Abstract
Benzo[a]pyrene (B[a]P) is a well-known genotoxic polycylic aromatic compound whose toxicity is dependent on signaling via the aryl hydrocarbon receptor (AHR). It is unclear to what extent detrimental effects of B[a]P exposures might impact future generations and whether transgenerational effects might be AHR-dependent. This study examined the effects of developmental B[a]P exposure on 3 generations of zebrafish. Zebrafish embryos were exposed from 6 – 120 hours post fertilization (hpf) to 5 and 10 μM B[a]P and raised in chemical- free water until adulthood (F0). Two generations were raised from F0 fish to evaluate transgenerational inheritance. Morphological, physiological and neurobehavioral parameters were measured at two life stages. Juveniles of the F0 and F2 exhibited hyper locomotor activity, decreased heartbeat and mitochondrial function. B[a]P exposure during development resulted in decreased global DNA methylation levels and generally reduced expression of DNA methyltransferases in wild type zebrafish, with the latter effect largely reversed in an AHR2-null background. Adults from the F0 B[a]P exposed lineage displayed social anxiety-like behavior. Adults in the F2 transgeneration manifested gender-specific increased body mass index (BMI), increased oxygen consumption and hyper-avoidance behavior. Exposure to benzo[a]pyrene during development resulted in transgenerational inheritance of neurobehavioral and physiological deficiencies. Indirect evidence suggested the potential for an AHR2-dependent epigenetic route.
Keywords: zebrafish, benzo[a]pyrene, transgenerational, neurobehavioral, developmental toxicity, physiological deficits, aryl hydrocarbon receptor
Graphical abstract
Introduction
Benzo[a]pyrene (B[a]P) is the most well-characterized member of the polycyclic aromatic hydrocarbon (PAH) family. It is formed nearly anytime that organic matter is combusted and thus found in cigarette smoke, diesel exhaust, grilled and broiled foods, coal tar, and as a byproduct of many industrial processes (ATSDR, 1995; Fang et al., 2004). Because B[a]P is ubiquitous in the environment and in many diets (Howsam and Jones, 1998), human contact is unavoidable. Adverse effects of B[a]P include carcinogenicity, mutagenicity, cardiovascular disease, impaired lung function, neurological effects in children and adults, decreased oxygen saturation levels, artery thickness and blood pressure (Perera et al., 1999; Perera et al., 2006; Gunes et al., 2007; Tang et al., 2007; Geerts et al., 2008; McCallister et al., 2008; D Jung, 2009; Edwards et al., 2010; Incardona et al., 2011; Genies, 2013; Jung et al., 2014; Smargiassi et al., 2014; Gerger et al., 2015; Huang et al., 2015). B[a]P has been detected in human placenta, umbilical cord blood, maternal blood and breast milk samples worldwide (Madhavan and Naidu, 1995; Perera et al., 1998; Perera et al., 2006) and B[a]P exposures produce developmental and reproductive toxicity, and behavior effects in multiple generation animal studies (Tracey et al., 2013; Corrales et al., 2014b; Vignet et al., 2015).
Epigenetic changes are non-genotoxic mechanisms of chemical bioactivity. The major epigenetic alteration modes are DNA methylation and histone/chromatin structural modification. Each of these mechanisms can readily alter the transcriptome independent of changes to the genome. A substantial body of evidence now indicates that epigenetic alterations have an important role in chemical toxicity including carcinogenesis (Chappell et al., 2016). In zebrafish, exposure to benzo[a]pyrene was shown both to induce genome-wide transcriptional changes in embryos (Fang et al., 2015) and gene-specific and genome-wide reductions in DNA methylation (Fang et al., 2013). In mice prenatal exposure to a PAH mixture impaired neurocognitive function and increased anxiety-like behaviors accompanied by methylation changes to the brain derived neurotrophic factor 1 promoter (Miller et al., 2016). In addition, murine oral exposure to BaP at a peri-pubertal stage had a transgenerational effect on testes morphology and sperm function (Mohamed el et al., 2010). Recent epidemiology studies suggested that PAH exposures may lead to epigenetic changes that are associated with a greater risk of disease in subsequent generations (Perera and Herbstman, 2011; Corrales et al., 2014b). In human cohorts, global reductions in DNA methylation cord blood were associated with prenatal exposure to elevated PAH levels (Herbstman et al., 2012). Overall, these studies provide converging evidence implicating epigenetic changes in mediating the effects of PAHBecause of its environmental ubiquity, strong concerns about the possibility of heritable epigenetic changes from B[a]P exposure have focused much attention on this topic. Key to addressing these concerns is the efficient use of a readily tractable animal model. With efforts to reduce animal testing, the zebrafish model has become the premier alternative vertebrate model. Rapid development, large clutch sizes and short generation time make it ideal for generational studies (Sipes et al., 2011; Baker et al., 2014; Corrales et al., 2014b). One can also assess cardiorespiratory function in juvenile and adult zebrafish with a swim tunnel respirometer to identify treatment effects on fitness (Thomas and Janz, 2011; Masse et al., 2013; Gerger et al., 2015). Early stage zebrafish exhibit a highly reproducible larval photomotor response (LPR) behavior of higher swim activity in the dark than the light. (MacPhail et al., 2009; Truong et al., 2012; Noyes et al., 2015). We have demonstrated that developmental chemical exposure results in detectable differences in learning and memory retention in adult zebrafish using an active avoidance test (Truong et al., 2014; Knecht et al., 2017).
Previously, we reported that developmental exposure to B[a]P resulted in hyperactivity in larval zebrafish and compromised learning in adult animals (Knecht et al., 2017). We also observed decreased cardiorespiratory fitness in adult animals. In this study, we sought to investigate trans-generational effects in larval and adult zebrafish (Figure 1) from developmental exposure to B[a]P. We evaluated behavioral and fitness effects in subsequent generations following F0 developmental exposure to 5 and 10 μM B[a]P. By assessing as many complex endpoints as was feasible, we maximized our opportunities to detect chemical-bioactivity. Our focus on the transgenerational impacts of B[a]P necessitated probing subtle endpoints like respiratory fitness and behavior that are easily confounded by morphological abnormalities at higher B[a]P concentrations. Here we present evidence that mitochondrial dysfunction, hyper locomotor activity, decreased heart rate, reduced respiratory fitness and learning and social behavior deficits were manifested epigenetically across three generations.
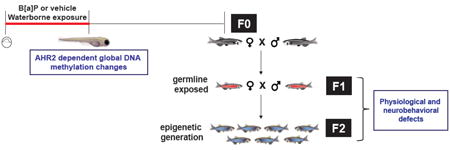
Figure 1. Overview of epigenetic study.
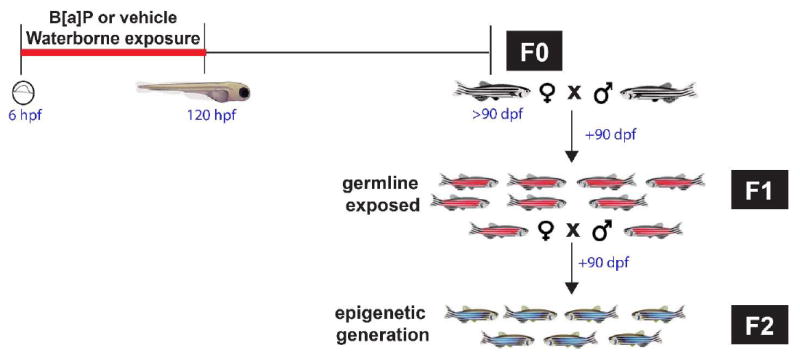
Embryonic zebrafish were developmentally exposed to 0, 5 and 10 μM Benzo[a]pyrene (B[a]P) from 6 - 120 hours post fertilization, then raised until adulthood (>90 days post fertilization; dpf) in chemical- free water [F0]. The F0 cohort was spawned to create 2 subsequent generations. Epigenetic inheritance was evaluated in F2.
Materials and Methods
Zebrafish care
Adult zebrafish were maintained with a water temperature of 28+/- 1°C on a recirculating system with a 14h light: 10h dark photoperiod at the Oregon State University Sinnhuber Aquatic Research Laboratory (SARL). All experiments were conducted with the wild type Tropical 5D or AHR2-null (ahr2hu3335) strains. The ahr2hu3335 line, with a point mutation in ahr2 (ahr2hu3335 strain) is from the Hubrecht Institute (Goodale et al., 2012). Fish husbandry, reproductive techniques, and adult assays were conducted according to Institutional Animal Care and Use Committee protocols at Oregon State University. All embryos used in exposure experiments were collected following group spawning of adult zebrafish as described previously (Kimmel 1995, Reimers 2006).
Benzo[a]pyrene developmental exposure
Benzo[a]pyrene powder standard was purchased from Sigma Aldrich and dissolved in dimethyl sulfoxide (DMSO) to make a stock concentration of 10 mM. For exposure experiments, dilution stocks were made in 100% DMSO, and exposure solutions were prepared with a 1:1000 dilution in embryo medium (Westerfield, 2000). Zebrafish embryos were statically exposed to 5 μM (1.25 ppm), 10 μM (2.5 ppm), or 0.1% DMSO vehicle from 6 - 120 hours post fertilization (hpf) in foil covered 96 well plates. We note that zebrafish are not capable of activating B[a]P metabolism into toxic intermediates via AHR signaling, prior to 6 hpf, because 12 hpf is the earliest expression of zfAHR2, thus it is unlikely that an earlier exposure window would yield any B[a]P impacts on the methylome (Andreasen et al., 2002). These concentrations were selected based on concentrations measured in soil and sediments (ATSDR, 1995; MDEP, 2002) and other zebrafish studies demonstrating no morphological phenotype toxicity, including pericardial edema (Corrales et al., 2014b; Fang et al., 2015; Knecht et al., 2017). For adult studies, exposed zebrafish (F0) at 120 hpf were rinsed several times in fishwater then placed on the recirculating water system and raised to adulthood at a density of 28 mL/larva. From 120 hpf to 45/90 dpf, larvae from each original exposure plate were housed as separate cohorts. After 45/90 dpf, fish were mixed within treatments and housed at a density of ~175-200 mL/adult zebrafish.
Production of B[a]P F1 and F2
To generate B[a]P F1 embryos, a total of 32 males and 32 females randomly selected from each exposure group (0.1% DMSO, 5 μM and 10 μM B[a]P) of the F0 cohorts were group spawned between 6-8 months. F1 embryos were pooled and kept in embryo medium in plastic petri dishes until 5 dpf, then placed on the recirculating fish water system and raised to adulthood in the same density as described for the F0 generation. To generate B[a]P F2 embryos, the same procedure as for F1 embryos was followed with the F1 and F2 cohorts, respectively.
Larval Photo-motor Response (LPR)
The Viewpoint Zebrabox (Viewpoint Behavior Technology) was used to evaluate photoinduced larval locomotor activity in 120hpf larvae. F2 B[a]P 5 and 10 μM embryos were plated one per well in 96-well plates, containing 100 μl of embryo medium. At 5 dpf, each plate was placed in a Zebrabox and the video tracking protocol on the Viewpoint Zebrabox software (version 3.2, Viewpoint Life Science, Lyon, France) was used to track total larval movement over light-dark cycles. The assay consisted of 3 min. light and dark alternating periods, with a total of four light-dark transitions. The integration time was set to 6 seconds and raw data files were processed using custom R scripts (R Core Team, 2016) to average the total distance traveled for each integration, and computed area under the curve. Any mortalities or animals with malformations at 120 hpf were excluded from the data analysis, and statistical outliers were identified by fitting observed values to a double-truncated gamma distribution for each assay, then removed per individual time point measurement. The overall area under the curve of the light and dark periods were calculated for the three light-dark cycles (the first cycle was treated as acclimation) and compared to the vehicle using a Kolmogorov-Smirnov test (p<0.01).
Heartbeat Assay
Heartbeat counts for 6 days post fertilization (dpf) larvae were determined by manually counting the total ventricle beats in 30 seconds at room temperature using a microscope with a 2x objective and a handheld counter and multiplied by 2 to determine heartbeats/min. For the F2, animals, a digital video of the heart was captured and the analysis was automated. We note here that the temperature the F2 animals were kept at for the video capture was 3 degrees lower (23°C) than the manually counted F0 animals (26°C) due to constraints of the automated platform. The effect of the 3°C temperature difference on baseline heartrate (Barrionuevo and Burggren, 1999) is noted in the Results. Larvae with malformations at 6 dpf were not evaluated and 35 - 40 larvae per treatment were measured. A one-way ANOVA with Tukey post hoc test (significance of p<0.05) was conducted to assess the treatment effect.
Oxygen Consumption at 24 hpf
A XF24 extracellular flux analyzer (Seahorse Bioscience) was used to measure the oxygen consumption rate of zebrafish embryos in vivo, modified from (Knecht et al., 2013). B[a]P F2 and embryos were collected at 4 hpf, rinsed in fishwater, and kept in plastic petri dishes in embryo medium until 24 hpf. Zebrafish embryos were then placed, four embryos per well, in 450 μL of unbuffered fish water (0.003% sea salt) in a XF24 islet plate. Oxygen consumption and extracellular acidification rates were measured before and after the addition of 50 μL of 500 μg/mL oligomycin, 55.6 μL of 25 μM FCCP, 61.6 μL of 25 μM Antamycin A, and 67 μL of 65 μM NaAzide. There were 8 measurements before and after each chemical injection, with each measurement consisting of 3 min of mixing, 2 min of waiting, and 3min of measuring. There were four to six wells per treatment, and total AUC values for OCR were calculated for baseline (before injections) and maximum oxygen capacity (following FCCP injection) using the Seahorse XF Reader software and exported as an excel file for statistical analysis and graphing. B[a]P F2 embryos were compared to the F2 control embryos, respectively, using a one-way ANOVA, p<0.05.
Quantification of Global DNA Methylation
To isolate both RNA and DNA from the same biological samples, the Zymo ZR-Duet MiniPrep (Zymo Research, Irvine, CA, Product #: D7001) was used from pools of n=8 zebrafish at both 24 and 120 hpf, according to manufacturer protocol. The DNA samples were eluted and used in the MethylFlash™ Global DNA Methylation Quantification Kit (Epigentek Group, Farmingdale, NY, Product # P-2094) to quantify the percent of methylated cytosine in the genomic DNA. The calculations were made according to manufacturer and Fang et al (Fang et al., 2013). Two-way ANOVA was used to determine the effect of treatment and time on Dnmt expression (p<0.05).
Quantitative Measure of Dnmt using real time PCR
RNA isolated from Zymo ZR-Duet was used as input for gene expression of 6 Dnmt transcripts from embryos exposed from 6-24 and 6-120 hpf embryos from the F0 lineage using either the wild type 5D Tropical or AHR2-null (ahr2hu3335). The ahr2hu3335 line, with a point mutation in ahr2 (ahr2hu3335 strain) is from the Hubrecht Institute. This line was identified from a library of N-ethyl-N-nitrosourea (ENU)-mutagenized zebrafish using the TILLING method and characterized by Goodale et al (Goodale et al., 2012). Gene-specific primers (IDT) are provided in Supplemental Table 1. Applied Biosystems Power SYBR Green RNA-to-CT 1-Step Kit (Catalog # 4389986) was used for all qRT-PCR experiments. Each experiment consists of 10 μL reactions consisting of 5.08 μL SYBR Green PCR one-step master mix and reverse transcription mix 0.4 μL of each 10 mM primer in 0.1% Triton X, 4.2 μL H2O and 2 μg of cDNA in 0.1% Triton X, which were dispensed using the PCR program on the HP D300 Digital Dispenser. The temperature program was used as described by the manufacturer. qRT-PCR analysis was performed with StepOne software (Applied Biosystems), using the Δ ΔCT method with genes normalized to β-actin (Pfaffl, 2001). Three biological replicates of 8 embryos each were analyzed by comparing B[a]P treated to vehicle control over time with a two-way ANOVA (p<0.05) following normalization with β-actin, using R (Team, 2011).
BMI Measurements
Fish were euthanized in accordance with protocols approved by the Oregon State University Institutional Animal Care and Use Committee (IACUC). Weight (mg) and length (cm) were measured and converted to kg and meters, respectively. To compute BMI, the weight (g) was divided by the length squared (m2)(Riu et al., 2014). A one-way ANOVA with Tukey post hoc test (significance of p<0.05) was conducted to assess the treatment effect.
Adult Behavior
To further investigate the adult behavior effects of developmental B[a]P, we tested B[a]P F0 and F2 cohorts for a suite of endpoints. We analyzed differences in social behavior in B[a]P F0 and F2 adults. Fish with observable morphological deficits were removed prior to statistical analysis for the adult behavior assays. Statistical outliers were identified by fitting observed values to a double-truncated gamma distribution for each assay, then removed per individual time point measurement. Following these quality control steps, statistical significance was determined using the Kolmogorov-Smirnov test to compare statistics from an exposed group to those of the corresponding control group. The statistics were computed as the time-averaged differences between an individual time series and the corresponding mean control response over the specified time interval (Reif et al., 2016).
To assess social interactions (shoaling), 48 fish per treatment group (12 groups of 4 adult zebrafish; 2 males and 2 females), 12-16 months old, were tested over a 3 day period beginning at 1 p.m. each day, approximately 30 minutes after the mid-day feeding. For the assay, the fish were co-housed in 1.8 L shoaling tanks (30 cm L x 10 cm W x 15 cm H) and allowed free swim for 1 hour following a 10 min acclimation period. The shoaling behavior was captured using Q-See cameras and Noldus (Leesburg, Virginia) Media Recorder software. The Viewpoint Zebrabox software was used to analyze videos and track the movement of the 4 individual fish within the shoal to compute the inter-individual distance (iid) and nearest-neighbor distance (nnd) parameters. The nearest-neighbor distance is the average of the nearest fish to each of the four fish in the shoal, and the inter-individual distance is the average distance between all four fish to each of the remaining 3 fish within the shoal, both measuring the tightness of the shoaling group. After 1 hour of shoaling, each fish was separated into a new 1.8L tank and recorded for an additional hour and total swim distance was calculated for the entire hour. All parameters were calculated per 1 min. time bins.
The zebrafish visual imaging system (zVIS) was used to measure innate predator avoidance and startle responses from 48 adult fish (24 male and 24 female) per treatment group. The fish were tested once in the assay over a 3-day period with the time of testing consistently beginning at 9 a.m., approximately 30 minutes after the morning feeding. The zVIS system consists of an array of 8 tanks (12 cm x 12 cm) with only single side views of video projections on LCD monitors. Each tank was filled with 750 mL of fish water. This format allowed individual fish to visualize a single predator fish video on the monitor (Eddins et al., 2010). For the predator test, zebrafish were expected to move away from the screen area. Within EthoVision, arenas were created that covered the rims of the square tanks. The arenas were subdivided into three zones: close, middle, and far, the close designating the region nearest the monitor. EthoVision tracked the velocity of the fish and the frequency spent in each zone. zVIS was also used to analyze the fish response to an audio startle stimulus generated by an electric solenoid below each tank. All 8 tanks received a simultaneous tap. The vehicle and B[a]P lineage fish received a total of 10 taps with a 20 second interval in between. Statistical differences between treatment and controls were determined by two-way ANOVA with repeated measures and Tukey HSD post hoc test (p<0.05).
Adult Oxygen Consumption Rates
An AutoResp swim tunnel and Fibox fiber optic oxygen probe (Loligo Systems, Denmark) was used to measure the oxygen consumption rate in groups of 8 male or female adult zebrafish. Oxygen consumption experiments were initiated 2 hours after feeding. Eight animals per group were weighed prior to the experiment and then placed in a small swim tunnel in a 2.5 L tank with an oxygen sensor connected to the AutoResp software. Oxygen consumption rate measurements (O2 mg/kg/hr) were taken every 10 min. consisting of a 240 s flush period in which water was renewed in the swim tunnel with a flush pump, then turned off to create a closed system during a 60s wait period, and a 300s measure period, as described in Masse (Masse et al., 2013). The zebrafish were given a 30 min acclimation period followed by a 20 min free swim at a water flow of 5 cm/sec before swimming against a water current of 10, 20, 30 and 40 cm/sec. The AutoResp software was used to calculate oxygen consumption rates from the slope of oxygen depletion per total weight of the fish. Two measurements were taken at each swimming speed, with the mean value used in statistical analysis. Four to 6 assays were run for each exposure cohort, with all data points included in analysis having an R2 value of 0.8 or higher. Statistical differences between treatment and controls were determined by two-way ANOVA with repeated measures and Tukey HSD post hoc test, p<0.05.
Results
B[a]P alters larval stage heart rate and mitochondrial respiration transgenerationally
The F0 5 and 10 μM B[a]P larvae exhibited decreased ventricle heart rate (Figure 2a). Compared to the vehicle control cohort (with an average of 152 beats per min), there was a significant 25% decrease (128 and 132, respectively). In the epigenetic F2 generation, similar depression of heart rate was observed. The F2 5 and 10 uM B[a]P groups exhibited significant decreases in heart rate of 7 and 14%, respectively compared to F2 controls. The rather dramatic baseline difference in heart rate between F0 and the other generations was potentially due to consistent overestimation by the F0 technique of visual counting and extrapolating and the temperature variability (Barrionuevo and Burggren, 1999). Adoption of a digital motion analysis technique by the time the F2 measurements were made obviated reliance on extrapolation (Pylatiuk et al., 2014).
Figure 2. Larval behavioral, heartbeat and mitochondrial function in F0 and epigenetic generations F2.
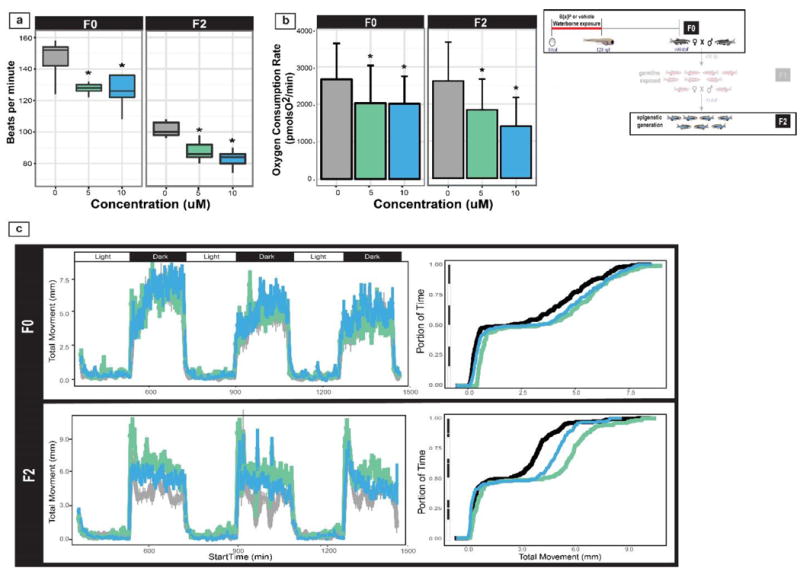
The inset (top right) illustrates the generation(s) discussed. (a) Heartrate differences for the F0 and F2 5 and 10 μM B[a]P and controls groups larvae. Note that while the relative treatments effects are conserved across generations, baseline heartrates differ because F2 animals were assayed 3°C cooler than the F0 animals (see Methods for details) (b) The oxygen consumption rate was measured in 24 hpf larvae in F0, and F2. * denotes statistical significance based on a one-way ANOVA (p < 0.05). (c) 5 day LPR response in the F2 larvae assessed in the light-dark assay.
The F0 and F2 larval mitochondrial respiration was evaluated at 24 – 26 hpf (Figure 2b). The F0 5 and 10 μM B[a]P groups showed significantly decreased mitochondrial respiration at 24 - 26 hpf. Baseline oxygen consumption rates (OCR) associated with the 5 and 10 μM B[a]P exposures were significantly decreased 23.8 and 24.5%, respectively, compared to vehicle controls, and the maximum oxygen capacity (following FCCP injection) was significantly reduced 21.6 and 17.9%, respectively compared to vehicle controls. To determine if the mitochondrial respiration effect would persist in the epigenetic generation, 26 hpf F2 embryos were similarly evaluated. The F2 5 and 10 μM B[a]P groups had significantly decreased oxygen consumption rates (21.5% and 14% lower OCR compared to F2 controls, respectively).
B[a]P associated hyperactive larval behavior is transgenerational
Previously, we demonstrated that direct developmental exposures to 5 and 10 μM B[a]P produced hyperactivity in only the dark periods of the three light-dark cycles in a larval photomotor response assay (LPR). To test our hypothesis that B[a]P larval neurotoxicity persists transgenerationally, the larvae from the first epigenetic generation were evaluated in the 24 minutes LPR assay. The F2 5 and 10 μM B[a]P groups exhibited a statistically significant increase in total distance moved in all intervals, but this strong response was driven by the increase distance moved in the dark (p< 0.005) (Figure 2c) and demonstrated that this larval behavioral phenotype was heritable to the first transgeneration.
Effect of B[a]P on global DNA methylation and on Dnmt gene expression
We hypothesized that neurophysiological effects associated with B[a]P exposure would be concomitant with methylation changes detectable in zebrafish DNA. Global DNA methylation and DNA methyltransferase (Dnmt) mRNA expression were measured in developing F0 5 and 10 μM B[a]P exposed larvae (Figure 3). There was no change in percent cytosine methylation at 24 hpf, but at 120 hpf a significant decrease in 5-methylcytosine levels was detected for both the 5 and 10 μM B[a]P groups (Figure 3a, 45 and 57%, respectively). To determine if DNA methyltransferase (Dnmt) gene expression was altered, the mRNA levels of six Dnmts were evaluated. We adapted the nomenclature based on a recent molecular evolution and phylogenetic analysis (Campos et al., 2012). B[a]P exposure at 5 μM was associated with significant reduction in Dnmt1 expression at both 24 and 120 hpf (Figure 3b). B[a]P exposure selectively altered the expression of 5 Dnmt3 genes; most notably, Dnmt3a2 was significantly reduced by exposure to 5 and 10 μM B[a]P at both developmental time points. Relative to vehicle exposed larvae, the expression of Dnmt3b2 was elevated by B[a]P exposure at 24 hpf, and it was reduced at 120 hpf. Dnmt3a1 and Dnmt3b1 expression were not affected at 24 hpf, but were significantly reduced by 120 hpf in the 5 and 10 μM B[a]P groups. It is important to note that at these concentrations of B[a]P all zebrafish embryos develop phenotypically indistinguishable from unexposed zebrafish.
Figure 3. Global DNA methylation and Dnmt expression at 24 and 120 hpf in F0 embryos exposed to B[a]P.
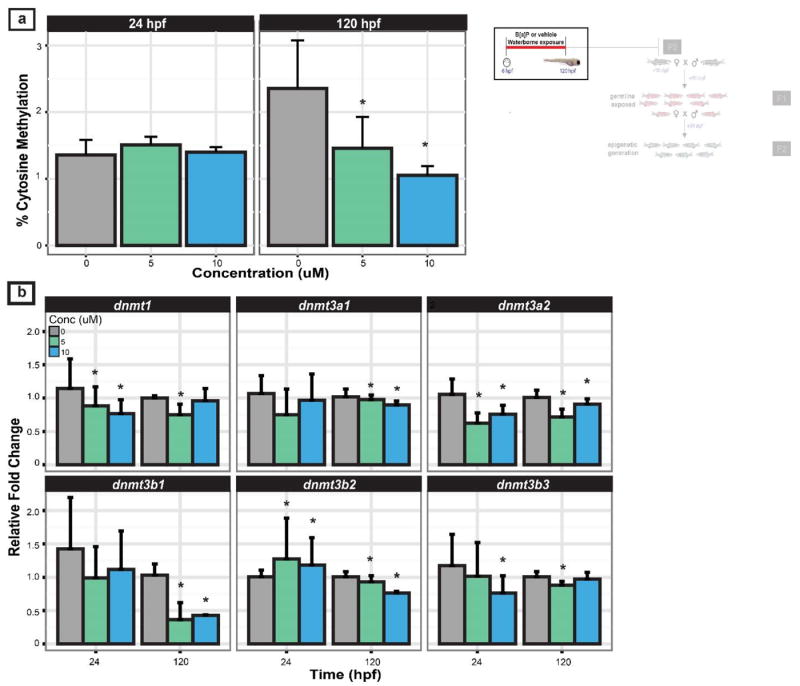
The inset (top right) illustrates the generation(s) discussed. (a) Global DNA methylation for F0 5 and 10 μM B[a]P and F0 control groups. (b) Dnmt expression measured at 24 and 120 hpf. Beta-actin was used as an internal standard. All values represent mean + standard error of mean (S.E.M; n=3) of fold change relative to control. Two-way ANOVA was used to determine the effect of treatment and time on Dnmt expression. A Tukey’s post-hoc test was used to determine statistical significance denoted by asterisks from the vehicle lineage (p<0.05).
The role of AHR2 in Dnmt gene expression
There is ample evidence that some PAHs and xenobiotics bind to the aryl hydrocarbon receptor (AHR) and that, for these ligands, the ensuing toxicity is usually AHR dependent. To determine whether altered expression of the Dnmt genes was AHR-dependent, we exposed ahr2-null (ahr2hu3335) embryos to 0 and 10 μM B[a]P and evaluated the expression of seven DNA methyltransferases. At 24 hpf, the 7 Dnmt genes exhibited an even stronger reduction in expression in the ahr2-null background than in the wild type background following exposure to 10 uM B[a]P (Figure 4, left panel, within the gray shaded polygon). However, by 120 hpf, B[a]P exposure in ahr2-null background resulted in mostly elevated expression of the Dnmt 3B family members (Figure 4, right panel, outside the gray shaded polygon), while in the functional ahr2 background, those transcripts were mostly reduced.
Figure 4. Dnmt expression in wild type (WT) and ahr2hu2334 embryos exposed to 0 and 10 μM B[a]P.
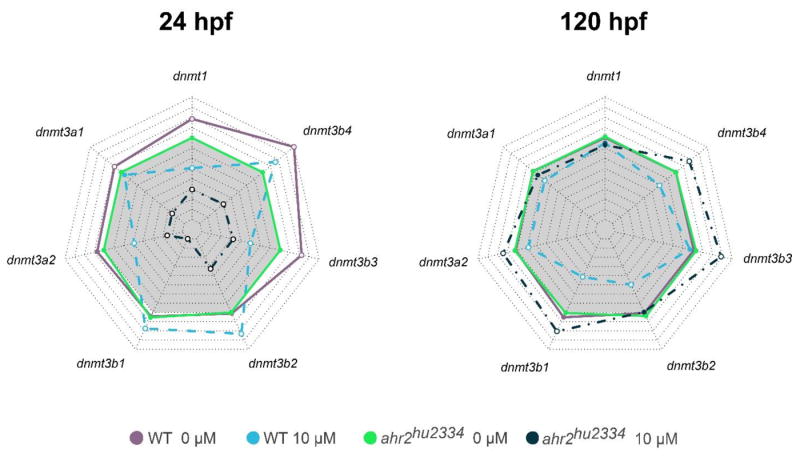
Gene expression changes were evaluated in WT and ahr2-null embryos at 24 (left panel) and 120 hpf (right panel). Beta-actin was used as an internal standard, and all values represent fold change relative to the controls. Two-way ANOVA with a Tukey Post Hoc was used to determine statistical significance. Points filled with white represent statistical significance (p<0.05). The gray shaded polygon represents 1-fold change. Any points within the gray represent repression of the gene.
B[a]P exposure leads to heritable changes in adult BMI and fitness
The adults raised from developmental (6 - 120 hpf) exposure to B[a]P were evaluated for changes to their body mass index (BMI), which was calculated by dividing the body weight (g) by the square of the body length (cm). The BMI was significantly lower for both B[a]P concentrations in the males, but only for 5 μM B[a]P in the females. In the next two subsequent generations, the average female BMI significantly increased relative to the vehicle control, while the male BMI was no different from the vehicle group by the time the F2 generation was reached (Figure 5a). To evaluate whether the BMI changes influenced zebrafish fitness, the amount of oxygen consumption was measured after exercising at 5 different swim speeds. As swim speed increased gradually, the oxygen consumption rate (OCR) at each speed and the B[a]P group’s ability to maintain the basal OCR was compared to the vehicle group’s performance. As Figure 5b illustrates, for the F0 (directly exposed to B[a]P), both concentrations of B[a]P significantly increased OCR. There were 55.9% and 59.6% increases in baseline OCR for groups treated with 5 and 10 μM B[a]P, respectively. The F1 5 and 10 μM B[a]P groups exhibited 16.4% and 30% increases in baseline OCR, respectively, and a ~32% increase at the highest swimming speeds. The F2 10 μM B[a]P group had an increased baseline OCR of 27.5% and exhibited significantly increased oxygen consumption over that of the F2 control animals at the highest swim speeds. This trend persisted into the transgeneration, suggesting that the B[a]P associated decrease in fitness was inherited.
Figure 5. Physiological characteristics and fitness in F0 and F2 cohort.
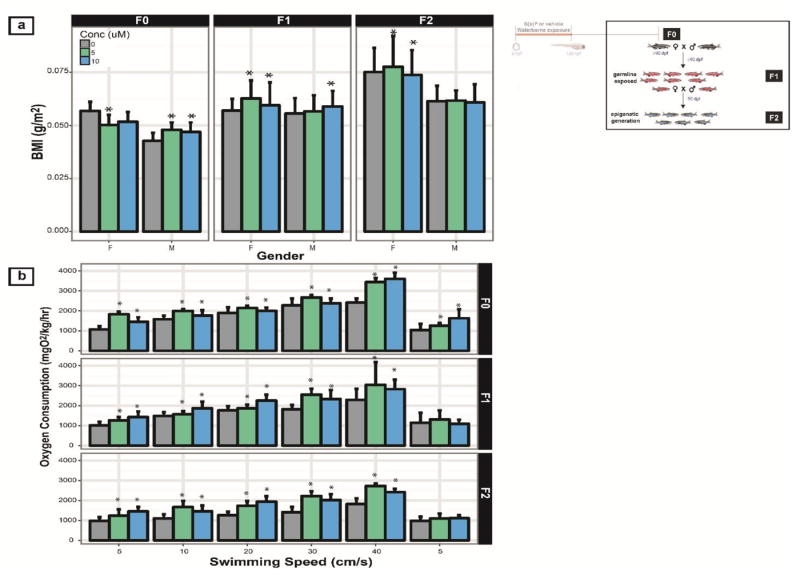
The inset (top right) illustrates the generation(s) discussed. (a) The BMI for adult zebrafish from F0 and F2 were assessed to identify potential gender differences, and (b) the oxygen consumption rate over 5 speeds were measured. * denotes statistical significance relative to vehicle (p < 0.05, two-way ANOVA).
Heritable changes in adult zebrafish behavior
Building on prior studies where developmental exposures low concentration B[a]P resulted in impaired learning in adult animals in an active avoidance test, we evaluated learning and memory, anxiety, and social behaviors in the F0 and ensuing generations in the present study. To evaluate social perception, adult shoaling behavior was evaluated via the inter-individual distance (iid) and nearest neighbor distance (nnd) measures. While the F0 10 μM B[a]P group was associated with significantly increased iid and nnd, indicative of less coherent shoaling, the F1 and F2 progeny of the B[a]P exposed animals did not differ from the control animals in their shoaling behavior (Figure 6).
Figure 6. Persistent neurobehavioral deficits in F0, F1 and F2 cohort.
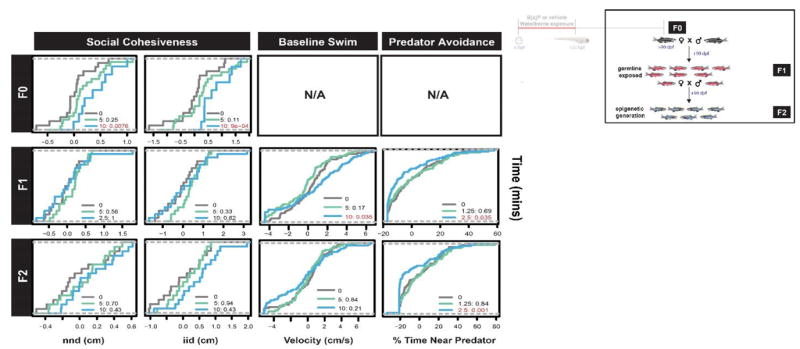
The inset (top right) illustrates the generation(s) discussed. F0 were evaluated in 1 neurobehavioral assay with 2 endpoints (nnd and iid) using 24 males and 24 females per treatment group. The N/A values denote assays that were not available at the time the F0 generation was tested. The cumulative distribution function (cdf) is illustrated for each lineage, with red text denoting statistically significant differences from the referent group (see Methods). The assays measured nearest neighbor distance (nnd), inter-individual distance (iid), the swimming velocity, and the preference for avoidance (% Time Near Predator). The gray line in each panel represents the vehicle lineage, with colored treatment lines indicating increases (right shift) or decreases (left shift) along the cumulative distribution for each assay.
To evaluate anxiety related behaviors, adult zebrafish predator avoidance, swim pattern in isolation, and startle response were examined. Unfortunately, during the propagation of the B[a]P lineage, these assays were not available until the F1. The F1 5 and 10 μM B[a]P lineages exhibited hyperactive swimming behavior in isolation relative to the vehicle adults (data not shown). These same F1 animals also exhibited a hyperactive startle response that was not present in the F2 (Figure 6). The F1 10 μM B[a]P group exhibited hyper avoidance behavior measured as significantly less time spent (12.7% compared to 17% in the vehicle control) proximal to the predator image than did vehicle control fish. The F2 generation also exhibited social anxiety- like behavior. The F2 5 μM B[a]P group spent 25% more time away from the predator image compared to the vehicle controls. Direct and indirect exposure to B[a]P in the F0 and the F1 generations, respectively was associated with hyperactivity in larvae and adults, but was not manifest in the F2 transgeneration. Instead, predator avoidance was enhanced in the F2 transgeneration.
Discussion
Investigation of potential epigenetic inheritance of B[a]P effects in zebrafish stems from our overlapping goals of 1) understanding the role of the aryl hydrocarbon receptor (AHR) in disease and 2) using the developmental zebrafish model as an experimental link to growing epidemiological evidence associating legacy hydrocarbon contamination with childhood obesity and neurological deficits. There is strong evidence that epigenetics is a biological mechanism for transmission of agent effects across generations. That environmental contaminants can induce non-genetic alterations in germ cells and be transmitted to subsequent generations is radically reshaping our understanding of how environmental health affects biology. Epigenetic transgenerational inheritance has been observed in plants, worms, flies, fish, rodents, pigs and humans (Pembrey et al., 2014; Skinner, 2014; Wei et al., 2015; Hanson and Skinner, 2016). A range of environmental factors including toxicants, nutrition and stress promote epigenetic transgenerational inheritance of disease and phenotype variation (Skinner et al., 2015).
A sizeable body of literature from rodents and fish models offers direct evidence that developmental PAH exposure can result in neurologic and cardiovascular deficits and a predilection for obesity in later life. As the prototypical PAH, much of this evidence is derived from various B[a]P exposure regimens. It is important to recognize the benefit of evaluating behavioral and fitness phenotypes at levels that occur below overt toxicity, such as malformation or mortality, as the responses are more likely to be related to effects occurring at environmental exposures.
To date, multigenerational studies in fish have primarily focused on the reproductive measures of endocrine disruptors, most commonly bisphenol A (Sohoni et al., 2001; Staples et al., 2011), nonylphenol (Holdway et al., 2008), 2,3,7,8-tetrachlorodibenzo-p-dioxin (King Heiden et al., 2009) and 17α-ethinylestradiol (Zha et al., 2008). But important work has recently been reported with PAHs. Exposure of a medaka F0 generation to an environmentally relevant concentration of B[a]P (1 ug/L) resulted in impaired bone formation that persisted to at least the F3 generation and the identities of the dyregulated target genes suggested that ancestral B[a]P exposure most likely perturbed chordoblast, chondroblast and osteoblast differentiation (Seemann et al., 2015; Seemann et al., 2017). The F1 and F2 zebrafish larval locomotor activity, similar to the endpoint we report, was potentiated up or down depending on the PAH mixture that the F0 was exposed to (Vignet et al., 2015). Transgenerational inheritance (F2 and beyond in fish) of deformities was reported following exposure of the F0 zebrafish generation to dietary B[a]P (Corrales et al., 2014b). A follow up B[a]P study by the same group demonstrated decreased global methylation and altered promoter methylation among a panel of 21 orthologous zebrafish genes known for their role in human diseases (Corrales et al., 2014a). A transcriptomic study by the same group, using water-borne B[a]P exposure of zebrafish F0 adults and their F1 progeny, out to 96 hours post fertilization, reported that many disease pathways were predicted to be activated in the larvae, including organismal death, growth failure, abnormal morphology of embryonic tissue, congenital heart disease, and adverse neuritogenesis, consistent with the phenotypic outcomes of BaP exposure (Fang et al., 2015). Members of the Dnmt3 family are required for proper neurogenesis and found in the zebrafish brain and retina (Rai et al., 2010; Hu et al., 2014) and Dnmt3a is critically important in regulating embryonic cardiomyocyte gene expression, morphology and function(Fang et al., 2016).We note that these phenotype outcomes and global DNA methylation changes are consistent with some of the transgenerational B[a]P phenotypes we have reported. Most recently another group reported that dietary PAH administered to parent zebrafish at a concentration similar to environmental measures, resulted in F2 offspring with aberrant photomotor behavior and evidence of endocrine disruption (Vignet et al., 2015).
The current study evaluated transgenerational effects of B[a]P exposure in zebrafish. We showed that some of the effects of developmental exposure to waterborne B[a]P in the F0 were also manifested in the F2 transgeneration as decreased mitochondrial respiration, hyperactive larval behavior and fitness deficit. A summary of the results is shown in Table 1. Adult F2 animals were not different from the vehicle control animals in shoaling behavior or startle response, but did recapitulate the anxiety- like/freezing behavior of the B[a]P exposure lineage in the predator avoidance assay. These changes may have been programmed into the maternal genome during the F0 B[a]P exposure and manifested as long-lasting neurotoxicity and compromised physical fitness.
Table 1.
Summary results by assay in B[a]P exposed F0 and unexposed trans (F2) generations relative to control animal responses.
| Assay, age, figure | F0 – B[a]P exposed (5 & 10 μM) | F2 – non-exposed transgeneration |
|---|---|---|
| Heartrate | Depressed (P<0.05) | Depressed (P<0.05) |
| 120 hpf, Fig. 2a | ||
| Oxygen consumption | Depressed (P<0.05) | Depressed (P<0.05) |
| 24 hpf, Fig. 2b | ||
| Larval photomotor response | Hyperactive (P<0.05) | Hyperactive (P<0.05) |
| 120 hpf, Fig. 2c | ||
| % Cytosine methylation | No differences at 24 hpf Decreased (P<0.05) at 120 hpf |
|
| 24 & 120 hpf, Fig. 3a | ||
| DNA methyltransferase transcription, 24 & 120 hpf, Fig. 3b | Mixed responses at 24 hpf Depressed (P<0.05) at 120 hpf |
|
| AhR dependency of Dnmt transcription, 24 & 120 hpf, Fig. 4 | Generally depressed (P<0.05) at 24 hpf in ahr2-null background. | |
| Generally enhanced (P<0.05) at 120 hpf in ahr2-null background | ||
| Body mass index-sex specific Adult, Fig. 5a | Increased (P<0.05) in males | Modest increase (P<0.05) in females |
| Adult oxygen consumption with Exercise Fig. 5b | Increased (P<0.05) | Increased (P<0.05) |
| Adult behavioral deficits Fig. 6 | Altered social behavior (P<0.05) | Altered predator avoidance (P<0.05) |
While we did not directly demonstrate Ahr2-dependent epigenetic inheritance of methylation markers, we did uncover indirect evidence. Upon initial B[a]P exposure during development, global DNA methylation levels were altered and the expression of a number of DNA methyltransferases was aberrant. B[a]P exposure of wild type animals was associated with reduced transcript abundance of a suite of Dnmt3 family genes but B[a]P in the absence of a functional AHR2 receptor resulted in considerably stronger reduction of Dnmt family transcription. Our preliminary hypothesis was that B[a]P ligand binding and activation of an AHR2/ARNT1 complex might downregulate expression via interaction with aryl hydrocarbon response elements in the Dnmt promoters. Thus, we anticipated that absence of the AHR2 would prevent the B[a]P associated downregulation of Dnmt transcription. Clearly, a different mechanism of B[a]P associated transcriptional regulation of Dnmt genes may be at work, perhaps via B[a]p’s ability to substantially modify histone acetylation (Sadikovic and Rodenhiser, 2006) and modulate expression of regulatory non-coding RNAs (Brevik et al., 2012). Reduction in global DNA methylation levels detected at 120 hpf would suggest that B[a]P did interfere with DNA methyltransferases or its substrate S-adenosyl methionine (Kamstra et al., 2015). The combination of altered global methylation levels and misregulated expression of Dnmts provides indirect evidence that the transgenerational inheritance of B[a]P associated neurobehavioral and physiological deficits were due, at least in part, to epigenetic changes in methylation patterns of the zebrafish genome.
Conclusions
This manuscript establishes an initial understanding that developmental exposure of zebrafish to the prototypical PAH, B[a]P, leads to transgenerational changes in behavior and fitness. A plausible mechanism for this phenomenon is via inheritance of B[a]P elicited epigenetic changes in the F2 transgeneration, a mechanism for which there is now substantial scientific precedence from similar xenobiotics. We hypothesize that other toxic xenobiotics that bind and activate the AHR could produce persisting impacts by a similar mechanism. An important next step is to determine the patterns of DNA methylation in wild type and ahr2-null backgrounds and establish how exactly the AHR2 is involved. This will enable both a broader and deeper understanding of the mechanism by which epigenetic inheritance of similar xenobiotic toxicity occurs.
Supplementary Material
Highlights.
Developmental exposure to benzo[a]pyrene results in transgenerational effects
Neurobehavioral and physiological functions impacted across multiple generations
B[a]P decreases global DNA methylation and DNA methyltransferase expression
Behavioral methylation and gene changes are AHR2-dependent
Acknowledgments
The authors would also like to thank Lindsey St. Mary, Jane La Du, and Kimberly Hayward from the Tanguay laboratory for the technical assistances and SARL for the fish husbandry. This work was supported by NIEHS grants P42 ES016465, and P30 ES000210. Pacific Northwest National Laboratory is a multi-program laboratory operated by Battelle for the U.S. Department of Energy under Contract DE-AC05-76RL01830.
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol Sci. 2002;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- ATSDR, A.f.T.S.a.D.R. Services, UDoHaH, (Ed) Public Health Service; Atlanta, GA: 1995. Toxicological profile for Polycyclic Aromatic Hydrocarbons (PAHs) [Google Scholar]
- Baker TR, King-Heiden TC, Peterson RE, Heideman W. Dioxin induction of transgenerational inheritance of disease in zebrafish. Mol Cell Endocrinol. 2014;398:36–41. doi: 10.1016/j.mce.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo WR, Burggren WW. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am J Physiol. 1999;276:R505–513. doi: 10.1152/ajpregu.1999.276.2.R505. [DOI] [PubMed] [Google Scholar]
- Brevik A, Lindeman B, Brunborg G, Duale N. Paternal Benzo[a]pyrene Exposure Modulates MicroRNA Expression Patterns in the Developing Mouse Embryo. Int J Cell Biol. 2012;2012 doi: 10.1155/2012/407431. 407431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos C, Valente LMP, Fernandes JMO. Molecular evolution of zebrafish dnmt3 genes and thermal plasticity of their expression during embryonic development. Gene. 2012;500:93–100. doi: 10.1016/j.gene.2012.03.041. [DOI] [PubMed] [Google Scholar]
- Chappell G, Pogribny IP, Guyton KZ, Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: A systematic literature review. Mutation research. Reviews in mutation research. 2016;768:27–45. doi: 10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Fang X, Thornton C, Mei W, Barbazuk WB, Duke M, Scheffler BE, Willett KL. Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure. Comp Biochem Physiol C Toxicol Pharmacol. 2014a;163:37–46. doi: 10.1016/j.cbpc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Thornton C, White M, Willett KL. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat Toxicol. 2014b;148:16–26. doi: 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, C Y, Collins LB, Swenberg JA, Di Giulio RT. Effects of benzo[a]pyrene on mitochondrial and nuclear DNA damage in Atlantic killifish (Fundulus heteroclitus) from a creosote-contaminated and reference site. Aquatic Toxicology. 2009;95:44–51. doi: 10.1016/j.aquatox.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, Flak E, Li Z, Wang S, Rauh V, Perera F. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118:1326–1331. doi: 10.1289/ehp.0901070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang GC, Chang CN, Wu YS, Fu PP, Yang IL, Chen MH. Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in central Taiwan, Taichung. Science of the Total Environment. 2004;327:135–146. doi: 10.1016/j.scitotenv.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Fang X, Corrales J, Thornton C, Clerk T, Scheffler BE, Willett KL. Transcriptomic Changes in Zebrafish Embryos and Larvae Following Benzo[a]pyrene Exposure. Toxicol Sci. 2015;146:395–411. doi: 10.1093/toxsci/kfv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Poulsen RR, Wang-Hu J, Shi O, Calvo NS, Simmons CS, Rivkees SA, Wendler CC. Knockdown of DNA methyltransferase 3a alters gene expression and inhibits function of embryonic cardiomyocytes. FASEB J. 2016;30:3238–3255. doi: 10.1096/fj.201600346R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Thornton C, Scheffler BE, Willett KL. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environ Toxicol Phar. 2013;36:40–50. doi: 10.1016/j.etap.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;28:2296–2302. doi: 10.1161/ATVBAHA.108.173229. [DOI] [PubMed] [Google Scholar]
- Genies C, Maitre Anne, Lefebvre Emmanuel, Jullien Amandine, Chopard-Lallier Marianne, Douki Thierry. The Extreme Variety of Genotoxic Response to Benzo[a]pyrene in Three Different Human Cell Lines from Three Different Organs. PLoS One. 2013;8:e78356. doi: 10.1371/journal.pone.0078356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerger CJ, Thomas JK, Janz DM, Weber LP. Acute effects of beta-naphthoflavone on cardiorespiratory function and metabolism in adult zebrafish (Danio rerio) Fish physiology and biochemistry. 2015;41:289–298. doi: 10.1007/s10695-014-9982-z. [DOI] [PubMed] [Google Scholar]
- Goodale BC, La Du JK, Bisson WH, Janszen DB, Waters KM, Tanguay RL. AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS One. 2012;7:e29346. doi: 10.1371/journal.pone.0029346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes T, Koklu E, Yikilmaz A, Ozturk MA, Akcakus M, Kurtoglu S, Coskun A, Koklu S. Influence of maternal smoking on neonatal aortic intima-media thickness, serum IGF-I and IGFBP-3 levels. Eur J Pediatr. 2007;166:1039–1044. doi: 10.1007/s00431-006-0376-9. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Skinner MK. Developmental origins of epigenetic transgenerational inheritance. Environ Epigenet. 2016;2 doi: 10.1093/eep/dvw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjodin A, Li Z, Camann D, Perera FP. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 2012;120:733–738. doi: 10.1289/ehp.1104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdway DA, Hefferman J, Smith A. Multigeneration assessment of nonylphenol and endosulfan using a model Australian freshwater fish, Melanotaenia fluviatilis. Environ Toxicol. 2008;23:253–262. doi: 10.1002/tox.20329. [DOI] [PubMed] [Google Scholar]
- Howsam M, Jones KC. Sources of PAHs in the environment, Anthropogenic compounds, Part I: PAHs and Related Compounds. Springer-Verlag; Berlin: 1998. pp. 137–174. [Google Scholar]
- Hu N, Strobl-Mazzulla PH, Bronner ME. Epigenetic Regulation in Neural Crest Development. Developmental biology. 2014;396:159–168. doi: 10.1016/j.ydbio.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Zuo Z, Zhang Y, Wang C. Toxicogenomic analysis in the combined effect of tributyltin and benzo[a]pyrene on the development of zebrafish embryos. Aquat Toxicol. 2015;158:157–164. doi: 10.1016/j.aquatox.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Linbo TL, Scholz NL. Cardiac toxicity of 5-ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicol Appl Pharmacol. 2011;257:242–249. doi: 10.1016/j.taap.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Jung KH, Liu B, Lovinsky-Desir S, Yan B, Camann D, Sjodin A, Li Z, Perera F, Kinney P, Chillrud S, Miller RL. Time trends of polycyclic aromatic hydrocarbon exposure in New York City from 2001 to 2012: assessed by repeat air and urine samples. Environ Res. 2014;131:95–103. doi: 10.1016/j.envres.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamstra JH, Alestrom P, Kooter JM, Legler J. Zebrafish as a model to study the role of DNA methylation in environmental toxicology. Environmental science and pollution research international. 2015;22:16262–16276. doi: 10.1007/s11356-014-3466-7. [DOI] [PubMed] [Google Scholar]
- King Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AL, Goodale BC, Truong L, Simonich MT, Swanson AJ, Matzke MM, Anderson KA, Waters KM, Tanguay RL. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol Appl Pharmacol. 2013;271:266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht AL, Truong L, Simonich MT, Tanguay RL. Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish. Neurotoxicol Teratol. 2017;59:27–34. doi: 10.1016/j.ntt.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology. 2009;30:52–58. doi: 10.1016/j.neuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Madhavan ND, Naidu KA. Polycyclic aromatic hydrocarbons in placenta, maternal blood, umbilical cord blood and milk of Indian women. Hum Exp Toxicol. 1995;14:503–506. doi: 10.1177/096032719501400607. [DOI] [PubMed] [Google Scholar]
- Masse AJ, Thomas JK, Janz DM. Reduced swim performance and aerobic capacity in adult zebrafish exposed to waterborne selenite. Comp Biochem Physiol C Toxicol Pharmacol. 2013;157:266–271. doi: 10.1016/j.cbpc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, Hood DB. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology. 2008;29:846–854. doi: 10.1016/j.neuro.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDEP, M.D.o.E.P. Protection, MDoE. Boston, MA: 2002. Background Levels of Polycyclic Aromatic Hydrocarbons and Metals in Soil. [Google Scholar]
- Miller RL, Yan Z, Maher C, Zhang H, Gudsnuk K, McDonald J, Champagne FA. Impact of prenatal polycyclic aromatic hydrocarbon exposure on behavior, cortical gene expression and DNA methylation of the Bdnf gene. Neuroepigenetics. 2016;5:11–18. doi: 10.1016/j.nepig.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed el SA, Song WH, Oh SA, Park YJ, You YA, Lee S, Choi JY, Kim YJ, Jo I, Pang MG. The transgenerational impact of benzo(a)pyrene on murine male fertility. Hum Reprod. 2010;25:2427–2433. doi: 10.1093/humrep/deq205. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci. 2015;145:177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey M, Saffery R, Bygren LO Network in Epigenetic, E. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet. 2014;51:563–572. doi: 10.1136/jmedgenet-2014-102577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(Suppl 3):451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Whyatt RM, Jedrychowski W, Rauh V, Manchester D, Santella RM, Ottman R. Recent developments in molecular epidemiology: A study of the effects of environmental polycyclic aromatic hydrocarbons on birth outcomes in Poland. Am J Epidemiol. 1998;147:309–314. doi: 10.1093/oxfordjournals.aje.a009451. [DOI] [PubMed] [Google Scholar]
- Pylatiuk C, Sanchez D, Mikut R, Alshut R, Reischl M, Hirth S, Rottbauer W, Just S. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish. 2014;11:379–383. doi: 10.1089/zeb.2014.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, Jones DA. Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Biol Chem. 2010;285:4110–4121. doi: 10.1074/jbc.M109.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif DM, Truong L, Mandrell D, Marvel S, Zhang G, Tanguay RL. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch Toxicol. 2016;90:1459–1470. doi: 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu A, McCollum CW, Pinto CL, Grimaldi M, Hillenweck A, Perdu E, Zalko D, Bernard L, Laudet V, Balaguer P, Bondesson M, Gustafsson JA. Halogenated bisphenol-A analogs act as obesogens in zebrafish larvae (Danio rerio) Toxicol Sci. 2014;139:48–58. doi: 10.1093/toxsci/kfu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikovic B, Rodenhiser DI. Benzopyrene exposure disrupts DNA methylation and growth dynamics in breast cancer cells. Toxicol Appl Pharmacol. 2006;216:458–468. doi: 10.1016/j.taap.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Seemann F, Jeong CB, Zhang G, Wan MT, Guo B, Peterson DR, Lee JS, Au DW. Ancestral benzo[a]pyrene exposure affects bone integrity in F3 adult fish (Oryzias latipes) Aquat Toxicol. 2017;183:127–134. doi: 10.1016/j.aquatox.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Seemann F, Peterson DR, Witten PE, Guo BS, Shanthanagouda AH, Ye RR, Zhang G, Au DW. Insight into the transgenerational effect of benzo[a]pyrene on bone formation in a teleost fish (Oryzias latipes) Comp Biochem Physiol C Toxicol Pharmacol. 2015;178:60–67. doi: 10.1016/j.cbpc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Sipes NS, Padilla S, Knudsen TB. Zebrafish: as an integrative model for twenty-first century toxicity testing. Birth Defects Res, Part C. 2011;93:256–267. doi: 10.1002/bdrc.20214. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Endocrine disruptor induction of epigenetic transgenerational inheritance of disease. Mol Cell Endocrinol. 2014;398:4–12. doi: 10.1016/j.mce.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Guerrero-Bosagna C, Haque MM. Environmentally induced epigenetic transgenerational inheritance of sperm epimutations promote genetic mutations. Epigenetics. 2015;10:762–771. doi: 10.1080/15592294.2015.1062207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smargiassi A, Goldberg MS, Wheeler AJ, Plante C, Valois MF, Mallach G, Kauri LM, Shutt R, Bartlett S, Raphoz M, Liu L. Associations between personal exposure to air pollutants and lung function tests and cardiovascular indices among children with asthma living near an industrial complex and petroleum refineries. Environmental Research. 2014;132:38–45. doi: 10.1016/j.envres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas) Environ Sci Technol. 2001;35:2917–2925. doi: 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
- Staples CA, Tilghman Hall A, Friederich U, Caspers N, Klecka GM. Early life-stage and multigeneration toxicity study with bisphenol A and fathead minnows (Pimephales promelas) Ecotoxicol Environ Saf. 2011;74:1548–1557. doi: 10.1016/j.ecoenv.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Tang D, Simonich MT, Innes RW. Mutations in LACS2, a long-chain acyl-coenzyme A synthetase, enhance susceptibility to avirulent Pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant physiology. 2007;144:1093–1103. doi: 10.1104/pp.106.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. Vienna, Austria: 2011. [Google Scholar]
- Thomas JK, Janz DM. Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol. 2011;102:79–86. doi: 10.1016/j.aquatox.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Mandrell D, Mandrell R, Simonich M, Tanguay RL. A rapid throughput approach identifies cognitive deficits in adult zebrafish from developmental exposure to polybrominated flame retardants. Neurotoxicology. 2014;43:134–142. doi: 10.1016/j.neuro.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155:269–274. doi: 10.1016/j.cbpc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignet C, Joassard L, Lyphout L, Guionnet T, Goubeau M, Le Menach K, Brion F, Kah O, Chung BC, Budzinski H, Begout ML, Cousin X. Exposures of zebrafish through diet to three environmentally relevant mixtures of PAHs produce behavioral disruptions in unexposed F1 and F2 descendant. Environmental science and pollution research international. 2015;22:16371–16383. doi: 10.1007/s11356-015-4157-8. [DOI] [PubMed] [Google Scholar]
- Wei Y, Schatten H, Sun QY. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum Reprod Update. 2015;21:194–208. doi: 10.1093/humupd/dmu061. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) University of Oregon; Eugene, OR: 2000. The zebrafish book. [Google Scholar]
- Zha J, Sun L, Zhou Y, Spear PA, Ma M, Wang Z. Assessment of 17alpha-ethinylestradiol effects and underlying mechanisms in a continuous, multigeneration exposure of the Chinese rare minnow (Gobiocypris rarus) Toxicol Appl Pharmacol. 2008;226:298–308. doi: 10.1016/j.taap.2007.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


