CSE are localized in the cytosol whereas 3-MST is localized in the mitochondria. Current knowledge regarding the fate of H2S following synthesis is limited. However, in many tissues and within the mitochondria, H2S undergoes several oxidation steps to form thiosulfate, sulfite and finally sulfate (Fig. 2). In the cytosol, it can be methylated by thiol-S-methyltransferase to form methanethiol and a less toxic compound dimethylsulfide (Fig. 2) [105, 106]. In addition, because of its strong reducing capacity, H2S can be utilized for scavenging members of the reactive oxygen species [107, 108].
Physiological Effects and Mechanism of Action of H2S
The principal physiological effects of H2S are diverse depending on the organ/tissue. The recognized targets for H2S include enzymes, intracellular signaling proteins, transcriptional factors and ion channels. In the vascular system, H2S has been shown to dilate several vessels such as aorta, portal vein, gastric artery [102, 103, 109] mesenteric artery [110], and human internal mammary artery [111]. The mechanism by which H2S induces vasodilation is attributed to the opening of vascular smooth muscle KATP channels as evidenced by a similar result seen using pinacidil, an agonist of KATP channels [102, 103]. Further confirmation of the involvement of KATP channels was demonstrated by observing increased electrical conductance across the channels in aortic and vascular smooth muscle cells following H2S treatment [103]. Despite these results, the exact mechanism of how KATP channels are activated by H2S still remains to be elucidated.
Exogenous administration of sodium hydrogen sulfide (NaHS) has been shown to act directly on smooth muscle cells causing a reduction in blood pressure independent of endothelial function [103, 112]. Conversely, a reduction in the expression of CSE and thus H2S has been implicated in hypoxic and high blood flow induced models of pulmonary hypertension [113, 114]. Furthermore, CSE knockout mice have shown a variety of pathological effects including severe hypertension, decreased endothelium-mediated vasorelaxation and delayed wound healing secondary to inhibition of angiogenesis [104, 115].
In a study involving children with essential hypertension, Chen et al. found an inverse relationship between plasma homocysteine and hydrogen sulfide suggesting a deficiency or functional impairment of H2S generating enzymes [116]. In spontaneously hypertensive rats, H2S treatment prevented the development of myocardial hypertrophy and fibrosis and also reduced ROS production [117]. The abolishment of these effects by glibenclamide suggested the involvement of ATP-sensitive potassium channels [117]. H2S treatment has also been shown to modulate aortic remodeling in spontaneously hypertensive rats by decreasing type I collagen accumulation [118]. In in vitro studies on vascular smooth muscle cells, NaHS treatment attenuated Angiotensin-II induced collagen synthesis by inhibiting phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) [118]. The antiatherogenic effect of H2S treatment in the aorta of apoE(-/-) mice has been attributed to the inhibition of expression and secretion of ICAM-1 [119]. Although H2S treatment inhibits platelet aggregation, the exact mechanism is still not clear. However, current evidence suggests that platelet activation involves disulfide metabolism and because H2S reduces disulfide bonds in proteins, it is possible that the anti aggregating effect could be secondary to modulation of disulfide metabolism [120].
Modulation of Inflammation by Hydrogen Sulfide
H2S has a dual role in inflammation. In endotoxic shock and pancreatitis, H2S exhibited a pro-inflammatory role by increasing myeloperoxidase activity and upregulation of TNFα [121, 122]. However, other studies suggest a protective role in lipopolysaccharide-induced inflammation evidenced by decreased myeloperoxidase activity, reduction of IL-1β and TNFα and increase in IL-10 [123, 124]. Indeed, H2S-releasing drugs have shown beneficial role in ulcerative colitis by reducing leukocyte infiltration and production of inflammatory cytokines such as TNFα and IFN-γ [125]. The anti-inflammatory effect of H2S treatment was demonstrated to be due to the activation of KATP channels and includes modulation of leukocyte-endothelial interaction, reduction of inflammatory cell infiltration and edema [126]. In ischemia/reperfusion injury of kidney and heart, NaHS treatment prevented the degradation and translocation of NF-κB [127, 128]. A reduction in the activation of NF-κB translates to decreased production of inflammatory cytokines and chemokines by downregulation of their respective genes.
Hydrogen Sulfide and Oxidative Stress
H2S has a protective role during oxidative stress and its effects have been attributed to the opening of KATP and Cl-channels and upregulation of glutathione [129]. A similar mechanism has been shown to offer mitochondrial protection from oxidant stress [130]. Interestingly, several studies involving HHcy have also shown beneficial effects of H2S therapy. In a rat model of HHcy, oxidative stress resulted in significant impairment of CSE activity causing an imbalance in Hcy and H2S leading to myocardial injury, whereas, treatment with H2S protected the myocardium from HHcy-induced damage to mitochondrial respiratory chain and from endoplasmic reticulum stress [108]. A previous study from our lab has showed that H2S supplementation protects the kidneys from HHcy induced damage by reducing apoptosis, normalization of matrix metalloproteinase activity and also by scavenging ROS [131]. In another study, we demonstrated that the administration of H2S protected brain endothelial cells from methionine-induced oxidative injury. Similarly, Yan et al. showed that HHcy causes vascular smooth muscle cell injury in a time and dose-dependent manner and treatment with H2S (30 or 50 μM) reduced superoxide, hydrogen peroxide, and peroxynitrite formation thereby reducing cell damage [132].
Cross-Talk Between Hydrogen Sulfide and Nitric Oxide in the Endothelial Cells
Since H2S and NO are both synthesized by the endothelial cells, it is possible that some of their functions are mediated by constant interaction between these molecules. Studies have suggested that this cross-talk may occur prior to the production of NO at the level of nitric oxide synthase (NOS), or NO can modulate CSE activity and the subsequent production of H2S which interacts with NO to form a nitrosothiol species. Supporting evidence for such nitrosothiol-like species was demonstrated by Whiteman et al. who showed that treatment of RAW264.7 cells with NaHS in the presence of NO donors resulted in the formation of new molecule which did not induce cyclic guanosine monophosphate (cGMP) normally seen after cells are exposed to NO [133]. In aortic smooth muscle cells, NO has been shown to increase the expression and activity of CSE [103]. The effect of H2S on NOS has shown mixed results. In some studies, NaHS was shown to inhibit nNOS and iNOS more than eNOS [134]. In another study, NaHS inhibited protein expression of eNOS but not nNOS or iNOS in isolated rat aortic preparation and human umbilical vein endothelial cells [135]. The observation that KATP channel agonist, pinacidil showed a similar effect whereas, its antagonist, glibenclamide blocked the inhibition of eNOS activity suggested an important role of these channels in H2S regulated eNOS activity [135]. Kubo et al. demonstrated that NaHS played a dual role causing contraction and vasodilation in the mouse and rat aorta [136]. In the rat aorta, vasoconstriction was secondary to direct inhibition of eNOS by H2S decreasing NO production, whereas, vasorelaxation involved both KATP channel-dependent and -independent mechanisms [136]. In in vitro studies using lipopolysaccharide treated macrophages, H2S was shown to induce heme oxygenase-1 (HO-1) expression which suppressed iNOS thereby decreasing NO production [137]. In addition, treatment with carbon monoxide a product of (HO-1) inhibited phosphorylation of IkB, a necessary step for the activation of NF-κB suggesting that H2S-induced reduction in NO involves HO-1/CO pathway [137]. In contrast to the above studies, under hypoxic conditions, NaHS has been reported to stimulate NO production by increasing NOS expression and activity and also by mediating nitrite reduction to NO via xanthine oxidase pathway [138]. Additionally, H2S treatment was shown to increase endothelial hypoxia-inducible factor (HIF) -1α and VEGF mediated angiogenesis in ischemic tissues by NO-dependent mechanism [138]. Another recent report also indicated that NaHS treatment increases NO production in endothelial cells by phosphorylation of eNOS; furthermore, NaHS was shown to promote angiogenesis as evidenced by the formation of endothelial cell tube and proliferation by both NO-dependent and -independent mechanisms [139].
Researchers have also investigated the effect of NO on H2S synthesis in normal and disease conditions. Sodium ni-troprusside (SNP) was shown to upregulate CBS expression in brain tissues increasing H2S production by NO-independent mechanism [140]. Treatment of vascular tissues with SNP, a NO donor, significantly increased H2S production in aorta, rat tail artery and mesenteric artery [103]. In the rat aorta, SNP-induced increase in H2S appears to be mediated by cGMP pathway [141]. In a rat model of endotoxic shock, treatment with nitroflurbiprofen, a NO donor, suppressed liver H2S synthesis along with other proinflammatory molecules in a dose-dependent manner to reduce inflammation [142].
Taken together, the above studies suggest a complex interaction between H2S and NO with distinct effects depending on tissues and animal models. Nevertheless, as research in this area progresses and more data becomes available with better measurement techniques, it will help us to better understand the underlying mechanisms.
CONCLUSIONS AND FUTURE PERSPECTIVES
To summarize, this review provides the evidence to support the role of HHcy in causing endothelial dysfunction and outlines several important mechanisms by which it occurs. HHcy-induced ROS production decreases NO production and bioavailability triggering increased redox signaling. Impaired NO production during HHcy can also occur due to inhibition of DDAH causing ADMA accumulation. HHcy-induced damage to mitochondrial DNA causes dysfunction and enhances mitochondrial biogenesis which may form a vicious cycle to worsen oxidative stress. In addition, HHcy and ROS activate NF-κB triggering inflammation which is accompanied by the activation of coagulation system. The production of adhesion molecules, cytokines and chemokines ensues eventually leading to endothelial dysfunction.
A deficiency of CBS enzyme causes HHcy leading to the development of premature peripheral, cardiac and cerebrovascular disease. This genetic defect can also result in low levels of H2S. In recent years, H2S has been increasingly studied and several functions have been identified including vasodilation, anti-inflammation, anti atherogenesis, proangiogenesis and organ protection. Due to the fact that H2S is a potent vasodilator, it is possible that its deficiency can predispose to the development of cardiovascular disease such as hypertension. Whether low levels of H2S are causative, contributory or incidental effect on the pathological conditions requires further study. Although H2S is reported to have a dual role as a pro- and anti-inflammatory agent, the underlying mechanisms are still not clear. Further studies are required to better understand the signaling mechanism including its effects on other molecular targets. Because NO and H2S are produced by the same cells and released into similar environment, it is likely that they may interact with each other to potentiate or suppress their own biological effect. Current data regarding the cross-talk between NO and H2S have shown controversial results requiring careful interpretation. Finally, studies are required to delineate the role of gasotransmitters in the presence or absence of each other to understand their complex interaction during normal and pathological conditions.
Acknowledgments
This study was supported by NIH grant HL104103 (to Dr. Utpal Sen).
ABBREVIATIONS
- ADMA
Asymmetric dimethylarginine
- CBS/CβS
Cystathionine β-synthase
- CSE/CTH
Cystationine γ-lyase
- cGMP
Cyclic guanosine-3′,5-monophosphate
- DDAH
Dimethylarginine dimethylaminohydrolase
- eNOS
Endothelial nitric oxide synthase
- ESRD
End-stage renal disease
- H2S
Hydrogen sulfide
- Hcy
Homocysteine
- HHcy
Hyperhomocysteinemia
- HO-1
Heme oxygenase-1
- ICAM-1
Intracellular adhesion molecule-1
- IL
Interleukin
- IkB alpha
I-kappa-B-alpha
- iNOS
Inducible nitric oxide synthase
- MAPK
Mitogen-activated phosphokinase
- MCP-1
Monocyte chemoattractant protein-1
- MTHFR
Methylenetetrahydrofolate reductase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NaHS
Sodium hydrogen sulfide
- NF-κB
Nuclear transcription factor κB
- nNOS
Neuronal nitric oxide synthase
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PLP
Pyridoxal-5′-phosphate
- ROS
Reactive oxxygen species
- SAM
S-adenosylmethionine
- TNFα
Tumour necrosis factor alpha
- VCAM
Vascular cell adhesion molecule
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflict of interest.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. British journal of pharmacology. 1988;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusting GJ, Moncada S, Mullane KM, Vane JR. Implications of prostacyclin generation for modulation of vascular tone. Clinical science and molecular medicine Supplement. 1978;4:195s–198s. doi: 10.1042/cs055195s. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet. 1977;1(8001):18–20. doi: 10.1016/s0140-6736(77)91655-5. [DOI] [PubMed] [Google Scholar]
- 6.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 7.Raz A, Minkes MS, Needleman P. Endoperoxides and thromboxanes. Structural determinants for platelet aggregation and vasoconstriction. Biochimica et biophysica acta. 1977;488(2):305–311. doi: 10.1016/0005-2760(77)90188-6. [DOI] [PubMed] [Google Scholar]
- 8.Tziros C, Freedman JE. The many antithrombotic actions of nitric oxide. Current drug targets. 2006;7(10):1243–1251. doi: 10.2174/138945006778559111. [DOI] [PubMed] [Google Scholar]
- 9.Momi S, Monopoli A, Alberti PF, Falcinelli E, Corazzi T, Conti V, Miglietta D, Ongini E, Minuz P, Gresele P. Nitric oxide enhances the anti-inflammatory and anti-atherogenic activity of atorvastatin in a mouse model of accelerated atherosclerosis. Cardiovascular research. 2012;94(3):428–438. doi: 10.1093/cvr/cvs100. [DOI] [PubMed] [Google Scholar]
- 10.Tsihlis ND, Oustwani CS, Vavra AK, Jiang Q, Keefer LK, Kibbe MR. Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10. Cell biochemistry and biophysics. 2011;60(1-2):89–97. doi: 10.1007/s12013-011-9179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallance P, Chan N. Endothelial function and nitric oxide: clinical relevance. Heart. 2001;85(3):342–350. doi: 10.1136/heart.85.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vascular health and risk management. 2007;3(6):853–876. [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120(13):1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. Journal of hypertension. 2013 doi: 10.1097/HJH.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 15.Grammas P, Martinez J, Miller B. Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert reviews in molecular medicine. 2011;13:e19. doi: 10.1017/S1462399411001918. [DOI] [PubMed] [Google Scholar]
- 16.Hunt BJ, Jurd KM. Endothelial cell activation. A central pathophysiological process. Bmj. 1998;316(7141):1328–1329. doi: 10.1136/bmj.316.7141.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 18.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA : the journal of the American Medical Association. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 19.Homocysteine Studies, C. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA : the journal of the American Medical Association. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 20.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. The New England journal of medicine. 1997;337(4):230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 21.Handy DE, Zhang Y, Loscalzo J. Homocysteine downregulates cellular glutathione peroxidase (GPx1) by decreasing translation. The Journal of biological chemistry. 2005;280(16):15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Werstuck GH, Lhotak S, de Koning AB, Sood SK, Hossain GS, Moller J, Ritskes-Hoitinga M, Falk E, Dayal S, Lentz SR, Austin RC. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteine-mic apolipoprotein E-deficient mice. Circulation. 2004;110(2):207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 23.Jakubowski H, Zhang L, Bardeguez A, Aviv A. Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circulation research. 2000;87(1):45–51. doi: 10.1161/01.res.87.1.45. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annual review of pharmacology and toxicology. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 25.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxidants & redox signaling. 2012;17(1):68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, Murohara T, Predmore BL, Gojon G, Sr, Gojon G, Jr, Wang R, Karusula N, Nicholson CK, Calvert JW, Lefer DJ. H(2)S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127(10):1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, Dickhout JG, Lhotak S, Meng QH, Wang R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation. 2013;127(25):2523–2534. doi: 10.1161/CIRCULATIONAHA.113.002208. [DOI] [PubMed] [Google Scholar]
- 28.Selhub J. Homocysteine metabolism. Annual review of nutrition. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 29.Ilhan N, Kucuksu M, Kaman D, Ilhan N, Ozbay Y. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Archives of medical research. 2008;39(1):125–130. doi: 10.1016/j.arcmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Tsai MY, Welge BG, Hanson NQ, Bignell MK, Vessey J, Schwichtenberg K, Yang F, Bullemer FE, Rasmussen R, Graham KJ. Genetic causes of mild hyperhomocysteinemia in patients with premature occlusive coronary artery diseases. Atherosclerosis. 1999;143(1):163–170. doi: 10.1016/s0021-9150(98)00271-8. [DOI] [PubMed] [Google Scholar]
- 31.Urreizti R, Asteggiano C, Bermudez M, Cordoba A, Szlago M, Grosso C, de Kremer RD, Vilarinho L, D'Almeida V, Martinez-Pardo M, Pena-Quintana L, Dalmau J, Bernal J, Briceno I, Couce ML, Rodes M, Vilaseca MA, Balcells S, Grinberg D. The p.T191M mutation of the CBS gene is highly prevalent among homocystinuric patients from Spain, Portugal and South America. Journal of human genetics. 2006;51(4):305–313. doi: 10.1007/s10038-006-0362-0. [DOI] [PubMed] [Google Scholar]
- 32.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. Journal of inherited metabolic disease. 2011;34(1):75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YI. Folic acid fortification and supplementation--good for some but not so good for others. Nutrition reviews. 2007;65(11):504–511. doi: 10.1111/j.1753-4887.2007.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Robinson K, Mayer EL, Miller DP, Green R, van Lente F, Gupta A, Kottke-Marchant K, Savon SR, Selhub J, Nissen SE, et al. Hyperhomocysteinemia and low pyridoxal phosphate. Common and independent reversible risk factors for coronary artery disease. Circulation. 1995;92(10):2825–2830. doi: 10.1161/01.cir.92.10.2825. [DOI] [PubMed] [Google Scholar]
- 35.Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. Journal of the American Society of Nephrology : JASN. 2001;12(10):2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- 36.Guttormsen AB, Ueland PM, Svarstad E, Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney international. 1997;52(2):495–502. doi: 10.1038/ki.1997.359. [DOI] [PubMed] [Google Scholar]
- 37.Garibotto G, Sofia A, Valli A, Tarroni A, Di Martino M, Cappelli V, Aloisi F, Procopio V. Causes of hyperhomocysteinemia in patients with chronic kidney diseases. Seminars in nephrology. 2006;26(1):3–7. doi: 10.1016/j.semnephrol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.van Guldener C, Kulik W, Berger R, Dijkstra DA, Jakobs C, Reijngoud DJ, Donker AJ, Stehouwer CD, De Meer K. Homocysteine and methionine metabolism in ESRD: A stable isotope study. Kidney international. 1999;56(3):1064–1071. doi: 10.1046/j.1523-1755.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 39.van Guldener C. Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(5):1161–1166. doi: 10.1093/ndt/gfl044. [DOI] [PubMed] [Google Scholar]
- 40.van Guldener C, Janssen MJ, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD. Folic acid treatment of hyperhomocysteinemia in peritoneal dialysis patients: no change in endothelial function after long-term therapy. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 1998;18(3):282–289. [PubMed] [Google Scholar]
- 41.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. Journal of the American Society of Nephrology : JASN. 2004;15(2):420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 42.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM Veterans Affairs Site, I. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;298(10):1163–1170. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 43.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Bmj. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald DS, Wald NJ, Morris JK, Law M. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. Bmj. 2006;333(7578):1114–1117. doi: 10.1136/bmj.39000.486701.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. American journal of nephrology. 2008;28(2):254–264. doi: 10.1159/000110876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terwecoren A, Steen E, Benoit D, Boon P, Hemelsoet D. Ischemic stroke and hyperhomocysteinemia: truth or myth? Acta neurologica Belgica. 2009;109(3):181–188. [PubMed] [Google Scholar]
- 47.Tyagi SC, Smiley LM, Mujumdar VS. Homocyst(e)ine impairs endocardial endothelial function. Canadian journal of physiology and pharmacology. 1999;77(12):950–957. [PubMed] [Google Scholar]
- 48.Tyagi SC, Smiley LM, Mujumdar VS, Clonts B, Parker JL. Reduction-oxidation (Redox) and vascular tissue level of homocyst(e)ine in human coronary atherosclerotic lesions and role in extracellular matrix remodeling and vascular tone. Molecular and cellular biochemistry. 1998;181(1-2):107–116. doi: 10.1023/a:1006882014593. [DOI] [PubMed] [Google Scholar]
- 49.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 50.Nauseef WM. Biological roles for the NOX family NADPH oxidases. The Journal of biological chemistry. 2008;283(25):16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109(2):227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 52.Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, Wakisaka M, Kawahara T, Rokutan K, Ibayashi S, Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke; a journal of cerebral circulation. 2005;36(5):1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- 53.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 54.Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow YL, O K. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circulation research. 2004;94(1):28–36. doi: 10.1161/01.RES.0000108264.67601.2C. [DOI] [PubMed] [Google Scholar]
- 55.Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free radical biology & medicine. 2004;36(12):1532–1541. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Medical hypotheses. 2011;77(6):1088–1093. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. The Journal of clinical investigation. 1996;98(1):5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sen U, Mishra PK, Tyagi N, Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell biochemistry and biophysics. 2010;57(2-3):49–58. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. American journal of physiology Heart and circulatory physiology. 2005;289(6):H2649–2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 60.Weiss N, Zhang YY, Heydrick S, Bierl C, Loscalzo J. Over-expression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawle P, Foresti R, Green CJ, Motterlini R. Homocysteine attenuates endothelial haem oxygenase-1 induction by nitric oxide (NO) and hypoxia. FEBS letters. 2001;508(3):403–406. doi: 10.1016/s0014-5793(01)03117-9. [DOI] [PubMed] [Google Scholar]
- 62.Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104(21):2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 63.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Molecular cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Austin RC, Sood SK, Dorward AM, Singh G, Shaughnessy SG, Pamidi S, Outinen PA, Weitz JI. Homocysteine-dependent alterations in mitochondrial gene expression, function and structure. Homocysteine and H2O2 act synergistically to enhance mitochondrial damage. The Journal of biological chemistry. 1998;273(46):30808–30817. doi: 10.1074/jbc.273.46.30808. [DOI] [PubMed] [Google Scholar]
- 65.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106(5):544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 66.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circulation research. 2007;100(4):460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 67.Chou YF, Yu CC, Huang RF. Changes in mitochondrial DNA deletion, content, and biogenesis in folate-deficient tissues of young rats depend on mitochondrial folate and oxidative DNA injuries. The Journal of nutrition. 2007;137(9):2036–2042. doi: 10.1093/jn/137.9.2036. [DOI] [PubMed] [Google Scholar]
- 68.Perez-de-Arce K, Foncea R, Leighton F. Reactive oxygen species mediates homocysteine-induced mitochondrial biogenesis in human endothelial cells: modulation by antioxidants. Biochemical and biophysical research communications. 2005;338(2):1103–1109. doi: 10.1016/j.bbrc.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 69.Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. The international journal of biochemistry & cell biology. 2005;37(4):822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxidants & redox signaling. 2007;9(11):1941–1958. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 71.Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereo-specific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(3):508–513. doi: 10.1161/01.ATV.0000201039.21705.dc. [DOI] [PubMed] [Google Scholar]
- 72.Silverman MD, Tumuluri RJ, Davis M, Lopez G, Rosenbaum JT, Lelkes PI. Homocysteine upregulates vascular cell adhesion molecule-1 expression in cultured human aortic endothelial cells and enhances monocyte adhesion. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(4):587–592. doi: 10.1161/01.atv.0000014221.30108.08. [DOI] [PubMed] [Google Scholar]
- 73.Wang G, Woo CW, Sung FL, Siow YL, O K. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(11):1777–1783. doi: 10.1161/01.atv.0000035404.18281.37. [DOI] [PubMed] [Google Scholar]
- 74.Babior BM. NADPH oxidase: an update. Blood. 1999;93(5):1464–1476. [PubMed] [Google Scholar]
- 75.Babior BM. The NADPH oxidase of endothelial cells. IUBMB life. 2000;50(4-5):267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 76.Robinson JM, Ohira T, Badwey JA. Regulation of the NADPH-oxidase complex of phagocytic leukocytes. Recent insights from structural biology, molecular genetics, and microscopy. Histochemistry and cell biology. 2004;122(4):293–304. doi: 10.1007/s00418-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 77.Dai J, Li W, Chang L, Zhang Z, Tang C, Wang N, Zhu Y, Wang X. Role of redox factor-1 in hyperhomocysteinemia-accelerated atherosclerosis. Free radical biology & medicine. 2006;41(10):1566–1577. doi: 10.1016/j.freeradbiomed.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 78.Gerdes VE, Hovinga HA, ten Cate H, Macgillavry MR, Leijte A, Reitsma PH, Brandjes DP, Buller HR Amsterdam Vascular Medicine, G. Homocysteine and markers of coagulation and endothelial cell activation. Journal of thrombosis and haemo-stasis : JTH. 2004;2(3):445–451. doi: 10.1111/j.1538-7836.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- 79.Kyrle PA, Stumpflen A, Hirschl M, Bialonczyk C, Herkner K, Speiser W, Weltermann A, Kaider A, Pabinger I, Lechner K, Eichinger S. Levels of prothrombin fragment F1+2 in patients with hyperhomocysteinemia and a history of venous thromboem-bolism. Thrombosis and haemostasis. 1997;78(5):1327–1331. [PubMed] [Google Scholar]
- 80.Al-Obaidi MK, Philippou H, Stubbs PJ, Adami A, Amersey R, Noble MM, Lane DA. Relationships between homocysteine, factor VIIa, and thrombin generation in acute coronary syndromes. Circulation. 2000;101(4):372–377. doi: 10.1161/01.cir.101.4.372. [DOI] [PubMed] [Google Scholar]
- 81.Nishinaga M, Ozawa T, Shimada K. Homocysteine, a thrombogenic agent, suppresses anticoagulant heparan sulfate expression in cultured porcine aortic endothelial cells. The Journal of clinical investigation. 1993;92(3):1381–1386. doi: 10.1172/JCI116712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, Malinow MR, Heistad DD. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. The Journal of clinical investigation. 1996;98(1):24–29. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palareti G, Coccheri S. Lowered antithrombin III activity and other clotting changes in homocystinuria: effects of a pyridoxine-folate regimen. Haemostasis. 1989;19(Suppl 1):24–28. doi: 10.1159/000216092. [DOI] [PubMed] [Google Scholar]
- 84.Rodgers GM, Conn MT. Homocysteine, an atherogenic stimulus, reduces protein C activation by arterial and venous endothelial cells. Blood. 1990;75(4):895–901. [PubMed] [Google Scholar]
- 85.Hayashi T, Honda G, Suzuki K. An atherogenic stimulus homocysteine inhibits cofactor activity of thrombomodulin and enhances thrombomodulin expression in human umbilical vein endothelial cells. Blood. 1992;79(11):2930–2936. [PubMed] [Google Scholar]
- 86.Harpel PC, Chang VT, Borth W. Homocysteine and other sulfhydryl compounds enhance the binding of lipoprotein(a) to fibrin: a potential biochemical link between thrombosis, atherogenesis, and sulfhydryl compound metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10193–10197. doi: 10.1073/pnas.89.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hajjar KA. Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. The Journal of clinical investigation. 1993;91(6):2873–2879. doi: 10.1172/JCI116532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weiss N, Heydrick S, Zhang YY, Bierl C, Cap A, Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(1):34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 89.Zeng XK, Remick DG, Wang X. Homocysteine induces production of monocyte chemoattractant protein-1 and interleukin-8 in cultured human whole blood. Acta pharmacologica Sinica. 2004;25(11):1419–1425. [PubMed] [Google Scholar]
- 90.Zeng X, Dai J, Remick DG, Wang X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circulation research. 2003;93(4):311–320. doi: 10.1161/01.RES.0000087642.01082.E4. [DOI] [PubMed] [Google Scholar]
- 91.Wang G, O K. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radicals. The Biochemical journal. 2001;357(Pt 1):233–240. doi: 10.1042/0264-6021:3570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacological reviews. 2000;52(3):349–374. [PubMed] [Google Scholar]
- 93.Guo X, Dudman NP. Homocysteine induces expressions of adhesive molecules on leukocytes in whole blood. Chinese medical journal. 2001;114(12):1235–1239. [PubMed] [Google Scholar]
- 94.Alvarez-Maqueda M, El Bekay R, Monteseirin J, Alba G, Chacon P, Vega A, Santa Maria C, Tejedo JR, Martin-Nieto J, Bedoya FJ, Pintado E, Sobrino F. Homocysteine enhances superoxide anion release and NADPH oxidase assembly by human neutrophils. Effects on MAPK activation and neutrophil migration. Atherosclerosis. 2004;172(2):229–238. doi: 10.1016/j.atherosclerosis.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Cai Y, Zhang C, Nawa T, Aso T, Tanaka M, Oshiro S, Ichijo H, Kitajima S. Homocysteine-responsive ATF3 gene expression in human vascular endothelial cells: activation of c-Jun NH(2)-terminal kinase and promoter response element. Blood. 2000;96(6):2140–2148. [PubMed] [Google Scholar]
- 96.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circulation research. 2007;100(1):27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 97.Riba R, Nicolaou A, Troxler M, Homer-Vaniasinkam S, Naseem KM. Altered platelet reactivity in peripheral vascular disease complicated with elevated plasma homocysteine levels. Atherosclerosis. 2004;175(1):69–75. doi: 10.1016/j.atherosclerosis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 98.Durand P, Prost M, Blache D. Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids. Atherosclerosis. 1996;121(2):231–243. doi: 10.1016/0021-9150(95)06724-8. [DOI] [PubMed] [Google Scholar]
- 99.Leoncini G, Bruzzese D, Signorello MG. Activation of p38 MAPKinase/cPLA2 pathway in homocysteine-treated platelets. Journal of thrombosis and haemostasis : JTH. 2006;4(1):209–216. doi: 10.1111/j.1538-7836.2005.01708.x. [DOI] [PubMed] [Google Scholar]
- 100.Leoncini G, Bruzzese D, Signorello MG. A role for PLCgamma2 in platelet activation by homocysteine. Journal of cellular biochemistry. 2007;100(5):1255–1265. doi: 10.1002/jcb.21123. [DOI] [PubMed] [Google Scholar]
- 101.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16(13):1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 102.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochemical and biophysical research communications. 1997;237(3):527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 103.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. The EMBO journal. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weisiger RA, Pinkus LM, Jakoby WB. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochemical pharmacology. 1980;29(20):2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- 106.Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. The Journal of clinical investigation. 1999;104(8):1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? Journal of neurochemistry. 2004;90(3):765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 108.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino acids. 2008;34(4):573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 109.Kubo S, Kajiwara M, Kawabata A. Dual modulation of the tension of isolated gastric artery and gastric mucosal circulation by hydrogen sulfide in rats. Inflammopharmacology. 2007;15(6):288–292. doi: 10.1007/s10787-007-1590-4. [DOI] [PubMed] [Google Scholar]
- 110.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. American journal of physiology Heart and circulatory physiology. 2004;287(5):H2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 111.Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. The Journal of pharmacology and experimental therapeutics. 2008;324(2):876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- 112.Ahmad FU, Sattar MA, Rathore HA, Abdullah MH, Tan S, Abdullah NA, Johns EJ. Exogenous hydrogen sulfide (H2S) reduces blood pressure and prevents the progression of diabetic nephropathy in spontaneously hypertensive rats. Renal failure. 2012;34(2):203–210. doi: 10.3109/0886022X.2011.643365. [DOI] [PubMed] [Google Scholar]
- 113.Chunyu Z, Junbao D, Dingfang B, Hui Y, Xiuying T, Chaoshu T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochemical and biophysical research communications. 2003;302(4):810–816. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 114.Yanfei W, Lin S, Junbao D, Chaoshu T. Impact of L-arginine on hydrogen sulfide/cystathionine-gamma-lyase pathway in rats with high blood flow-induced pulmonary hypertension. Biochemical and biophysical research communications. 2006;345(2):851–857. doi: 10.1016/j.bbrc.2006.04.162. [DOI] [PubMed] [Google Scholar]
- 115.Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(51):21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L, Ingrid S, Ding YG, Liu Y, Qi JG, Tang CS, Du JB. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chinese medical journal. 2007;120(5):389–393. [PubMed] [Google Scholar]
- 117.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. American journal of physiology Heart and circulatory physiology. 2007;293(4):H2093–2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 118.Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31(8):1619–1630. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(2):173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 120.Zagli G, Patacchini R, Trevisani M, Abbate R, Cinotti S, Gensini GF, Masotti G, Geppetti P. Hydrogen sulfide inhibits human platelet aggregation. European journal of pharmacology. 2007;559(1):65–68. doi: 10.1016/j.ejphar.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 121.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(9):1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 122.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(6):623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 123.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free radical biology & medicine. 2007;42(5):706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 124.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macro-phages. Antioxidants & redox signaling. 2010;12(10):1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, Santucci L, Cirino G, Wallace JL. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. British journal of pharmacology. 2007;150(8):996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(12):2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 127.Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Laboratory investigation; a journal of technical methods and pathology. 2008;88(10):1038–1048. doi: 10.1038/labinvest.2008.73. [DOI] [PubMed] [Google Scholar]
- 128.Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26(2):154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 129.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxidants & redox signaling. 2006;8(3-4):661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 130.Kimura Y, Goto Y, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxidants & redox signaling. 2010;12(1):1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 131.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. American journal of physiology Renal physiology. 2009;297(2):F410–419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochemical and biophysical research communications. 2006;351(2):485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 133.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and biophysical research communications. 2006;343(1):303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 134.Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, Kawabata A. Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology. 2007;241(1-2):92–97. doi: 10.1016/j.tox.2007.08.087. [DOI] [PubMed] [Google Scholar]
- 135.Geng B, Cui Y, Zhao J, Yu F, Zhu Y, Xu G, Zhang Z, Tang C, Du J. Hydrogen sulfide downregulates the aortic L-arginine/nitric oxide pathway in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293(4):R1608–1618. doi: 10.1152/ajpregu.00207.2006. [DOI] [PubMed] [Google Scholar]
- 136.Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A. Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology. 2007;232(1-2):138–146. doi: 10.1016/j.tox.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 137.Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free radical biology & medicine. 2006;41(1):106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 138.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. Journal of the American Heart Association. 2012;1(5):e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Altaany Z, Yang G, Wang R. Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. Journal of cellular and molecular medicine. 2013;17(7):879–888. doi: 10.1111/jcmm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eto K, Kimura H. A novel enhancing mechanism for hydrogen sulfide-producing activity of cystathionine beta-synthase. The Journal of biological chemistry. 2002;277(45):42680–42685. doi: 10.1074/jbc.M205835200. [DOI] [PubMed] [Google Scholar]
- 141.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Canadian journal of physiology and pharmacology. 2003;81(9):848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- 142.Anuar F, Whiteman M, Siau JL, Kwong SE, Bhatia M, Moore PK. Nitric oxide-releasing flurbiprofen reduces formation of proinflammatory hydrogen sulfide in lipopolysaccharide-treated rat. British journal of pharmacology. 2006;147(8):966–974. doi: 10.1038/sj.bjp.0706696. [DOI] [PMC free article] [PubMed] [Google Scholar]