Abstract
Fertilization is a multi-step process that begins with plasma membrane interactions that enable sperm - oocyte binding followed by fusion of the sperm and oocyte plasma membranes. Once membrane fusion has occurred, sperm incorporation involves actin remodeling events within the oocyte cortex that allow the sperm head to penetrate the cortical actin layer and gain access to the ooplasm. Despite the significance for reproduction, the control mechanisms involved in gamete binding, fusion, and sperm incorporation are poorly understood. While it is known that proline - rich tyrosine kinase 2 (PYK2 or PTK2b) kinase activity plays an important role in fertilization, its specific function has not been addressed. The present study made use of a zona-free mouse oocyte fertilization assay to investigate the relationship between PYK2 activity and sperm - oocyte binding and fusion, as well as localized changes in actin polymerization and sperm incorporation. In this assay, the majority of bound sperm had no apparent effect on the oocyte and only a few became incorporated into the ooplasm. However, a subset of bound sperm were associated with a localized response in which PYK2 was recruited to the oocyte cortex where it frequently co-localized with a ring or disk of f-actin. The frequency of sperm-oocyte binding sites that exhibited this actin response was reduced in pyk2−/− oocytes and the pyk2−/− oocytes proved less efficient at incorporating sperm, indicating that this protein kinase may have an important role in sperm incorporation. The response of PYK2 to sperm-oocyte interaction appeared unrelated to gamete fusion since PYK2 was recruited to sperm - binding sites under conditions where sperm - oocyte fusion was prevented and since PYK2 suppression or ablation did not prevent sperm - oocyte fusion. While a direct correlation between the PYK2 response in the oocyte and the successful incorporation of individual bound sperm remains to be established, these findings suggest a model in which the oocyte is not a passive participant in fertilization, but instead responds to sperm contact by localized PYK2 signaling that promotes actin remodeling events required to physically incorporate the sperm head into the ooplasm.
Keywords: Fertilization, PYK2, Sperm incorporation, actin
1. Introduction
In mammals, fertilizing sperm undergo a sequence of interactions with the oocyte, including binding to and penetration of the zona pellucida, followed by binding to and fusion with the oocyte plasma membrane. Gamete fusion occurs at the tips of oocyte microvilli in contact with the sperm head (Runge et al., 2007; Talbot and Chacon, 1982) and is followed by a sperm incorporation process where the sperm head is drawn through the cortical actin layer into the ooplasm. The fusion and incorporation events correlate temporally with initiation of cytoplasmic Ca2+ oscillations that represent the primary driver of egg activation in most animal models examined (Runft et al., 2002; Swann et al., 2004). However, the question of whether sperm-oocyte contact itself induces a signaling event in the oocyte has not been resolved. Early work using reciprocal crosses between sea urchin and starfish gametes demonstrated that oocyte microvilli exhibited a ‘sperm-engulfing response’ to bound sperm that occurred independent of gamete fusion or Ca2+ transients (Kyozuka and Osanai, 1988). This result provided evidence that oocytes respond to sperm-oocyte contact irrespective of membrane fusion with the sperm, however the molecular mechanism of this response remained unknown. Potential signaling mechanisms proposed to act during sperm-oocyte interaction include membrane depolarization (Lynn et al., 1988), or activation of Src-family kinases (O’Neill et al., 2004; Townley et al., 2009) which have been observed in non-mammalian oocytes. However, recent studies have demonstrated activation of the proline-rich tyrosine kinase 2 (PYK2 or PTK2b) at the site of sperm-oocyte interaction in the zebrafish and mouse oocyte, raising the possibility that this pathway may represent a conserved response of the oocyte to sperm (Luo et al., 2014; Sharma and Kinsey, 2013).
PYK2 is a member of the focal adhesion kinase (FAK) family that can be activated by cell surface stimuli such as integrin (Eleniste and Bruzzaniti, 2012) or protocadherin oligomerization (Suo et al., 2012), as well as ligand binding to transmembrane growth factor receptors (Perez et al., 2011; Selitrennik and Lev, 2015). FAK-family kinases can also respond to cytoplasmic signals such as oxidative stress (Felty, 2011; Martel-Gallegos et al., 2013), but PYK2 is unique in its ability to respond to intracellular Ca2+, which promotes trans - autophosphorylation and activation of kinase activity in many, but not all cell types (Collins et al., 2010; Orr and Murphy-Ullrich, 2004; Takahashi, 2004; Walkiewicz et al., 2015). PYK2 shares many functions with and can compensate for FAK (Sulzmaier et al., 2014) but differs from FAK in the level to which it is involved with dynamic events such as cell process formation (Gil-Henn et al., 2007; Li et al., 2014), neuronal synapse function (Bartos et al., 2010) and Ca2+ homeostasis (Chen et al., 2011; Zhu et al., 2014). While fak−/− mice die during embryonic development, pyk2−/− mice were described as fertile with no gross anatomical abnormalities, although accelerated breeding studies were not reported (Okigaki et al., 2003). However, pyk2 ablation did result in a wide range of defects in macrophages including impaired Ca2+ signaling, reduced lamellipodial contractile activity, and overall migratory activity (Okigaki et al., 2003; Owen et al., 2007). Other negative consequences of pyk2 ablation include defective osteoclast podosome structure (Ray et al., 2012) and reduced keratinocyte migration during wound repair (Koppel et al., 2014).
PYK2 is localized to the cortical cytoplasm of zebrafish (Sharma and Kinsey, 2013), rat (Meng et al., 2006), and mouse oocytes (McGinnis et al., 2013) and is activated transiently at the site of sperm-oocyte interaction (Sharma and Kinsey, 2013). Inhibitor studies indicated that PYK2 activity was required for sperm incorporation into mouse oocytes (Luo et al., 2014), however the stimulus for PYK2 activation and its downstream functions remained unknown. The goal of the present study was to define the temporal sequence and precise subcellular location of PYK2 activation at the sperm binding site, test whether the PYK2 response required gamete fusion or could be triggered simply by sperm-oocyte contact, and test whether PYK2 is required for sperm-oocyte fusion or sperm incorporation. The results demonstrate that PYK2 is recruited and activated early during the interaction between sperm and oocyte. The PYK2 response can be induced by sperm contact, and neither requires nor enables membrane fusion. Instead, PYK2 plays an important role in actin remodeling events required for sperm incorporation.
2. Methods
2.1 Gamete handling and in vitro fertilization
CF1, C57Bl/6, and B6D2F1 mice were obtained from (Charles River Laboratories, O’Fallon, MO; Harlan Laboratories, Tampa FL: and from Envigo orp., Indianapolis, IN), while pyk2−/− mice (Pfizer, (New York, NY)) were transferred from the laboratory of M. Pepper, (University of Washington, Seattle, WA), and genotyped as described elsewhere (Lang et al., 2011). Animals were housed in a temperature and light cycle controlled room and experiments were conducted in accordance with the ‘Guide for the Care and use of Laboratory Animals’ (Institute of Laboratory Animal Resources (U.S.) Committee on Care and Use of Laboratory Animals 1996; National Research Council (U.S.) 2011). Experimental procedures were approved by the University of Kansas Medical Center IACUC committee. In vitro fertilization was carried out under sperm-limiting conditions (Gardner and Evans, 2006) to slow down the rate of fertilization so that changes in PYK2 localization during the different phases of sperm-oocyte interaction could be detected. Sperm were isolated from the cauda epididymis of B6D2F1 males, transferred to a swim up column containing 0.4 ml of modified Tyrode’s medium (Summers et al., 2000) containing 5 mg/ml BSA, then incubated for 3 hours at 37°C, 5% CO2 in air to induce capacitation. Oocytes were collected from female mice 4–5 weeks of age that were stimulated with 5 IU of PMSG (Intervet, Inc. Millsborough, DE) followed 48 hours later by 5 IU hCG (Sigma Aldrich, St. Louis, MO). Cumulus-oocyte complexes were collected 15–16 hours post-hCG, washed in FHM (HEPES buffered KSOM, Specialty Media Inc., Phillipsburgh, NJ) containing 4mg/ml BSA, then cumulus cells were removed by treatment with 30 IU/ml hyaluronidase (Sigma-Aldrich) and the zona pellucida was removed by treatment with acidic Tyrode’s (pH 2.3) for 5 – 15 seconds followed by several washes in FHM. The oocytes were allowed to recover for 1 hour in KSOMAA (EMD Millipore, Billerica, MA) containing 15mg/ml BSA at 37°C, in a 5% CO2 / air atmosphere. Groups of 20–30 oocytes were transferred to 500ul of KSOMAA with 15mg/ml BSA under oil. In vitro fertilization was carried out under low sperm concentrations (2 × 104/ml final) in order to limit the number of sperm bound per oocyte.
In some experiments, PYK2 activity was suppressed in WT zona-free oocytes by incubation with the PYK2 inhibitor PF04594755, or DMSO as a solvent control as described previously (Luo et al., 2014). Briefly, groups of 10–20 oocytes were incubated in KSOMAA + 15mg/ml BSA containing 0.1% DMSO or 10µM PF04594755 for 30 minutes. The treated oocytes were washed through two drops of normal medium before transfer to 500ul of KSOMAA + 15mg/ml BSA and addition of sperm.
2.2 Dominant-negative eGFP construct
An eGFP-linked dominant-negative construct encoding the N-terminal ERM domain (aa1–370) of PYK2 was amplified from the human PYK2 transcript variant 1 cDNA (Origene, Rockville, MD) using the following primers: (forward; CCCAAGCTTATGTCTGGGGTGTCCGAGCCC); (reverse; CCCAAGCTTGCTGTTCCGCTTCTCACCATCTT) which contained a Hind III site used to ligate the product into the N-terminal cloning site of pEGFP-N1 (Clontech, Mountain View, CA). The PERM-eGFP construct was excised with Hind III and Xba I and cloned into pSP64 (Promega, Madison, WI). Complimentary RNA (cRNA) was prepared with the mMessage machine SP6 transcription kit (ThermoFisher, Grand Island, NY). Expression of the eGFP and PERM-eGFP constructs in oocytes was carried out by microinjection of MII-stage oocytes with approximately 5pl of cRNA (3.5 µg/ml), followed by a 4hour incubation in FHM + 4mg/ml BSA to allow protein expression which was confirmed by monitoring eGFP fluorescence via confocal microscopy.
2.3 Analysis of Sperm-oocyte fusion
A dye transfer method was used to differentiate sperm that were bound to the oocyte surface from those which had fused with the oocyte plasma membrane (Hinkley et al., 1986; Lawrence et al., 1997). Zona-free oocytes were pre-incubated in KSOMAA + 15mg/ml BSA containing the DNA binding dye DRAQ5 (1uM) (Cell Signaling, Danvers, MA) for 15 minutes, then washed through 5 drops of KSOM + 15mg/ml BSA prior to addition of capacitated sperm at a final concentration of 2 × 104/ml. Samples of oocytes with bound sperm were fixed in 2% formaldehyde in PBS at 20, 30, or 40 minutes post-insemination. After addition of fixative, oocytes were quickly (within 10 minutes to minimize post-fixation permeabilization) examined by confocal fluorescence microscopy to quantify the fraction of oocytes with bound sperm that exhibited DRAQ5 labeling of the sperm nucleus, indicating that membrane fusion had occurred.
2.4 Microscopy
Samples for immunofluorescence were fixed for 1–2 hours in 2% formaldehyde, 0.5% saturated picric acid, in PBS containing 40µM phenylarsine oxide and 100µM sodium orthovanadate as phosphatase inhibitors. Oocytes were washed three times in glycine blocking buffer (PBS containing 0.1% Triton X-100; 3 mg/ml BSA, and 0.15M glycine with phenylarsine oxide and orthovanadate as above), then further incubated for 1 hour in blocking buffer without glycine. Primary antibodies diluted in blocking buffer were incubated with oocytes overnight at 4°C. Alexa 568-phalloidin (ThermoFisher Scientific, Grand Island, NY) was usually included with the primary antibody for detection of filamentous actin (f-actin). Labeled oocytes were washed three times with blocking buffer prior to incubation with secondary antibody with DRAQ5 (5µM) for detection of DNA. Finally, oocytes were washed three times in blocking buffer without phenylarsine oxide and mounted under a coverslip. Confocal microscopy was performed with a Nikon TE2000 confocal microscope. A rabbit anti-PYK2 antibody (Sigma Aldrich, St. Louis, MO) directed against aa 991–1009 of human PYK2 was used at (25ug/ml). The specificity of this antibody was demonstrated by its failure to label pyk2−/− oocytes (Figure 1E). A phospho-specific anti-PYK2 PY579 (ThermoFisher, Grand Island, NY) was used (at 1:200 dilution) and the specificity of this antibody for the phosphorylated form of PYK2 was demonstrated through a kinase inhibitor study (Luo et al., 2014). The secondary antibody used was alexa fluor 488-goat anti-rabbit IgG (ThermoFisher, Grand Island, NY).
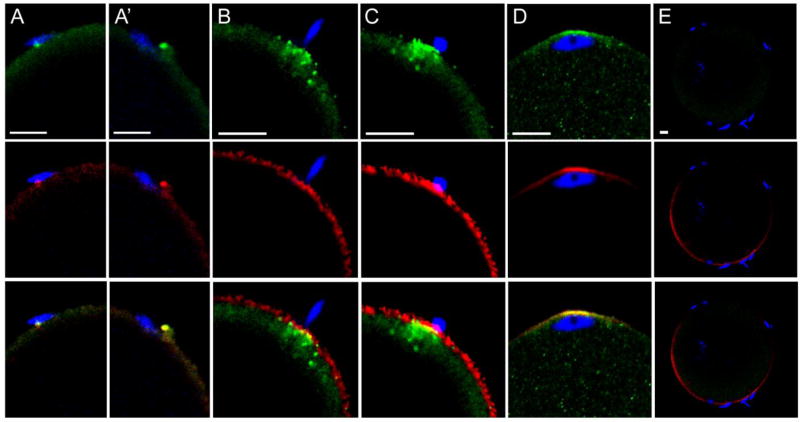
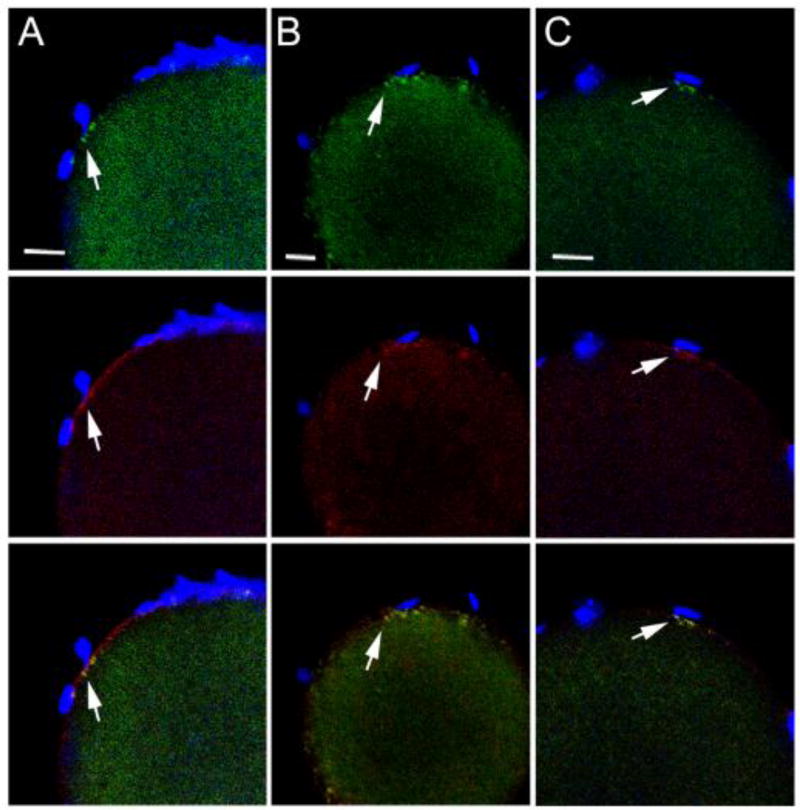
Fig. 1.
PYK2 recruitment at the site of sperm-oocyte contact. Zona-free oocytes were incubated with a limiting concentration of sperm and samples were fixed at 30 (A,A’), 45 (B, C), and 60 (D) m.p.i., then processed for confocal immunofluorescence. The distribution of anti- PYK2 protein (green) is shown in the top panels, while f-actin detected by alexa 568-phalloidin (red) is shown in the middle row. Sperm chromatin was detected with DRAQ5 (blue) and the combined images containing all three channels are displayed in the bottom row. Specificity of the anit-PYK2 antibody is seen in column (E) where a pyk2−/− oocyte collected at 45 m.p.i. was labeled under identical conditions. Magnification is indicated by the bar’ which represents 5µm.
Fluorescence quantitation used to measure PYK2 or f-actin content within the oocyte cortex underlying sperm binding sites was performed by linescan analysis with Metamorph 6.2 (Molecular Devices, Downington, PA). Z-stack images were recorded at 0.35µm intervals from oocytes fixed and labeled with Alexa 568-phalloidin as described above. Z-planes containing 1 or more sperm heads attached to the egg surface were selected for analysis. A measurement region (8 pixels or 1.3µm in width) was drawn over the entire cortical region of the oocyte including the cortical actin layer and plasma membrane. The region was converted into a line, and fluorescence intensity around the region was recorded in the red, green, and blue channels using the linescan measurement tool. The data was smoothed by calculating a 5 pixel moving average and graphed using SigmaPlot 11(Systat Software, San Jose, CA). The position of bound sperm along the X axis was identified by the inflection points on either side of the peak observed within the blue channel, and these × values were used to calculate the integrated fluorescence intensity within the same region of the green or red channel to get alexa 488 goat anti-rabbit IgG or alexa 568-phalloidin fluorescence associated with the sperm binding site. This data was expressed relative to the baseline calculated between the two × values to get the relative integrated fluorescence intensity.
2.5 Statistics
Differences in the mean frequency with which oocytes fused with and incorporated sperm during IVF assays was analyzed by Student’s t-test. Relative integrated fluorescence intensity (alexa 568-phalloidin) associated with sperm binding sites from wt and pyk2−/− oocytes was compared by one way Analysis of Variance (ANOVA) (Systat Software).
3. Results
3.1 Sperm-oocyte contact induces recruitment and activation of PYK2
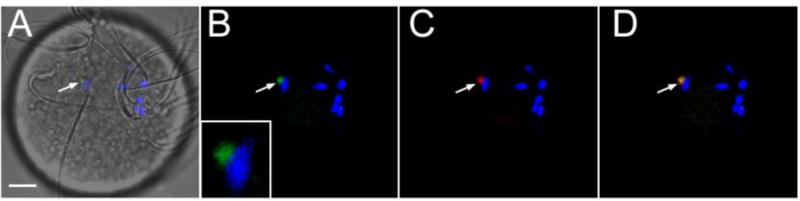
In order to establish the sequence of PYK2 recruitment and activation at the site of sperm-oocyte contact, a time series of oocytes fixed at different stages of sperm-oocyte interaction was analyzed by confocal immunofluorescence microscopy with antibodies to PYK2 and the activated/phosphorylated (Y579) form of PYK2. An IVF protocol utilizing zona-free oocytes and a limiting concentration of sperm was used to slow the rate of fertilization and increase the likelihood that different stages of the response to sperm contact would be observed. The earliest response of the oocyte to sperm contact appeared at 30 minutes post-insemination (m.p.i.) as small foci of concentrated PYK2 that appeared adjacent to the bound/fused sperm, and often were associated with microvilli adjacent to the sperm head (Fig. 1A, A’, top). In most cases, the PYK2 foci co-localized with condensations of filamentous actin (f-actin), which was detected with alexa 568-phalloidin (red) (Fig. 1A, A’, middle, bottom). Similar foci were detected using the phospho-specific antibody (anti-PYK2-PY579) indicating at least some of the PYK2 in these foci was activated (Fig. 2). The number of PYK2 foci in the oocyte cortex underlying sperm heads increased by 45 m.p.i. (Fig. 1B, top) and in some cases, a plaque of concentrated PYK2 was visible immediately under the sperm head (Fig. 1C, top). In these cases, PYK2 appeared to underlie a disk of f-actin (Fig. 1C, middle) and partially co-localize with it (Fig. 1C, bottom). The above responses by the oocyte appeared to occur at only one or two of the sperm that were bound to the oocyte plasma membrane at any given time. In order to further define the frequency of the oocyte response, the PYK2 and f-actin associated with individual sperm binding sites was quantified by measuring fluorescence intensity as described in “Methods”. Analysis of 91 sperm binding sites examined on 39 oocytes revealed that only 13 (14%) of sperm binding sites were associated with a robust (>50% above adjacent cortex) increase in PYK2 content (Table 1). While most of the 13 sperm binding / fusion sites with a >50% increase in PYK2 content exhibited some f-actin accumulation, the amount of f-actin was varied with 9 of the 13 sites exhibited an increase in f-actin >20%, and only 5 of the sites exhibited an increase in f-actin content greater than 50% above the adjacent cortex (similar to Fig. 1C). By 60 m.p.i., most oocytes had incorporated a sperm head which had penetrated the cortical actin layer and was positioned underneath an f-actin cap that co-localized with PYK2 (Fig. 1D). Interestingly, binding of capacitated sperm to somatic cells (ARPE-19 retinoblastoma cells and COS cells) did not induce accumulation or activation of PYK2 detectable by the methods used above (not shown).
Fig. 2.
PYK2 activation at the site of sperm-oocyte contact. Zona-free oocytes incubated with sperm for 30 minutes and processed for immunofluorescence as above, were labeled with anti PYK2 PY579 (green), alexa 568-phalloidin (red), and DRAQ5 (blue). The position of an aggregation of phosphorylated (activated) PYK2 and f-actin in close proximity to one of the bound sperm is indicated by the arrows. Magnification is indicated by the bar, which represents 10 µm.
Table 1.
Frequency of PYK2 and actin foci formation at sperm binding/fusion sites
| PYK2 > 50% | f-actin > 50% | PYK2 > 50% & f-actin > 50% |
PYK2 > 50% & f-actin > 20% |
|
|---|---|---|---|---|
| # of sperm binding / fusion sites | 13 | 7 | 5 | 9 |
Zona free oocytes incubated with capacitated sperm for 45 minutes (four different experiments) were fixed and labeled with anti-PYK2, together with alexa 568-phalloidin and DRAQ5 to label the cortical actin layer and sperm nuclei. Confocal images including the entire oocyte circumference were recorded to include each sperm binding / fusion site (n=91) and fluorescence intensity within the oocyte cortex was quantified by circumferential linescan analysis in the green (anti-PYK2), red (alexa 568- phalloidin), and far-red (DRAQ5) channels. The integrated relative fluorescence intensity of the sperm binding / fusion site was calculated relative to that of the adjacent oocyte cortex where no sperm were bound. The number of sperm binding / fusion sites where PYK2 or f-actin fluorescence was >50% relative to the adjacent cortex is presented in the left two columns. The number of sites where PYK2 fluorescence was >50% relative to the adjacent cortex and f-actin fluorescence was greater than 50% or 20% is presented in the two columns at right.
3.2 Is gamete fusion required for PYK2 recruitment?
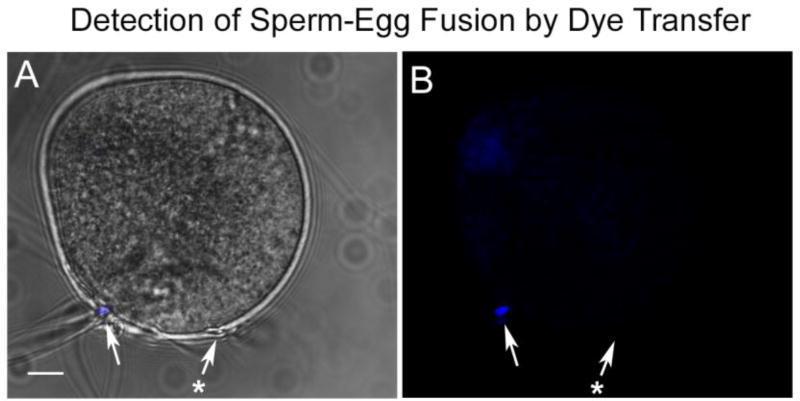
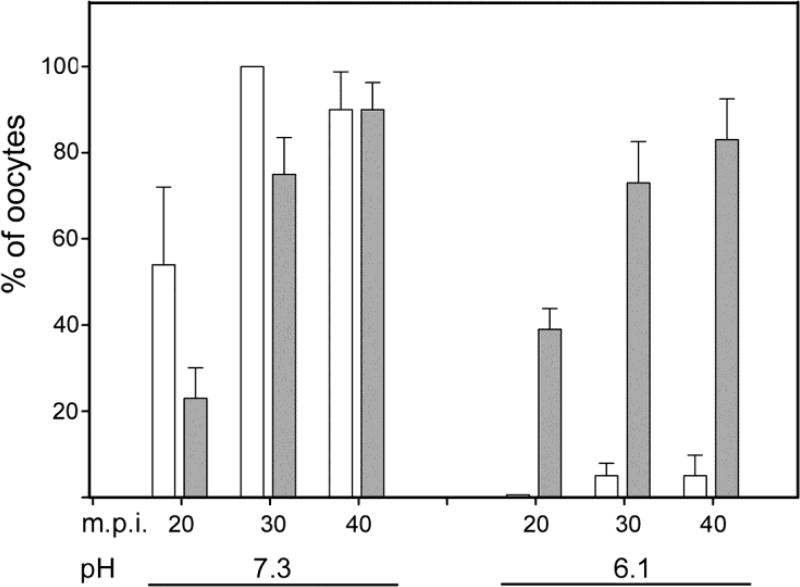
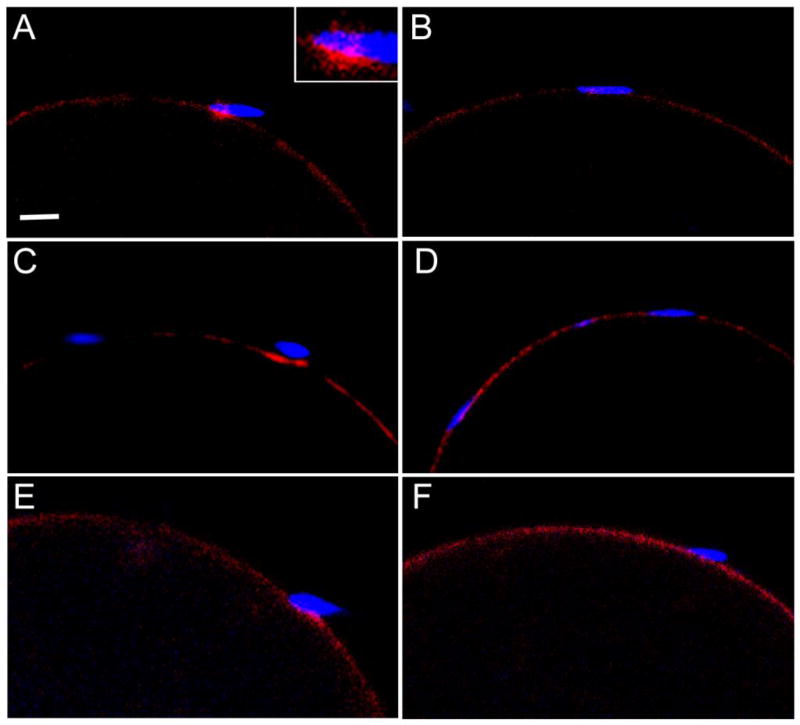
Observation of the IVF process revealed that most zona-free oocytes had bound multiple sperm within the first 15 minutes post-insemination, so the timing of PYK2 recruitment and activation presented in Fig. 1 and Fig. 2 indicated that kinase activation begins after the sperm and oocyte had been in contact for 15 or 20 minutes. This raised the question of whether the PYK2 response is triggered by cell surface contact or by gamete fusion and subsequent PLCζ– induced Ca2+ release. The role of membrane fusion in recruitment of PYK2 to the site of sperm-oocyte contact was tested by performing IVF under conditions that prevented sperm-oocyte fusion. Earlier work in hamsters and mice demonstrated that lowering the pH of IVF medium to pH 6.1 reversibly blocked gamete fusion, but had no effect on sperm-oocyte binding (Lawrence et al., 1997; Yanagimachi, 1980). In order to monitor sperm-oocyte fusion, zona-free oocytes were pre-incubated with the DNA binding compound DRAQ5, then washed extensively to remove unincorporated dye. The labeled oocytes were then subjected to IVF with capacitated sperm at pH 7.3 (control) or 6.1 (experimental). Samples were fixed at 20, 30, and 40 m.p.i., and half of each group was fixed in formaldehyde and immediately imaged by confocal microscopy to determine whether sperm-oocyte fusion (Fig. 3) had occurred. The other half of the samples was processed for immunofluorescence to detect PYK2 recruitment near bound sperm. Under normal IVF conditions (pH 7.3), the fraction of oocytes that showed evidence of fusion with sperm (Fig. 4, white bars) was similar to that exhibiting PYK2 foci (Fig 4. grey bars) in close proximity to bound sperm (Fig. 4 left). However, this correlation was lost when IVF was performed at pH 6.1 where PYK2 foci formed normally in most oocytes, but sperm-egg fusion was almost completely inhibited (Fig. 4 right). Oocytes incubated at pH 6.1 in the absence of sperm did not exhibit any changes in PYK2 distribution. The morphology of PYK2 recruitment observed at sperm binding sites in oocytes inseminated at pH 6.1 (Fig. 5) was similar to that observed in oocytes inseminated at pH 7.3 (Fig. 1).
Fig. 3.
Detection of Sperm-oocyte fusion by dye transfer. Zona-free oocytes were pre-labeled with DRAQ5, washed, and incubated with capacitated sperm for 30 minutes. They were then fixed in formaldehyde and imaged by confocal microscopy to determine whether DRAQ5 had diffused into bound sperm indicating the presence of intact fusion channels between sperm and oocyte. The presence of a fused sperm is indicated by the white arrow, and an example of a sperm that did not fuse with the oocyte is indicated by the arrow with (*). Magnification is indicated by the bar, which represents 10µm.
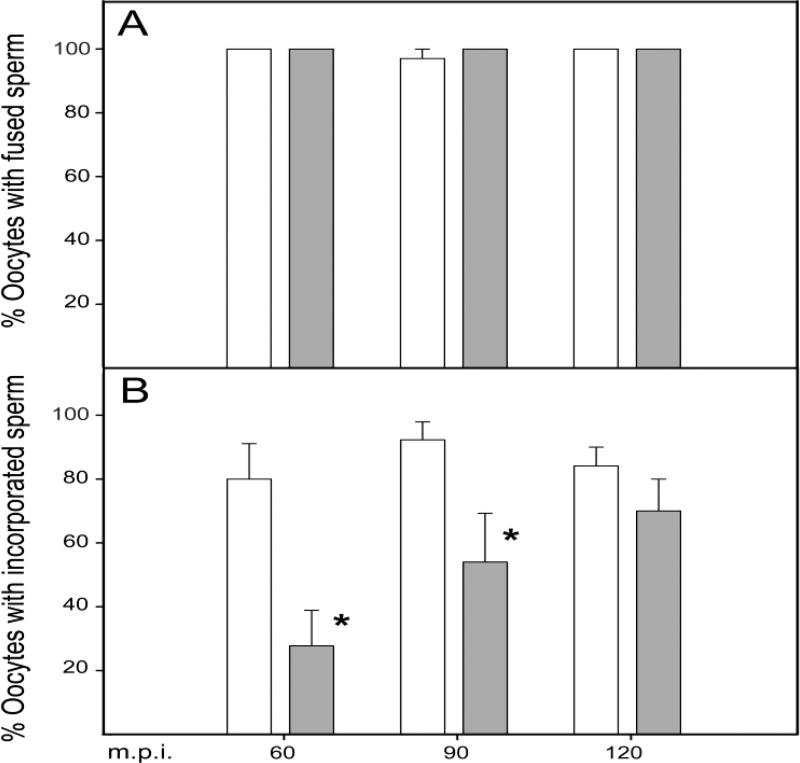
Fig. 4.
PYK2 recruitment to sperm binding sites does not require sperm–oocyte fusion. Zona-free CF-1 oocytes were pre-loaded with DRAQ5, then washed and incubated in KSOMaa + 15mg/ml BSA at pH 6.1 or at pH 7.3, as indicated under the X-axis. Capacitated sperm were added and samples were fixed at 20, 30, and 40 minutes post-insemination. For dye diffusion analysis, groups of 10 oocytes collected at each time point were fixed in formaldehyde to maintain membrane integrity and immediately imaged by confocal microscopy with the 633nm laser to identify sperm heads that had accumulated DRAQ5 from the oocyte. Duplicate samples for immunofluorescence detection of PYK2 were fixed in 2% formaldehyde with picric acid, and processed through immunolabeling of PYK2. The percent of oocytes that had fused with at least one sperm is indicated by white bars. The percent of oocytes that exhibited PYK2 foci in close proximity to bound sperm is indicated by grey bars. Values along the Y axis represent the mean of 4 experiments +/− SEM.
Fig. 5.
Recruitment of PYK2 at sperm binding sites of oocytes cultured at pH 6.1. Zona-free oocytes were pre-loaded with DRAQ5, then washed and incubated in KSOMaa + 15mg/ml BSA at pH 6.1, as in Fig 3. Capacitated sperm were added and samples were fixed with 2% formaldehyde containing picric acid at 20 (A), 30 (B), and 40 (C) minutes post-insemination, then prepared for immunofluorescence detection of PYK2. PYK2 protein detected by alexa 488-anti-rabbit IgG is represented in the green channel (top row), while f-actin detected by alexa 568-phalloidin is seen in the red channel (middle row). Sperm chromatin labeled with DRAQ5 is seen in the blue channel. Sperm binding sites where PYK2 accumulation was observed are indicated by the arrows. Magnification is indicated by the bar, which represents 10µm.
3.3 Role of PYK2 in sperm-oocyte fusion, and incorporation
Our previous study demonstrated that pharmacological suppression of PYK2 activity in mouse oocytes had no effect on sperm-oocyte binding, but significantly reduced the frequency of fertilization following zona-free IVF under sperm-limiting conditions (Luo et al., 2014). However, the question of whether PYK2 plays a role in membrane fusion or in the physical incorporation of the sperm head into the ooplasm was not addressed. In order to resolve this question, we compared the rate of sperm-oocyte fusion and sperm incorporation in WT and in pyk2−/− oocytes. The pyk2−/− oocytes bound sperm in numbers that were not significantly different from that of WT oocytes (Table 2), and the fraction of pyk2−/− oocytes that exhibited dye transfer to sperm (indicating membrane fusion) was not significantly different from WT controls at any point during the 120 minute incubation with sperm (Fig. 6A). However, pyk2−/− oocytes incorporated sperm more slowly, with the result that the percent of oocytes with incorporated sperm was significantly lower than that of WT oocytes (28% vs 81% at 60mp.i. and 54% vs 92% at 90 m.p.i.) as seen in Fig. 6B. By 120 m.p.i., pyk2−/− oocytes did eventually incorporate sperm with a frequency similar to that of WT oocytes.
Table 2.
Sperm bound to zona-free oocytes during IVF
| Maternal genotype |
(n) | Sperm bound / oocyte 60 m.p.i. |
90 m.p.i. | 120 m.p.i. |
|---|---|---|---|---|
| WT | 124 | 8.8 +/− 2.2 | 12.6 +/− 3.4 | 11.9 +/− 1.5 |
| pyk2−/− | 193 | 9.4 +/− 5.2 | 16.2 +/− 3.5 | 17.2 +/− 4.4 |
Zona-free oocytes recovered from C57BL/6 (WT) or pyk2−/− females were incubated with limiting concentrations of sperm as described in “Materials and Methods”. At 60, 90, and 120 m.p.i., oocytes were fixed, labeled with DRAQ5 to detect chromatin and alexa 568-phalloidin to detect the cortical actin layer, then imaged by confocal fluorescence to identify oocytes with bound sperm heads. (n) represents the number of oocytes analyzed. Values represent the mean of four experimental groups +/− standard deviation.
Fig. 6.
Role of PYK2 in gamete fusion and sperm incorporation. The timing and extent of sperm-oocyte fusion was quantified in WT (white bars) and pyk2−/− (grey bars) oocytes pre-loaded with DRAQ5 as in Fig. 3. Samples of oocytes were collected at 60, 90, and 120 m.p.i. to correlate with the sperm incorporation timecourse (below). Sperm heads bound to the oocyte surface that accumulated DRAQ5 from the oocyte were considered to have fused with the oocyte plasma membrane (panel A). Values represent the mean of 4 experiments including 116 WT oocytes and 126 pyk2−/− oocytes. The timing and extent of sperm incorporation was quantified in separate experiments (Panel B) where WT (white bars) and pyk2−/− (grey bars) oocytes were fixed and labeled with DRAQ 5 to label all sperm heads and with alexa 568-phalloidin to label the cortical actin layer. Sperm heads that were located within the oocyte cytoplasm and which showed evidence of nuclear decondensation were considered to be incorporated. Values in panel B represent the mean of 5 groups including 123 WT oocytes and 133 pyk2−/− oocytes +/− SEM. (*) f-test indicated that the WT and pyk2−/− means were significantly different (P=0.01).
3.4 Role of PYK2 in actin remodeling at the site of sperm-oocyte contact
The observation that PYK2 foci located near bound sperm frequently co-localized with condensations of f-actin suggested that PYK2 might play a role in inducing accumulation of f-actin at the site of sperm-oocyte interaction. This was tested by evaluating the effect of different methods of suppressing PYK2 kinase activity, as well as the effect of pyk2 gene ablation on the distribution of actin within the oocyte cortex underlying bound sperm. The catalytic activity of the PYK2 protein was inhibited in oocytes by pre-treatment with the specific inhibitor PF04594755 or by expression of a dominant-negative construct encoding the Ezrin – Radixin - Moesin (ERM) domain of human PYK2 ligated to eGFP (PERM-eGFP). This fusion protein binds to native PYK2 and blocks activation of the kinase (Riggs et al., 2011). In order to test for possible non-catalytic functions of the PYK2 protein, pyk2−/− oocytes treated with WT sperm were also examined. As seen in Fig. 7, control oocytes incubated with the solvent control DMSO (panel A) exhibited significant condensations of f-actin under the heads of some bound sperm. These actin condensations often included structures that appeared to represent the actin core of microvilli or filopodia (panel A inset). However, oocytes treated with PF04594755 prior to sperm addition rarely exhibited f-actin condensations (Fig. 7B). Oocytes injected with cRNA encoding eGFP as a control also responded to bound sperm by formation of condensations of f-actin (Fig. 7C) similar to those observed in untreated oocytes (Fig 1C). However, these condensations were very rarely seen in oocytes injected with cRNA encoding the inhibitory PERM-eGFP construct (Fig. 7D). Similarly, oocytes from WT females exhibited actin accumulation at some sperm-binding sites (Fig. 7E) while pyk2−/− oocytes appeared to respond to bound sperm less frequently, and those responses that did occur appeared to involve smaller regions of f-actin than observed in WT oocytes (Fig 7F).
Fig. 7.
Role of PYK2 in actin polymerization at sperm-oocyte binding sites. Oocytes in which PYK2 was suppressed by different methods were incubated with capacitated sperm, then fixed at 45 m.p.i. to determine whether they could respond to sperm binding by accumulation of t-actin in the cortical actin layer. The distribution of actin at sites of sperm contact was detected by labeling with alexa 568-phalloidin to detect t-actin (red) and DRAQ5 to detect sperm DNA (blue). PYK2 activity was suppressed in WT oocytes by three independent methods. The effect of chemical inhibition is shown in panels A and B, where oocytes were treated with 0.1% DMSO as a solvent control (A) or with the inhibitor PF0454799 at 10uM for 30 minutes, then washed prior to addition of sperm (B). The effect of the dominant-negative PERM fusion construct was tested by injecting oocytes with cRNA encoding eGFP as a control (C), or with cRNA encoding the inhibitory PERM-eGFP construct (D). Panels E and F demonstrate the effect of pyk2 knockout on the response of oocytes to bound sperm with examples of sperm bound to a WT oocyte (E) and a pyk2 −/− oocyte (F). Magnification is indicated by the bar which represents 5 µm.
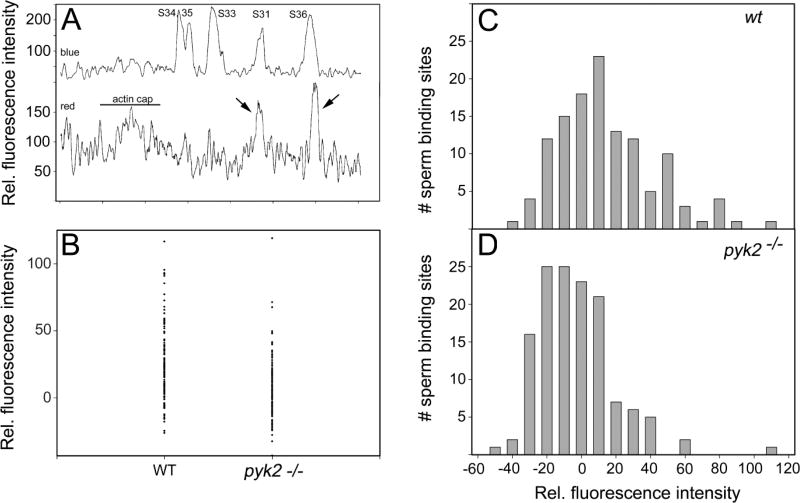
The above subjective observations prompted us to quantify the impact of PYK2 on the frequency and extent to which sperm-oocyte binding induced an increase in f-actin within the cortical actin layer. Zona-free oocytes from WT and pyk2−/− females were incubated with WT capacitated sperm for 45 minutes, then fixed and labeled with alexa 568-phalloidin to detect f-actin and DRAQ5 to detect sperm DNA. Serial confocal Z-stack images were recorded to image the cortical actin layer throughout the oocyte. DRAQ5 and alexa 568-phalloidin fluorescence was quantified by circumferential linescan analysis of the oocyte cortex & plasma membrane within each Z-plane that included a bound sperm. Fig 8A shows a typical scan with the blue channel (top) containing five distinct peaks representing the position of bound sperm heads. The lower trace represents fluorescence within the red channel indicating alexa 568- phalloidin bound to f-actin. Two of the sperm heads (S31 and S36) overlap high-amplitude peaks of alexa 568 fluorescence (arrows) representing significant concentrations of f-actin that were not present at sperm S33–35. Analysis of 7 WT oocytes produced 123 quantifiable sperm binding sites, while 7 pyk2−/− oocytes had 134 quantifiable bound sperm binding sites. The alexa 568-phalloidin fluorescence in the cortex underneath each bound sperm was calculated by integrating peak intensity within the red channel and expressed relative to the baseline calculated from fluorescence in the immediately adjacent cortex. Sperm binding sites of both WT and pyk2−/− oocytes exhibited a spectrum of alexa 568-phalloidin fluorescence intensities within the cortical actin layer (Fig 8B), with binding sites of some WT oocytes distributed in a tail with higher relative fluorescence values that were less abundant within the pyk2−/− group. The mean relative integrated alexa 568 fluorescence of sperm binding sites from WT oocytes was significantly different (higher) than that of pyk2−/− oocytes (Table 3). The difference in the oocyte response to sperm binding is better seen in histograms prepared from WT (Fig 8C) and pyk2−/− oocytes (Fig 8D) where bars represent the number of sperm binding sites that exhibited given levels of integrated relative fluorescence in the red channel. The majority of sperm binding sites in both WT and pyk2−/− oocytes were not associated with increased f-actin content (x-axis near 0). However, a subset of sperm binding sites were associated with increased alexa 568-phalloidin binding (x-axis > 0) and appear as a ‘tail’ extending to the right side of the distribution. This ‘tail’ was much larger in the WT oocytes, where 18.7% of sperm binding sites were associated with an increase in relative alexa-568 fluorescence of > 50%. However, only 2.2% of sperm binding sites in the pyk2−/− oocytes exhibited an increase of >50%.
Fig. 8.
PYK2 is required for enhanced actin polymerization at a subset of sperm-oocyte binding sites. Zona-free oocytes collected from WT and PYK2 −/− females were incubated with capacitated sperm for 45 minutes as described in “Materials and Methods” then fixed and labeled with alexa 568-phalloidin to detect f-actin (red) and DRAQ5 to detect sperm DNA (blue). The distribution of bound sperm and f-actin in the oocyte cortex was recorded by Z-stack confocal imaging and quantified by linescan analysis as described in “Materials and Methods”. Panel A demonstrates the distribution of sperm heads (labeled S31, 33, 34, 35 and 36) detected by DRAQ5 fluorescence in the blue channel (Y axis, upper trace) around the perimeter of a representative optical section through a WT oocyte. The corresponding alexa 568-phalloidin fluorescence is expressed in the red channel (Y axis, lower trace). Instances where sperm binding sites are associated with increased alexa 568-fluorescence are indicated by arrows. “actin cap” represents the thickening of the cortical actin layer that normally occurs over the MII spindle. Panel B demonstrated the relative integrated alexa 568-phalloidin fluorescence (Y axis) calculated for each sperm binding site (n=123) from 7 WT oocytes and (n=134) from 7 pyk2−/− oocytes. “0” representing no change relative to the adjacent cortex and positive values representing an increase in fluorescence intensity relative to the adjacent cortex. Panels C and D display the number of sperm binding sites associated with given levels of relative integrated alexa 568-phalloidin fluorescence (X axis).
Table 3.
F-actin accumulation at sperm binding sites on WT and pyk2 −/− oocytes
| Maternal genotype |
Oocytes examined |
binding sites examined (n) |
integrated rel. fluorescence at bound sperm
|
||
|---|---|---|---|---|---|
| mean | SD | median | |||
| WT | 7 | 123 | 124.2 | +/− 27.7 | 121.2 * |
| pyk2−/− | 7 | 134 | 106.9 | +/− 22.6 | 104.7 |
Zona-free oocytes were incubated with capacitated WT sperm for 45 minutes, then fixed and labeled with alexa 568-phalloidin to detect f-actin and with DRAQ5 to detect DNA allowing localization of sperm heads. The alexa 568 fluorescence associated with the cortical actin layer and/or the plasma membrane was quantified by circumferential linescan analysis of the oocyte at optical Z planes where bound sperm heads were evident. The integrated alexa 568 fluorescence associated with the region of oocyte cortex underlying each sperm binding site was calculated relative to a baseline intersecting the immediately adjacent regions of cortex.
The mean relative integrated relative fluorescence of the two groups was compared by one way ANOVA, which demonstrated that the difference in median values was significant (P=< 0.001).
4. Discussion
4.1 Proposed sequence of events during fertilization
Fertilization in vertebrates and many invertebrates begins with sperm binding to the tips of oocyte microvilli (Kyozuka and Osanai, 1988; Talbot and Chacon, 1982) where oocyte proteins interact with sperm surface proteins to promote gamete fusion through an as yet unknown mechanism. While the sperm binding and fusion events may seem to occur without active participation of the oocyte, several lines of evidence indicate that the oocyte does respond to sperm that have bound to, or are in the early stages of fusing with the plasma membrane. The earliest response of the oocyte observed morphologically involves elongation and increased f-actin content of microvilli in contact with or in close proximity to the sperm head (Cline and Schatten, 1986; Kyozuka and Osanai, 1988; Phillips and Shalgi, 1982). These microvilli form additional contact sites with the sperm, increasing the total surface contact area. Other forms of the oocyte response to sperm contact include depolarization of the plasma membrane potential in sea urchin and Xenopus oocytes (Longo et al., 1994; Longo et al., 1986), and the sperm-induced proteolysis of the oocyte membrane protein uroplakin III which triggers activation of SRC-family PTKs in Xenopus oocytes (Hasan et al., 2011 ; Sakakibara et al., 2005). The above examples provide evidence that sperm-oocyte contact can trigger biochemical responses in the oocyte that may result from trans-interactions between sperm and oocyte surface proteins. The recent finding that PYK2 was activated at the site of sperm-oocyte interaction in zebrafish and mouse oocytes (McGinnis et al., 2013; Sharma and Kinsey, 2013) raised the possibility that this kinase may represent a conserved example of “outside-in” signaling between sperm and oocyte.
The present study sought to establish the function of PYK2 activity at the site of sperm-oocyte contact and determine whether it was induced by gamete contact or fusion. The approach made use of a zona-free IVF protocol with limiting sperm concentrations to examine oocytes at different stages during the PYK2 response to sperm. The results led us to propose a sequence of events beginning with recruitment and activation of PYK2 in contact with, or in close proximity to bound sperm. The physical proximity between PYK2 foci and the sperm head suggests that the initial response may result from direct contact between the sperm and the tips of oocyte microvilli. However, in some cases, PYK2 foci were present in microvilli a short distance from the sperm head. This might be a result of the slow nature of formaldehyde fixation, which could allow cell processes to retract during fixation as we have observed in zebrafish oocytes (unpublished). However, it could also reflect signaling across distance, possibly a result of localized calcium influx (Miao and Williams, 2012), or via the exchange of membrane vesicles between sperm and oocyte (Barraud-Lange et al., 2012; Miyado et al., 2008). The initial PYK2 response was followed by accumulation of PYK2 in the oocyte cortex under the sperm head indicating that the initial PYK2 signal was amplified and propagated beyond the initial point of origin. The more advanced examples of PYK2 recruitment appeared as a disk or plaque located directly underneath the sperm head. Only a small fraction (approximately 14%) of sperm binding sites were associated with a robust (>50% relative to adjacent cortex) accumulation of PYK2 sufficient to form a plaque-like structure. Most (approximately 70% (9/13)) of the plaque-like PYK2 structures exhibited significant accumulation of f-actin >20% relative to adjacent cortex, but only 38% (5/13) exhibited an accumulation of f-actin >50% higher than adjacent cortex. The results indicate that as more PYK2 is recruited, the amount of f-actin associated with the sperm binding site increases significantly. The next step would presumably involve penetration of the actin plaque by the sperm head, but we were unable to observe any examples where a sperm head was fixed during passage through the cortical actin layer, suggesting that this event may occur quickly or be refractory to formaldehyde fixation. However, we did observe multiple examples where the sperm head was fully incorporated underneath the oocyte cortical actin layer and had often begun the process of nuclear de-condensation. In these cases, the cortical actin layer overlying the sperm nucleus exhibited increased f-actin content forming a ‘cap’ that was also enriched in PYK2 protein. During anaphase, activated PYK2 became concentrated in wider regions of the oocyte cortex as described previously (Luo et al., 2014), and once the male pronucleus formed, PYK2 was no longer associated with the oocyte cortex.
4.2 Regulation of PYK2 by cell surface signals at sperm-oocyte contact
The observation that PYK2 recruitment and activation begins in close proximity to bound sperm raises the question of whether the trigger for the PYK2 response involves the interaction of sperm and oocyte surface proteins, or of sperm cytoplasmic factors delivered into the oocyte following membrane fusion. The possibility that sperm-oocyte fusion might be required for PYK2 recruitment and activation was tested by performing IVF at low pH (pH 6.1), which prevents formation of fusion pores between sperm and oocyte (Lawrence et al., 1997; Yanagimachi, 1980). The fact that low pH almost completely blocked sperm-oocyte fusion, but did not block formation of PYK2 foci associated with bound sperm, demonstrated that the cell-surface interaction between sperm and oocyte membrane components is sufficient to induce the initial stages of PYK2 recruitment and activation.
The interaction between sperm and oocyte surface proteins has been the object of numerous studies which defined a subset of oocyte proteins that are important for fertilization; including integrins (Evans, 2012; He et al., 2003; Runge et al., 2007; Ziyyat et al., 2006), cadherins (Takezawa et al., 2011), and GPI-linked proteins such as JUNO (Bianchi and Wright, 2015). In other cell types, PYK2 is known to respond to cell-cell or cell-matrix contact induced by protocadherin or integrin clustering (Suo et al., 2012; Yu et al., 2013), so there is reason to speculate that oocyte integrins or cadherins could transduce a signal that leads to PYK2 activation. JUNO is another oocyte surface protein recently identified as the oocyte receptor for the sperm protein IZUMO (Bianchi et al., 2014). GPI-linked proteins, including the folate receptors, are known to form complexes with SRC- and FAK-family PTKs in other systems (Miotti et al., 2000; Stefanova et al., 1991; Zhang et al., 2014), which subsequently develop into ‘signaling microclusters’ where PYK2 and Src-family kinases reach a concentration sufficient for transphosphorylation and PYK2 activation to occur (Steblyanko et al., 2015). Future studies will test the role of different sperm and oocyte proteins in PYK2 activation at the site of sperm – egg interaction.
The question of why only a small subset of bound sperm induce PYK2 recruitment and activation remains unanswered. The experimental conditions used here typically resulted in 5 to 25 sperm bound to the oocyte plasma membrane for up to 30 minutes before evidence of a PYK2 response was observed in proximity to one or two sperm per oocyte. While it appears that only a small subset of sperm that have bound to or fused with the oocyte plasma membrane elicit the PYK2 response, it is also possible that the response is highly transient and we only catch a small fraction on sperm that are actually signaling with the oocyte when the chemical fixative halted the action. Live cell imaging with fluorescent probes may enable resolution of this issue. It is also possible that few sperm are of sufficient quality to induce PYK2 activation, or that a series of molecular events must occur successfully before PYK2 activation is induced. For example, recent studies have detailed a series of rearrangements between JUNO and IZUMO that occur at the molecular level and at a microscopic level within the egg plasma membrane underneath and adjacent to the bound sperm (Inoue et al., 2015). It is possible that such rearrangements must reach a certain threshold before PYK2 in the oocyte cortex could respond. Another possibility is that the molecular components of the membrane block to polyspermy may become active (even before sperm incorporation) and present a higher threshold that other sperm must overcome to achieve a response by PYK2.
4.3 Role of PYK2 in sperm-oocyte fusion and sperm incorporation
Sperm-oocyte fusion is thought to begin with formation of transient fusion pores (Kumakiri et al., 2003) which initially are reversible, but still allow diffusion of dyes and possibly proteins between sperm and oocyte. The possibility that PYK2 activity might be play a role in sperm-oocyte fusion was tested by comparing the rate at which WT sperm fuse with WT and PYK2 −/− oocytes. Dye diffusion analysis demonstrated that PYK2-suppressed oocytes were able to fuse with WT sperm at a frequency similar to that of controls, indicating that PYK2 activity is not required for membrane fusion. Sperm incorporation occurs after sperm-oocyte fusion pores become stabilized and expanded to create permanent cytoplasmic continuity with the oocyte. The incorporation process requires actin-mediated events enabled by rho-family GTPases without which bound sperm can reportedly detach even after temporary fusion with the oocyte (Kumakiri et al., 2003). Our analysis using the zona-free IVF assay under sperm-limiting conditions revealed that pyk2−/− oocytes incorporated sperm at a lower rate than WT controls, even though gamete binding and fusion occurred normally. The fact that pyk2−/− oocytes still became fertilized, just at a lower rate, differs from our results using the PYK2 inhibitor (PF04594755) that was much more effective in blocking sperm incorporation (Luo et al., 2014). This difference could reflect side effects of the inhibitor, or it could be that the inhibitor produced a pool of catalytically inactive PYK2 in the oocyte that exerted a dominantnegative effect. In any case, the ability of pyk2−/− oocytes to incorporate sperm well enough to achieve fertility demonstrates that alternate pathways can compensate for the loss of pyk2 function. Similar, partially penetrant phenotypes have been observed in other pyk2−/− cell types such as macrophages (Okigaki et al., 2003), keratinocytes (Koppel et al., 2014), and astrocytes (Giralt et al., 2016). In some cell types, FAK is thought to compensate for PYK2 suppression, but our observation that FAK inhibition or ablation produced no inhibitory effect on fertilization (Luo et al., 2014) suggests that this not the case in the oocyte.
4.4 Role of PYK2 in actin remodeling in the oocyte cortex
The fact that PYK2 was co-localized with f-actin-enriched structures in microvilli and within the cortical actin layer suggested that this kinase may play some role in actin remodeling. Functional studies using the protein kinase inhibitor PF04594755, the dominant-negative PERM-eGFP construct, and pyk2−/− oocytes all suggested that suppression of PYK2 activity greatly reduced the extent to which f-actin accumulated at the site of sperm-oocyte interaction. Quantitative analysis of Z-stack images confirmed that the amount of f-actin associated with sperm binding sites was significantly lower in pyk2−/− oocytes. This analysis also showed that both WT and pyk2−/− oocytes did not respond (as detected by f-actin accumulation) to most bound sperm. However, WT oocytes did respond to a small subset of bound sperm with significant accumulation of f-actin in the cortex and in cell surface protrusions near bound/fused sperm. The frequency with which pyk2−/− oocytes exhibited similar f-actin accumulations at sperm binding sites was substantially lower. Since the dye transfer experiments reveled that pyk2−/− oocytes fused with most bound sperm within 45 m.p.i., it seems that f-actin accumulation was more dependent on PYK2 activation than on gamete fusion. Kumakiri and co-workers (Kumakiri et al., 2003) reported a similar phenomenon in response to Rho-family G protein inhibition, where gamete fusion occurred but sperm incorporation was greatly reduced. Together these studies indicate that membrane fusion alone is not sufficient for successful fertilization, and suggest that actin remodeling events represent an active role of the oocyte required for fertilization.
The data are consistent with a model in which cell surface interaction between sperm and oocyte triggers PYK2 recruitment and activation in the form of small foci within oocyte microvilli that induces actin remodeling leading to microvillus elongation and movement. The activity of these microvilli would establish additional contact sites with the sperm that would not only increase the strength of sperm binding, but could stabilize contact to an extent that would favor the coalescence of stable fusion pores to establish complete cytoplasmic continuity between sperm and oocyte. In addition to actin associated with cell processes, the recruitment and activation of PYK2 protein was also associated with an increased density of f-actin within the cortical actin layer underneath the bound sperm head, which took the form of a plaque in WT oocytes, but occurred on a smaller scale in PYK2 −/− oocytes. This plaque would initially seem to present a barrier to sperm incorporation, but similar structures have been reported at the site of invasive f-actin foci formed by fusion-competent myoblasts (Sens et al., 2010) suggesting that they may have a role in stabilizing cell fusion sites. These observations demonstrate that the important functions of PYK2, like that of Rho GTPases (Kumakiri et al., 2003), occur after membrane fusion had already begun, and are related to the sperm incorporation process rather than sperm-oocyte fusion.
4.5 Conclusion
The current study demonstrates that PYK2 is recruited to the oocyte cortex in response to sperm binding and plays a role in sperm incorporation, probably through actin-mediated events needed to incorporate the sperm head. Since pyk2−/− mice can reproduce, alternate signaling proteins (FAK, Rho GTPases, etc.) must also have the ability to induce these actin-mediated events to a level sufficient for fertilization in vivo, even though IVF experiments indicate that it must be less efficient in the absence of PYK2. The results of this study have important implications for our understanding of fertilization. First, the concept that surface contact between sperm and oocyte is sufficient (in the absence of membrane fusion) to induce a biochemical response in the oocyte may explain how surface proteins not directly involved with membrane fusion may still play an important role in fertilization. Secondly, the observation that multiple sperm may fuse (at least transiently) with the oocyte, while few induce PYK2 activation, f-actin accumulation, and become incorporated, indicates that the oocyte is not passive during fertilization, but must respond via actin remodeling events involved in sperm incorporation. By inference, one might speculate that the ability of a sperm to induce the PYK2 response within the oocyte represents another challenge that must be met in order to propagate the species. Therefore, characteristics that could give an individual sperm an advantage in triggering PYK2 activation may represent another aspect of gamete quality not previously considered.
Highlights.
sperm-oocyte contact triggers recruitment of PYK2 to the sperm-contact site
PYK2 recruitment does not require membrane fusion
PYK2 activity is not required for gamete fusion but is required for sperm incorporation
PYK2 foci associated with bound sperm are co-localized with condensations of f-actin.
Acknowledgments
We acknowledge the support of the compound transfer program at Pfizer Corporation for providing PF4594755. Pfizer, together with Dr. Marion Pepper at the University of Washington provided the pyk2−/− mouse line. This work was supported by the National Institutes of Health (RO1 HD062860), and (P20 GM104936).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barraud-Lange V, Chalas Boissonnas C, Serres C, Auer J, Schmitt A, Lefevre B, Wolf JP, Ziyyat A. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilising ability of Cd9-deleted oocytes. Reproduction. 2012;144:53–66. doi: 10.1530/REP-12-0040. [DOI] [PubMed] [Google Scholar]
- Bartos JA, Ulrich JD, Li H, Beazely MA, Chen Y, Macdonald JF, Hell JW. Postsynaptic clustering and activation of Pyk2 by PSD-95. J Neurosci. 2010;30:449–463. doi: 10.1523/JNEUROSCI.4992-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Wright GJ. Cross-species fertilization: the hamster egg receptor, Juno, binds the human sperm ligand, Izumo1. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2015;370:20140101. doi: 10.1098/rstb.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline CA, Schatten G. Microfilaments during sea urchin fertilization: fluorescence detection with rhodaminyl phalloidin. Gamete Res. 1986;14:277–291. doi: 10.1002/mrd.1120140402. [DOI] [PubMed] [Google Scholar]
- Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. J Leukoc Biol. 2010;87:691–701. doi: 10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- Eleniste PP, Bruzzaniti A. Focal adhesion kinases in adhesion structures and disease. Journal of signal transduction. 2012;2012:296450. doi: 10.1155/2012/296450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP. Sperm-egg interaction. Annu Rev Physiol. 2012;74:477–502. doi: 10.1146/annurev-physiol-020911-153339. [DOI] [PubMed] [Google Scholar]
- Felty Q. Redox sensitive Pyk2 as a target for therapeutics in breast cancer. Front Biosci (Landmark Ed) 2011;16:568–577. doi: 10.2741/3706. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reproduction, fertility, and development. 2006;18:53–61. doi: 10.1071/rd05122. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(−/−) mice. The Journal of cell biology. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Coura R, Girault JA. Pyk2 is essential for astrocytes mobility following brain lesion. Glia. 2016;64:620–634. doi: 10.1002/glia.22952. [DOI] [PubMed] [Google Scholar]
- Hasan AK, Fukami Y, Sato K. Gamete membrane microdomains and their associated molecules in fertilization signaling. Molecular reproduction and development. 2011;78:814–830. doi: 10.1002/mrd.21336. [DOI] [PubMed] [Google Scholar]
- He ZY, Brakebusch C, Fassler R, Kreidberg JA, Primakoff P, Myles DG. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Developmental biology. 2003;254:226–237. doi: 10.1016/s0012-1606(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Hinkley RE, Wright BD, Lynn JW. Rapid visual detection of sperm-egg fusion using the DNA-specific fluorochrome Hoechst 33342. Developmental biology. 1986;118:148–154. doi: 10.1016/0012-1606(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Inoue N, Hagihara Y, Wright D, Suzuki T, Wada I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat Commun. 2015;6:8858. doi: 10.1038/ncomms9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel AC, Kiss A, Hindes A, Burns CJ, Marmer BL, Goldberg G, Blumenberg M, Efimova T. Delayed skin wound repair in proline-rich protein tyrosine kinase 2 knockout mice. Am J Physiol Cell Physiol. 2014;306:C899–909. doi: 10.1152/ajpcell.00331.2013. [DOI] [PubMed] [Google Scholar]
- Kumakiri J, Oda S, Kinoshita K, Miyazaki S. Involvement of Rho family G protein in the cell signaling for sperm incorporation during fertilization of mouse eggs: inhibition by Clostridium difficile toxin B. Developmental biology. 2003;260:522–535. doi: 10.1016/s0012-1606(03)00273-2. [DOI] [PubMed] [Google Scholar]
- Kyozuka K, Osanai K. Sperm-engulfing response of sea urchin egg surfaces inseminated with acrosome-reacted starfish sperm. Gamete Res. 1988;21:169–177. doi: 10.1002/mrd.1120210207. [DOI] [PubMed] [Google Scholar]
- Lang D, Glukhov AV, Efimova T, Efimov IR. Role of Pyk2 in cardiac arrhythmogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H975–983. doi: 10.1152/ajpheart.00241.2011. [DOI] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- Li HY, Cui XY, Wu W, Yu FY, Yao HR, Liu Q, Song EW, Chen JQ. Pyk2 and Src mediate signaling to CCL18-induced breast cancer metastasis. J Cell Biochem. 2014;115:596–603. doi: 10.1002/jcb.24697. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Cook S, McCulloh DH, Ivonnet PI, Chambers EL. Stages leading to and following fusion of sperm and egg plasma membranes. Zygote. 1994;2:317–331. doi: 10.1017/s0967199400002148. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Lynn JW, McCulloh DH, Chambers EL. Correlative ultrastructural and electrophysiological studies of sperm-egg interactions of the sea urchin, Lytechinus variegatus. Developmental biology. 1986;118:155–166. doi: 10.1016/0012-1606(86)90083-7. [DOI] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Carlton C, Beggs HE, Kinsey WH. PTK2b function during fertilization of the mouse oocyte. Biochemical and biophysical research communications. 2014;450:1212–1217. doi: 10.1016/j.bbrc.2014.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn JW, McCulloh DH, Chambers EL. Voltage clamp studies of fertilization in sea urchin eggs. II. Current patterns in relation to sperm entry, nonentry, and activation. Developmental biology. 1988;128:305–323. doi: 10.1016/0012-1606(88)90294-1. [DOI] [PubMed] [Google Scholar]
- Martel-Gallegos G, Casas-Pruneda G, Ortega-Ortega F, Sanchez-Armass S, Olivares-Reyes JA, Diebold B, Perez-Cornejo P, Arreola J. Oxidative stress induced by P2×7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochim Biophys Acta. 2013;1830:4650–4659. doi: 10.1016/j.bbagen.2013.05.023. [DOI] [PubMed] [Google Scholar]
- McGinnis LK, Luo J, Kinsey WH. Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption. Molecular reproduction and development. 2013;80:260–272. doi: 10.1002/mrd.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XQ, Zheng KG, Yang Y, Jiang MX, Zhang YL, Sun QY, Li YL. Proline-rich tyrosine kinase2 is involved in F-actin organization during in vitro maturation of rat oocyte. Reproduction. 2006;132:859–867. doi: 10.1530/rep.1.01212. [DOI] [PubMed] [Google Scholar]
- Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Molecular reproduction and development. 2012;79:742–756. doi: 10.1002/mrd.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotti S, Bagnoli M, Tomassetti A, Colnaghi MI, Canevari S. Interaction of folate receptor with signaling molecules lyn and G(alpha)(i-3) in detergent-resistant complexes from the ovary carcinoma cell line IGROV1. Journal of cell science 113 Pt. 2000;2:349–357. doi: 10.1242/jcs.113.2.349. [DOI] [PubMed] [Google Scholar]
- Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12921–12926. doi: 10.1073/pnas.0710608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill FJ, Gillett J, Foltz KR. Distinct roles for multiple Src family kinases at fertilization. Journal of cell science. 2004;117:6227–6238. doi: 10.1242/jcs.01547. [DOI] [PubMed] [Google Scholar]
- Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. The Journal of cell biology. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J, Torres RA, Rocic P, Cismowski MJ, Weber DS, Darley-Usmar VM, Lucchesi PA. PYK2 signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2011;301:C242–251. doi: 10.1152/ajpcell.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM, Shalgi R. Sperm penetration into rat ova fertilized in vivo. J Exp Zool. 1982;221:373–378. doi: 10.1002/jez.1402210313. [DOI] [PubMed] [Google Scholar]
- Ray BJ, Thomas K, Huang CS, Gutknecht MF, Botchwey EA, Bouton AH. Regulation of osteoclast structure and function by FAK family kinases. J Leukoc Biol. 2012;92:1021–1028. doi: 10.1189/jlb.0512259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs D, Yang Z, Kloss J, Loftus JC. The Pyk2 FERM regulates Pyk2 complex formation and phosphorylation. Cell Signal. 2011;23:288–296. doi: 10.1016/j.cellsig.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Developmental biology. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Developmental biology. 2007;304:317–325. doi: 10.1016/j.ydbio.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Sato K, Yoshino K, Oshiro N, Hirahara S, Mahbub Hasan AK, Iwasaki T, Ueda Y, Iwao Y, Yonezawa K, Fukami Y. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. The Journal of biological chemistry. 2005;280:15029–15037. doi: 10.1074/jbc.M410538200. [DOI] [PubMed] [Google Scholar]
- Selitrennik M, Lev S. PYK2 integrates growth factor and cytokine receptors signaling and potentiates breast cancer invasion via a positive feedback loop. Oncotarget. 2015;6:22214–22226. doi: 10.18632/oncotarget.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens KL, Zhang S, Jin P, Duan R, Zhang G, Luo F, Parachini L, Chen EH. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. The Journal of cell biology. 2010;191:1013–1027. doi: 10.1083/jcb.201006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. PYK2: a calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Developmental biology. 2013;373:130–140. doi: 10.1016/j.ydbio.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steblyanko M, Anikeeva N, Campbell KS, Keen JH, Sykulev Y. Integrins Influence the Size and Dynamics of Signaling Microclusters in a Pyk2-dependent Manner. The Journal of biological chemistry. 2015;290:11833–11842. doi: 10.1074/jbc.M114.614719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MC, McGinnis LK, Lawitts JA, Raffin M, Biggers JD. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Human reproduction. 2000;15:1791–1801. doi: 10.1093/humrep/15.8.1791. [DOI] [PubMed] [Google Scholar]
- Suo L, Lu H, Ying G, Capecchi MR, Wu Q. Protocadherin clusters and cell adhesion kinase regulate dendrite complexity through Rho GTPase. J Mol Cell Biol. 2012;4:362–376. doi: 10.1093/jmcb/mjs034. [DOI] [PubMed] [Google Scholar]
- Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127:431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Pyk2/CAKbeta signaling: a novel Ca(2+)-dependent pathway leading to cardiac hypertrophy. J Mol Cell Cardiol. 2004;36:791–793. doi: 10.1016/j.yjmcc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Takezawa Y, Yoshida K, Miyado K, Sato M, Nakamura A, Kawano N, Sakakibara K, Kondo T, Harada Y, Ohnami N, Kanai S, Miyado M, Saito H, Takahashi Y, Akutsu H, Umezawa A. beta-catenin is a molecular switch that regulates transition of cell-cell adhesion to fusion. Sci Rep. 2011;1:68. doi: 10.1038/srep00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P, Chacon RS. Ultrastructural observations on binding and membrane fusion between human sperm and zona pellucida-free hamster oocytes. Fertil Steril. 1982;37:240–248. doi: 10.1016/s0015-0282(16)46047-4. [DOI] [PubMed] [Google Scholar]
- Townley IK, Schuyler E, Parker-Gur M, Foltz KR. Expression of multiple Src family kinases in sea urchin eggs and their function in Ca2+ release at fertilization. Developmental biology. 2009;327:465–477. doi: 10.1016/j.ydbio.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Walkiewicz KW, Girault JA, Arold ST. How to awaken your nanomachines: Site-specific activation of focal adhesion kinases through ligand interactions. Prog Biophys Mol Biol. 2015;119:60–71. doi: 10.1016/j.pbiomolbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Miyashiro LH, Yanagimachi H. Reversible inhibitio of sperm-egg fusion by low pH. Devel. Growth, and Differentiation. 1980;22:281–288. doi: 10.1111/j.1440-169X.1980.00281.x. [DOI] [PubMed] [Google Scholar]
- Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, Sheetz MP. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell reports. 2013;5:1456–1468. doi: 10.1016/j.celrep.2013.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, March ME, Lane WS, Long EO. A signaling network stimulated by beta2 integrin promotes the polarization of lytic granules in cytotoxic cells. Sci Signal. 2014;7:ra96. doi: 10.1126/scisignal.2005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen L, Zhao P, Zhou H, Zhang C, Yu S, Lin Y, Yang X. Store-operated Ca 2+ entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. Journal of experimental & clinical cancer research : CR. 2014;33:98. doi: 10.1186/s13046-014-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyyat A, Rubinstein E, Monier-Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP. CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. Journal of cell science. 2006;119:416–424. doi: 10.1242/jcs.02730. [DOI] [PubMed] [Google Scholar]