Abstract
Background
Geographic and demographic variation in buprenorphine and methadone treatment use in U.S. cities has not been assessed. Identifying variance in opioid maintenance is essential to improving treatment access and equity.
Purpose
To examine the differential uptake of buprenorphine treatment in comparison to methadone treatment between 2004 and 2013 in neighborhoods in New York City characterized by income, race and ethnicity.
Methods
Social area (SA) analysis of residential zip codes of methadone and buprenorphine patients in NYC, which aggregated zip codes into five social areas with similar percentages of residents below poverty, identifying as Black non-Hispanic and as Hispanic, to examine whether treatment rates differed significantly among social areas over time. For each rate, mixed model analyses of variance were run with fixed effects for social area, year and the interaction of social area by year.
Results
Buprenorphine treatment increased in all social areas over time with a significantly higher rate of increase in the social area with the highest income and the lowest percentage of Black, Hispanic, and low-income residents. Methadone treatment decreased slightly in all social areas until 2011 and then increased bringing rates back to 2004 levels. Treatment patterns varied by social area.
Conclusions
Buprenorphine treatment rates are increasing in all social areas, with slower uptake in moderate income mixed ethnicity areas. Methadone rates have remained stable over time. Targeted investments to promote public sector buprenorphine prescription may be necessary to reduce disparities in buprenorphine treatment and to realize its potential as a public health measure.
Keywords: Buprenorphine, Methadone, Treatment disparities, Racial disparities
1. Introduction
The dramatic rise in the non-medical use of prescription opioids and of heroin in the U.S. over the past decade has had major health consequences: as of 2012, drug overdose became the leading cause of injury death in the U.S., most of these from opioids (Centers for Disease Control and Prevention (CDC, 2014). At the same time, it is estimated that 80% of people dependent on heroin or prescription opioids do not receive treatment (Stancliff et al., 2012). Buprenorphine has emerged as a clinical and public health intervention; a partial opioid agonist, it has comparable effectiveness to methadone in treating heroin and prescription opioid dependence (Mattick et al., 2013). In order to expand access to treatment, in 2002 the U.S. Food and Drug Administration approved the prescription of buprenorphine for opioid maintenance treatment by general physicians in their offices, as an alternative to regulated methadone clinics with directly observed dosing requirements (Wakhlu, 2009).
In Western European countries such as France, buprenorphine provision is seen as a public health measure and promoted by the government among low-income, ethnic minority heroin injecting patients, reducing their HIV and overdose rates (Lovell, 2006; Emmanuelli and Desenclos, 2005). In the U.S., buprenorphine patients have historically been privately insured prescription opioid users. As of 2005, buprenorphine patients were likely to be White (92% compared to 53% of methadone patients), employed (56% vs 29% of methadone patients), to have some college education (56% vs 19% of methadone patients), and to be prescription opioid dependent (75%) rather than heroin dependent (Stanton et al., 2006). Although no nationally representative data on the socioeconomic status or ethnicity/race of buprenorphine patients has been published since the Stanton et al. study, recent city and state-level analysis have found similar racial and socioeconomic trends (Hansen et al., 2013; Stein et al., 2015a,b),
The demographic patterns of buprenorphine treatment in the U.S. may be explained by Congress’ desire to address the growth in suburban and rural prescription opioid and heroin use, which was enhanced by targeted marketing of new prescription opioids in those areas beginning in the 1990s (Zee, 2009). Members of Congress passed legislation enabling office-based buprenorphine maintenance that would be more palatable to a growing population of suburban, opioid dependent people than methadone clinics (Egan et al., 2010). Additionally, the manufacturer advertised to consumers primarily over the internet, and helped the Substance Abuse and Mental Health Services Administration launch a web-based physician locator to help patients find buprenorphine certified prescribers (Hansen and Roberts, 2012), which may have favored literate and more affluent consumers. The certification requirement for eight hours of training itself contributed to a shortage of public sector buprenorphine prescribers (Barry et al., 2009).
Heroin and other opioid use remains an endemic cause of overdose death, HIV and hepatitis C infection among Blacks and Hispanics (CDC African Americans, 2015; CDC Latinos, 2015; CDC Health Disparities, 2015; Khlifi et al., 2009; Mack, 2013). Buprenorphine has been shown to improve treatment retention and health outcomes among low-income, socially marginalized populations (Hersh et al., 2011; Stancliff et al., 2012) including formerly incarcerated patients (Lee et al., 2012; Magura et al., 2009) who often express a preference for buprenorphine over methadone (Mitchell et al., 2012). Disparities in access to buprenorphine represent a missed opportunity to protect the health of these populations.
To address the uneven distribution of buprenorphine, all states now include some form of Medicaid coverage for buprenorphine (Rinaldo and Rinaldo, 2013), and certain major cities, including Baltimore and Boston, actively promote buprenorphine treatment in public clinics (Schwartz et al., 2013; Hersh et al., 2011). Beginning in 2005, New York City’s public hospitals publicized buprenorphine among patients, encouraged physicians to become certified, and offered buprenorphine in outpatient and inpatient settings (personal communication with Peter Coleman, M.D., June 24, 2013). New York City is instructive with regard to buprenorphine access; while it has the greatest number of opioid dependent residents of any U.S. city, with estimates ranging from 92,000 to 200,000 (Frank, 2000), only an estimated 11–23% have initiated treatment (McNeely et al., 2012). Their untreated opioid dependence has significant consequences; injection drug use is a primary reason that New York has the highest number of new HIV cases in the U.S. (Centers for Disease Control and Prevention (CDC, 2012).
Despite New York City’s promotion of buprenorphine in public clinics, as of 2007, buprenorphine patients were significantly more likely to live in high income, predominantly White areas of New York City, while methadone patients were significantly more likely to live in low-income, predominantly Black or Hispanic areas (Hansen et al., 2013). The question raised in this paper is whether these treatment patterns persist. Theories of the health impact of new health technologies in socially stratified societies predict that they increase disparities in health and health care, due to uptake among consumers that have the economic, cultural and social capital to access them. In the absence of countervailing public investments, new technologies are used by more affluent, educated patients, and the use of these technologies further increases disparities in disease detection, treatment access, and mortality based on education and income (Glied and Lleras-Muney, 2008; Tehranifar et al., 2009). This compounds pre-existing health inequalities: Link and Phelan (1995) have argued that socioeconomic inequalities fundamentally cause health disparities by putting people “at risk of risk”.
This study traces geographic changes in buprenorphine maintenance treatment (BMT) and methadone maintenance treatment (MMT) across neighborhoods with markedly different demographics and income levels, over a period of regulatory changes and increases in opioid use. In 2007, a Federal amendment raised the legal limit of buprenorphine patients per provider from 30 to 100. In 2012, a Federal regulatory change allowed methadone clinics to dispense take-home buprenorphine. By 2013, New York City reported an unprecedented rise in opioid overdose deaths (Siegler et al., 2014). In analyses in which usage rates of buprenorphine and of methadone are the dependent variables and usage is examined over constructed social areas defined by their income levels and ethnic mix, the hypotheses tested were: 1) usage of methadone has declined and of buprenorphine has increased in all social areas; 2) the variation among neighborhoods defined by income, race/ethnicity in buprenorphine and methadone usage persists over time.
2. Method
2.1. Data
The buprenorphine treatment rate was determined from data collected by the Federal Drug Enforcement Agency (DEA) on the number of buprenorphine prescriptions written from 2004 to 2013 by residential postal ZIP code of the patient treated. Data were supplied by the New York State Bureau of Narcotics Enforcement. Although independent verification of the completeness of this data is not available, it is the most complete publicly available data set on buprenorphine prescriptions because retail pharmacies are federally mandated to report the age, address and date of service for each buprenorphine prescription recipient to the DEA. MMT rates were based on data from the New York State Office of Alcoholism and Substance Abuse Services (OASAS) that accounted for all patients receiving MMT in New York City methadone clinics in 2004–2013 by patients’ ZIP code. Estimates of missing data in this set are unavailable. MMT programs do not record ZIP codes for homeless individuals not in shelters. Otherwise, missing data is expected to be rare since all New York City methadone maintenance clinics are regulated by OASAS, and this data set is linked to reimbursements and is used for budgeting purposes by the state payer. Ethnic/racial data (Black non-Hispanic and/or Hispanic), and proportion of the population who are living less than two times below the poverty level, were obtained from 2010 US Census ZIP Code Tabulation area reports. Census ZIP codes were subsequently matched back to their postal ZIP equivalents (Grubesic and Matisziw, 2006). After exclusion of codes denoting water areas, non-residential areas, and areas with populations of less than 200, the sample consisted of 179 New York City ZIP codes.
2.2. Measures
Annual BMT and MMT usage rates were calculated for each ZIP code. For both BMT and MMT rates, anyone who began or ended treatment within the year was included. The buprenorphine treatment rate is the annual number of buprenorphine prescriptions written to patients residing in each ZIP code divided by the number of persons residing in the ZIP code times 10,000. Because individual prescriptions usually provide a 30 day supply of medication, prescriptions were annualized by dividing the count of 30-day prescriptions by 12. This divisor is somewhat arbitrary but does not impact the analyses that were used. The MMT rate was defined as the annual number of people enrolled for any period of time in methadone clinics operating in a ZIP code divided by the number of persons residing in the ZIP code times 10,000. BMT and MMT rates have different units of measurement so are not directly comparable. They were used to examine relative patterns of variation in use across neighborhoods but buprenorphine and methadone rates were not compared with each other in the same ZIP code.
2.3. Formation of social areas
Persons receiving buprenorphine prescriptions or enrolled in methadone clinics were identified by the ZIP code of their residential addresses. ZIP codes were aggregated based on similarities in ZIP code census data on race, ethnicity and percent with income below two times the poverty level (Araujo et al., 2010; Bocquier et al., 2011; Chau, 2010; Smith et al., 2010) resulting in areas in which persons are homogeneous with respect to income and race/ethnicity. These areas are called social areas (SAs) as they capture social features of a population. Social areas are not necessarily geographically contiguous but are homogeneous neighborhoods with common characteristics. For example in its application in this study, socials areas distinguish high income predominantly white areas from poor black or poor Hispanic areas. Analyzing data based on social areas is an established public health research approach to examine inequalities in health particularly useful when residential level data but not individual level data are available for each subject (Das-Munshi et al., 2010; Gottfredson et al., 1991; Scott-Samuel, 1977; Shevky and Bell, 1995). Examining usage rates by social areas is also advantageous when examining variables that interact in ways that make univariate analysis difficult to interpret − in this case, the variables income, race and ethnicity. The method was previously used in a paper by the authors to examine disparities in buprenorphine and methadone treatment in 2007 (Hansen et al., 2013).
2.4. Statistical analyses
ZIP codes were clustered into five social areas based on the three census variables using Ward’s minimum-variance method in the SAS 9.3 Cluster procedure (Heye and Leuthold, 2005) following the approach described in Hansen et al. (2013). Each variable was also individually clustered into three categories which were used to describe the social area. To examine whether rates differed significantly among social areas and whether rates varied over year, for each rate, mixed model analyses of variance were run with fixed effects for social area, year and an interaction of social area by year. The random component had a random intercept effect for zip code and a subject block for years within zip code. Since raw means for MMT usage suggested a decrease in rates over time followed by an increase, both linear and quadratic models for the variable year were fit to the methadone data. For consistency, this was also done for buprenorphine even though its raw means only indicated an increase in usage. If the quadratic term in the model significantly differed from zero, two separate linear models were run for the years of decrease and another for years of increase. If the quadratic term did not differ from zero, the results would be based on a linear model. In these models, to examine whether rates significantly changed at the family wise error rate of 5% over years, model-based estimates of the slope of rates were individually tested using a Bonferroni adjusted p value for significance (p < 0.01), for being different from zero. Slopes were also contrasted among social areas for statistical differences (Bonferroni adjusted, p < 0.005). Race, ethnicity and income were not introduced as control variables in analysis of variance since the social areas encapsulate these variables. In addition, a model was run to examine change from 2004 to 2013. All study analyses were conducted in SAS 9.3-SAS/STAT 12.1 (SAS, 2008). While insurance data might have been informative, no zip code level data were available on insurance. However, in the U.S. income level and ethnicity are correlated with percent persons with insurance coverage (Adams et al., 2009; Liao et al., 2011).
3. Results
3.1. Social area analyses
The five social areas identified were characterized by their mean percent of having a demographic characteristic that by using cluster analysis had been classified into low (L), medium (M), or high (H) mean percent of the characteristic. Cut-points for percent in poverty were L < 34% and for H > 47%; L and H cut-points for Black, non-Hispanic 19% and 51% and for Hispanic 31% and 53%. SD denotes standard deviations (See Table 1).
Table 1.
Demographic characteristics of social areas in New York City based on 2010 Census Data.a
| SocialArea (SA) | # (%) Zip codes in area | Mean% (SD) of demographic characteristic | |||
|---|---|---|---|---|---|
| Poverty level | Black non-Hispanic | Hispanic | Social Area label | ||
| 1 | 47 (26) | 15.0 (5.1) | 3.4 (2.3) | 9.1 (3.7) | (L,L,L) |
| 2 | 57 (32) | 34.2 (9.2) | 11.0 (9.5) | 20.6 (7.7) | (M,L,L) |
| 3 | 35 (20) | 38.4 (12.8) | 69.0 (16.3) | 17.3 (9.7) | (M,H,L) |
| 4 | 18 (10) | 44.1 (9.2) | 10.2 (5.9) | 54.3 (13.5) | (M,L,H) |
| 5 | 22 (12) | 60.2 (9.9) | 34.8 (6.6) | 60.6 (10.5) | (H,M,H) |
Zip codes analyzed were grouped into five social areas with similar demographic characteristics including percent of residents below poverty, percent Black non-Hispanic, and percent Hispanic. Each of the five social areas received a descriptive label based on these characteristics: e.g. low percentage of poverty, low percentage Black non-Hispanic, and low percentage Hispanic was labeled L,L,L.
3.2. Rate analyses
3.2.1. Unadjusted means
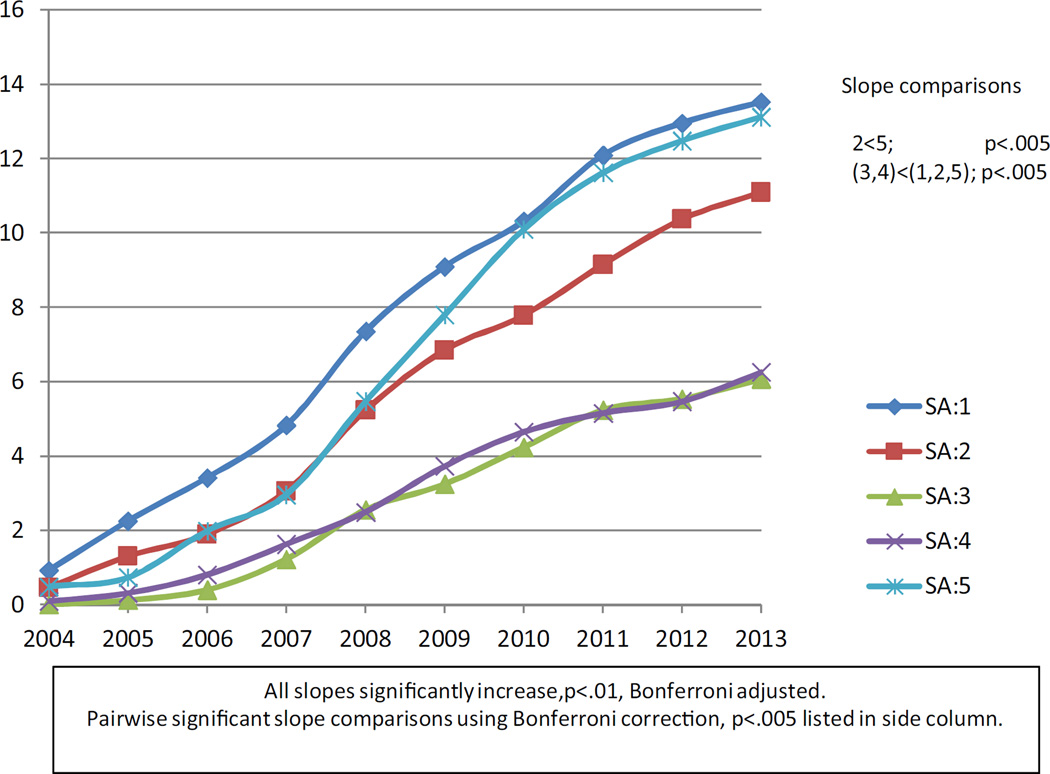
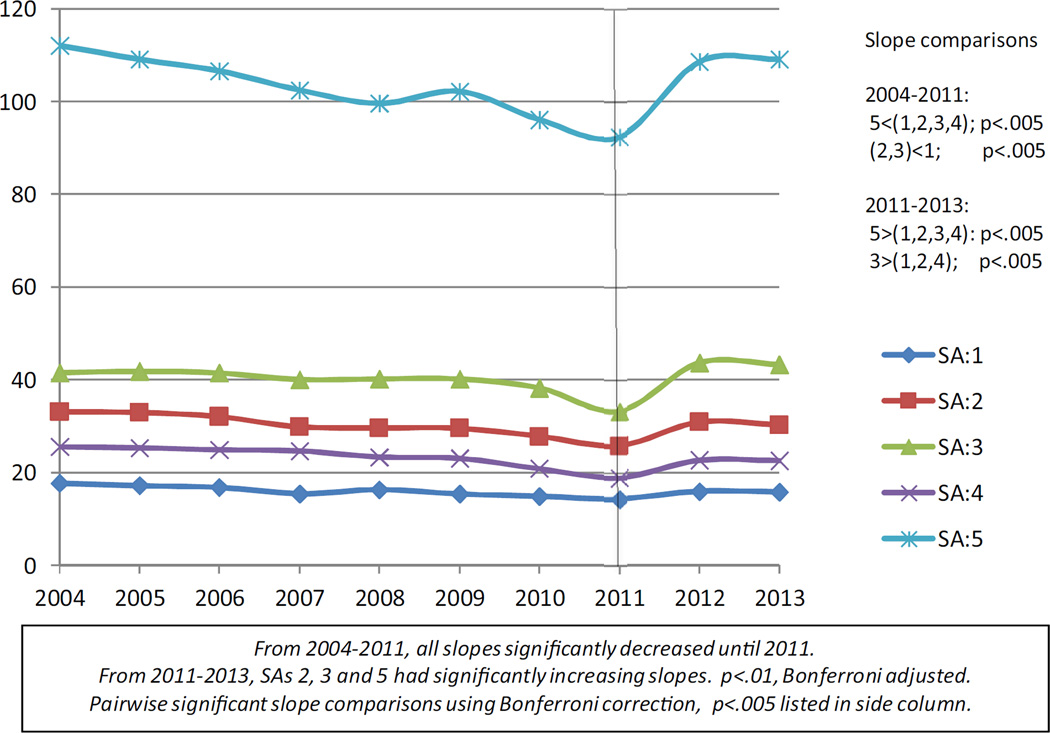
Figs. 1 and 2 plot the unadjusted means of the BMT and MMT rates respectively over years for each social area. These figures visually allow us to examine whether; 1) MMT usage has declined and BMT usage has increased in all SAs; 2) the variation among neighborhoods (SAs) defined by income, race/ethnicity in BMT and MMT usage persists over time. We see that while BMT rates did increase over time, MMT decreased until 2011 and increased thereafter partially supporting hypothesis 1. Hypothesis 2 is supported by the figures which show rates for SAs retaining their relative positions over time. However, the rates of decrease or increase did show some variation both for methadone and buprenorphine. Statistical analyses were conducted to back up these visualization observations.
Fig. 1.
Unadjusted Buprenorphine Rates: 2004–2013.
Fig. 2.
Unadjusted Methadone Rates: 2004–2013.
3.2.2. Statistical analyses
The quadratic regression model for methadone had a significant coefficient for its quadratic term reflecting a decrease in treatment rate followed by an increase. Subsequently, separate linear regression models were fit for 2004–2011 and for 2011–2013, the time point cut suggested by the empirical data. For buprenorphine, one model was run for 2004–2013. Table 2 displays the buprenorphine and MMT slopes estimated by the models. For 2004–2011, each model based MMT slope was significantly different from zero indicating a significant decrease in rates in that period and these slopes differed among social areas. The slope for SA 5 decreased at a greater rate than that of all other slopes. For 2011–2013, SAs 2, 3 and 5 had significantly increasing slopes. Pairwise contrasts indicated that the rate of increase in SA 5 significantly exceeded that of all other SAs with SA 3 showing a faster rate of increase than rates in SAs 1, 2 and 4. The quadratic regression model for buprenorphine did not have a significant quadratic term. Thus analyses are based on a single linear regression model. All rates of change were increasing and significantly different from zero. SAs 3 and 4 had the lowest rates of change which were significantly lower than that of social areas 1, 2, and 5. In addition the BMT treatment rate of change of SA 5 was significantly greater than that of SA 2.
Table 2.
Buprenorphine and methadone maintenance treatment slopes.
| Social Area (SA) | Buprenorphine 2004–2013 | Methadone 2004–2011 | 2011–2013 | |||
|---|---|---|---|---|---|---|
| Slope | Sig. | Slope | Sig | Slope | Sig. | |
| 1 (L, L, L) | 1.51 | * | −0.45 | * | 0.73 | * |
| 2 (M,L,L) | 1.28a | * | −1.00c | * | 2.22 | * |
| 3 (M,H,L) | 0.77b | * | −0.94c | * | 4.98e | * |
| 4 (M,L,H) | 0.75b | * | −0.90 | * | 1.81 | |
| 5.(H,M,H) | 1.62 | * | −2.60d | * | 8.33f | * |
Pairwise contrast significant using Bonferroni adjustment to account for 10 comparisons, p < 0.005.
2 < 5.
(3,4) < (1,2,5).
(2,3) < 1.
5 < (1,2,3,4).
3 > (1,2,4).
5 > (1,2,3,4).
Slope significant using Bonferroni adjustment to account for 5 tests, p < 0.01.
Table 3 displays the mean usage rates by social areas contrasting years 2004 and 2013. MMT rates within each social area did not statistically differ between these two years. BMT rates by 2013 ranged from 6.1 to 13.5 per 10,000. In 2013, the lowest BMT treatment rates were in SAs 3 and 4, the moderate income areas with sizable black and Hispanic populations. The highest BMT rates were seen in both the lowest and highest income areas.
Table 3.
Least Square Meana annual buprenorphine and methadone.maintenance treatment use rates per 10,000 by social area for 2004 and 2013.
| Social Area (SA) | 2004 Buprenorphine LSM (SE) |
2004 vs 2013 Sig. Contrast* |
2013 Buprenorphine LSM (SE) |
2004 Methadone LSM (SE) |
2004 vs 2013 Sig. Contrasta |
2013 Methadone LSM (SE) |
|---|---|---|---|---|---|---|
| 1 (L,L,L) | 0.92(1.0) | * | 13.5(1.0) | 17.7(4.2) | 15.9(4.2) | |
| 2 (M,L,L) | 0.46(0.9) | * | 11.1(0.9) | 33.1(3.8) | 30.3(3.8) | |
| 3 (M,H,L) | 0.00(1.1) | * | 6.1(1.1)b | 41.5(4.9)d | 43.3(4.9)d | |
| 4 (M,L,H) | 0.09(1.6) | * | 6.3(1.6)c | 25.6(6.8) | 22.6(6.8) | |
| 5.(H,M,H) | 0.49(1.4) | * | 13.1(1.4) | 112.1(6.1)e | 109.2(6.2)e |
Significance of pair wise comparisons between SAs using Bonferroni adjustment to account for 10 comparisons within each year, (p < 0.005).
Least square means (LSM) = raw means and standard errors (SE) from model fit.
3 < (1,2,5).
4 < (1,5).
3 > 1.
5 > (1,2,3,4).
Comparison of LSMs of 2004 vs. 2013 significant using Bonferroni adjustment to account for 5 comparisons, (p < 0.01).
4. Discussion
Over time, there was a steady growth in buprenorphine treatment rates in all New York City social areas (SAs) studied. This is consistent with national studies showing that the number of buprenorphine prescriptions per capita has increased overall (Stein et al., 2015a,b). Methadone rates modestly declined until 2011, but by 2013 had returned to 2004 levels. In 2013, in the high income, predominately white SA 1, the buprenorphine treatment rate was high in contrast to a low methadone treatment rate. In SA 5, the poorest social area with the highest percentage of Hispanic residents, the BMT treatment rate was also high, but so was the MMT treatment rate. Predominantly white SA 2 of moderate income also had relatively high rates of buprenorphine treatment, and a relatively high methadone treatment rates.
The increase in buprenorphine treatment in SAs 1 and 2, could reflect the rapid uptake by private physicians prescribing this treatment in predominately white well off areas. The increase in buprenorphine treatment in the poorest area (SA5) might well indicate the uptake of buprenorphine by public health clinicians. The fact that MMT rates were maintained in all SAs indicates that methadone is still preferred by physicians for some and continues to be used in well-established treatment programs. By 2013, social areas 3 and 4 with moderate incomes and with predominately black and predominately Hispanic residents respectively had the lowest rates of buprenorphine treatment, and had over time, slow rates of growth in its usage, and relatively low rates of methadone treatment. Our data do not allow us to determine if this was due to lower demand for treatment of opioid dependence in those areas, to lower levels of physician adoption of these treatments, to lower levels of health insurance coverage, or to other unidentified factors disproportionately affecting those areas.
One exception to the pattern of disparate geographic dissemination of buprenorphine is the high treatment rate in social area 5, made up of low-income, primarily Hispanic/Latino neighborhoods concentrated in the South Bronx. This exception may be explained by the early, active efforts of South Bronx addiction treatment advocates, including those affiliated with Montefiore Hospital and Einstein School of Medicine, to integrate buprenorphine into both primary care and outpatient substance abuse clinics that served primarily low-income and Medicaid insured patients (personal communication with Ernest Drucker, PhD, April 2, 2011). This network of providers, many of whom were oriented to HIV prevention and public health outreach, were particularly receptive to using buprenorphine, (Cunningham et al., 2006, 2008) and beginning in the early 2000s attempted to integrate it in diverse clinical sites, including methadone clinics (Whitley et al., 2007). The impact of their efforts demonstrates the value of promoting buprenorphine accessibility among public sector providers.
Overall, however, buprenorphine treatment access is still unevenly distributed across residential social areas of New York City a decade after its introduction as a novel opioid treatment technology. Methadone treatment initially decreased after buprenorphine’s approval but has recently shown modest increases back to its 2004 levels, indicating that it is still a widely used treatment. While buprenorphine prescriptions are increasing in all social areas in New York City, the rate of increase is uneven but there is promise of substantial uptake in the poorest areas.
The fact that two of the three social areas with the fastest rate of increase in buprenorphine treatment were the highest income areas with the highest percentage of white residents − SAs 1 and 2 − is consistent with theories of fundamental causes of disease and of stratified diffusion of new technologies. In the case of SA 5, the low income predominantly Hispanic social area concentrated in the Bronx, targeted investments in buprenorphine provision by a safety net healthcare network dedicated to HIV prevention appear to have counteracted this trend. In SAs 3 and 4, however, black and Hispanic populations that are already at higher risk of opiate related morbidity may be left without buprenorphine as a treatment option, and thereby be at even higher relative risk. BMT is covered by Medicaid in New York, therefore disparate access to buprenorphine is likely due to the shortage of public sector buprenorphine prescribers rather than to a lack of prescription coverage (Ducharme and Abraham, 2008). Shortages of prescribers may be due to the pressures of large patient caseloads assigned to public sector physicians, the lack of incentives to get trained and certified in buprenorphine prescription among salaried public sector physicians, and physician discomfort with pharmaceutical management of opioid maintenance patients in conditions where they lack time and mentoring (Hutchinson et al., 2014; Thomas et al., 2008; Turner et al., 2005; Walley et al., 2008).
The increase in disparities in buprenorphine prescriptions by social area after the Federal regulatory changes of 2007 that raised the limit of buprenorphine patients per prescriber indicates that factors other than patient limits may determine disparities. One limiting factor may be Medicaid accepting prescribers; our 2012 comparison of the register of buprenorphine-certified providers produced by the US Substance Abuse and Mental Health Services Administration (SAMHSA), with the New York City Medicaid provider network, showed that less than 10% of certified buprenorphine providers in New York City accept Medicaid (Hansen et al., 2013).
The finding that MMT rates are relatively constant in the same time period that BMT rates are increasing may suggest that buprenorphine attracts new patients into treatment that were not previously on MMT. These buprenorphine patients come from more affluent areas of New York City that have the highest rates of prescription opioid misuse. For example, while prescription opioid overdose rates increased 65% across all boroughs between 2005 and 2011, in Staten Island – an affluent, suburban borough with the highest percentage of White residents in New York City – increased 261% in the same time period (personal communication with Marc N. Gourevitch, MD, March 11, 2009). Not displayed, but in our data set, Staten Island had the largest increase in buprenorphine prescriptions between 2004 and 2013 and had the highest buprenorphine prescription rates in New York City. In our interviews with community physicians in Staten Island, we learned that many of them sought buprenorphine certification in order to address a local crisis of opioid overdose, among patients who had not previously received substance abuse treatment (Mendoza et al. (2016) Mendoza et al., in press). This indicates that in higher income neighborhoods, private practice physicians have taken the initiative to make buprenorphine more widely available to patients who are not otherwise in treatment.
One factor that may influence differences in treatment rates by SAs is the geographic distribution of buprenorphine prescribers and methadone clinics in New York City. Local availability may influence the likelihood that opioid dependent patients will receive a specific treatment. Our interviews with Staten Island and Bronx based prescribers and administrators (cited above) do indicate that local initiatives to increase treatment availability have an impact on local treatment utilization. In addition it is notable that Staten Island of SA 1, which has high rates of BMT and low rates of MMT utilization, has fewer local MMT programs than other SAs. However the addresses of buprenorphine prescribers and methadone clinics cannot in themselves settle this question since patients in New York City have low cost, accessible public transportation to freely cross boundaries between social areas and boroughs, frequently see providers outside of their neighborhoods, and public facilities in New York City do not have official catchment areas. In addition, the SAMHSA roster of buprenorphine certified physicians is incomplete with outdated addresses, and in using it to locate prescribers for interviews, we found that many prescribers had multiple offices in different boroughs but reported only one address to SAMHSA. Therefore, an accurate geographic mapping of buprenorphine prescribers would require original data beyond available administrative sources.
This study has other limitations. One cannot infer without some error the demographic background of individual opioid maintenance patients from the characteristics of their neighborhoods due to the risk of ecological fallacy. Second, unmeasured neighborhood characteristics that may influence MMT and BMT rates, such as insurance coverage rates or the prevalence of opioid dependence, may be unevenly geographically distributed and therefore confound the relationships between treatment rates and the demographics of social areas. Third, differences between the units of measurement by which BMT and MMT rates are reported precludes their direct comparison. Accordingly, we compared only geographic patterns of these treatments over time. Finally, the patterns of treatment that this study identified in New York City may not be generalizable to other U.S. cities.
Several interventions could be considered in light of these findings. Institutional incentives for public clinics to train and certify physicians in buprenorphine management could enhance public sector access to buprenorphine. For example, the New York City municipal hospital with the largest number of buprenorphine outpatients dramatically expanded its BMT capacity when it received grant funding for primary care-based buprenorphine and funds to offer buprenorphine free of charge to uninsured patients (personal communication with Marc N. Gourevitch, MD, March 11, 2009). Other studies report that public clinic participation in clinical trials is a predictor of adoption of buprenorphine (Roman et al., 2010), suggesting that outside resources (such as training and clinical support from research staff) foster dissemination of new technologies for underserved populations.
This analysis points to the need for more research on uptake of medically assisted treatment for opioid dependence among low-income and ethnic minority communities that demonstrate moderate increases in utilization of the more recently approved buprenorphine, but at much lower rates than affluent predominantly White communities, suggesting continued barriers to care. The study indicated that recent methadone treatment rates are at the same levels as those in the beginning of the study period, while buprenorphine treatment has increased significantly in all social areas. Income, racial and ethnic composition of neighborhoods remain strongly correlated with buprenorphine and methadone usage over time, and geographic disparities in BMT relative to MMT, by social areas with differing income levels and ethnic/racial makeup, have persisted over time. In order to appropriately tailor policy and practice interventions to enhance the equity of treatment for opioid dependence and the access of communities strongly affected by opioid dependence to treatment, further research is needed to determine what distinguishes new patients being treated with office based BMT who would not otherwise have sought MMT, and which factors determine the slower uptake of buprenorphine in low-income, predominantly ethnic minority communities, including the availability of prescribers, insurance coverage, and the quality and acceptability of buprenorphine treatment as opposed to methadone treatment in those communities. Our findings suggest that disparities in treatment for opioid dependence will persist without targeted interventions and investments informed by such research.
Acknowledgments
We gratefully acknowledge the assistance of Dawn Lambert-Wacey of the New York State Office of Alcoholism and Substance Abuse for her help accessing the methadone usage data for this study, as well as the New York State Bureau of Narcotics for assistance in accessing buprenorphine usage data. Thanks also to the thoughtful feedback of three anonymous reviewers, and to Sonia Mendoza and Jennifer Hernandez for copy editing. This study was supported by NIH award DA032674-05 (to Hansen), the Robert Wood Johnson Foundation Health Policy Investigator Award (to Hansen), the New York University Department of Psychiatry statistical analysis unit, and the New York State Office of Mental Health Center of Excellence in Culturally Competent Mental Health.
Role of funding source
This work was supported by NIHDA032674 (to the first author), and by the Robert Wood Johnson Health Policy Investigator Award Program (to the first author). Additional support was granted by the Nathan Kline Institute’s Center of Excellence in Culturally Competent Mental Health, funded by the New York State Office of Mental Health.
Footnotes
Contributors
Helena Hansen conceived the paper idea and led the writing of the paper. Carole Siegel designed the quantitative approach and led the statistical analysis. Joseph Wanderling and Danae DiRocco analyzed and interpreted data as well as assisted with writing and revising the paper.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Adams PR, Martinez ME, Vickerie JL. Summary health statistics for the U.S. population: national health interview survey 2009. Vital Health Stat. 2009;10:1–115. [PubMed] [Google Scholar]
- Araujo EM, Costa Mda C, Oliveira NF, et al. Spatial distribution of mortality by homicide and social inequalities according to race/skin color in an intra-urban Brazilian space. Rev. Bras. Epidemiol. 2010;13:549–560. doi: 10.1590/s1415-790x2010000400001. [DOI] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Hansen H, O’Connor PG, Schottenfeld RS, Fiellin DA. Integrating buprenorphine treatment into office-based practice: a qualitative study. J. Gen. Intern. Med. 2009;24:218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquier A, Cortaredona S, Nauleau S, Jardin M, Verger P. Prevalence of treated diabetes: geographical variations at the small-area level and their association with area-level characteristics. A multilevel analysis in Southeastern France. Diabetes Metab. 2011;37:39–46. doi: 10.1016/j.diabet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) CDC Fact Sheet: HIV among African Americans. [accessed 03. 30. 15];2015a Available at: http://www.cdc.gov/nchhstp/newsroom/docs/CDC-HIV-AA-508.pdf.
- Centers for Disease Control (CDC) CDC Fact Sheet: HIV among Latinos. [accessed 03. 30. 15];2015b Available at http://www.cdc.gov/nchhstp/newsroom/docs/CDC-HIV-Latinos-508.pdf.
- Centers for Disease Control (CDC) Health Disparities in HIV/AIDS, Viral Hepatitis, STDs, and TB. [accessed 03. 30. 15];2015c Available at http://www.cdc.gov/nchhstp/healthdisparities/AfricanAmericans.html.
- Centers for Disease Control and Prevention (CDC) Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 17. 2012
- Centers for Disease Control and Prevention (CDC) Prescription Drug Overdose in the United States: Fact Sheet. [accessed 10. 17. 14];2014 Available at: http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html.
- Chau KL. Ecological analysis of health care utilization for China’s rural population: association with a rural county’s socioeconomic characteristics. BMC Public Health. 2010;10:664. doi: 10.1186/1471-2458-10-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CO, Sohler NL, McCoy K, Kunins HV. Attending physicians’ and residents’ attitudes and beliefs about prescribing buprenorphine at an urban teaching hospital. Fam. Med. 2006;38:336–340. [PubMed] [Google Scholar]
- Cunningham C, Giovanniello A, Sacajiu G, Whitley S, Mund P, Beil R, Sohler N. Buprenorphine treatment in an urban community health center: what to expect. Fam. Med. 2008;40:500–506. [PMC free article] [PubMed] [Google Scholar]
- Das-Munshi J, Becares L, Dewey ME, Stansfeld SA, Prince MJ. Understanding the effect of ethnic density on mental health: multi-level investigation of survey data from England. BMJ. 2010;341:c5367. doi: 10.1136/bmj.c5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Subst. Abuse Treat. Prev. Policy. 2008;3:17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JE, Casadonte P, Gartenmann T, Martin J, MacCance-Katz EF, Netherland J, Renner JA, Weiss L, Saxon AJ, Fiellin DA. The Physician Clinical Support System-Buprenorphine (PCSS-B): a novel project to expand/improve buprenorphine treatment. J. Gen. Intern. Med. 2010;25:936–941. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuelli J, Desenclos JC. Harm reduction interventions, behaviours and associated health outcomes in France, 1996–2003. Addiction. 2005;100:1690–1700. doi: 10.1111/j.1360-0443.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- Frank B. 2000. An overview of heroin trends in New York City: past, present and future. Mt. Sinai J. Med. 2000;67:340–346. [PubMed] [Google Scholar]
- Glied S, Lleras-Muney A. Technological innovation and inequality in health. Demography. 2008;45:741–761. doi: 10.1353/dem.0.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredson DC, McNeil RJ, Gottfredson GD. Social area influences on delinquency: a multilevel analysis. J. Res. Crime Delinq. 1991;28:197–226. [Google Scholar]
- Grubesic TH, Matisziw TC. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int. J. Health Geogr. Dec. 2006;5:58. doi: 10.1186/1476-072X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Roberts S. Two tiers of biomedicalization: buprenorphine: methadone and the biopolitics of addiction stigma and race. Adv. Med. Soc. 2012;14:79–102. [Google Scholar]
- Hansen H, Siegel CE, Case BG, Bertollo DN, DiRocco D, Galanter M. Variation in use of buprenorphine and methadone treatment by racial, ethnic, and income characteristics of residential social areas in New York City. J. Behav. Health Serv. Res. 2013;40:367–377. doi: 10.1007/s11414-013-9341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh D, Little SL, Gleghorn A. Integrating buprenorphine treatment into a public healthcare system: the San Francisco Department of Public Health’s office-based buprenorphine pilot program. J. Psychoactive Drugs. 2011;43:136–145. doi: 10.1080/02791072.2011.587704. [DOI] [PubMed] [Google Scholar]
- Heye C, Leuthold H. Theory-based social area analysis: an approach considering the conditions of a post-industrial society; 14th European Colloquium on Theoretical and Quantitative Geography, 2005; 2005. [accessed 03. 28. 13]. Available at: http://www.sotomo.ch/media/publis/ch_hl_2005_socialarea.pdf. [Google Scholar]
- Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Ann. Fam. Med. 2014;12:128–133. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlifi M, Zun L, Johnson G, Harbison RJ. Etiological characterization of acute poisonings in the emergency department. J. Emerg. Trauma Shock. 2009;2:159–163. doi: 10.4103/0974-2700.50878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Grossman E, Truncali A, Rotrosen J, Rosenblum A, Magura S, Gourevitch MN. Buprenorphine-naloxone maintenance following release from jail. Subst. Abuse. 2012;33:40–47. doi: 10.1080/08897077.2011.620475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Bang D, Cosgrove S, Dulin R, Harris Z, Taylor A, White S, Yatabe G, Liburd L, Giles W Division of adult and community health, National center for chronic disease prevention and health promotion, Centers for disease control and prevention (CDC) Surveillance of Health Status in Minority Communities—racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) Risk Factor Survey United Statesv 2009. MMWR Surveill. Summ. 2011;60:1–44. [PubMed] [Google Scholar]
- Link BG, Phelan J. Social conditions as fundamental causes of disease. J. Health Soc. Behav. 1995;(Spec. No):80–94. [PubMed] [Google Scholar]
- Lovell A. Addiction markets: high dose buprenorphine in France. In: Petryna A, Kleinman A, Lakoff A, editors. Global Pharmaceuticals. Durham: Duke University Press; 2006. [Google Scholar]
- Mack KA. Drug-induced deaths−United States 1999–2010. MMWR Suppl. 2013;62:161–163. [PubMed] [Google Scholar]
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, Rosenblum A. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99:222–230. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2013:CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- McNeely J, Gourevitch MN, Paone D, Shah S, Wright S, Heller D. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi: 10.1186/1471-2458-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza S, Rivera A, Hansen H. Shifting blame: buprenorphine prescribers, addiction treatment, and prescription monitoring in middle-class America. Transcult. Psychiatry. 2016 doi: 10.1177/1363461516660884. in press, ()in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Kelly SM, Gryczynski J, Myers CP, Jaffe JH, O’Grady Ke, Olsen YK, Schwartz RP. African American patients seeking treatment in the public sector: characteristics of buprenorphine vs methadone patients. Drug Alcohol Depend. 2012;122:55–60. doi: 10.1016/j.drugalcdep.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo SG, Rinaldo D. Report I: Availability Without Accessibility? State Medicaid Coverage And Authorization Requirements For Opioid Dependence Medications The Avisa Group. American Society for Addiction Medicine. 2013 http://www.asam.org/docs/default-source/advocacy/aaam implications-for-opioid-addiction-treatment final. [Google Scholar]
- Roman PM, Abraham AJ, Rothrauff TC, Knudsen HK. A longitudinal study of organizational formation innovation adoption, and dissemination activities within the National Drug Abuse Treatment Clinical Trials Network. J. Subst. Abuse Treat. 2010;38(Suppl. 1):S44–S52. doi: 10.1016/j.jsat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S.A.S. Institute, Inc. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- Schwartz RP, Gryczynski J, O’Grady KE, Sharfstein JM, Warren G, Olsen Y, Mitchell SG, Jaffe JH. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am. J. Public Health. 2013;103:917–922. doi: 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Samuel A. Social area analysis in community medicine. Br. J. Prev. Soc. Med. 1977;31:199–204. doi: 10.1136/jech.31.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevky E, Bell W. Social Area Analysis: Theory, Illustrative Application And Computational Procedures. Stanford: Stanford University Press; 1995. [Google Scholar]
- Siegler A, Tuazon E, Bradley O’Brien D, Paone D. Unintentional opioid overdose deaths in New York City, 2005–2010: a place-based approach to reduce risk. Int. J. Drug Policy. 2014;25:569–574. doi: 10.1016/j.drugpo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Smith MP, Olatunde O, White C. Inequalities in disability-free life expectancy by area deprivation: England 2001–04 and 2005–08. Health Stat. Q. 2010;48:36–57. doi: 10.1057/hsq.2010.20. [DOI] [PubMed] [Google Scholar]
- Stancliff S, Joseph H, Fong C, Furst T, Comer SD, Roux P. Opioid maintenance treatment as harm reduction tool for opioid-dependent individuals in New York City: the need to expand access to buprenorphine/naloxone in marginalized populations. J. Addict. Dis. 2012;31:278–287. doi: 10.1080/10550887.2012.694603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton A, McLeod C, Luckey B, et al. Substance Abuse and Mental Health Services Administration. Rockville: 2006. [Accessed 07. 05. 13]. SAMHSA/CSAT Evaluation Of The Buprenorphine Waiver Program: Expanding Treatment Of Opioid Dependence: Initial Physician And Patient Experiences With The Adoption Of Buprenorphine. Available at: http://buprenorphine.samhsa.gov/ASAM_06_Final_Results.pdf. [Google Scholar]
- Stein BD, Pacula RL, Gordon A, Burns R, Leslie D, Sorbero M, Bauhoff S, Mandell T, Dick A. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 2015a;93:561–583. doi: 10.1111/1468-0009.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Gordon AJ, Dick AW, burns RM, Pacula RL, Farmer CM, Leslie DL, Sorbero M. Supply of buprenorphine waivered physicians: the influence of state policies. J. Subst. Abuse Treat. 2015b;48:104–111. doi: 10.1016/j.jsat.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranifar P, Neugut AI, Phelan JC, Link BG, Liao Y, Desai M, Terry MB. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol. Biomarkers Prev. 2009;18:2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Reif S, Haq S, Wallack SS, Hoyt A, Ritter GA. Use of buprenorphine for addiction treatment: perspectives of addiction specialists and general psychiatrists. Psychiatr. Serv. 2008;59:909–916. doi: 10.1176/ps.2008.59.8.909. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Lin T, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch. Intern. Med. 2005;165:1769–1776. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- Wakhlu S. Buprenorphine: a review. J. Opioid Manag. 2009;5:59–64. doi: 10.5055/jom.2009.0007. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J. Gen. Intern. Med. 2008;23:1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley SD, Kunins HV, Arnsten JH, Gourevitch MN. Colocating buprenorphine with methadone maintenance and outpatient chemical dependency services. J. Subst. Abuse Treat. 2007;33:85–90. doi: 10.1016/j.jsat.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Zee V. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am. J. Public Health. 2009;99:221–227. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]