Abstract
The noncoding RNA Xist has been shown to be essential for X-chromosome inactivation and to coat the inactive X-chromosome (Xi). Thus, an important question in understanding the formation of Xi is whether the binding reaction of Xist is necessary for X-chromosome inactivation. In this article, we demonstrate the failure of X-chromosome silencing if the association of Xist with the X-chromosome is inhibited. The chromatin-binding region was functionally mapped and evaluated by using an approach for studying noncoding RNA function in living cells that we call peptide nucleic acid (PNA) interference mapping. In the reported experiments, a single 19-bp antisense cell-permeating PNA targeted against a particular region of Xist RNA caused the disruption of the Xi. The association of the Xi with macro-histone H2A is also disturbed by PNA interference mapping.
X-chromosome inactivation is an early developmental process occurring in female mammals to compensate for differences between male and female mammals in dosage of genes residing on the X-chromosome (1, 2, 4). In mammals, dosage compensation is achieved by the transcriptional silencing of genes on one of the two X-chromosomes in females (5). The inactivated X-chromosome (Xi) can be microscopically observed during interphase as a condensed body at the nuclear periphery (6). Moreover, the Xi has been shown to form a nuclear structure termed the macrochromatin body (MCB) known to be enriched for the variant histone, macrohistone H2A (7–10).
On the basis of the study of chromosomal translocations, an interval called the X inactivation center (XIC) of the X chromosome has been identified to control the process of inactivation (11, 12). The XIC has been shown to be a complex transcription unit consisting of at least two genes, Xist and Tsix, which are transcribed off the opposite strands of the same DNA duplex (3, 13–18). Although the function of Xist is not known, deletion of the gene leads to failure of X-inactivation, and female knockout mice die near gastrulation (19, 20). In embryonic stem (ES) cells, the Xist transcript supplied in an inducible manner by a transgene has been shown to be necessary and sufficient to cause silencing of genes in cis to the transgene (21).
The gene Xist is expressed exclusively from the Xi and shows several interesting features. First, both human and mouse XIST/Xist cDNAs are unusually long, 19.3 and 17.8 kb, respectively (22, 23). Second, the transcript does not seem to encode a protein (3, 16). Third, the Xist RNA physically associates with, or “coats,” the Xi (3). The silencing was always associated with coating of the chromosome. Two basic questions about Xist are: (i) How is the coating/binding process related to the structure of the Xist RNA; and (ii) is the binding reaction of Xist to Xi necessary for Xist function?
We have developed a technology that we term Peptide Nucleic Acid (PNA)-Interference Mapping (P-IMP) to define in living cells how specific regions of the Xist transcript contribute to X-inactivation. These experiments take advantage of the unique properties of PNAs. PNAs are nucleic acid mimics, which contain a pseudopeptide backbone, composed of charge neutral and achiral N-(2-aminoethyl) glycine units to which the nucleobases are attached via a methylene carbonyl linker (24–26). PNAs hybridize with high affinity to complementary RNA sequences forming PNA–RNA complexes via Watson–Crick or Hoogsteen binding (26). PNAs are not readily degraded and do not participate in or activate repair or degradative pathways for DNA or RNA, ensuring a very selective range of activity. In addition to the high thermal stability of complexes, PNA–RNA binding is highly sensitive to mismatches (27–30). The PNAs used for P-IMP were conjugated to transportan via a cleavable disulfide linkage. Transportan is a 27-aa chimeric peptide consisting of the N-terminal fragment of the neuropeptide galanin and the membrane-interacting wasp venom peptide mastoparan. The conjugation of PNA to transportan results in rapid energy-independent nonsaturable transport of the PNA–transportan conjugate across the plasma membrane (31–33).
Using P-IMP, we found that a group of PNAs complementary to a distinct repeat region in the first exon of Xist completely abolished binding of Xist to the X-chromosome and, in so doing, prevented formation of Xi.
Experimental Procedures
RNA folding experiments were performed by using mfold software, available at http://mfold2.wustl.edu/∼mfold/rna/form1.cgi. Many probable structures were generated by submission of sequence derived from the C region.
For PNA conjugate treatment, we plated 104 tetraploid fibroblast cells per well in eight-well Lab-Tek slides (Nalge) 12 h before treatment to allow for cell attachment. PNA conjugates were dissolved in water at 50 μM concentration. The PNA stock was diluted with DMEM with 10% vol/vol Cosmic Calf Serum (HyClone) to a concentration 1 μM. PNA conjugate and medium mixture (0.2 ml) was then added to the cells. Afterward, PNA conjugate treatment slides were cooled on ice and processed for RNA fluorescence in situ hybridization (FISH).
Diploid female ES cell (mWS244.6) culture and differentiation were accomplished by standard method (21). Cells (5 × 104 per well) were treated for 6 days with 1 μM PNA in the presence of 10−7 M retinoic acid. To maintain high PNA concentration, medium was changed every 12 h. PNAs used are indicated below and in the text.
To perform the TaqMan assay, total RNA was isolated by using Tri-Reagent (Sigma) according to the manufacturer's instructions. Total RNA was treated with 1 unit of RQ-DNase (Promega) per 10 μg of RNA. TaqMan quantitative PCR analysis was performed by using the EZ-RT-PCR Core Reagent from Applied Biosystems. Four hundred nanograms of total RNA was used for analysis. A standard curve was obtained by using serial dilutions of the known concentrations of pWS889, the plasmid containing the 3′ end region of Xist. Reverse-transcription reactions and quantification were done by using the ABI 7700 Sequence Analyzer (Applied Biosystems). For Xist, two different assays were performed at the 5′ and 3′ regions of the transcript; only data for the 3′ assay are presented (see Fig. 3, which is published as supplemental data on the PNAS web site, www.pnas.org), as both of the assays yielded substantially identical results.
Tsix
pWS1049: (GCCAAGGTGTAAGTAGACTAGCCACT) F. primer
pWS1048: (CGTGGCGGTGCAAACTAAA) R. primer
pWS1304: (6FAM-CTCAGCCCGTTCCATTCCTTTGTATTGTT-TAMRA) TaqMan probe
mouse Idh1
pWS1394: (ACCGCATGTACCAGAAAGGG) F. primer
pWS1395: (CTCGGGACCAGGCAAAAAT) R. primer
pWS1396: (6FAM-AGAGACGTCCACCAACCCCATTGCTT-TAMRA) TaqMan probe
mouse Dnmt3b
pWS1397: (CAGGTCTCGGAGACGTCGAG) F. primer
pWS1398: (CTTCCATGAAGTCGACGCTG) R. primer
pWS1399: (6FAM-TCGTCTTCAGCAAGCACGCCATG-TAMRA) TaqMan probe
mouse Hmg2
pWS1400: (GGGCAAAATGTCCTCGTACG) F. primer
pWS1401: (CGAGTCGGGATGCTTCTTCT) R. primer
pWS 1402: (6FAM-CAGACCTGCCGCGAGGAGCAC-TAMRA) TaqMan probe
5′ Xist assay
pWS1048: (CGTGGCGGTGCAAACTAAA) F. primer
pWS1049: (GCCAAGGTGTAAGTAGACTAGCCACT) R. primer
pWS1304: (6FAM-CTCAGCCCGTTCCATTCCTTTGTATTGTT-TAMRA) TaqMan probe
3′ Xist assay
pWS483: (AACAGTTAGGTCCCGGCTTT) F.primer
pWS831: (CTTTGCTTTTATCCCAGGCA) R. primers
pWS869: (6FAM-TCTGTGTGGAGCTTTGTGAAG-TAMRA) TaqMan probe
RNA-FISH was done according to refs. 22 and 23. RNA-FISH probes: for Xist, full length cDNA = pWS1081, and for β-actin, a murine BAC from Genome Systems (St. Louis) (assignment no. 324C19). Histone macroH2A1 immunofluorescent labeling was done according to Costanzi and Pehrson (9).
For actinomycin D treatment, we plated 104 cells per well in eight-well Lab-Tek slides (Nalge) 12 h before treatment to allow for cell attachment. Actinomycin D stock was diluted with DMEM with 10% vol/vol Cosmic Calf Serum (HyClone) to a final concentration of either 2.5 or 5 μg/ml. Two hundred microliters of actinomycin D solution was applied to each well. After treatment, slides were cooled on ice and processed for RNA-FISH.
Results
Experimental System.
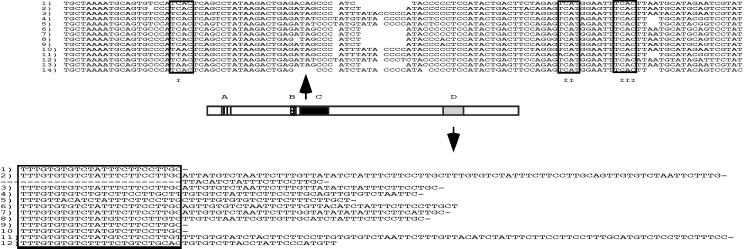
We decided to test whether the structure of Xi could be disrupted in living cells by the administration of sequence-specific PNAs against the Xist transcript. PNA–transportan conjugates were selected on the basis of a careful survey of the sequence composition of the Xist transcript. Analysis of the cDNA encoding Xist shows four repeated regions [Fig. 1 (3)]. This analysis reveals the striking repetitive structure of four distinct regions, termed A, B, C, and D. Further analysis of the C region revealed that it consists of 14 direct repeats of ≈110–120 bases. These repeats contain several conserved regions, in three locations (termed I, II, and III), in which the motif UCAY was observed. The sequence GAGUCAU observed in region II was very conserved from repeat to repeat, with the UCAU being invariant. Similarly, the sequence GAAUUUCACUU in region III was almost invariant throughout the C region.
Figure 1.
Description of murine Xist repetitive regions used in this study. Schematic line drawing of Xist. Repetitive sequence regions are colored and labeled with letters A, B, C, and D (3). The majority of Xist transcripts (>90%) do not contain the A region, and thus it was not investigated. Regions C and D are approximately the same size, and antisense oligomers for the C region are directed at the region labeled II and for the D region against the boxed motif.
These motifs have been observed in other systems where they are involved in RNA–protein interactions (34–36). Several lines of evidence, including x-ray crystallographic analysis, show that the NOVA2/NOVA1-binding site is UCAY, where Y stands for a pyrimidine. Furthermore, the sequence GAGUCAU was shown to be an optimal binding site for the NOVA2 protein by in vitro selection (SELEX) experiments (34–36).
We performed RNA folding experiments with sequences from the C-repeat region, by using the mfold software (see Experimental Procedures). The modeling experiments revealed that sequences from the C region consistently formed ordered structures, which we call RNA fingers. These RNA fingers have consensus hairpin structures seen in other systems (35).
These observations provided the rational basis for our design of targeting PNA conjugates (Table 1). Antisense PNAs specific for the C region were designed to span region II containing one of the UCAY sites in the RNA fingers. PNA conjugates were also directed at the B and D regions. Also used were the sense version of the Xist C-region-binding antisense PNA, an antisense Xist-binding PNA with three sequence mismatches, a set of three PNA conjugates (used as a mixture, bind at the Xist 3′ end) (22, 37), and a scrambled sequence control.
Table 1.
PNA used in this study
| 5′-sequence | Name | Orientation |
|---|---|---|
| Experimental PNA sequences directed against the C-region | ||
| aaattctatgactctggaa | #pWS1246 | Antisense |
| aaattccatgactctgtaa | #pWS1248 | Antisense |
| aaattccatgactctagaa | #pWS1250 | Antisense |
| Experimental PNA sequences directed against the B-region | ||
| ggggcaggggctggggca | #pWS1458 | Antisense |
| Experimental PNA sequences directed against the D-region | ||
| ggagaaatagacacacaaa | #pWS1380 | Antisense |
| Nonspecific control | ||
| cggactaagtccattgc | #pWS1252 | Scramble sequence |
| PNA control: Sense sequences directed against the C-region | ||
| ttccagagtcatagaattt | #pWS1247 | Sense |
| ttacagagtcatggaattt | #pWS1249 | Sense |
| PNA control: 3-bp mismatch on C region | ||
| aaattccacagctctggaa | #pWS1290 | Antisense |
| PNA control: Directed to the 3′ end (used as a cocktail) | ||
| ctgattgttgtcaattttattattc | #pWS1295 | Antisense |
| tttatgcaagaatgttaaac | #pWS1296 | Antisense |
| tcaatttggtctttcgttcctccag | #pWS1284 | Antisense |
To determine the effect of PNA on Xist binding to Xi, we treated tetraploid male and female murine C57BL/6 dermal fibroblasts with PNA conjugate under optimal conditions (see Experimental Procedures). We chose tetraploid cells because a measure of the effect of PNA administration would be more credible if it altered both copies of Xi. The cells were then fixed and hybridized to a full length Xist DNA probe to visualize the location of Xist RNA by RNA-FISH (see Experimental Procedures). The cells were then analyzed under an epifluorescence microscope for the association of Xist with Xi.
PNA Conjugates Do Not Alter Steady-State Levels of Xist RNA.
The first concern in this project was whether antisense PNA-conjugate administration would have an effect on the steady-state levels of RNA. Thus, we first designed experiments to determine the effect of C-region antisense PNA on Xist steady-state RNA levels. Total RNA was isolated from fibroblasts treated with PNA-conjugate for a variety of times. Quantitative reverse transcription–PCR (RT-PCR) was performed by using the TaqMan system (see Experimental Procedures and Fig. 3A). No difference in Xist RNA steady-states levels was observed after PNA treatment.
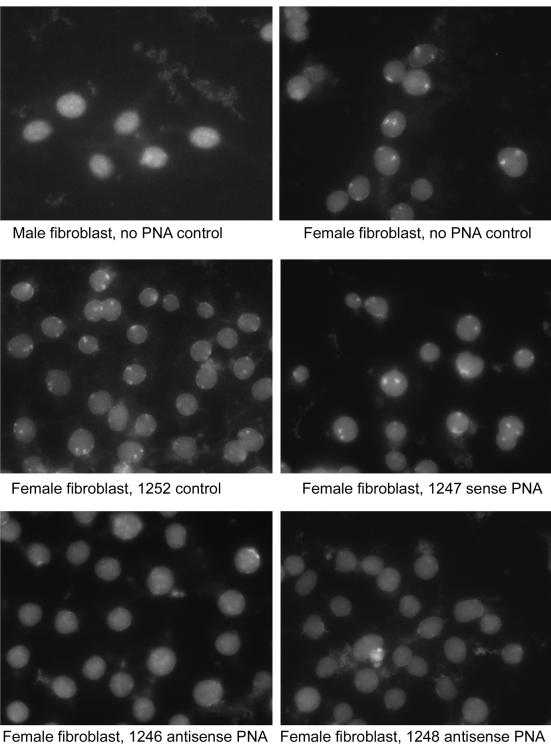
Specific PNA Conjugates Cause Loss of the Xist Body.
As steady-state levels of Xist did not change after PNA treatment, we wished to determine whether other aspects of Xist metabolism might have been altered by treatment. Therefore, RNA-FISH experiments on murine fibroblast cells were performed to identify the presence of the macromolecular complex called the Xist body, which has been shown to be congruent with Xi. PNA conjugates directed against the B, C, and D regions were compared. The results of these experiments were tabulated to yield the percentage of cells with Xist bodies after different PNA-conjugate treatments (Table 2). Representative microscopic fields are presented in Fig. 2. Cells were treated with the PNA conjugates, as indicated in Fig. 2, Table 2, and notes therein. The results of this experiment showed a clear effect of antisense PNA-conjugates directed to the C region of the Xist RNA. PNAs directed against the C region caused the loss of localized Xist RNA and the disappearance of the Xist body. Antisense PNAs against the B and D regions showed no effect. PNA conjugates, that also had no effect on Xist binding, were: C region sense PNA conjugates, scramble sequence PNA conjugate, mismatch C-region antisense PNA conjugate, and a mixture of antisense PNA conjugates directed to the 3′end of Xist.
Table 2.
Numerical results of RNA-FISH experiments depicted in Fig. 2
| PNA name | PNA description | No. cells with Xist body | No. cells without Xist body | Total no. cells | Percent cells with Xist body |
|---|---|---|---|---|---|
| pWS1246 | Xist antisense “C” region | 12 | 540 | 552 | 2.1 |
| pWS1248 | Xist antisense “C” region | 44 | 526 | 570 | 7.7 |
| pWS1250 | Xist antisense “C” region | 0 | 505 | 505 | 0 |
| pWS1290 | Xist antisense “C” region with 3bp-mismatch | 468 | 72 | 546 | 86.6 |
| pWS1295/1296/1284 | Xist antisense to 3′ end | 476 | 53 | 529 | 89.9 |
| pWS1249 | Xist sense “C” region | 345 | 167 | 512 | 67.3 |
| pWS1247 | Xist sense “C” region | 427 | 179 | 606 | 70.4 |
| pWS1458 | Xist antisense “B” region | 479 | 59 | 538 | 89.0 |
| pWS1380 | Xist antisense “D” region | 451 | 53 | 504 | 89.5 |
| pWS1252 | Scramble sequence | 429 | 106 | 535 | 80.1 |
| Female cells no PNA | N/A | 501 | 50 | 551 | 90.9 |
| Male cells no PNA | N/A | 0 | 510 | 510 | 0 |
The images presented in Fig. 2 are representative of the response of cells in the experiment. To quantitate the response of cells to PNA-transportan treatment, we counted the cells with Xist bodies. The total number of cells counted and number of cells with and without Xist bodies, as well as the percentages, are presented. Treatment time was 36 h.
Figure 2.
RNA-FISH of fibroblast cells from various PNA treatments. Female and male fibroblastic cells were treated for 36 h as indicated. After treatment, cells were fixed and processed for RNA-FISH .
To evaluate the mechanism of action of PNA on Xist body loss, we decided to compare the effects of PNA treatment to the effects of the RNA polymerase inhibitor actinomycin D. To evaluate the presence of Xist body loss after each treatment, RNA-FISH was performed. The time course of antisense C-region PNA conjugate activity was compared with matched cell cultures treated with actinomycin D. Results were determined by counting percentage of cells with Xist bodies. The results of this experiment (see Fig. 3B) show that PNA conjugates exhibit a pronounced effect after 18 h of action, with complete activity after 24 h. In contrast, actinomycin D showed complete activity (loss of Xist body) in 6 h at 5 μg/ml.
Specific PNA Conjugates Cause Loss of the Histone mH2A Body [Macrochromatin Body (MCB)].
Previously, it has been shown that histone mH2A forms associates with the Xi (9). To establish whether the MCB would disappear after PNA treatment, experiments using the antisense C-region PNA conjugate were performed. PNA conjugate activity was evaluated by Immuno-FISH by using antibodies against the variant histone macrohistone H2A (9). The percentage of cells showing the presence of the macrohistone body was determined as a function of hours of PNA conjugate treatment. The results were plotted with RNA-FISH time-course results. In these experiments, the MCB was seen to disappear after C-region antisense PNA treatment (see Fig. 3B).
Xist Must Bind to the X-Chromosome to Function.
To show that Xist binding is essential for Xist function, diploid female ES cells were differentiated in vitro; during the course of differentiation PNA conjugates were added to the media. After the experimental course, samples were processed for analysis.
The effect of PNA administration on the steady-state levels of Xist and Tsix was measured before or during differentiation. The results of PNA (pWS1248) administration were evaluated by quantitative RT-PCR (TaqMan) for Xist and Tsix. Xist was up-regulated normally in the presence of PNA (see Fig. 3C). Tsix was down-regulated as previously reported (14, 15) (see Fig. 3C). Similarly, PNA administration does not alter expression for genes at autosomal locations. Genes at autosomal locations were evaluated by quantitative RT-PCR. The loci evaluated were Idh1:(Chromosome 1), Dnmt3b:(Chromosome 2), and Hmg2:(Chromosome 8) (see Fig. 3C).
PNA-conjugate treatment does not alter the capacity of female ES cells to differentiate normally, as manifested by the appearance of the Xist body. Table 3 shows the effect of either scramble PNA (pWS1252) or mismatch PNA (pWS1290) on the formation of the Xist body. Treated cultures showed the same number of Xist bodies as untreated cultures. Further, in Table 3, 60% is a measure of the percentage of female ES cells that have differentiated during the experimental time course of 6 days.
Table 3.
Effect of control PNAs on Xist body formation in female ES cells
| PNA | No signal | Xist double dot | Xist body | Number nuclei | Percent Xist bodies |
|---|---|---|---|---|---|
| (−) | 17 | 196 | 318 | 531 | 60 |
| 1290 | 32 | 207 | 359 | 598 | 60 |
| 1252 | 20 | 208 | 307 | 535 | 57 |
We wished to test whether PNA administration by itself could alter the capacity of ES cells to differentiate normally and form Xist bodies. We evaluated scrambled sequence (pWS1252) or mismatched sequence (pWS1290) PNAs for their capacity to prevent Xist body formation during differentiation of female ES cells (see Experimental Procedures). Female ES cells were differentiated and treated with PNA–transportan conjugates for 6 days. The cells were then fixed and assayed for the number of Xist bodies by RNA-FISH.
We evaluated the specific effect of Xist antisense PNA administration on coating/binding of Xist in ES cell culture. Treated and untreated female ES cells at day 6 of differentiation were examined by RNA-FISH. PNA-untreated and differentiated ES cells show the expected Xist body and a single site of expression for Pgk1 and Mecp2 loci. In contrast, PNA-treated and differentiated ES cells do not show a Xist body and exhibit two sites of expression for Pgk1 and loci. When transcription at the murine β-actin locus on Ch 4 was determined by RNA-FISH, no difference between untreated ES cells and ES cells treated with PNA pWS1248 was observed. The RNA-FISH data are tabulated in Tables 3–6. In Tables 3–6, over 500 interphase nuclei from treated and untreated cultures were examined after differentiation and scored for the presence of Xist body and the suppression of transcription from either Pgk1, Mecp2, or β-actin loci.
Table 6.
Effect of PNA administration on autosomal expression measured by RNA-FISH
| β-Actin single dot | β-Actin double dot | Number nuclei | Percent single dot | Percent double dot | |
|---|---|---|---|---|---|
| (−)PNA | 16 | 530 | 546 | 3 | 97 |
| (+)PNA | 20 | 550 | 570 | 3 | 97 |
PNA conjugate-treated cells were evaluated for alterations in β-actin expression. Actin mRNA expression was evaluated for single or double sites of expression and then tabulated.
Discussion
Here we report the first functional demonstration that the large nontranslated RNA, Xist, can be organized into functional domains. These studies also confirm that the function of Xist is mediated through its binding to X-chromosome. It is also apparent from the kinetic studies in this report that macro histone H2A and Xist are intimately associated in a macromolecular complex on Xi.
The process of Xist-mediated gene repression observed in X-chromosome inactivation is thought to be a special case of a general developmental pathway for chromatin reorganization directed at transcriptional repression (38, 39). As the nontranslated RNA Xist has been shown to be necessary and sufficient to initiate the silencing pathway (21), it is of great interest to discern how the nontranslated RNA Xist interacts with the X-chromosome to initiate silencing. One of the most striking qualities of the Xist transcript is its localization to the Xi. To make the connection between the structure of Xist, its binding to the X-chromosome, and silencing of gene expression, a functional analysis of the Xist transcript is vital. Here we report evidence for the existence of a functional domain in Xist responsible for Xist coating/binding to Xi. We argue that this functional domain appears to be encoded in the repetitive sequence in the large first exon (C region, Fig. 1).
The data presented here definitively demonstrate that C-region antisense PNA-conjugates can alter Xist coating/binding. PNA conjugates directed to other regions of Xist do not alter its localization. Perfectly matched PNAs against the C region can bind a maximum of seven times; if one or two mismatches are allowed, they can bind a maximum of 14 times. D-region PNA can bind to six perfectly matched target sites, and if mismatches are permitted to at least eight sites. Thus, the amounts of PNA bound to the C and D regions are similar. Nonetheless, antisense PNAs directed against the Xist B and D regions, and 3′ end have no effect on Xist coating. In addition, the pWS1290 PNA conjugate, which represents a 3-bp mismatch of the active antisense PNA (pWS1248) PNA conjugate, has no effect on coating/binding.
Time-course results show striking differences between the kinetics of PNA action and actinomycin D inhibition on Xist body loss. In our experiments, complete loss of Xist bodies was observed 6 h after actinomycin D treatment, whereas for PNA treatment, this period was 18–24 h. In the context of no change in Xist RNA expression (see Fig. 3), PNA treatment is governed by a slow kinetic path, which remains to be defined. We postulate potential steric factors that might contribute to the inaccessibility of Xist to PNA action.
Under conditions where the intracellular levels of steady-state Xist RNA do not change (see Fig. 3), the slow step in Xist body loss is comparable to the kinetics of MCB loss. The variant histone mH2A has been shown to be associated with the Xi during development (8, 9). Previous attempts at describing the association between the Xist body and the MCB required the recombinational loss of the Xist gene and subsequent loss of Xist expression to measure the association between Xist body and the MCB (7). The current kinetic study does not suffer this limitation, as PNA uptake is essentially instantaneous, with high concentrations of PNA available in the nucleus in minutes; further, the rate of change for Xist and the MCB can be monitored from the inception of PNA administration. The data presented here would suggest that the binding of Xist and the MCB to the Xi are equally destabilized by antisense PNA against the C region. This simultaneous destabilization suggests concerted breakdown of a macromolecular structure by PNA action. The kinetics of dissociation for Xist and macrohistone H2A imply that the MCB and Xist are in intimate association in the Xi. The loss of the MCB relative to Xist is the predicted result if one were to suppose a hierarchical structure where Xist represents a foundation on which proteins like mH2A might associate with Xi.
Two lines of evidence support the conclusion that PNA activity is caused by the interference with a particular RNA structure. The theoretical folding of the Xist RNA by mfold algorithm predicts highly stable hairpins. Thus PNA activity against these structures is based on the specificity, not the accessibility, of PNA to Xist RNA. Secondly, unique kinetics of MCB disruption by PNA interference is different from actinomycin D time-course, implying that dissociation of Xist and the MCB is coupled with, and not attributable to, an alteration in Xist metabolism per se.
Coating/binding of Xist is necessary for X-inactivation. During female development, a number of changes occur within the XIC. On Xi, Xist expression is up-regulated by an unknown mechanism, and Tsix expression is silenced. On Xa, expression of both Xist and Tsix is suppressed. Formally, it has not been proven which of these steps is required for the biochemical process of silencing. Using antisense PNA conjugates that can bind only to the Xist transcript, we demonstrate that, despite the ordered progression of developmental stages in differentiating female ES cells, if up-regulated Xist cannot bind to the Xi, there is no chromosomal silencing. Thus, the described experiments distinguish between the expression of Xist and the correct localization of the transcript with respect to function.
One of the major limitations for analysis of the nuclear compartment and Xist function within it has been the lack of a functional technology that would specifically connect DNA sequence-based knowledge to the biochemistry of the nucleus. One of the goals of the present work was to make the connection between Xist sequence information and its function. The technology described here, P-IMP, is a functionally based technology to probe the RNA–RNA and RNA–protein interactions that occur in the living cell. P-IMP experiments can be envisaged for a variety of processes that involve nontranslated RNAs, including splicing, telomere formation and maintenance, gene imprinting, and chromatin-mediated gene silencing. It will be exciting to envisage a high-throughput functional genomic analysis of the nuclear compartment by P-IMP.
Supplementary Material
Table 4.
Effects of PNA administration on X-linked gene expression as measured by RNA-FISH experiments
| Mecp2 single dot | Mecp2 double dot | Number nuclei | Percent single dot | Percent double dot | (+) Xist bodies | (−) Xist bodies | Number nuclei | Percent Xist bodies | |
|---|---|---|---|---|---|---|---|---|---|
| (−)PNA | 288 | 264 | 552 | 52 | 48 | 332 | 220 | 552 | 60 |
| (+)PNA | 65 | 415 | 480 | 13 | 87 | 50 | 430 | 480 | 10 |
To quantitate the response of cells to PNA–transportan (pWS1248) treatment, we counted the cells with Xist bodies, one or two sites of expression for either Pgk1 or Mecp2, as measured by RNA–FISH. The total number of cells counted, number of cells with and without Xist bodies, and the number of cells with one or two spots of expression for Mecp2 are presented.
Table 5.
Effects of PNA administration on X-linked gene expression as measured by RNA-FISH experiments
| Pgk1 single dot | Pgk1 double dot | Number nuclei | Percent single dot | Percent double dot | (+) Xist bodies | (−) Xist bodies | Number nuclei | Percent Xist bodies | |
|---|---|---|---|---|---|---|---|---|---|
| (−)PNA | 241 | 277 | 518 | 46 | 53 | 295 | 223 | 518 | 57 |
| (+)PNA | 122 | 396 | 518 | 23 | 76 | 64 | 454 | 518 | 12 |
To quantitate the response of cells to PNA–transportan (pWS1248) treatment, we counted the cells with Xist bodies, one or two sites of expression for either Pgk1 or Mecp2, as measured by RNA–FISH. The total number of cells counted, number of cells with and without Xist bodies, and the number of cells with one or two spots of expression for Pgk1 are presented.
Acknowledgments
We thank Ralph A. Casale and Eric G. Anderson for their dedication and skill in the preparation of the PNA conjugates. We acknowledge the support of U.S. Army Prostate Cancer Research Award DAMD17–99-1–9032 and National Institutes of Health Grants R21 CA81732 and RO1 GM61079 (to W.M.S.).
Abbreviations
- MCB
macrochromatin body
- PNA
peptide nucleic acid
- P-IMP
PNA Intereference Mapping
- Xi
the inactive X-chromosome
- ES
embryonic stem
- FISH
fluorescence in situ hybridization
- RT-PCR
reverse transcription–PCR
- MCB
macrochromatin body
- Xa
the active x-chromosome
- BAC
bacterial artificial chromosome
- Ch
chromosome
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Brockdorff N. Curr Opin Genet Dev. 1998;8:328–333. doi: 10.1016/s0959-437x(98)80090-7. [DOI] [PubMed] [Google Scholar]
- 2.Brockdorff N, Duthie S M. Cell Mol Life Sci. 1998;54:104–112. doi: 10.1007/s000180050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown C J, Hendrich B D, Rupert J L, Lafreneire R G, Xing Y, Lawrence J, Willard H F. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 4.Lyon M F. Nature (London) 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 5.Gartler S M, Chen S H, Fialkow P J, Giblett E R, Singh S. Nat New Biol. 1972;236:149–150. doi: 10.1038/newbio236149a0. [DOI] [PubMed] [Google Scholar]
- 6.Barr M L, Bertram E G. Nature (London) 1949;163:676–677. doi: 10.1038/163676a0. [DOI] [PubMed] [Google Scholar]
- 7.Csankovszki G, Panning B, Bates B, Pehrson J R, Jaenisch R. Nat Genet. 1999;22:323–324. doi: 10.1038/11887. [DOI] [PubMed] [Google Scholar]
- 8.Mermoud J E, Costanzi C, Pehrson J R, Brockdorff N. J Cell Biol. 1999;147:1399–1408. doi: 10.1083/jcb.147.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costanzi C, Pehrson J R. Nature (London) 1998;393:599–601. doi: 10.1038/31275. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen T P, Mastrangelo M A, Eden A, Pehrson J R, Jaenisch R. J Cell Biol. 2000;150:1189–1198. doi: 10.1083/jcb.150.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell L B, Mongomery C S. Genetics. 1965;52:470–471. [Google Scholar]
- 12.Russell L B. Science. 1963;140:976–978. doi: 10.1126/science.140.3570.976. [DOI] [PubMed] [Google Scholar]
- 13.Lee J T, Strauss W M, Dausman J A, Jaenisch R. Cell. 1996;86:83–94. doi: 10.1016/s0092-8674(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee J T, Davidow L S, Warshawsky D. Nat Genet. 1999;21:400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 15.Lee J T, Lu N. Cell. 1999;99:47–57. doi: 10.1016/s0092-8674(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 16.Brockdorff N, Ashworth A, Kay G F, McCabe V M, Norris D P, Cooper P J, Swift S, Rastan S. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- 17.Borsani G, Tonlorenzi R, Simmler M C, Dandolo L, Arnaud D, Capra V, Grompe M, Pizzuti A, Muzny D, Lawrence C. Nature (London) 1991;351:325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- 18.Brown C J, Ballabio A, Rupert J L, Lafreniere R G, Grompe M, Tonorenzi R, Willard W F. Nature (London) 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 19.Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R. Genes Dev. 1997;11:156–166. doi: 10.1101/gad.11.2.156. [DOI] [PubMed] [Google Scholar]
- 20.Penny G D, Kay G F, Sheardown S A, Rastan S, Brockdorff N. Nature (London) 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 21.Wutz A, Jaenisch R. Mol Cell. 2000;5:695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y K, Ontiveros S D, Chen C, Strauss W M. Proc Natl Acad Sci USA. 1999;96:6829–6834. doi: 10.1073/pnas.96.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong Y K, Ontiveros S D, Strauss W M. Mamm Genome. 2000;11:220–224. doi: 10.1007/s003350010040. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen P E, Egholm M, Berg R H, Buchardt O. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 25.Egholm M, Buchardt O, Nielsen P E, Berg R H. J Am Chem Soc. 1992;114:1895–1897. [Google Scholar]
- 26.Egholm M, Buchardt O, Christensen L, Behrens C, Freier S M, Driver D A, Gerg R H, Kim S K, Norden B, Nielsen P E. Nature (London) 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 27.Verheijen J C, Van der Marel G A, Van Boom J H, Bayly S F, Player M R, Torrence P F. Bioorg Med Chem. 1999;7:449–455. doi: 10.1016/s0968-0896(98)00258-2. [DOI] [PubMed] [Google Scholar]
- 28.Bonham M A, Trown S, Boyd A L, Brown P H, Bruckenstein D A, Hanvey J C, Thomson S A, Pipe A, Hassman F, Bisi J E, et al. Nucleic Acids Res. 1995;23:1197–1203. doi: 10.1093/nar/23.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer G, Ross J. Mol Cell Biol. 1989;9:1996–2006. doi: 10.1128/mcb.9.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlmann E. Biol Chem. 1998;379:1045–1052. [PubMed] [Google Scholar]
- 31.Pooga M, Lindgren M, Hallbrink M, Brakenhielm E, Langel U. Ann NY Acad Sci. 1998;863:450–453. doi: 10.1111/j.1749-6632.1998.tb10721.x. [DOI] [PubMed] [Google Scholar]
- 32.Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, Kahl U, Hao J X, Xu X J, Wiesenfeld-Hallin Z, et al. Nat Biotechnol. 1998;16:857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 33.Pooga M, Hallbrink M, Zorko M, Langel U. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Jensen K B, Musunuru K, Lewis H A, Burley S K, Darnell R B. Proc Natl Acad Sci USA. 2000;97:5740–5745. doi: 10.1073/pnas.090553997. . (First Published May 16, 2000; 10.1073/pnas.090553997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis H A, Chen H, Edo C, Buckanovich R J, Yang Y Y, Musunuru K, Zhong R, Darnell R B, Burley S K. Struct Folding Des. 1999;7:191–203. doi: 10.1016/S0969-2126(99)80025-2. [DOI] [PubMed] [Google Scholar]
- 36.Lewis H A, Musunuru K, Jensen K B, Edo C, Chen H, Darnell R B, Burley S K. Cell. 2000;100:323–332. doi: 10.1016/s0092-8674(00)80668-6. [DOI] [PubMed] [Google Scholar]
- 37.Memili E, Hong Y-K, Kim D K, Ontiveros S D, Strauss W M. Gene. 2001;266:131–137. doi: 10.1016/s0378-1119(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 38.Wolffe A P, Matzke M A. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 39.Bird A P, Wolffe A P. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.