Abstract
Objective
To characterize in rice rats: (a) periodontitis (PD) progress with feeding of standard laboratory rat chow (STD) during ages 4–80 weeks; and (b) PD progress with feeding of a high sucrose-casein (H-SC) diet during young adulthood.
Methods
One group (N = 12) was euthanized at age 4 weeks (Baseline). Four groups (N = 8–16) consumed a STD diet from baseline and were necropsied at ages 22, 30, 52, and 80 weeks. Three groups (N= 10–16) consumed an H-SC diet from baseline. Two were necropsied at ages 22 and 30 weeks, respectively. The third switched to the STD diet at age 22 weeks and was necropsied at age 30 weeks. All mandibles/maxillae were assessed by histometry for degree of periodontal inflammation (PD Score), alveolar crest height (ACH, mm), and horizontal alveolar bone height (hABH, mm2).
Results
In STD diet rats aged ≥30 weeks, all endpoints were worse (P < 0.05) than at Baseline. In H-SC diet rats aged ≥22 weeks, all endpoints were worse than at Baseline (P < 0.05). At age 22 weeks, all endpoints were worse in the H-SC group than in the STD group (P < 0.05). By age 30 weeks, the STD and H-SC groups did not differ.
Conclusions
1) STD diet fed rice rats develop moderate/severe PD by age 30 weeks; 2) an H-SC diet accelerates moderate/severe PD development; and 3) switching to a STD diet does not halt/reverse PD that was accelerated by an H-SC diet. These data further clarify use of the rice rat as a PD model.
Keywords: High sucrose-casein diet, Alveolar crest height, MicroCT, Mandible, Maxilla
1. Introduction
Tissue level characteristics of human periodontitis (PD) that illustrate its severity are well known (Loe, 1983; Page, 1986; Tonetti & Mombelli, 1999). The thoroughness and specificity of epidemiologic evaluation of human PD has increased during the past decade, with a new emphasis on detecting individuals with severe PD (SP), that now afflicts 11 % of the world’s population (Kassebaum et al., 2014). In fact, the US National Health and Nutrition Examination Survey (NHANES) III 1988–1994, that screened two quadrants and two sites/tooth per patient, had revealed an overall PD prevalence of only 16.1% in people over age 20 (Borrell & Talih, 2012). NHANES 2009–2010’s most recent evaluation of US PD prevalence not only focused on people over age 30 and screened four quadrants and six sites/tooth per patient, but also categorized PD as mild, moderate, or severe. It reported their respective US prevalence as 8.7%, 30.0% and 8.5% (Thornton-Evans, Eke, & Wei, 2013). Given the current US population, this translates into approximately 15 million cases of SP, indicating sufficient prevalence to warrant investigation of small animal models that specifically address SP.
Though several well-standardized, highly-respected laboratory rat models for study of both induction processes and early and intermediate phase human PD now exist (Oz & Puleo, 2011; Graves, Kang, Andriankaja, & Wada, 2012; Struillou, Boutigny, Soueidan, & Layrolle, 2010), a recent review summarizes the current status of the field as follows: “A simple and reproducible model that truly mimics human pathogenesis of periodontal disease has yet to be discovered” (Oz & Puleo, 2011). This statement implies that additional preclinical models, that ultimately comprise a group that together faithfully mimics all severities of human PD at the tissue level, could enable experiments that test phase-specific therapeutic approaches that are as yet, neither ethical nor proven safe in humans.
Existing laboratory rat models for mild and moderate human PD (Oz & Puleo, 2011; Graves et al., 2012; Struillou et al., 2010) include ligature-induced localized PD (Cesar Neto, de Souza, Barbieri, & Sallum, 2004; Lohinai, Benedek, & Feher, 1998), LPS-injection-induced localized PD (Dumitrescu, Abd-El-Aleem, Morales-Aza, & Donaldson, 2004; Nakamura, Ukai, & Yoshimura, 2010), and bacterial-inoculation-induced PD (Li et al., 2010; Lohinai et al., 1998; Okada, Hamada, & Kim, 2010). Each, with a known initiation date and requirement for ongoing maintenance or injection/inoculation, causes development of either localized or generalized tissue level PD similar to moderate PD in humans and permits studies of both the induction phase itself, and of preventive or remedial treatments of 2–8 weeks duration. The rice rat (Oryzomys palustris), is now known to be susceptible to induction of progressive SP by feeding of a powdered diet high in sucrose and casein (H-SC) with no requirement for mechanical manipulation to initiate/maintain the condition (Gupta & Shaw, 1956; Ryder, 1980; Aguirre, Akhter, & Kimmel, 2012a). As in human PD itself and the other small animal PD models, marginal gingivitis is the initial pathologic finding (Leonard, 1979), followed by widespread plaque accumulation and gingival ulceration. Unlike experimental PD models that focus on mild and moderate PD, substantial soft tissue destruction and alveolar bone loss with loss of functional tooth support routinely occur in rice rats following the moderate PD phase (Gupta & Shaw, 1956; Gotcher and Jee, 1981). However, unlike SP in humans that classically progresses over many years, rice rat SP develops within 12–18 weeks of starting the H-SC diet (Leonard, 1979).
We recently confirmed that rice rats consuming a pelleted H-SC diet develop PD, including worsening of both alveolar crest height (ACH) and horizontal (h) alveolar bone height (ABH) (Aguirre et al., 2012a). Though this study (Aguirre et al., 2012a) also disclosed a trend for rice rats consuming a pelleted standard (STD) diet to develop tissue level PD, there are currently no long-term studies of PD in STD diet-fed rice rats because of the perceived requirement to feed a H-SC diet to induce PD in rice rats. The STD diet-fed rice rat may have potential as a pre-clinical model that, without dietary intervention, not only complements existing rat models of mild and moderate PD, but also evolves continuously into SP in a sufficiently-compressed timeframe to enable practical studies that address human SP.
We report here an experiment that investigates three new topics: a) ACH and hABH from STD diet-fed rice rats that includes the first evaluation of their periodontal condition at ages 4 weeks, 52 weeks, and 80 weeks (Gupta & Shaw, 1956; Aguirre et al., 2012a; Gotcher & Jee, 1981); b) systemic metabolic condition of rice rats that consumed a H-SC diet for 26 weeks; and c) a method to reverse H-SC-diet-induced PD. We hypothesize that rice rats fed a: a) STD diet from weaning develop durable SP by age 30 weeks; b) H-SC diet from weaning develop SP more rapidly than STD diet rats; c) H-SC diet from weaning for 26 weeks have abnormal serum glucose, insulin, and cholesterol; and d) H-SC diet long enough from weaning to develop moderate PD and then switched to a STD diet, experience stabilization or healing of PD.
2. Materials and methods
2.1. Animals, diets and experimental groups
Ninety-two male rice rats were physically examined and weighed immediately after weaning (age 4 weeks). Clinically normal, healthy rats (body weight (BW) ≥30 g and body condition score ≥3.0) (Ullman-Cullere & Foltz, 1999) were weight-randomized into eight groups (Fig. 1). One group was sacrificed at age 4 weeks (Baseline; N = 12). The other seven groups were fed either a pelleted STD diet (Harlan 8604 Teklad Rodent Diet; Tampa, FL USA) or a pelleted H-SC diet (TestDiet #5SXA AIN-93G, Purina Feed; Richmond, IN, USA). The composition of the H-SC diet was based on the formula of the powdered Harvard high sucrose 700 diet and the ration 100 diet (Gotcher and Jee, 1981; Gupta & Shaw, 1956; Ryder, 1980; Auskaps, Gupta, & Shaw, 1957).
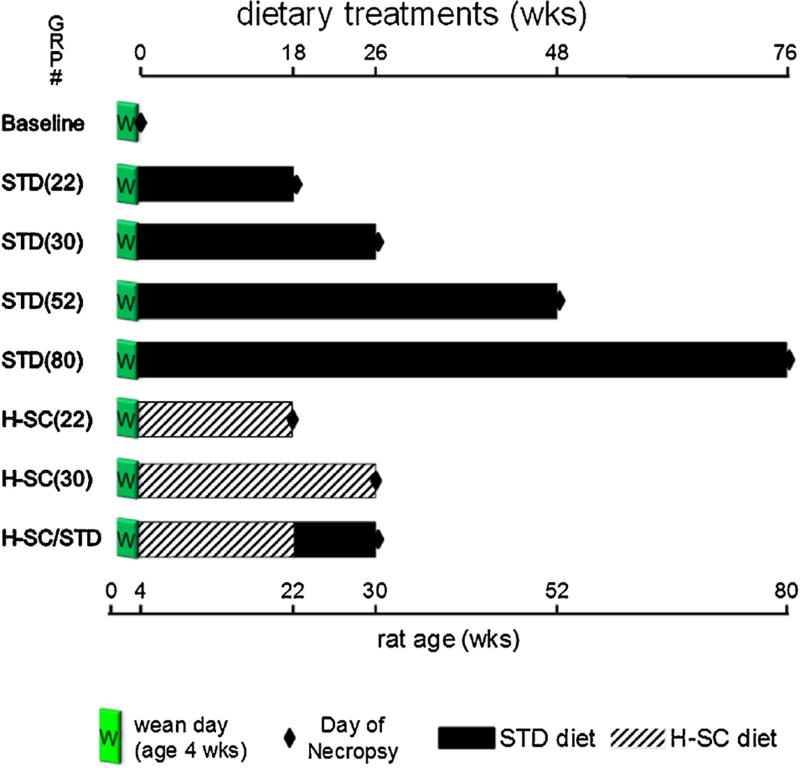
Fig. 1.
Experimental Design. One group was necropsied at age four weeks (Baseline). Four groups (STD [22], STD [30], STD [52], and STD [80]), were fed standard laboratory chow, starting at age four weeks and necropsied at ages 22, 30, 52, and 80 weeks. Three groups were fed the H-SC diet starting at age four weeks. Two of those groups (H-SC [22] and H-SC [30]) were necropsied after 18 or 26 weeks. The third group (H-SC/STD) switched from H-SC to STD diet at age 22wks and was necropsied at age 30 weeks.
Four groups were fed the STD diet for 18, 26, 48, or 76 weeks and then necropsied [STD (22) (N = 12); STD (30) (N = 16); STD (52) (N = 11); and STD (80) (N = 8), respectively], more than encompassing a duration adequate for H-SC diet-fed rice rats to develop mild or moderate PD (Aguirre et al., 2012a; Aguirre, Akhter, & Kimmel, 2012b). Two groups were fed the H-SC diet for 18 or 26 weeks and then necropsied [H-SC (22) (N = 11) and H-SC (30) (N = 10), respectively]. The eighth group was fed the H-SC diet for 18 weeks, switched to the STD diet for 8 weeks and necropsied [HSC/STD (N = 12)].
Rats were housed (2–5 rats per cage) in static filter top cages (area: 143 in2) with pine shavings as bedding and continuous access to food and water. The housing room was maintained at 68–79 °F with an average humidity of 30–70% and a 14:10 h light:dark cycle. The Animal Care Services resource at the University of Florida (UF) is an AAALAC-accredited animal care and use program. Adequate measures were taken at all times to minimize pain and discomfort in all animals. The protocol was approved by the UF Institutional Animal Care and Use Committee (IACUC).
2.2. Terminal bleeding, euthanasia, serum testing and tissue collection/handling
Rats were euthanized by CO2 inhalation followed by thoracotomy. Blood samples from STD (30), H-SC (30) and H-SC/STD rats were obtained by cardiac puncture at necropsy. Whole blood was centrifuged at 3000RPM for five minutes to obtain serum, and stored at −80 °C. A comprehensive metabolic panel (CMP) test was performed that included: serum glucose, cholesterol, total protein, albumin (ALB), alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (creat), calcium (Ca) and phosphorus (P) using a clinical chemistry system (Alfa Wassermann, Diagnostic Technologies, LLC, West Caldwell, NJ). Serum insulin levels were measured using the Rat Insulin ELISA Kit (80-INSRT-E01; ALPCO Diagnostics, Inc.; Salem, NH), following manufacturer’s instructions.
Maxillae and mandibles were carefully stripped of musculature, leaving an intact periosteum. Left mandibles were placed in 10% phosphate-buffered formalin (pH 7.4) for 48 h for assessment of periodontal status (PD Score, Table 1 and Fig. 2A), and ACH (see below). Right mandibles and maxillae were fixed in 70% ethanol. Right mandibles were processed to evaluate ACH (Aguirre et al., 2012b; Verma, Rajapakse, & Meka, 2010a; Verma, Bhattacharyya, & Sevilla, 2010b) in the interdental region between the first (M1) and second molars (M2), and interradicular and interdental alveolar bone parameters by MicroCT, followed by the evaluation of hABH by morphometry (see below). Right maxillae were processed to determine hABH. MicroCT and hABH analyses were done in all groups of age 30 weeks or less.
Table 1.
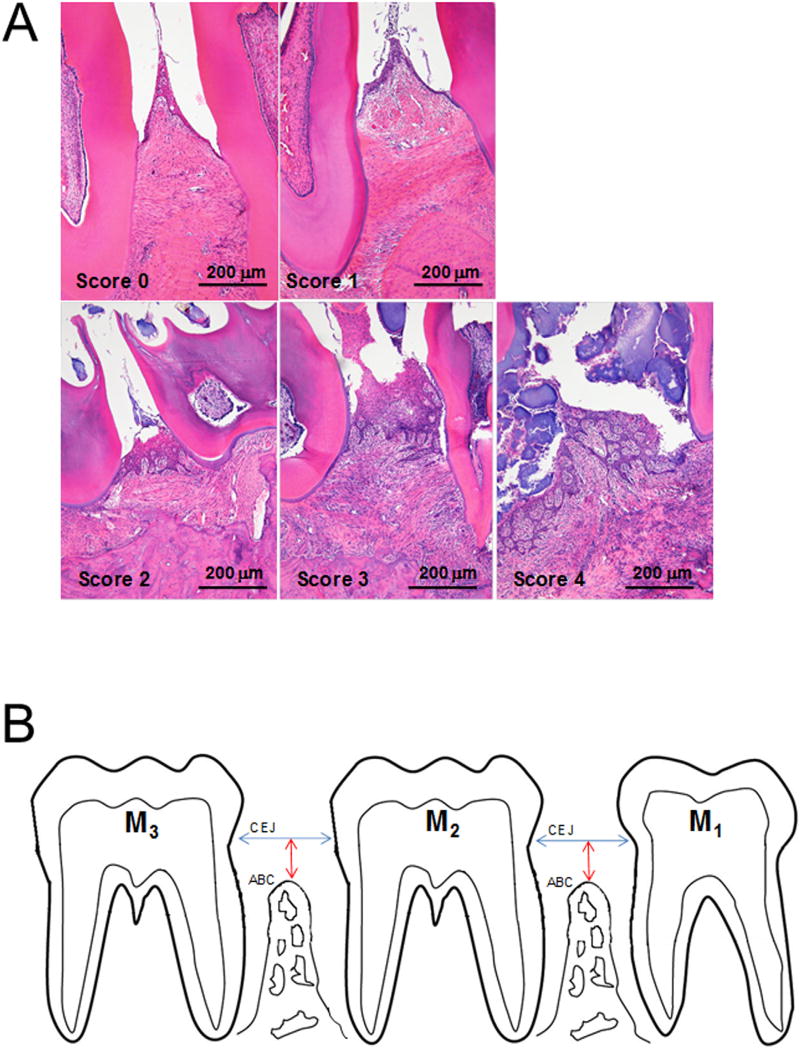
Inflammation scoring system (PD Score) for Periodontal Status.
| Score | Degree | Lesions |
|---|---|---|
| 0 | Absence | None |
| 1 | Slight | Gingivitis: slight hyperplasia of GE, intraepithelial inflammatory cell infiltration. Accumulation of bacterial plaque. No substantial changes in the LP, PDL or ABC. |
| 2 | Mild | Mild gingival hyperplasia, inflammatory cell infiltration of the GE and LP, accumulation of bacterial plaque. Slight disruption of the PDL. No substantial changes in ABC. |
| 3 | Moderate | Erosion/ulceration and hyperplasia of the GE and accumulation of bacterial plaque. Moderate inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and ABC resorption. |
| 4 | Severe | Ulceration and hyperplasia of the GE. Severe inflammatory cell infiltration of the LP, disruption of the PDL, migration of the junctional epithelium, and severe ABC resorption and osteolysis. |
GE: gingival epithelium, LP: lamina propria, PDL: periodontal ligament, ABC: alveolar bone crest.
Fig. 2.
Inflammation scoring system (PD Score). Representative photomicrographs of interdental regions representing the five inflammation PD Scores (0–4) are shown. 0 corresponds to no histologic lesions, 1 to slight, 2 to mild, 3 to moderate, and 4 to severe inflammatory periodontal lesions. Details of histopathologic features are listed in Table 1. B. Schematic view of orientation used to obtain histologic sections for PD Scoring (histopathologic analysis) and histometric measurement of alveolar crest height (ACH). The mandible was sectioned in the parasagittal (mesio-distal) plane. Molar (M) 1, 2 and 3. Cementoenamel junction (CEJ), alveolar bone crest (ABC). Interdental alveolar crest height (ACH) was determined by measuring the perpendicular distance from a line between the CEJs of adjacent teeth to the ABC between M1-M2 and between M2-M3 (Verma et al., 2010b; Hausmann et al., 1989a; Hausmann et al., 1989b). A larger value signals worse ACH.
2.3. PD score (qualitative histologic analysis) and ACH by histometry
After decalcification in 5% formic acid for two weeks, left mandibles were processed as before with slight modifications (Aguirre et al., 2012a; Verma et al., 2010a; Verma et al., 2010b). Tissues were trimmed, dehydrated in increasing concentrations of ethanol up to 100%, paraffin-embedded, sectioned in the mesiodistal plane (Fig. 2B) using a Microm HM 325 microtome (GMI Inc, Ramsey, MN), and sectioning was performed from lingual to buccal. The block was oriented so as to obtain sections that simultaneously included the mesial and distal roots of all molars. The first two adequately-aligned 5 µm thickness sections that contained alveolar process tissue from near the lingual surface of the molars were stained with hematoxylin and eosin (H & E) and used for quantitative analysis. Fifty consecutive 20 µm sections were then discarded to obtain two more 5 µm thickness sections adjacent to the buccal surface of the molars. Lingual and buccal sections were used to determine PD Score as before (Table 1; Fig. 2A) (Aguirre et al., 2012a) and ACH. A higher PD Score signals worse disease.
ACH was determined by measuring the distance from a line between the cementoenamel junctions (CEJ) of adjacent teeth to the crest of the interdental alveolar bone between M1-M2 and between M2-M3 (Hausmann, Allen, Dunford, & Christersson, 1989a; Hausmann, Allen, Christersson, & Genco, 1989b; Hausmann, Allen, & Clerehugh, 1991), with the OsteoMeasure System (OsteoMetrics,Inc., Decatur, GA), at a magnification of 100X (Fig. 2B). Thus, a larger number signals worse ACH. When no interdental alveolar bone was present, a default “worst” value for ACH of 1500 µm that was obtained in a pilot study (data not shown), was used. All PD score and histometric ACH measurements were done in a randomized, blinded manner.
2.4. Alveolar crest height (ACH) and interdental/interradicular microarchitecture
MicroCT analyses of the right mandible of rats from Baseline, STD (22), H-SC (22), STD (30), H-SC (30) and HSC/STD groups were performed (Aguirre et al., 2012a; Aguirre et al., 2012b; Hausmann et al., 1989a; Hausmann et al., 1989b; Hausmann et al., 1991; Park, Abramson, & Taba, 2007). Mandibles were trimmed with a disc to remove the coronoid process and reduce the mesio-distal dimension to ~7.5 mm. A MicroCT scan (8 µm pixel resolution [PR]; ScanCo 40; ScanCo Medical; Bruttisellen, Germany) in an approximate mesio-distal plane through the mandibular molars was completed. It yielded a series of ~140 consecutive 8 µm slices that create a serial lingual-to-buccal view of the mandible, with individual slices replicating a view similar to that in human dental radiographs (Fig. 3A and B). Ten slices spaced at an ~80 µm interval spanning the lingual-to-buccal dimension that showed both the distal root of M1 and the mesial root of M2, with a clear view of the M1-M2 interdental bone, were obtained. ACH was measured in the M1-M2 interdental space (Aguirre et al., 2012b) in each of the ten slices (Fig. 3A–B) (Hausmann et al., 1989a; Hausmann et al., 1989b; Hausmann et al., 1991). ACH for each rat was the mean of the ten measured slices. When no M1-M2 interdental alveolar bone was present, a default “worst” ACH value of 2500 µm that was determined in a pilot study (data not shown), was assigned. The default worst value for histometric ACH (1500 µm) is less than the default worst value for MicroCT ACH, because MicroCT ACH was measured on ten sections throughout the buccolingual dimension of the M1-M2 interdental region, including the actual root apex where the root appears longer, while histometric ACH was measured only on sections from near either the lingual or buccal surfaces, where the roots appear shorter. A 3D reconstruction of the mandibular scan was done to display horizontal alveolar bone on the buccal and lingual aspects of M1-M3 (Fig. 6) (Liu, Wiswall, & Rutledge, 2008). All MicroCT measurements were performed in a blinded, randomized manner.
Fig. 3.
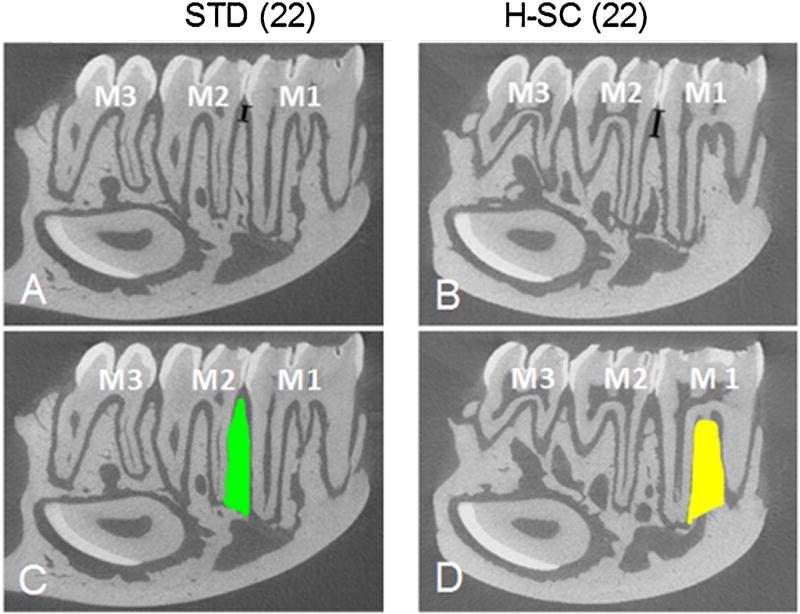
MicroCT Slices (8 µm thick) from right mandibles of representative STD(22) and H-SC(22) rats. MicroCT analysis was performed from a series of ~140 consecutive parasagittal slices and from a 3D reconstruction (Fig. 7). Note that ACH is greater [black line between the CEJ and ABC between M1-M2 in H-SC(22) rat (B) compared to a STD (22) rat (A), signifying worse disease with the H-SC diet. In addition to ACH, traditional microarchitectural values of BV/TV, Tb.N, Tb.Sp, and Conn.D (Bouxsein et al., 2010) of the mandibular alveolar bone were assessed in the M1M2 interdental (C) and interradicular (D) regions. The interdental region was defined as the alveolar bone area located between M1-M2 (green area in C). The interradicular region was defined as the alveolar bone area located between the mesial and distal roots of M1 (yellow area in D).
Fig. 6.
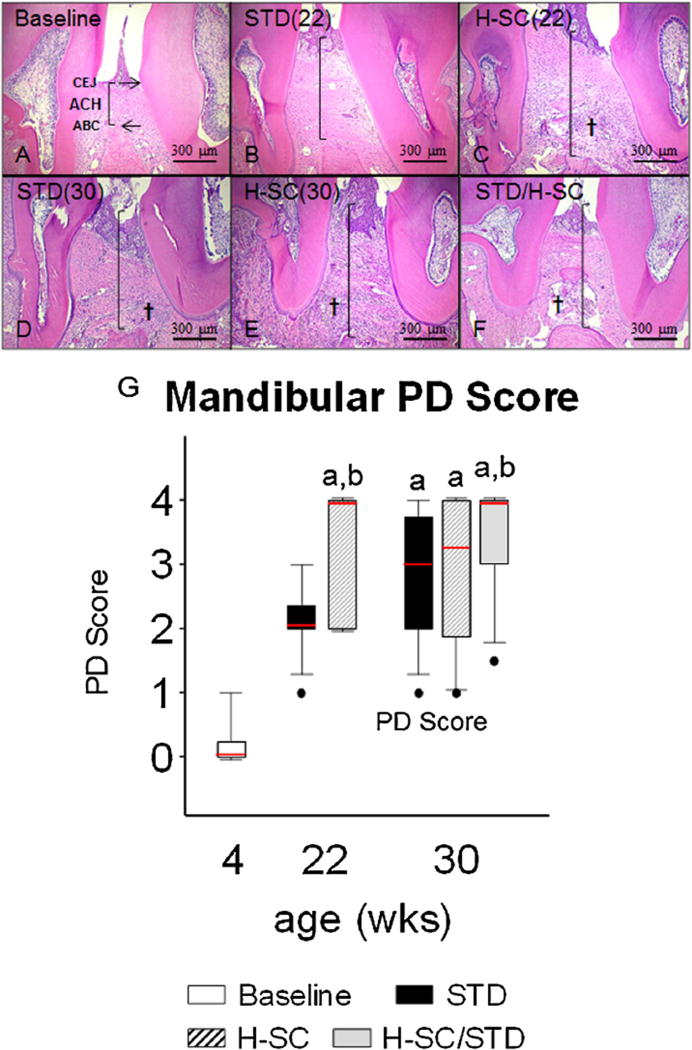
Alveolar Crest Height and Periodontitis (PD) Score in representative animals from groups aged 30 weeks or less. Photomicrographs of histologic sections from the interdental region between the first and second mandibular molars of rats from groups: Baseline (A), age 22 weeks with standard diet [STD (22)] (B); age 22 weeks with H-SC diet [H-SC(22)](C); age 30 weeks with STD diet [STD (30)] (D); age 30 weeks with H-SC diet [H-SC (30)] (E); and age 30 weeks after H-SC diet for 18 weeks, then STD diet for 8 weeks (H-SC/STD) (F). Note worse alveolar crest height (ACH) at ages 22 and 30 weeks (B–F) than at Baseline (A). Also note that whereas the inflammatory reaction in periodontal tissues (†) is more pronounced and ACH is worse in H-SC than in STD rats at age 22 weeks (C vs B), the STD and H-SC diets have comparable inflammation and ACH (D vs. E) by age 30 weeks. In (A), note CEJ and ABC. Vertical black lines demonstrate ACH. † Inflammatory cell infiltration of the lamina propria and periodontal ligament (PDL). Scale Bars = 300 µm. Mandibular PD Score (G). Box-whisker plot data represent Mean ± SEM. a: different from Baseline; b: different from STD (22) (P < 0.05).
Traditional MicroCT microarchitectural analyses of the M1 interradicular and M1-M2 interdental alveolar bone regions were performed using the same series of ~140 consecutive slices and 3D reconstructions from the 8 µm PR scans. The interdental region was defined coronally by a horizontal line between the CEJs and apically by a horizontal line connecting the apices of the distal root of M1 with the mesial root of M2 (Fig. 3C). The interradicular region was defined as the alveolar bone area bounded apically by a line between the apices of the mesial and distal roots of M1, and elsewhere by the interface of M1 with the interradicular alveolar bone (Fig. 3D). The following parameters were assessed: Total volume [TV (mm3)], bone volume/total tissue volume [BV/TV (%)], apparent bone mineral density [BMD (mg/cm3)], connectivity density [Conn.D (1/mm3)], structure model index (SMI), trabecular number [Tb.N (mm−3)], trabecular separation [TB.SP (mm)], trabecular thickness [Tb.Th. (mm)] See Bouxsein et al. for more details (Bouxsein et al., 2010).
2.5. Horizontal alveolar bone height (hABH) by morphometry
Right maxillae and mandibles, the latter after MicroCT scanning, were autoclaved at 121°C for 20 min, defleshed, immersed in 3% (vol/vol) hydrogen peroxide overnight, washed with deionized water, air dried, and stained for 1 min in an aqueous solution of 0.1% (wt/vol) methylene blue to delineate the CEJ (Verma et al., 2010a; Verma et al., 2010b; Rajapakse, O’Brien-Simpson, Slakeski, Hoffmann, & Reynolds, 2002). Digital images of both lingual and buccal surfaces of M1-M3 for each quadrant studied were captured with a stereo dissecting microscope (SteReo Discovery V8; Carl Zeiss MicroImaging, Inc., Thornwood, NY) at 10X magnification, after superimposition of buccal and lingual cusps to ensure consistent alignment. The line tool was used to measure hABH, using the CEJ as the coronal-most boundary, the coronal-most aspect of the alveolar bone as the apical boundary, the mesial aspect of M1 as the mesial boundary, and the distal aspect of M3 as the distal boundary. hABH for each quadrant was calculated using AxioVision LE 29A software version 4.6.3 (Carl Zeiss, Germany). A larger number signals worse hABH.
2.6. Sample size calculation and reproducibility
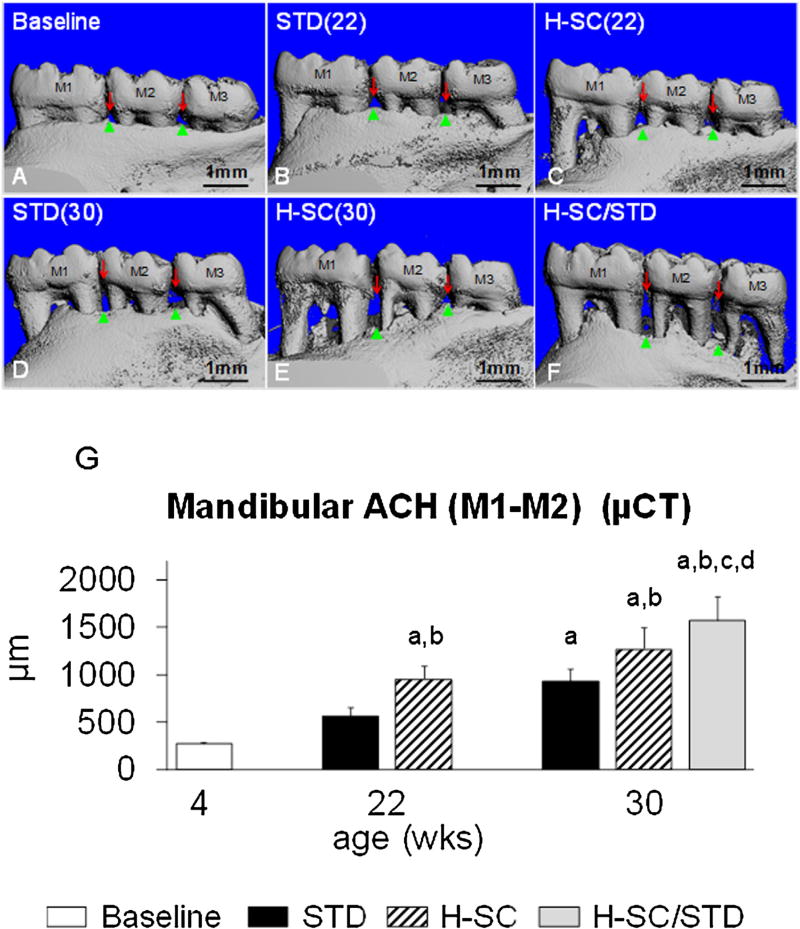
A group of rice rats with a healthy periodontium (Baseline) has M1M2 MicroCT ACH of 270±40 µm (Mean±SD). A group with mild/moderate PD [STD (22)] has ACH of 550 ± 300 µm. One with moderate PD [H-SC (22)] has ACH of 950 ± 420 µm, while one with SP (H-SC/STD) has ACH of 1570 ± 850 µm (Fig. 7G).
Fig. 7.
Alveolar crest height (ACH) and horizontal alveolar bone height (hABH) in representative animals from groups aged 30 weeks or less. Lingual view of 3D-reconstructed MicroCT images of mandibles from Baseline [A], STD (22) [B], H-SC (22) [C], STD (30) [D], H-SC (30) [E], and H-SC/STD [F] groups. Red arrows delineate the CEJ. Green arrows depict the coronal border of the alveolar bone. Note worse ACH at the M1-M2 and M2-M3 interdental spaces between with the H-SC diet at age 22 weeks (C) compared to Baseline (A). Also note that ACH is worse in H-SC (C) than in STD (B) rats at age 22 weeks, and continues to be worse in H-SC (E) and H-SC/STD (F) rats at age 30 weeks than in STD diet rats (D). ACH in the M1-M2 interdental space measured by MicroCT (G). Baseline; STD (22); H-SC (22); STD (30), H-SC (30), and H-SC/STD groups are shown. (Mean ± SEM). Superscript letters indicate a significant difference between groups (P < 0.05); a: different from Baseline; b: different from STD (22); c: different from H-SC (22); d: different from STD (30).
Sample size calculation methods, using the above data, were applied (Rosner, 2011). Assuming α = 0.05 and power of 0.8, and a combined mean SD (170), the sample size to find a significant ACH difference (P <. 05 0.05) between rats with no PD and mild/moderate PD is 7. With a combined SD of 360, the sample size to find a significant ACH difference between rats with mild/moderate PD and moderate PD is 13. With a combined SD of 640, the sample size to find a significant ACH difference between rats with moderate PD and SP is 17.
We selected one sample each from STD (22), H-SC (22), and H-SC/STD, that represented the mean of each group, as examples of mild/moderate periodontitis, moderate periodontitis, or SP, respectively. We evaluated samples for PD Score, ACH [mandibular M1M2 (histo)], and mandibular lingual hABH. The same experienced observer evaluated/measured those samples six times over a three week period, in random order mixed with five other samples. The coefficients of variation (CVs) for PD Score, ACH, and mandibular lingual hABH were 0.19, 90, and 0.05, respectively.
2.7. Statistical analysis
Group data are expressed as Mean ± Standard Error (SEM). Two ANOVAs were conducted to address hypotheses concerning periodontal status during long-term feeding of the STD diet and dietary effects on periodontal status in rats age 30 weeks and younger. The first ANOVA involved the five standard diet groups: Baseline, STD (22), STD (30), STD (52), and STD (80), for PD Score and ACH in the mandibular M1-M2 interdental region by histo (ACHM1M2histo). When the ANOVA was significant, the Holm-Sidak test was applied to intercompare all groups. The second ANOVA involved the groups necropsied at age 30 weeks or less: Baseline, STD (22), STD (30), H-SC (22), H-SC (30), and H-SC/STD. When the ANOVA was significant, the Holm-Sidak test was applied to make only the following nine pre-planned comparisons, for all variables: a) Baseline vs. STD (22); b) Baseline vs. H-SC (22); c) STD (22) vs. H-SC (22); d) Baseline vs. STD (30); e) Baseline vs. H-SC (30); f) H-SC (22) vs. H-SC (30); g) STD (30) vs. H-SC (30); h) STD (30) vs. H-SC/STD; and i) H-SC (30) vs. H-SC/STD. For a) and d) that were addressed in the first ANOVA, PD Score and ACHM1M2histo were not included. When ANOVA assumptions regarding data normality were not met, the non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons was used. Regardless of the test, P < 0.05 was considered statistically significant.
3. Results
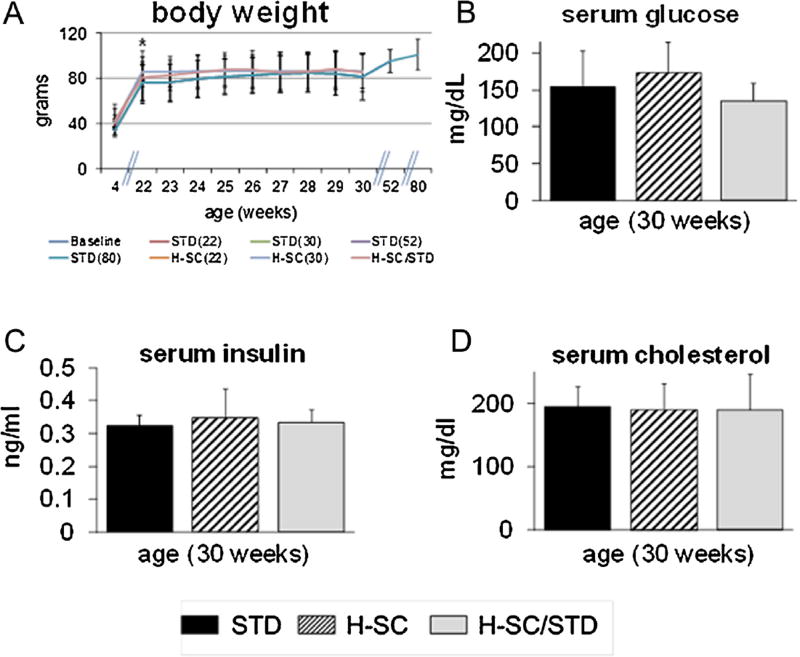
Rats gained significant BW during ages 4–22 weeks (Fig. 4A) and achieved a numerical BW plateau between ages 22 and 80 weeks. No significant BW differences were ever observed among the dietary groups. In addition, no significant intergroup differences were seen in any CMP parameter (Table 2, Fig. 4B–D), particularly glucose, insulin, and cholesterol, at age 30 weeks. No problems concerning animal welfare, including periodontal or periapical abscess, occurred at any time.
Fig. 4.
Body weight (BW) throughout study and serum glucose, insulin and cholesterol (Mean ± SEM) at age 30 weeks. No significant BW differences among groups were observed at any time (A). No differences in serum glucose, insulin and cholesterol were observed (B, C, and D). The asterisk in (A) denotes a significant difference between rats aged 4 weeks and rats of all groups at age 22 weeks (P < 0.05).
Table 2.
Comprehensive Metabolic Panel (age 30 weeks).
| Diet Group Endpoint (units) |
STD | H-SC | H-SC/STD |
|---|---|---|---|
| ALT (IU/l) | 125.7 ± 35.8 | 139.3 ± 57.3 | 107.6 ± 26.9 |
| AST (IU/l) | 153.8 ± 42.2 | 125.5 ± 36.2 | 181.3 ± 142.2 |
| Ca (mg/dl) | 10.2 ± 0.9 | 10.7 ± 1.2 | 10.1 ± 0.7 |
| P (mEq/l) | 7.2 ± 0.8 | 7.7 ± 3.1 | 7.2 ± 1.4 |
| BUN (mg/dl) | 24.6 ± 2.6 | 28.3 ± 2.0 | 25.8 ± 3.8 |
| Creat (mg/dl) | 0.49 ± 0.07 | 0.50 ± 0.06 | 0.48 ± 0.05 |
| T Prot (g/l) | 5.8 ± 0.4 | 6.6 ± 0.7 | 6.5 ± 0.5 |
| Alb (g/l) | 3.8 ± 0.3 | 3.9 ± 0.4 | 3.9 ± 0.4 |
ALT: alanine transaminase; AST: aspartate transaminase; CA: calcium; P: phosphorus; BUN: blood urea nitrogen; CREAT: creatinine; T. PROT: total protein; ALB: albumin. STD: standard diet age 4–30wks; H-SC: high sucrose and casein diet age 4–30wks; H-SC/STD: H-SC diet from age 4–22wks and then switched to STD diet for 8 weeks. Values are Mean ± SEM of 7–8 rats/grp.
3.1. Age-related periodontal findings with standard diet
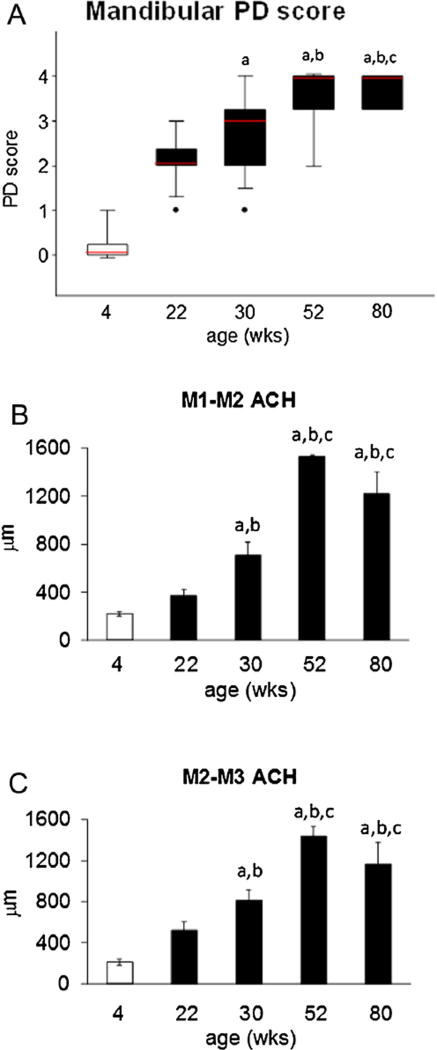
PD Score was significantly worse in STD diet rats aged 30 weeks and older than in Baseline rats (P < 0.05) (Fig. 5A). PD Score was significantly worse in STD diet rats aged 52 weeks and older than in STD diet rats aged 22 weeks (P < 0.05). PD Score at age 80 weeks was significantly worse than in STD diet rats aged 30 weeks (P < 0.05) (Fig. 5A).
Fig. 5.
Periodontitis (PD) Score and histometric Alveolar Crest Height (ACH) in the M1-M2 and M2-M3 interdental regions of the mandible of rats fed standard laboratory chow. PD Score (A), ACH at M1-M2 (B), and ACH at M2-M3 (C). Box-whisker plot data (A) and plots in (B) and (C) are Mean±SEM. a: different from Baseline; b: different from STD (22); c: different from STD (30) (P < 0.05).
ACH in the mandibular M1-M2 interdental region by histo-metry (ACHM1M2histo) was significantly worse in STD diet rats of age 30 weeks and older than in Baseline rats (P < 0.05) (Fig. 5B). ACHM1M2histo was significantly worse in STD diet rats aged 30 weeks than in rats aged 22 weeks. (P < 0.05) (Fig. 5B). ACHM1M2-histo was significantly worse in STD diet rats aged 52 weeks and older than in rats aged 22 and 30 weeks. (P < 0.05) (Fig. 5B). ACHM1M2histo did not differ significantly between STD diet rats aged 52–80 weeks (Fig. 5B).
Similarly, ACH in the mandibular M2-M3 interdental region by histometry (ACHM2M3histo) was significantly worse in STD diet rats of age 30 weeks and older than in Baseline rats (P < 0.05) (Fig. 5C). ACHM2M3histo was significantly worse in STD diet rats aged 30 weeks than in rats aged 22 weeks. (P < 0.05) (Fig. 5C). ACHM2M3histo was significantly worse in STD diet rats aged 52 weeks and older than in rats aged 22 and 30 weeks. (P < 0.05) (Fig. 5C). ACHM2M3histo did not differ significantly among STD diet rats aged 52 and 80 weeks (Fig. 5C).
3.2. Baseline vs. age 22wks
ACH in the mandibular M1-M2 interdental region by MicroCT (ACHM1M2 µCT) was significantly worse in H-SC (22) rats than in both Baseline and STD (22) rats (P < 0.05) (Fig. 7G). ACHM1M2 µCT in STD (22) rats did not differ from that in Baseline rats (Fig. 7G). ACHM1M2 µCT in all rats aged 30 weeks was worse than that in Baseline rats (P < 0.05) (Fig. 7G). Interradicular BV/TV, BMD and Conn.D were significantly higher in STD (22) rats than in Baseline rats (P < 0.05) (Table 4). Both mandibular buccal hABH (mnbhABH) and maxillary buccal hABH (mxbhABH) were significantly worse in STD (22) rats than in Baseline rats (P < 0.05) (Table 3).
Table 4.
Microarchitectural Endpoints of Interdental and Interradicular Alveolar Bone.
| GRP | Baseline | STD(22) | H-SC(22) | STD(30) | H-SC(30) | H-SC/STD |
|---|---|---|---|---|---|---|
| N | 10 | 8 | 10 | 11 | 10 | 9 |
| Symbol | A | b | f | c | g | h |
| Interdental Region | ||||||
| TV (mm3) | 0.154 ± 0.008 | 0.173 ± 0.017 | 0.164 ± 0.018 | 0.111 ± 0.011g | 0.194 ± 0.018 | 0.158 ± 0.010 |
| BV/TV (%) | 47.1 ± 0.9 | 46.0 ± 5.6 | 21.1 ± 7.3 | 33.1 ± 6.8 | 27.4 ± 8.4 | 24.2 ± 7.6 |
| Apparent BMD (mg/cm3) | 471.0 ± 35.5 | 497.0 ± 129.8 | 280.0 ± 204.1 | 392.2 ± 194.7 | 307.5 ± 231.8 | 281.8 ± 201.6 |
| Conn.D (mm−3) | 82.7 ± 9.4 | 226.3 ± 62.6a | 94.9 ± 30.7 | 272.2 ± 82.4a | 108.4 ± 28.4b | 104.7 ± 34.0b |
| SMI | 0.994 ± 0.654 | 2.089 ± 1.064 | 3.181 ± 0.508 | 3.877 ± 0.995 | 2.645 ± 0.648 | 4.990 ± 0.61a |
| Tb.N (mm−3) | 9.81 ± 0.28 | 11.09 ± 0.22 | 10.26 ± 0.56 | 10.32 ± 0.43 | 10.01 ± 0.46 | 9.57 ± 0.42 |
| Tb.Sp (mm) | 0.089 ± 0.003 | 0.087 ± 0.004 | 0.105 ± 0.008 | 0.094 ± 0.009 | 0.104 ± 0.006 | 0.109 ± 0.007 |
| Tb.Th (mm) | 0.118 ± 0.003 | 0.099 ± 0.010 | 0.067 ± 0.012a | 0.083 ± 0.010 | 0.077 ± 0.013 | 0.072 ± 0.016 |
| Interradicular Region | ||||||
| TV (mm3) | 0.185 ± 0.012 | 0.238 ± 0.012 | 0.263 ± 0.011a,c | 0.200 ± 0.012g | 0.253 ± 0.014a | 0.254 ± 0.012a |
| BV/TV (%) | 48.9 ± 1.5 | 67.5 ± 2.36a | 47.3 ± 4.9b | 59.0 ± 3.8 | 44.20 ± 6.2b | 41.4 ± 7.1b |
| Apparent BMD (mg/cm3) | 481.0 ± 49.5 | 699.0 ± 68.4 | 508.9 ± 149.3 | 610.7 ± 115.6 | 459.3 ± 187.3 | 431.1 ± 200.8 |
| Conn.D (mm−3) | 138.8 ± 28.5 | 388.1 ± 52.0a | 233.1 ± 36.8 | 379.1 ± 90.4a | 143.4 ± 21.2b | 194.8 ± 20.7b |
| SMI | 0.426 ± 0.402 | 0.341 ± 0.971 | 1.752 ± 0.570 | 1.530 ± 0.948 | 1.873 ± 0.401 | 3.090 ± 0.921 |
| Tb.N (mm−3) | 9.87 ± 0.27 | 10.43 ± 0.13 | 10.46 ± 0.23 | 10.48 ± 0.37 | 10.38 ± 0.33 | 10.13 ± 0.20 |
| Tb.Sp (mm) | 0.092 ± 0.005 | 0.069 ± 0.005 | 0.088 ± 0.006 | 0.079 ± 0.006 | 0.092 ± 0.005 | 0.099 ± 0.008b |
| Tb.Th (mm) | 0.113 ± 0.003 | 0.128 ± 0.002 | 0.121 ± 0.004 | 0.126 ± 0.003 | 0.116 ± 0.007 | 0.113 ± 0.007 |
Symbol: superscript letters indicate groups from which group differs (P < 0.05); Mean ± SEM; TV: Total volume; BV/TV: bone volume/total tissue volume; BMD: bone mineral density; Conn.D: connectivity density; SMI: Structure model index; Tb.N: trabecular number; TB.Sp: trabecular separation; Tb.Th: trabecular thickness (Bouxsein et al., 2010).
Table 3.
Alveolar Crest Height and horizontal Alveolar Bone Height Measurements.
| Endpoint Group |
Symbol | Man ACH M1M2 (Histo) (mm) |
Man ACH M2M3 (Histo) (mm) |
Man Buccal hABH (mm2) |
Max Lingual hABH (mm2) |
Max Buccal hABH (mm2) |
|---|---|---|---|---|---|---|
| Baseline | a | 0.20 ± 0.02 | 0.21 ± 0.03 | 0.52 ± 0.04 | 0.69 ± 0.06 | 0.83 ± 0.08 |
| STD (22) | b | 0.37 ± 0.05 | 0.52 ± 0.08 | 1.33 ± 0.14a | 1.13 ± 0.13 | 1.55 ± 0.10a |
| STD (30) | c | 0.71 ± 0.11a | 0.80 ± 0.01a | 2.22 ± 0.38a,b | 3.19 ± 0.34a,b | 2.78 ± 0.38a,b |
| STD (52) | d | 1.53 ± 0.14a | 1.43 ± 0.09a | – | – | – |
| STD (80) | e | 1.10 ± 0.19a | 1.17 ± 0.21a | – | – | – |
| H-SC (22) | f | 1.11 ± 0.19a,b | 1.10 ± 0.19a,b | 3.30 ± 0.66a,b | 1.71 ± 0.33a | 2.20 ± 0.33a |
| H-SC (30) | g | 0.93 ± 0.19a | 0.91 ± 0.02a | 3.67 ± 0.67a,b | 3.04 ± 0.49a,b,f | 2.25 ± 0.46a |
| H-SC/STD | h | 1.26 ± 0.12a,c | 1.33 ± 0.11a,c | 4.16 ± 0.55a,c | 4.14 ± 0.51 | 4.16 ± 0.55a,c,g |
Man: Mandible. Max: Maxilla. ACH: alveolar crest height. M1M2: molar 1-molar 2 interdental región; M2M3: molar 2-molar 3 interdental region. Lingual: lingual surface. Buccal: buccal surface. hABH: horizontal alveolar bone height. (Mean±SEM). Superscript letters indicate groups from which group differs (P < 0.05).
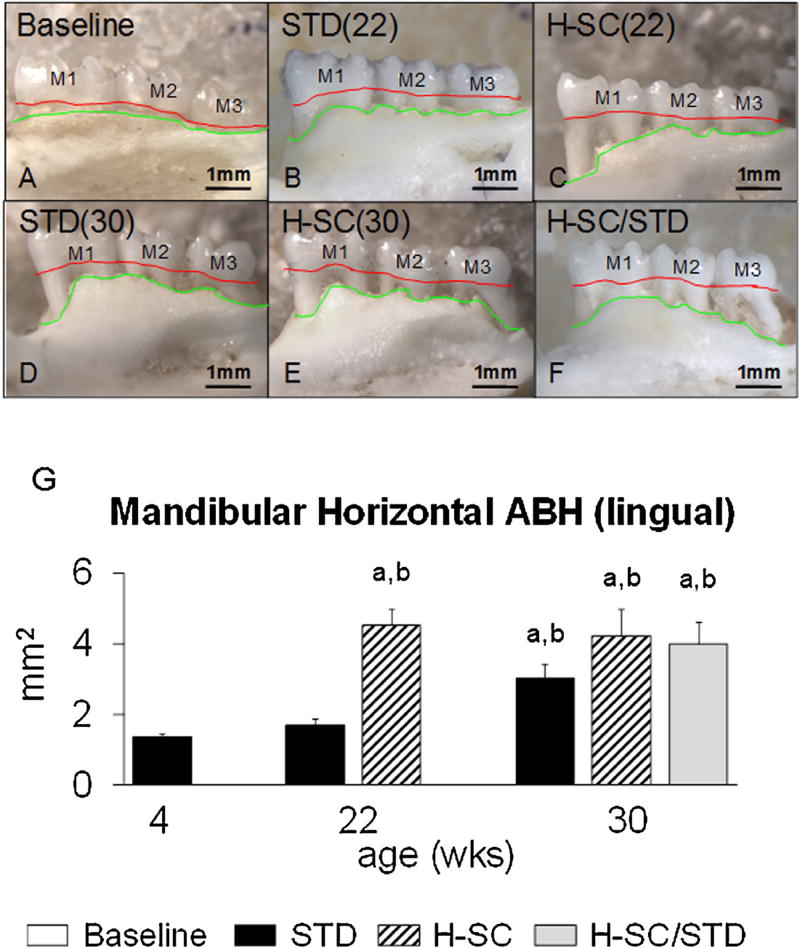
PD Score was significantly worse in H-SC (22) rats than in Baseline rats (P < 0.05) (Fig. 5G). All measures of ACH and hABH were significantly worse in H-SC (22) than in Baseline rats (P < 0.05) (Figs. 7 G and 8, Table 3).
Fig. 8.
Mandibular horizontal alveolar bone height (hABH) of representative animals from all groups. Photographs from the lingual aspect of the defleshed mandible from (Base) [A]; STD (22) [B]; H-SC (22) [C]; STD (30) [D]; H-SC (30) [E]; and H-SC/STD [F] groups. Red lines delineate the CEJ, the coronal boundary of the hABH measurement area. Green lines depict the coronal border of the alveolar bone and the apical boundary of the hABH measurement area. Note the worse hABH in H-SC (22) [C], but not in STD (22) [B] than in Base [A]. At age 22 weeks, hABH is also significantly worse in [C] than in [B]. By age 30 weeks, STD (30), H-SC (30), and H-SC/STD rats have comparable hABH [D-F]. M1, M2, and M3: first, second, and third molars, respectively. Lingual mandibular hABH (G). Superscript letters indicate a significant difference between groups (P < 0.05); a: different from Baseline; b: different from STD (22).
3.3. STD diet vs. H-SC diet (age 22wks)
PD Score and ACHM1M2 µCT were significantly worse in H-SC (22) rats than STD (22) rats (P < 0.05) (Figs. 6 G and 7 G). Interradicular BV/TV was significantly worse in H-SC (22) rats than in STD (22) rats (P < 0.05) (Table 4). All mandibular hABH measures were also significantly worse in H-SC (22) than STD (22) rats (P < 0.05) (Fig. 8 and Table 3).
3.4. Baseline vs. STD (30wks)
All ACH and hABH measures were significantly worse in STD (30) rats than in Baseline rats (P < 0.05) (Figs. 7 and 8, and Table 3). In addition, interradicular Conn.D was significantly higher in STD (30) rats than in Baseline rats (P < 0.05) (Table 4).
3.5. Age 22wks vs. age 30wks
All hABH measures were significantly worse in STD (30) rats than in STD (22) rats (P < 0.0004 or better) (Fig. 8, Table 3). All ACH, interdental/interradicular microarchitecture, and hABH measures were similar in H-SC (30) and H-SC (22) rats, except maxillary lingual hABH (Figs. 7 and 8, Tables 3 and 4).
3.6. STD diet vs. H-SC diet (age 30wks)
The STD (30) group had comparable PD Score, ACH, hABH, and interdental/interradicular microarchitecture to that in the H-SC (30) group (Figs. 6–8, Tables 3 and 4).
3.7. H-SC/STD vs. STD diet or H-SC diet (age 30wks)
H-SC/STD rats had comparable PD score to both STD (30) and H-SC (30) rats (Fig. 6). ACHM1M2 µCT, ACHM1M2(histo), ACHM2M3 (histo), mxbhABH, and mnbhABH were significantly worse in H-SC/STD rats than in STD (30) rats (P < 0.05), but not significantly different from H-SC (30) rats (Figs. 7–8, Table 3). Interdental/interradicular microarchitectural parameters in H-SC/STD rats were comparable to those in STD (30) and H-SC (30) rats (Table 4).
4. Discussion
These data indicate that juvenile rice rats develop significant moderate/severe PD after six months of eating standard laboratory rat chow. This PD worsens somewhat by age 80 weeks, but creates no animal welfare issues. These observations bolster previous data (Aguirre et al., 2012a) by adding 58 additional weeks of observation and comparing inflammation and alveolar crest height directly to a periodontally-healthy (Baseline) group of rice rats. Unlike other small animal PD models that employ standard laboratory rat species that are generally highly resistant to the development of age-related PD (Cesar Neto et al., 2004; Dumitrescu et al., 2004; Li et al., 2010; Lohinai et al., 1998; Nakamura et al., 2010; Struillou et al., 2010), only a baseline group of juvenile rice rats appears to be PD-free.
In particular, documenting ACH, hABH, and PD Score in periodontally-healthy rice rats is crucial. ACH is usually expressed relative to the CEJ (Hausmann et al., 1989a; Hausmann et al., 1989b; Hausmann et al., 1991). hABH is actually a direct measure of exposed cementum. For both ACH and hABH, a larger value indicates more extensive alveolar bone loss. Though both measurements use the CEJ as a referent, it is expected that the interdental alveolar crest in a healthy periodontium is somewhat below the level of the CEJ (Hausmann et al., 1989a; Hausmann et al., 1989b; Hausmann et al., 1991). Our data show that the interdental alveolar crest is 200–300 µm below the CEJ in mandibular interdental regions of periodontally healthy juvenile rice rats, rather than right at the CEJ. Similarly, our hABH data also show that 0.5–1.3 mm2 of cementum is exposed at buccal and lingual surfaces of the molars in the mandible and maxilla of periodontally healthy juvenile rice rats. For both ACH and hABH, only values significantly larger than those above represent actual bone loss. In addition, including baseline tissue from periodontally-healthy juvenile rice rats in any experiment is helpful in a practical sense because it provides healthy, albeit not age-matched, tissue to incorporate into randomized blinded quantitative morphologic and biochemical evaluation of the periodontium from rice rats given treatments that may be eventually tested for their ability to prevent/treat PD.
We also performed standard MicroCT-based 3D microarchitectural analyses of the mandibular M1 interradicular and M1-M2 interdental alveolar bone regions. Unfortunately, these endpoints frequently failed to reveal significant differences in regions where more traditional local measures, such as ACH and hABH, found significant alveolar bone deterioration with increasing severity of periodontitis. These analyses, that usually assay a total volume of 5–10 mm3 in the long bone metaphysis, were limited to approximately 0.15–0.25 mm3 of total interradicular or interdental volume per rat, suggesting a possible sampling problem. It is also possible that bone loss caused by inflammatory processes affecting the periodontium is localized to the coronal portion of the alveolar bone in a manner that causes no microarchitectural changes to the apical portion of the alveolar bone, in rats with the degree of periodontitis observed in this experiment.
In the past, rice rats have seldom been fed standard laboratory chow during PD experiments. When viewed in the backdrop of groups fed standard laboratory chow, our data provide a new perspective about the role of the H-SC diet that is usually seen as required to initiate PD in rice rats (Gupta & Shaw, 1956; Leonard, 1979). As shown above, rice rats develop significant PD without eating a H-SC diet. Therefore, our data indicate that feeding rice rats a H-SC diet accelerates, rather than initiates, the onset of PD. Rice rats fed the H-SC diet for 18 weeks have the same PD severity as those fed the STD diet for 26 weeks. Furthermore, rice rats fed the H-SC and STD diets for 26 weeks have similar severity of PD. These data may also suggest that the H-SC diet accelerates the onset of PD only in individual rice rats that have a predisposition to develop PD.
Our data show that the H-SC diet can be fed to rice rats for at least 26 weeks without inducing obesity and metabolic disturbances characteristic of diabetes. Recent serum chemistry data for normal rice rats (Aguirre, Edmonds, & Zamora, 2015) indicate that CMP values are comparable to the normal range for other laboratory rodent species (Berglund, Li, & Poffenberger, 2008; Fox, Anderson, & Quimby, 2002). The H-SC diet has been a potential concern, because high sucrose can induce diabetes that would be a confounder for PD development (Moodley, Wood, & Shangase, 2013; Preshaw & Bissett, 2013). Long term excess sucrose feeding in rodents induces weight gain, hyperglycemia and glucose intolerance, with variable insulin responses (Hallfrisch, Lazar, Jorgensen, & Reiser, 1979; Lombardo, Drago, &Chicco, 1996). A high sucrose diet can also induce insulin resistance, hyperinsulinemia (Gutman, Basilico, Bernal, Chicco, & Lombardo, 1987) or hyper-triglyceridemia and hypertension without obesity or changes in fasting plasma glucose and leptin (Chevalier, Wiley, & Leveille, 1972; Agheli, Kabir, & Berni-Canani, 1998; London, Lala, & Berger, 2007). Diabetes alters wound healing (Meyer, 1996; Nolan, Damm, & Prentki, 2011) and is associated with higher PD prevalence and increased severity in humans (Leite, Marlow, & Fernandes, 2013; Watanabe, Petro, Shlimon, & Unterman, 2008). In Rattus norvegicus, a high sucrose diet can even induce diabetic-like nephropathy (Cohen & Rosenmann, 1971), something apparently absent from H-SC diet rice rats with their normal BUN and creatinine. All these data together suggest that the H-SC diet poses no systemic health risk. The ability to accelerate safely the development of rice rat PD adds a relevant option to shorten experiments, because many humans with severe PD are systemically healthy.
Our data also suggest that the STD diet rice rat could be used to test interventions that alter the rate of onset of SP. The lack of animal welfare problems in rice rats consuming a STD diet also suggests that long-term treatments intended to reverse PD could be undertaken without ethical concerns. The gradual onset of PD on the STD diet also produces an opportunity to initiate treatments of different severities of PD with interventions that could prevent (e.g., starting before inflammation has begun or alveolar bone loss has progressed) or treat (e.g., to reduce established inflammation or regenerate lost alveolar bone) tissue level PD. Hence, in this way, rice rats can model not only human SP, but also mild/moderate PD that progresses in the absence of routine oral hygiene practices.
Switching to a STD diet from a H-SC diet for eight weeks in rats with moderate/severe PD caused significantly worse maxillary buccal hABH than in rats that continued the H-SC diet, with no significant differences in the other seven endpoints. We interpret these data as showing that switching diets caused no reversal of PD. This minimal difference is not surprising considering that PD occurs in STD diet rice rats. We suggest that more powerful, non-mechanical tissue-level interventions such as antibiotics, perhaps targeting Aggregatibacter actinomycetemcomitans (Aa) (e.g., doxycycline), or TNF-α antagonists (Di et al., 2007) (etanercept, etc.), might slow the progress of rice rat PD (Bezerra, Brito, Ribeiro, & Rocha, 2002).
The dental field already has excellent models for studying the initiation and early progression phases of PD (Cesar Neto et al., 2004; Dumitrescu et al., 2004; Li et al., 2010; Lohinai et al., 1998; Nakamura et al., 2010; Struillou et al., 2010). All display gingival inflammation followed by plaque accumulation, periodontal ligament (PDL) involvement, and alveolar bone loss, making each well-suited for 2–8 week studies. In each, PD induction requires a specific event or ongoing oral intervention to commercially-available rodents that are otherwise quite PD-resistant (Struillou et al., 2010). These events/treatments are reasonable surrogates for events known to cause mild/moderate PD in humans (Bezerra et al., 2002), enabling their use in study of PD initiation and progression during multiple relevant treatment interventions (Cesar Neto et al., 2004; Okada et al., 2010; Bezerra et al., 2002). Mild-to-moderate PD in adult humans with its high prevalence (i.e., 39%) (Thornton-Evans et al., 2013;Corbet, Zee, & Lo, 2002) and available preclinical models (Graves et al., 2012; Oz & Puleo, 2011; Struillou et al., 2010), has drawn much attention because of the intrinsic appeal of studying treatments for less advanced PD. The PD Score, ACH data, and hABH data presented here indicate that the STD diet rice rat could represent a novel small animal model of SP at the tissue level. Severe periodontitis that occurs in 8.5% of US and 11% of worldwide adult humans, (Kassebaum et al., 2014; Thornton-Evans et al., 2013), remains one of the two major causes for tooth extraction in the many adults who forego preventive dental care (Petersen, 2008). 20–42% of teeth extracted worldwide in adults are periodontally-involved (McCaul, Jenkins, & Kay, 2001; Aida et al., 2006; Vignarajah, 1993; Ong, Yeo, & Bhole, 1996; Reich & Hiller, 1993). The high prevalence of SP in humans emphasizes the potential value of preclinical models of human SP. Periodontitis in STD diet rice rats could enable pre-clinical studies of treatments that may halt or reverse SP that is less well-addressed by current models.
Though the tissue level pathology of rice rat PD resembles that seen in humans with moderate/severe PD and the molecular and bacterial etiologies may differ, clues to the fundamental etiology of rice rat PD exist. Rice rats, like humans, harbor Aa, one of only a few bacteria capable of longer-term infection in the oral cavity of rats (Graves et al., 2012; Socransky, Mac Donald, Sawyer, & auskaps, 1960). Aa colonizes the tooth surface, invades the gingival connective tissue, and adheres to rat buccal epithelial cells. However, Aa is rarely found in commercially-available laboratory rat strains (e.g., Sprague-Dawley, Wistar, Fischer 344, Long-Evans, etc.) that are resistant to the development of PD (Struillou et al., 2010). Rice rats may also possess unique, as yet uninvestigated, genetic susceptibility to gradual PD development. Unlike other small PD animal models, PD in rice rats occurs without local mechanical manipulation, ongoing maintenance/replacement of irritant or periodic injection of or re-inoculation with pathogens. This relative convenience may compensate for its more deliberate onset of PD, making the rice rat model practical for investigators who lack the resources or skills to apply precise intraoral manipulations. In addition, compared to mouse PD models (Abe & Hajishengallis, 2013; Polak, Wilensky, & Shapira, 2009), rice rats are large enough to permit sampling periodontal tissues by both oral examination in vivo and post-mortem study. Though this study addresses tissue level pathology and provides no new information on the etiology of severe PD in the rice rat, it identifies general times at which various phases of PD appear, guiding the design of experiments that obtain tissue before or during such phases that might allow identifying molecular events or microbiologic environments that precede or accompany tissue level changes, facilitating comparison to similar human studies.
The current rice rat model of severe PD has significant practical limitations. It offers little chance to study surgical and oral hygiene interventions known to be effective in treating human PD. This animal species is not currently commercially available, requiring investigator-directed breeding and weaning. Though some evidence suggests an infectious PD etiology (Socransky et al., 1960; MacDonald, Socransky, & Sawyer, 1960; Dick, Shaw, & Socransky, 1968), the early microbiologic and molecular events surrounding rice rat PD remain undefined. Because there is currently no prospective measure to predict which individual rats will develop severe PD, rice rat PD experiments require group sizes of 13–15. The advantages of including a baseline group of rice rats to serve as a periodontally-healthy control that enables the most accurate evaluation of alveolar bone loss are apparent.
In conclusion, rice rats fed standard laboratory rat chow from weaning develop mild PD by age 22 weeks and moderate/severe PD by age 30 weeks that persists without animal welfare concerns through age 80 weeks. Feeding a H-SC diet from weaning accelerates the development of severe PD to age 22wks without creating systemic health risks. Switching to a standard diet for eight weeks after 18 weeks of H-SC diet feeding does not reverse the progress of PD. These findings support further examination of the rice rat for its utility in preclinical studies of the prevention and treatment of SP.
Acknowledgments
This research was supported by Amgen Inc. (Thousand Oaks, CA, USA). We thank Dr. Kent Edmonds, Department of Biology, Indiana University Southeast, and New Albany, IN for providing the original rice rat breeders.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Authors’ declaration of contributions
J.I Aguirre: Conception and design of study; acquisition of data; interpretation of data; drafting of manuscript; revising/adding important intellectual content; final approval of submitted version
M.P. Akhter: Acquisition of data; revising/adding important intellectual content; final approval of submitted version
K.G. Neuville: Acquisition of data; final approval of submitted version
C.R. Trcalek: Acquisition of data; final approval of submitted version
A.M. Leeper: Acquisition of data; final approval of submitted version
A.A. Williams: Acquisition of data; final approval of submitted version
M. Rivera: Acquisition of data; final approval of submitted version
L. Kesavalu: Acquisition of data; final approval of submitted version
H.Z. Ke: Interpretation of data; final approval of submitted version
M. Liu: Interpretation of data; revising/adding important intellectual content; final approval of submitted version
D.B. Kimmel: Conception and design of study; interpretation of data; revising/adding important intellectual content; final approval of submitted version
All authors have read and approved the final version of the article.
References
- Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. Journal of Immunological Methods. 2013;394(1–2):49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agheli N, Kabir M, Berni-Canani S, et al. Plasma lipids and fatty acid synthase activity are regulated by short-chain fructo-oligosaccharides in sucrose-fed insulin-resistant rats. Journal of Nutrition. 1998;128(8):1283–1288. doi: 10.1093/jn/128.8.1283. [DOI] [PubMed] [Google Scholar]
- Aguirre JI, Akhter M, Kimmel D, et al. Enhanced alveolar bone loss in a model of non-invasive periodontitis in rice rats. Oral Diseases. 2012;18(5):459–468. doi: 10.1111/j.1601-0825.2011.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JI, Akhter MP, Kimmel DB, et al. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. Journal of Bone and Mineral Research. 2012;27(10):2130–2143. doi: 10.1002/jbmr.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre JI, Edmonds K, Zamora B, et al. Breeding, Husbandry, Veterinary Care, and Hematology of Marsh Rice Rats (Oryzomys palustris), a small animal model for periodontitis. Journal of the American Association for Laboratory Animal Science. 2015;54(1):51–58. [PMC free article] [PubMed] [Google Scholar]
- Aida J, Ando Y, Akhter R, Aoyama H, Masui M, Morita M. Reasons for permanent tooth extractions in Japan. Journal of Epidemiology. 2006;16(5):214–219. doi: 10.2188/jea.16.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auskaps A, Gupta O, Shaw J. Periodontal disease in the rice rat: III. Survey of dietary influences. Journal of Nutrition. 1957;63(3):325–343. doi: 10.1093/jn/63.3.325. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra MM, Brito GA, Ribeiro RA, Rocha FA. Low-dose doxycycline prevents inflammatory bone resorption in rats. Brazilian Journal of Medical and Biological Research. 2002;35(5):613–616. doi: 10.1590/s0100-879x2002000500015. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Talih M. Examining periodontal disease disparities among U. S. adults 20 years of age and older: NHANES III (1988–1994) and NHANES 1999–2004. Public Health Reports. 2012;127(5):497–506. doi: 10.1177/003335491212700505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M, Boyd SK, Christiansen BA, Guldberg R, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-Computed tomogrraphy. Journal of Bone and Mineral Research. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Cesar Neto JB, de Souza AP, Barbieri D, Moreno H, Jr, Sallum EA, Nociti FH., Jr Matrix metalloproteinase-2 may be involved with increased bone loss associated with experimental periodontitis and smoking: A study in rats. Journal of Periodontology. 2004;75(7):995–1000. doi: 10.1902/jop.2004.75.7.995. [DOI] [PubMed] [Google Scholar]
- Chevalier MM, Wiley JH, Leveille GA. Effect of dietary fructose on fatty acid synthesis in adipose tissue and liver of the rat. Journal of Nutrition. 1972;102(3):337–342. doi: 10.1093/jn/102.3.337. [DOI] [PubMed] [Google Scholar]
- Cohen AM, Rosenmann E. Diffuse intercapillary glomerulosclerosis in sucrose-fed rats. Diabetologia. 1971;7(1):25–28. doi: 10.1007/BF02346250. [DOI] [PubMed] [Google Scholar]
- Corbet EF, Zee KY, Lo EC. Periodontal diseases in asia and oceania. Periodontology 2000. 2002;29:122–152. doi: 10.1034/j.1600-0757.2002.290107.x. [DOI] [PubMed] [Google Scholar]
- Di PR, Mazzon E, Muia C, et al. Effects of etanercept, a tumour necrosis factor-alpha antagonist, in an experimental model of periodontitis in rats. British Journal of Pharmacology. 2007;150(3):286–297. doi: 10.1038/sj.bjp.0706979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DS, Shaw JH, Socransky SS. Further studies on the microbial agent or agents responsible for the periodontal syndrome in the rice rat. Archives of Oral Biology. 1968;13(2):215–228. doi: 10.1016/0003-9969(68)90053-8. [DOI] [PubMed] [Google Scholar]
- Dumitrescu AL, Abd-El-Aleem S, Morales-Aza B, Donaldson LF. A model of periodontitis in the rat: Effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. Journal of Clinical Periodontology. 2004;31(8):596–603. doi: 10.1111/j.1600-051X.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- Fox JG, anderson LFM, Quimby FW. Laboratory animal medicine. 2. London: Elsevier; 2002. [Google Scholar]
- Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat: I. Morphometric and autoradiographic studies. Journal of Periodontal Research. 1981;16(3):275–291. doi: 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol. 2012;15:117–132. doi: 10.1159/000329675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta O, Shaw J. Periodontal disease in the rice rat. I: Anatomic and histopathologic findings. Oral Surgery, Oral Medicine, Oral Pathology. 1956;9(6):592–603. doi: 10.1016/0030-4220(56)90319-x. [DOI] [PubMed] [Google Scholar]
- Gutman RA, Basilico MZ, Bernal CA, Chicco A, Lombardo YB. Long-term hypertriglyceridemia and glucose intolerance in rats fed chronically an isocaloric sucrose-rich diet. Metabolism: Clinical and Experimental. 1987;36(11):1013–1020. doi: 10.1016/0026-0495(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Hallfrisch J, Lazar F, Jorgensen C, Reiser S. Insulin and glucose responses in rats fed sucrose or starch. American Journal of Clinical Nutrition. 1979;32(4):787–793. doi: 10.1093/ajcn/32.4.787. [DOI] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Dunford R, Christersson L. A reliable computerized method to determine the level of the radiographic alveolar crest. Journal of Periodontal Research. 1989a;24(6):368–369. doi: 10.1111/j.1600-0765.1989.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Christersson L, Genco RJ. Effect of x-ray beam vertical angulation on radiographic alveolar crest level measurement. Journal of Periodontal Research. 1989b;24(1):8–19. doi: 10.1111/j.1600-0765.1989.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Hausmann E, Allen K, Clerehugh V. What alveolar crest level on a bitewing radiograph represents bone loss? Journal of Periodontology. 1991;62(9):570–572. doi: 10.1902/jop.1991.62.9.570. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systemic review and meta-regression. Journal of Dental Research. 2014;93(11):1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite RS, Marlow NM, Fernandes JK. Oral health and type 2 diabetes. American Journal of the Medical Sciences. 2013;345(4):271–273. doi: 10.1097/MAJ.0b013e31828bdedf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard EP. Periodontitis: Animal model: Periodontitis in the rice rat (Oryzomys palustris) American Journal of Pathology. 1979;96(2):643–646. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Messina C, Bendaoud M, Fine DH, Schreiner H, Tsiagbe VK. Adaptive immune response in osteoclastic bone resorption induced by orally administered Aggregatibacter actinomycetemcomitans in a rat model of periodontal disease. Mol Oral Microbiology. 2010;25(4):275–292. doi: 10.1111/j.2041-1014.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- Liu XM, Wiswall AT, Rutledge JE, et al. Osteotropic beta-cyclodextrin for local bone regeneration. Biomaterials. 2008;29(11):1686–1692. doi: 10.1016/j.biomaterials.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe H. Principles of aetiology and pathogenesis governing the treatment of periodontal disease. International Dental Journal. 1983;33(2):119–126. [PubMed] [Google Scholar]
- Lohinai Z, Benedek P, Feher E, et al. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. British Journal of Pharmacology. 1998;123(3):353–360. doi: 10.1038/sj.bjp.0701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo YB, Drago S, Chicco A, et al. Long-term administration of a sucrose-rich diet to normal rats: Relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metabolism: Clinical and Experimental. 1996;45(12):1527–1532. doi: 10.1016/s0026-0495(96)90183-3. [DOI] [PubMed] [Google Scholar]
- London E, Lala G, Berger R, et al. Sucrose access differentially modifies 11beta-hydroxysteroid dehydrogenase-1 and hexose-6-phosphate dehydrogenase message in liver and adipose tissue in rats. Journal of Nutrition. 2007;137(12):2616–2621. doi: 10.1093/jn/137.12.2616. [DOI] [PubMed] [Google Scholar]
- MacDonald JB, Socransky SS, Sawyer SJ. Pathogenicity experiments with the flora of the periodontium in rice rats. Journal of Dental Research. 1960;39:861. doi: 10.1177/00220345600390041601. [DOI] [PubMed] [Google Scholar]
- McCaul LK, Jenkins WM, Kay EJ. The reasons for extraction of permanent teeth in Scotland: A 15-year follow-up study. British Dental Journal. 2001;190(12):658–662. doi: 10.1038/sj.bdj.4801068. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Diabetes and wound healing. Critical Care Nursing Clinics of North America. 1996;8(2):195–201. [PubMed] [Google Scholar]
- Moodley A, Wood NH, Shangase SL. The relationship between periodontitis and diabetes: A brief review. SADJ. 2013;68(6):260. [262-0, 264] [PubMed] [Google Scholar]
- Nakamura H, Ukai T, Yoshimura A, et al. Green tea catechin inhibits lipopolysaccharide-induced bone resorption in vivo. Journal of Periodontal Research. 2010;45(1):23–30. doi: 10.1111/j.1600-0765.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Okada Y, Hamada N, Kim Y, et al. Blockade of sympathetic b-receptors inhibits Porphyromonas gingivalis-induced alveolar bone loss in an experimental rat periodontitis model. Archives of Oral Biology. 2010;55(7):502–508. doi: 10.1016/j.archoralbio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Ong G, Yeo JF, Bhole S. A survey of reasons for extraction of permanent teeth in Singapore. Community Dentistry and Oral Epidemiology. 1996;24(2):124–127. doi: 10.1111/j.1600-0528.1996.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Oz HS, Puleo DA. Animal models for periodontal disease. Journal of Biomedicine and Biotechnology. 2011;2011:1–8. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC. Current understanding of the aetiology and progression of periodontal disease. International Dental Journal. 1986;36(3):153–161. [PubMed] [Google Scholar]
- Park C, Abramson Z, Taba MJ, et al. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. Journal of Periodontology. 2007;78(2):273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PE. World Health Organization global policy for improvement of oral health-world health assembly 2007. International Dental Journal. 2008;58(3):115–121. doi: 10.1111/j.1875-595x.2008.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Polak D, Wilensky A, Shapira L, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: Bone loss and host response. Journal of Clinical Periodontology. 2009;36(5):406–410. doi: 10.1111/j.1600-051X.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Bissett SM. Periodontitis: Oral complication of diabetes. Endocrinology and Metabolism Clinics of North America. 2013;42(4):849–867. doi: 10.1016/j.ecl.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Rajapakse PS, O’Brien-Simpson NM, Slakeski N, Hoffmann B, Reynolds EC. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infection and Immunity. 2002;70(5):2480–2486. doi: 10.1128/IAI.70.5.2480-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E, Hiller KA. Reasons for tooth extraction in the western states of Germany. Community Dentistry and Oral Epidemiology. 1993;21(6):379–383. doi: 10.1111/j.1600-0528.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Rosner B. Hypothesis testing: One-Sample inference. In: Talylor M, Seibert D, editors. Fundamentals of biostatistics. Boston, MA: Brooks/Cole; 2011. [Google Scholar]
- Ryder MI. Histological and ultrastructural characteristics of the periodontal syndrome in the rice rat: I. General light microscopic observations and ultrastructural observations of initial inflammatory changes. Journal of Periodontal Research. 1980;15(5):502–515. doi: 10.1111/j.1600-0765.1980.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Mac Donald JB, Sawyer SJ, auskaps AM. Quantitative studies of the bacterial flora of the periodontium in rice rats. Archives of Oral Biology. 1960;2:104–110. doi: 10.1016/0003-9969(60)90058-3. [DOI] [PubMed] [Google Scholar]
- Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: A review. Open Dentistry Journal. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Evans G, Eke P, Wei L, et al. Periodontitis among adults aged >/=30 years – United States, 2009–2010. MMWR Surveillance Summaries. 2013;62(Suppl 3):129–135. [PubMed] [Google Scholar]
- Tonetti MS, Mombelli A. Early-onset periodontitis. Annals of Periodontology. 1999;4(1):39–53. doi: 10.1902/annals.1999.4.1.39. [DOI] [PubMed] [Google Scholar]
- Ullman-Cullere MH, Foltz CJ. Body condition scoring: A rapid and accurate method for assessing health status in mice. Laboratory Animal Science. 1999;49(3):319–323. [PubMed] [Google Scholar]
- Verma RK, Rajapakse S, Meka A, et al. Porphyromonas gingivalis and treponema denticola mixed microbial infection in a rat model of periodontal disease. Interdisciplinary Perspectives on Infectious Diseases. 2010;2010:605125–605131. doi: 10.1155/2010/605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma RK, Bhattacharyya I, Sevilla A, et al. Virulence of major periodontal pathogens and lack of humoral immune protection in a rat model of periodontal disease. Oral Diseases. 2010;16(7):686–695. doi: 10.1111/j.1601-0825.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- Vignarajah S. Various reasons for permanent tooth extractions in a Caribbean population-Antigua. International Dental Journal. 1993;43(3):207–212. [PubMed] [Google Scholar]
- Watanabe K, Petro BJ, Shlimon AE, Unterman TG. Effect of periodontitis on insulin resistance and the onset of type 2 diabetes mellitus in Zucker diabetic fatty rats. Journal of Periodontology. 2008;79(7):1208–1216. doi: 10.1902/jop.2008.070605. [DOI] [PubMed] [Google Scholar]