Abstract
Background and Purpose
Preeclampsia (PEC) affects 3–8% of pregnancies, and increases risk of pregnancy-associated stroke (PAS). Data are limited on which women with PEC are at highest risk for PAS.
Methods
Using billing data from the 2003–2012 New York State Department of Health inpatient database, we matched women with PEC and PAS 1:3 to preeclamptic controls based on age and race/ethnicity. Pre-defined PAS risk factors included pregnancy complications, infection present on admission, vascular risk factors, prothrombotic states, and coagulopathies. We constructed multivariable conditional logistic regression models to calculate the odds ratios and 95% confidence intervals (OR, 95%CI) for independent risk factors for PAS.
Results
Among women aged 12–55 years admitted to New York State hospitals for any reason during the study period (n= 3,373,114), 88,857 had PEC, 197 of whom (0.2%) had PAS. In multivariable analysis, women with PEC and stroke were more likely than controls to have severe PEC or eclampsia (OR 7.2, 95%CI 4.6–11.3), infections present on admission (OR 3.0, 95%CI 1.6–5.8), prothrombotic states (OR 3.5, 95%CI 1.3–9.2), coagulopathies (OR 3.1, 95%CI 1.3–7.1), or chronic hypertension (OR 3.2, 95%CI 1.8–5.5). Additional analyses matched and stratified by severity of PEC confirmed these results.
Conclusions
Infections, chronic hypertension, coagulopathies, and underlying prothrombotic conditions increase PAS risk in women with PEC. These women may warrant closer monitoring.
INTRODUCTION
Preeclampsia (PEC) is a multisystem hypertensive disorder unique to pregnancy, characterized by widespread endothelial dysfunction and immune dysregulation.1 Approximately 36% of women with pregnancy-associated strokes (PAS) have comorbid PEC,2 and PEC increases stroke risk during the puerperium up to 6-fold.3 Among women with PAS, women with PEC are at increased risk of complications and death.4,5
Although PEC affects 3–8% of all pregnancies,3,6 the overall occurrence of PAS remains rare (34.2 per 100,000 deliveries).7 Older age, black race, and lack of private insurance are associated with PAS in women with PEC,4,8 and increased PEC severity is associated with an increased risk of cardiovascular events.9 The rarity of PAS makes it difficult to predict which PEC patients are at highest risk for cerebrovascular complications. The American Heart Association/American Stroke Association found insufficient evidence to make recommendations regarding prevention of stroke in pregnancy complicated by hypertensive disorders.10
We sought to identify modifiable risk factors that put women with PEC at highest risk of PAS.
METHODS
Study design and data description
We performed a case-control study using billing data, coded according to the International Classification of Diseases, Ninth Revision (ICD-9), from the 2003–2012 New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS) inpatient database. We identified all women aged 12–55 years old admitted with preeclampsia, including mild preeclampsia (642.4x [where “x” is any fifth digit from 0–9], 642.7x), severe preeclampsia (642.5x), or eclampsia (642.6x), from January 1, 2003 through December 31, 2012. Women without PEC were not included in the study, regardless of whether they had strokes during or after pregnancy. Cases were defined as women with PEC and PAS, including diagnosis codes for transient ischemic attack (TIA) or ischemic stroke, intracerebral hemorrhage (ICH), cerebral venous thrombosis, and codes specific for non-specified PAS. Pregnancy-specific stroke codes were validated using internal datasets (Supplemental Table I). In women with more than one stroke admission, we counted only the first admission. We characterized PAS in terms of timing and stroke subtype. Although stroke risk is increased up to 12 weeks after delivery,11 a 6-week postpartum time frame was chosen due to limitations of ICD-9 coding.12 Traumatic ICH and SAH and hospitalizations with a primary rehabilitation diagnosis (V57.89) were excluded. We matched each stroke case to 3 controls of the same age, race/ethnicity, and insurance status, selected randomly from the pool of women with PEC without PAS.
Pre-defined exposures of interest, selected based on previously described associations with PEC and/or PAS, included pregnancy-related variables, chronic vascular risk factors, comorbid prothrombotic states, coagulopathies/bleeding disorders, and infection of any type present on admission (POA), identified with a SPARCS-specific indicator.13,14 Infections acquired during the stroke hospitalization were not included. Coding details are in Supplemental Table II.
“Cerebral symptoms” are sufficient to classify preeclampsia as “severe.”15,16 Since all our cases likely had neurological symptoms, this could create selection bias, confusing the relationship between severity of PEC and stroke risk (i.e., women with stroke and PEC might by definition be coded as having severe PEC). We therefore conducted a separate analysis matching each case to 3 controls of the same age, race/ethnicity, and severity of PEC. Prespecified subgroup analyses stratified the severity-matched cohort into two groups: those with mild PEC and those with severe PEC or eclampsia.
Statistical analysis
We conducted univariable analyses comparing risk factors among cases and controls using chi square tests. Including only those PAS risk factors with p<0.2 from the univariable analysis,17 we calculated unadjusted and adjusted odds ratios and 95% confidence intervals (OR, 95% CI) using multivariable conditional logistic regression. Prespecified subgroup analyses included a sensitivity analysis excluding all cases and controls with eclampsia (642.6x), and a subgroup analysis of postpartum strokes. All analyses were completed with SAS version 9.3 (SAS Institute, Cary, NC). A p-value of <0.05 was set as the significance level.
Study approvals
Approval was obtained from the Institutional Review Board of Columbia University Medical Center to conduct the analyses. Requirement for consent was waived due to the public, de-identified nature of the data.
RESULTS
Baseline characteristics of women with PEC, with and without PAS
Among women aged 12–55 years admitted for any reason during the study period (n= 3,373,114), 88,857 had PEC, including eclampsia. Of these, 197 (0.2%) had PAS and were identified as cases, giving a cumulative incidence of PAS in women with PEC of 222/100,000 during the study period. In the unmatched sample, compared to the 88,660 women with PEC who did not have PAS, women with PEC and PAS were older, had higher proportion of black race, lower proportion of Hispanic ethnicity, and higher proportion of severe preeclampsia (42.1% versus 29%) or eclampsia (28.9% versus 2%) (Figure 1). Among cases, median age was 32 years (interquartile range 26–36); 57 women (28.9%) had mild preeclampsia, 83(42.1%) had severe PEC, and 57(28.9%) had eclampsia. The 197 stroke cases were matched with 591 non-stroke controls of the same age, race/ethnicity and insurance status (Figure 2).
Figure 1.
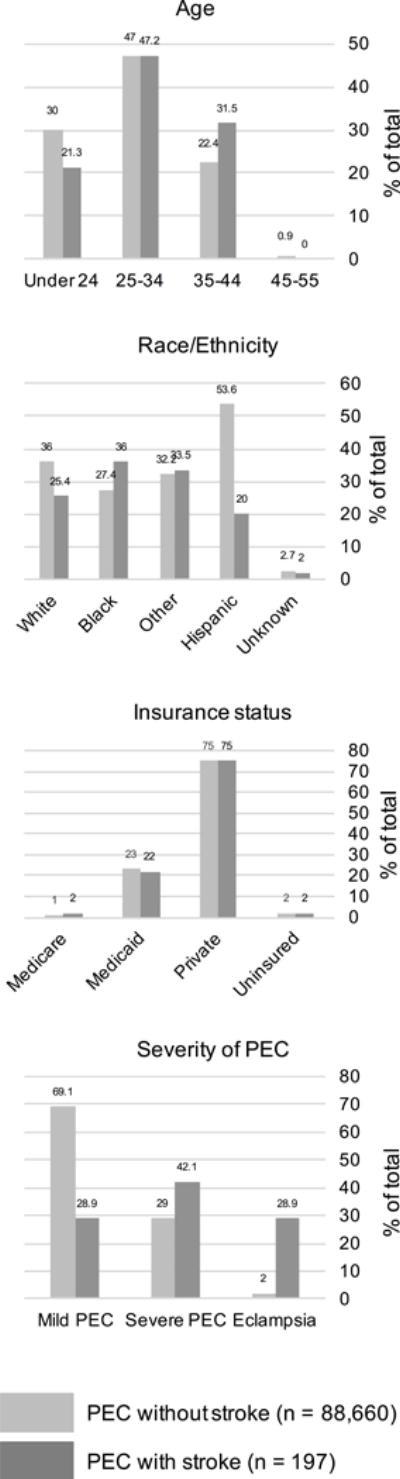
Baseline demographics of all women hospitalized with preeclampsia in New York State 2003-2012, with and without pregnancy-associated stroke
Cases (women with PEC and stroke, n=197) are represented in dark gray. The unmatched sample of all women with PEC and without stroke (n=88,660) is represented in light gray. All columns represent percentages of total number in category. PEC: preeclampsia.
Figure 2.
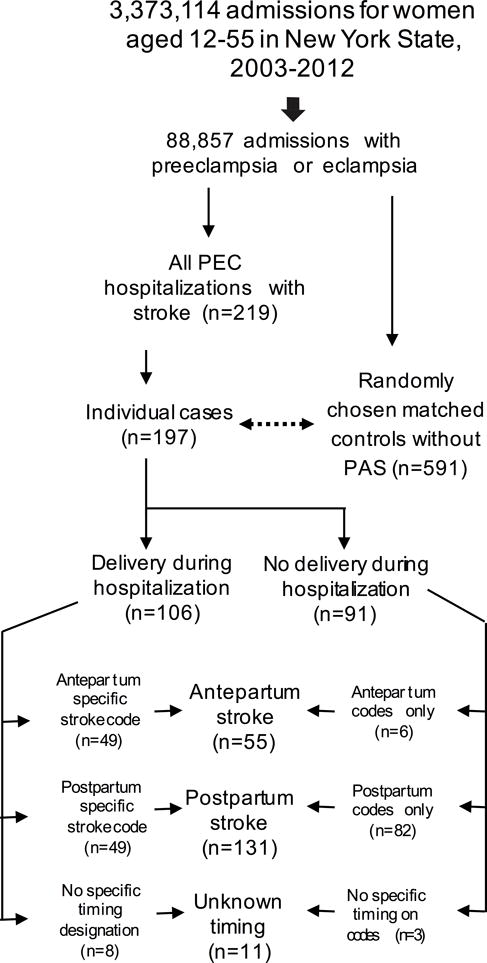
Patient selection, flow diagram
88,857 patients were admitted to hospitals in New York State between 2003 and 2012 with preeclampsia. Of these, 197 individual women had preeclampsia and stroke. Strokes were classified as postpartum if they occurred either during a postpartum admission, or during a delivery admission that included a pregnancy-specific stroke code with a fifth digit of 2 or 4 indicating a postpartum complication, in accordance with ICD-9 coding guidelines.12 If the pregnancy-specific stroke code had a fifth digit of 3 indicating an antepartum complication, and the admission included a delivery, the stroke was categorized as antepartum. PEC: preeclampsia.
Timing and characteristics of strokes in women with PEC and PAS
Among the 197 women with strokes, 55(27.9%) strokes occurred antepartum; 8(4.1%) occurred during the delivery hospitalization without further characterization in timing; 131(66.5%) occurred postpartum; and 3(1.5%) occurred during an admission without delivery, with no further characterization of timing. Of 131 postpartum strokes, 82(62.6%) occurred after discharge (Figure 2). Stroke types included 92(46.7%) hemorrhagic strokes, 26(13.1%) ischemic, 8(4.1%) TIA, 5(2.5%) cerebral venous thromboses, and 70(35.5%) non-specific PAS; 9 women had multiple subtypes (Supplemental Figure I). In-hospital mortality was 13.2% among cases, compared to 0.2% among controls. Details of discharge disposition/mortality are in Supplemental Table III.
Stroke risk factors in cases and controls matched on age/race-ethnicity/insurance
In univariable analysis, women with PAS had a higher proportion than controls of severe PEC or eclampsia, infections POA, chronic hypertension, prothrombotic states, coagulopathies, migraine, and heart disease (Table 1). The difference in infections between cases and controls was driven by genitourinary infections: 71% of infections in cases were genitourinary, compared with 39% of infections in controls, and genitourinary infections occurred in 10% of cases compared to 2% of controls (p<0.0001). After adjusting for other risk factors, significant risk factors for stroke were severe preeclampsia or eclampsia (OR7.2, 95%CI 4.6–11.3); infections POA (OR3.0, 95%CI 1.6–5.8); chronic hypertension (OR3.2, 95%CI 1.8–5.5); prothrombotic states (OR3.5, 95%CI 1.3–9.2); and coagulopathies (OR3.1, 95%CI 1.3–7.1) (Table 2).
Table 1.
Demographics and baseline characteristics in New York State women with pre-eclampsia, with and without pregnancy-associated stroke, 2003–2012.
| Prevalence of characteristics | Cases (PEC and PAS) | Controls (PEC without PAS) | p-value |
|---|---|---|---|
| n=197 (%) | n=591 (%) | ||
| Age | n/a (matched) | ||
| 24 and under | 42 (21) | 126 (21) | |
| 25-34 | 93 (47) | 279 (47) | |
| 35-44 | 62 (31) | 186 (31) | |
| Race/ethnicity | n/a (matched) | ||
| Non-Hispanic White | 50 (25) | 150 (25) | |
| Non-Hispanic Black | 71 (36) | 213 (36) | |
| Non-Hispanic Other | 33 (17) | 99 (17) | |
| Hispanic | 39 (20) | 117 (20) | |
| Unknown | 4 (2) | 12 (2) | |
| Insurance status | n/a (matched) | ||
| Uninsured | 4 (2) | 12 (2) | |
| Medicare | 43 (22) | 129 (22) | |
| Medicaid | 148 (75) | 444 (75) | |
| Private | 2 (1) | 6 (1) | |
| Severity of preeclampsia | p<0.0001 | ||
| Mild preeclampsia | 73 (37) | 435 (74) | p<0.0001 |
| Severe preeclampsia | 90 (46) | 157 (27) | p<0.0001 |
| Eclampsia | 57 (29) | 19 (3) | p<0.0001 |
| Chronic hypertension | 44 (22) | 81 (14) | p=0.004 |
| Prothrombotic states (includes following) | 26 (13) | 15 (3) | p<0.0001 |
| Hypercoagulable state | 15 (8) | 7 (1) | p<0.0001 |
| Sickle cell disease | 5 (3) | 1 (0) | p=0.005 |
| Systemic lupus erythematosus | 2 (1) | 0 (0) | p=0.06 |
| DVT/PE (acute, chronic, or history of prior) | 10 (5) | 7 (1) | p=0.003 |
| Infection present on admission (includes following) | 28 (14) | 33 (6) | p<0.0001 |
| Genitourinary infection | 20 (10) | 13 (2) | p<0.0001 |
| Chorioamnionitis | 4 (2) | 7 (1) | p=0.5 |
| Non-specified pregnancy related infection | 6 (3) | 17 (3) | p=1.0 |
| Respiratory infection | 0 (0) | 2 (0) | p=1.0 |
| Gastrointestinal infection | 1 (1) | 1 (0) | p=0.4 |
| Sexually transmitted infection (includes HIV) | 1 (1) | 7 (1) | p=0.7 |
| Sepsis | 3 (2) | 2 (0) | p=0.1 |
| Other infection | 4 (2) | 12 (2) | p=1.0 |
| Coagulopathy | 29 (15) | 16 (3) | p<0.0001 |
| Migraine | 6 (3) | 1 (0) | p=0.001 |
| Heart disease (includes following) | 6 (3) | 5 (1) | p=0.02 |
| Congestive heart failure | 1 (1) | 0 (0) | p=0.3 |
| Chronic ischemic heart disease | 1 (1) | 0 (0) | p=0.3 |
| Congenital heart disease | 1 (1) | 1 (0) | p=0.4 |
| Valvular heart disease | 3 (2) | 4 (1) | p=0.4 |
| Chronic renal disease | 1 (1) | 4 (1) | p=1.0 |
| Drug abuse or dependence | 4 (2) | 9 (2) | p=0.7 |
| Alcohol abuse | 4 (2) | 10 (2) | p=0.2 |
| HIV/AIDS (SPARCS Indicator) | 0 (0) | 1 (0) | p=1.0 |
| Diabetes | 7 (4) | 22 (4) | p=1.0 |
| Active smoking | 4 (2) | 8 (1) | p=0.5 |
| Obesity | 8 (4) | 15 (3) | p=0.3 |
| Gestational hypertension | 7 (4) | 21 (4) | p=1.0 |
| Multiple gestation | 4 (2) | 34 (6) | p=0.03 |
| Multigravida | 35 (18) | 137 (23) | p=0.1 |
Cases and controls matched on age, race/ethnicity, and insurance status. PEC: preeclampsia. PAS: pregnancy-associated stroke. DVT: deep vein thrombosis. PE: pulmonary embolism.
See Supplemental Table II for complete details of diagnostic codes.
Table 2.
Independent stroke risk factors in women with preeclampsia, with and without pregnancy-associated stroke, matched by age, race/ethnicity, and insurance status.
| Risk factors: multivariable analysis | Odds ratio (95% confidence interval) |
|
|---|---|---|
| Infection present on admission | Unadjusted |
2.7 (1.6–4.7) |
| Adjusted |
3.0 (1.6–5.8) |
|
| Chronic hypertension | Unadjusted |
1.9 (1.2–2.9) |
| Adjusted |
3.2 (1.8–5.5) |
|
| Prothrombotic statea | Unadjusted |
7.5 (3.5–16.1) |
| Adjusted |
3.5 (1.3–9.2) |
|
| Coagulopathyb | Unadjusted |
6.0 (3.1–11.3) |
| Adjusted |
3.1 (1.3–7.1) |
|
| Severe preeclampsia or eclampsia | Unadjusted |
6.6 (4.4–9.8) |
| Adjusted |
7.2 (4.6–11.3) |
|
| Heart diseasec | Unadjusted |
3.6 (1.1–11.8) |
| Adjusted | 1.7 (0.3–9.4) |
|
| Multiple gestation | Unadjusted | 0.3 (0.1–1.0) |
| Adjusted |
0.3 (0.1–0.9) |
|
| Multigravida | Unadjusted | 0.7 (0.5–1.1) |
| Adjusted | 1.1 (0.6–1.9) |
|
Includes primary hypercoagulable state, history of deep vein thrombosis or pulmonary embolism, systemic lupus erythematosis, sickle cell disease.
Includes bleeding diatheses and thrombocytopenia.
Includes congenital, valvular, and ischemic heart disease, and congestive heart failure.
See Supplemental Table II for complete details of diagnostic codes.
Demographics and risk factors in severity-matched cohort
When cases were matched to controls by PEC severity, similar risk factors emerged: infections POA (OR2.6, 95% 1.4–4.6); prothrombotic states (OR2.9, 95%CI 1.3–6.4); and chronic hypertension (OR4.2, 95%CI 2.4–7.4) conferred greater risk of stroke after adjusting for other risk factors (Table 3). In the severity-stratified subgroup analysis, after adjusting for other variables, chronic hypertension (OR3.0, 95%CI 1.4–6.3) and infection POA (OR4.5, 95%CI 1.6–12.6) were significant stroke risk factors in the mild subgroup (n=57). In the severe subgroup (n=140), chronic hypertension (OR8.4, 95%CI 3.1–22.5) and prothrombotic states (OR6.6, 95%CI 2.5–17.5) increased stroke risk; the association of infection POA did not reach statistical significance after adjusting for other risk factors (OR1.9, 95%CI 0.9–4.0; Supplemental Table IV). Demographics of mild versus severe cases and controls are shown in Supplemental Table V.
Table 3.
Investigation of risk factors for stroke, New York State women with preeclampsia, matched on severity of preeclampsia.
| Risk factors: multivariable analysis | All cases | Mild cases only | Severe cases only | |
|---|---|---|---|---|
| Odds ratio (95% CI) |
Odds ratio (95% CI) | Odds ratio (95% CI) |
||
| Chronic hypertension | Unadjusted | 4.8 (2.8–8.3) | 3.3 (1.6–6.5) | 8.6 (3.4–21.7) |
| Adjusted | 4.2 (2.4–7.4) | 3.0 (1.4–6.3) | 8.4 (3.1–22.5) | |
| Infection present on admission | Unadjusted | 2.8 (1.6–4.7) | 4.3 (1.7–10.6) | 2.1 (1.1–4.1) |
| Adjusted | 2.6 (1.4–4.6) | 4.5 (1.6–12.6) | 1.9* (0.9–4.0) | |
| Prothrombotic statea | Unadjusted | 3.6 (2.0–6.5) | n/a | 6.1 (3.1–12.2) |
| Adjusted | 2.9 (1.3–6.4) | n/a | 6.6 (2.5–17.5) | |
| Coagulopathyb | Unadjusted | 2.8 1.7–4.7 | n/a | 3.0 (1.7–5.4) |
| Adjusted | 1.5 (0.8–3.1) | n/a | 1.3 (0.5–2.9) | |
| Obesity | Unadjusted | n/a | 9.0 (1.8–44.6) | n/a |
| Adjusted | n/a | 4.9 (0.9–26.5) | n/a | |
| Heart diseasec | Unadjusted | 4.5 (1.3–15.9) | n/a | 3.8 (1.0–14.0) |
| Adjusted | 2.1 0.5–9.9 | n/a | 1.0 (0.2–5.4) | |
| Migraine | Unadjusted | 3.6 (1.1–11.8) | n/a | n/a |
| Adjusted | 3.6 (0.9–14.5) | n/a | n/a | |
| Multiple gestation | Unadjusted | 0.3 (0.1–0.8) | n/a | 0.3 (0.1–0.9) |
| Adjusted | 0.2 (0.1–0.9) | n/a | 0.2 (0.04–0.7) | |
| Multigravida | Unadjusted | 0.5 (0.3–0.7) | n/a | 0.3 (0.2–0.5) |
| Adjusted | 0.6 (0.4–0.9) | n/a | 0.3 (0.2–0.6) | |
Includes primary hypercoagulable state, history of deep vein thrombosis or pulmonary embolism, systemic lupus erythematosis, sickle cell disease.
Includes bleeding diatheses and thrombocytopenia.
Includes congenital, valvular, and ischemic heart disease, and congestive heart failure.
See Supplemental Table II for complete details of diagnostic codes.
Subgroup analyses
In the subgroup analysis excluding women with eclampsia (ICD-9 642.6x), results were similar: women with chronic hypertension (adjusted OR4.2, 95%CI 2.2–7.8), infection POA (adjusted OR3.7, 95%CI 1.8–7.7), and prothrombotic states (adjusted OR2.6, 95%CI 1.1–6.3), had higher risk of stroke (Supplemental Table VI). In the subgroup analysis including only postpartum stroke, fewer cases than controls delivered via cesarean section (52% versus 65%, p=0.01). However, after adjusting for other risk factors, there was no significant association between cesarean delivery and stroke risk (adjusted OR0.7, 95%CI 0.4–1.1). Otherwise, similar risk factors were found in women with postpartum stroke (Supplemental Tables VII–VIII).
DISCUSSION
In this case-control study in a diverse population of women with preeclampsia, we found that risk factors for PAS included infections (predominantly genitourinary), chronic hypertension, prothrombotic conditions and coagulopathies. The cumulative incidence of stroke in our preeclamptic population was 222/100,000, over six times the incidence of stroke in the overall pregnant population, consistent with prior studies.3,4 Two-thirds of cases of PAS (131 of 197) were diagnosed postpartum, consistent with prior research.7 More than 1 in 10 women with PEC and PAS died during their admission for stroke, likely reflecting the large proportion of hemorrhagic strokes.18 In comparison, overall US maternal mortality in 2011 was 17.8 per 100,000 deliveries, or 0.02%,5,19–21 emphasizing the importance of identifying women at the highest risk of PAS and targeting them for closer monitoring.
Infection
Infection is increasingly recognized as a trigger for stroke, particularly in young people.14,22 Proposed mechanisms include increased levels of inflammatory cytokines leading to platelet aggregation and impaired endothelial function. Infections may also provoke cardiac arrhythmias or dehydration-induced thrombosis; in one study, infections tripled the odds of peripartum cerebral venous thrombosis.23 The pathophysiology of PEC remains incompletely understood,1 although imbalance of pro- and anti-angiogenic factors and an increase in pro-inflammatory cytokines appear to play roles.24 Animal models of PEC have demonstrated impaired cerebral autoregulation, increased blood-brain barrier permeability, and neuronal hyperexcitability, mediated by elevated levels of tumor necrosis factor-alpha.25–28 In the setting of PEC-associated inflammation and puerperial hypercoagulability, superimposed infection could trigger stroke by exacerbating inflammation and coagulopathy.11
While we included only infections present on admission in our analysis, it is important to acknowledge that we cannot be certain that the infection preceded the stroke without a prospective study. Nevertheless, a possible association between infection and PEC-associated PAS has important clinical implications, as it may represent a modifiable risk factor. The difference in infections between cases and controls in our cohort was driven entirely by genitourinary infections. Symptoms of urinary infections such as dysuria or incontinence may go unrecognized in postpartum women, since similar symptoms may occur with normal postpartum recovery. Women with preeclampsia undergo postpartum blood pressure checks; screening for urinary tract infections at these visits, and monitoring those with infections more closely for neurological symptoms may be warranted. The role for prophylactic antibiotics could be considered on a case by case basis, and may be a target in clinical trials.
Chronic hypertension
The role of chronic hypertension in PEC-associated stroke is poorly characterized. Chronic hypertension shifts the cerebral autoregulatory curve,29 but is not associated with loss of dynamic cerebral autoregulatory capacity due to rapid adaptation of the cerebral vasculature.30 However, acute malignant hypertension in chronically hypertensive patients severely impairs dynamic cerebral autoregulation.31 Preeclampsia may cause impairment of cerebrovascular autoreactivity32 leading to hyperemia, hypertensive leukoencephalopathy, and/or the reversible cerebral vasocontriction syndrome (RCVS),33 all of which can be associated with stroke.34 RCVS is highly associated with postpartum stroke.35 Unfortunately, we were unable to assess investigate the role of RCVS and stroke in our population, since RCVS is not reliably captured by ICD-9 coding.
Prothrombotic conditions and coagulopathies
Women with an underlying propensity to thrombosis are at increased risk of stroke when pregnancy, itself a hypercoagulable state, is complicated by PEC.4,36 Only 2.5% of strokes in our cohort were due to cerebral venous thrombosis, suggesting that prothrombotic states may put women at risk of arterial thrombosis and hemorrhagic stroke as well. However, cerebral venous thrombosis, which may also present with hemorrhage, may be underdiagnosed. Women with underlying thrombophilia may have been on antithrombotic treatment during or after their pregnancy, increasing their ICH/SAH risk. Patients with PEC and a history of coagulopathy likely warrant increased vigilance in the peripartum period.
Other vascular risk factors
Heart disease has been identified as a risk factor for PAS in women with PEC.4 After adjusting for other risk factors, our results failed to show significant between-group differences in heart disease. Similarly, we found no significant differences in stroke risk comparing other traditional vascular risk factors such as diabetes mellitus, obesity, chronic renal disease, smoking and other substance abuse. In fact, proportions of these risk factors were low in both cases and controls. Our study may have been underpowered to detect between-group differences in some risk factors, since other studies have found otherwise; alternatively, the pathophysiology of PAS in women with PEC may bear little relation to traditional vascular risk factors.
Preeclampsia severity
PEC is regarded as a disease along a continuum; women with gestational hypertension may go on to develop PEC and then severe PEC.37 Women with severe PEC were overrepresented in our cases compared to the overall population of women with PEC; however, it is difficult to interpret this finding, since any “cerebral symptom” in a woman with PEC is considered a severe feature, creating a significant selection bias. Further complicating interpretation of our data is the updating of diagnostic criteria for severe PEC in 2013, after our study period.16 The lack of data on the HELLP syndrome (hemolysis, elevated liver enzymes and low platelets), a variant of preeclampsia, is a limitation of our study, as these women may be at particularly high stroke risk.38
Research in context: study strengths and limitations
Two prior population-based studies compared women with PEC and stroke to women with PEC without stroke.39,40 Neither study was designed to identify multiple independent risk factors for stroke in this population: one compared only mortality outcomes, and the other, methods of anesthesia during delivery. The ethnic and regional diversity of New York State increases the generalizability of our findings. Matching of cases and controls allowed for nuanced analysis of other risk factors. We conducted multiple subgroup analyses to explore possible biasing factors in our results.
Our study has limitations. Data were drawn from a large administrative database, and there may be coding errors. Some PEC cases may not have been formally diagnosed and thus not included in the study. Pregnancy-specific stroke codes do not distinguish between stroke subtypes, limiting the granularity of the data, although the majority of patients had additional codes to identify stroke subtypes with more precision (Supplemental Figure I). While the proportion of each of the “prothrombotic conditions” individually (hypercoagulable states, history of thromboembolic events, systemic lupus erythematosis and sickle cell disease) was higher in cases than controls, grouping them together may overestimate their effects. Diagnosis of prothrombotic conditions or coagulopathies may have occurred only after the stroke, leading to ascertainment bias. Although we included only infections present on admission for stroke, exact timing of infections in relation to stroke onset cannot be confirmed without a prospective study. Causality cannot be inferred from this observational study, and results should be interpreted cautiously.
In summary, urinary tract infections, chronic hypertension, prothrombotic conditions and coagulopathies increased stroke risk in women with preeclampsia. Infections may be an important treatable risk factor in this population; similarly, screening for coagulopathies and prothrombotic conditions may be warranted in women with preeclampsia. Prospective studies are needed to confirm these findings and develop interventions aimed at preventing strokes in this uniquely vulnerable group.
Supplementary Material
Acknowledgments
ECM receives support from an NIH NINDS StrokeNet Training Fellowship. RSM receives support from NIH NINDS 1U10 NS086728.
Footnotes
Disclosures: None.
References
- 1.Ahmed A, Rezai H, Broadway-Stringer S. Evidence-Based Revised View of the Pathophysiology of Preeclampsia. Adv Exp Med Biol. 2016:1–20. doi: 10.1007/5584_2016_168. [DOI] [PubMed] [Google Scholar]
- 2.Crovetto F, Somigliana E, Peguero A, Figueras F. Stroke during pregnancy and pre-eclampsia. Curr Opin Obstet Gynecol. 2013;25:425–432. doi: 10.1097/GCO.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 3.Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? The American Journal of Obstetrics & Gynecology. 2002;186:198–203. doi: 10.1067/mob.2002.119177. [DOI] [PubMed] [Google Scholar]
- 4.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstetrics & Gynecology. 2015;125:124–131. doi: 10.1097/AOG.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. European Journal of Obstetrics and Gynecology. 2013;171:266–270. doi: 10.1016/j.ejogrb.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Giannubilo SR, Landi B, Ciavattini A. Preeclampsia: what could happen in a subsequent pregnancy? Obstet Gynecol Surv. 2014;69:747–762. doi: 10.1097/OGX.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 7.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstetrics & Gynecology. 2005;106:509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 8.Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke. 2014;45:1947–1950. doi: 10.1161/STROKEAHA.114.005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42:982–989. doi: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a Thrombotic Event after the 6-Week Postpartum Period. N Engl J Med. 2014;370:1307–1315. doi: 10.1056/NEJMoa1311485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics ICD-9-CM official guidelines for coding and reporting. Washington (DC): US GPO; 2011. [Google Scholar]
- 13.Elkind MSV. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–57. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 14.Fullerton HJ, Hills NK, Elkind MSV, Dowling MM, Wintermark M, Glaser CA, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015;85:1459–1466. doi: 10.1212/WNL.0000000000002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstetrics & Gynecology. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. 2013:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 17.Bender R, Lange S. Adjusting for multiple testing–when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 18.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40:2068–2072. doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- 19.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67:424–429. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- 20.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, et al. Patient Characteristics and Outcomes After Hemorrhagic Stroke in Pregnancy. Circ Cardiovasc Qual Outcomes. 2015;8:S170–8. doi: 10.1161/CIRCOUTCOMES.115.002242. [DOI] [PubMed] [Google Scholar]
- 21.Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention: Reproductive Health. 2016 http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. Accessed April 12, 2017.
- 22.Elkind MSV, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, et al. Herpesvirus Infections and Childhood Arterial Ischemic Stroke: Results of the VIPS Study. Circulation. 2016;133:732–741. doi: 10.1161/CIRCULATIONAHA.115.018595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–1282. doi: 10.1161/01.str.31.6.1274. [DOI] [PubMed] [Google Scholar]
- 24.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 25.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma From Preeclamptic Women Increases Blood-Brain Barrier Permeability: Role of Vascular Endothelial Growth Factor Signaling. Hypertension. 2010;56:1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipolla MJ, Bishop N, Chan SL. Effect of Pregnancy on Autoregulation of Cerebral Blood Flow in Anterior Versus Posterior Cerebrum. Hypertension. 2012;60:705–711. doi: 10.1161/HYPERTENSIONAHA.112.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-alpha. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1425–31. doi: 10.1152/ajpregu.00372.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porcello Marrone LC, Gadonski G, de Oliveira Laguna G, Poli-de-Figueiredo CE, Pinheiro da Costa BE, Lopes MFT, et al. Blood-brain barrier breakdown in reduced uterine perfusion pressure: a possible model of posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2014;23:2075–2079. doi: 10.1016/j.jstrokecerebrovasdis.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976;53:720–727. doi: 10.1161/01.cir.53.4.720. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Witkowski S, Fu Q, Claassen JAHR, Levine BD. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–1155. doi: 10.1161/HYPERTENSIONAHA.106.084939. [DOI] [PubMed] [Google Scholar]
- 31.Immink RV, van den Born B-JH, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation. 2004;110:2241–2245. doi: 10.1161/01.CIR.0000144472.08647.40. [DOI] [PubMed] [Google Scholar]
- 32.Oehm E, Reinhard M, Keck C, Els T, Spreer J, Hetzel A. Impaired dynamic cerebral autoregulation in eclampsia. Ultrasound Obstet Gynecol. 2003;22:395–398. doi: 10.1002/uog.183. [DOI] [PubMed] [Google Scholar]
- 33.Oehm E, Hetzel A, Els T, Berlis A, Keck C, Will H-G, et al. Cerebral hemodynamics and autoregulation in reversible posterior leukoencephalopathy syndrome caused by pre-/eclampsia. Cerebrovasc Dis. 2006;22:204–208. doi: 10.1159/000093810. [DOI] [PubMed] [Google Scholar]
- 34.Ducros A. Reversible cerebral vasoconstriction syndrome. The Lancet Neurology. 2012;11:906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- 35.Razmara A, Bakhadirov K, Batra A, Feske SK. Cerebrovascular complications of pregnancy and the postpartum period. Curr Cardiol Rep. 2014;16:532. doi: 10.1007/s11886-014-0532-1. [DOI] [PubMed] [Google Scholar]
- 36.Fischer-Betz R, Specker C, Brinks R, Schneider M. Pregnancy outcome in patients with antiphospholipid syndrome after cerebral ischaemic events: an observational study. Lupus. 2012;21:1183–1189. doi: 10.1177/0961203312451335. [DOI] [PubMed] [Google Scholar]
- 37.Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. American Journal of Obstetrics and Gynecology. 2001;184:979–983. doi: 10.1067/mob.2001.112905. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa J, Ikeda T, Sekizawa A, Tanaka H, Nakata M, Murakoshi T, et al. Maternal Death Due to Stroke Associated With Pregnancy-Induced Hypertension. Circ J. 2015;79:1835–1840. doi: 10.1253/circj.CJ-15-0297. [DOI] [PubMed] [Google Scholar]
- 39.Gastrich MD, Gandhi SK, Pantazopoulos J, Zang EA, Cosgrove NM, Cabrera J, et al. Cardiovascular outcomes after preeclampsia or eclampsia complicated by myocardial infarction or stroke. Obstetrics & Gynecology. 2012;120:823–831. doi: 10.1097/AOG.0b013e31826ae78a. [DOI] [PubMed] [Google Scholar]
- 40.Huang C-J, Fan Y-C, Tsai P-S. Differential impacts of modes of anaesthesia on the risk of stroke among preeclamptic women who undergo Caesarean delivery: a population-based study. Br J Anaesth. 2010;105:818–826. doi: 10.1093/bja/aeq266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


