Key Points
Question
Is monitored anesthesia care feasible and as safe as general anesthesia for patients undergoing mechanical thrombectomy for acute posterior circulation strokes?
Findings
In this matched case-control study, patients who underwent the procedure with monitored anesthesia care had similar rates of successful reperfusion, good clinical outcomes, hemorrhagic complications, and mortality as patients that underwent the procedure under general anesthesia. The anesthesia modality was not associated with any significant changes in the modified Rankin scale score distribution at 90 days.
Meaning
Monitored anesthesia care is feasible and appears to be as safe and effective as general anesthesia for acute posterior circulation stroke endovascular therapy.
Abstract
Importance
No consensus regarding the ideal sedation treatment for stroke endovascular therapy has been reached, and practices remain largely based on local protocols and clinician preferences. Most studies have focused on anterior circulation strokes; therefore, little is known regarding the optimal anesthesia type for vertebrobasilar occlusion strokes.
Objective
To compare clinical and angiographic outcomes between monitored anesthesia care (MAC) and general anesthesia (GA) in patients presenting with vertebrobasilar occlusion strokes.
Design, Setting, and Participants
Retrospective, matched, case-control study of consecutive vertebrobasilar occlusion strokes treated with endovascular therapy at 2 academic institutions. The study took place between September 2005 and September 2015 at University of Pittsburgh Medical Center Stroke Institute, Pittsburgh, Pennsylvania, and between September 2010 and September 2015 at the Marcus Stroke and Neuroscience Center at Grady Memorial Hospital, Atlanta, Georgia. Patients requiring emergent intubation prior to endovascular therapy were excluded. The remaining patients were categorized into (1) MAC and (2) elective intubation for the procedure (elective GA). Patients who converted from MAC to GA during the procedure were included in the MAC group. The 2 groups were matched for age, baseline National Institutes of Health Stroke Scale score, and glucose levels. Baseline characteristics and outcomes were compared.
Main Outcomes and Measures
The primary outcome measure was the shift in the degree of disability among the 2 groups as measured by the modified Rankin scale at 90 days.
Results
A total of 215 patients underwent endovascular therapy for vertebrobasilar occlusion strokes during the study period. Thirty-nine patients were excluded owing to emergent pre–endovascular therapy intubation. Sixty-three patients had MAC (36%) and 113 patients had GA (64%). The conversion rate from MAC to GA was 13% (n = 8). After matching, 61 pairs of patients (n = 122) underwent primary analysis. The 2 groups were well balanced in terms of baseline characteristics. Median age was 69 years (interquartile range, 60-75 years) in the MAC group vs 67 years (interquartile range, 55.5-78.5 years) in the GA group (P = .83). Fifty-four percent of the patients in the MAC group were men vs 41% in the GA group (P = .44). When compared with the elective GA group, patients who underwent the procedure with MAC had similar rates of successful reperfusion, good clinical outcomes, hemorrhagic complications, and mortality. The modality of anesthesia was not associated with any significant changes in the modified Rankin scale score distribution (MAC: OR, 1.52; 95% CI, 0.80-2.90; P = .19).
Conclusions and Relevance
In endovascular therapy for acute posterior circulation stroke, MAC is feasible and appears to be as safe and effective as GA. Future clinical trials are warranted to confirm our findings.
This case-control study compares clinical and angiographic outcomes between monitored anesthesia care and general anesthesia in patients presenting with vertebrobasilar occlusion strokes.
Introduction
Endovascular therapy (ET) is increasingly used in acute ischemic stroke treatment and is now considered the gold standard for proximal anterior circulation occlusions presenting in the early time window. Prior studies have demonstrated that eventual patient outcome depends on both patient-specific factors (age, collaterals strength, and stroke burden on presentation) as well as procedural considerations (onset to treatment time and quality of reperfusion). Periprocedural considerations, such as anesthetic modality, also seem to have an effect on clinical outcomes. Previous studies have suggested that conscious sedation/monitored anesthesia care (MAC) is safe and feasible for intra-arterial therapy and that patients treated with MAC may actually have better outcomes than those treated with general anesthesia (GA). However, no consensus about the ideal sedation treatment has been reached, and practices remain largely based on local protocols and clinician preferences.
To date, most studies have focused on patients presenting with anterior circulation strokes. A significant proportion of patients with posterior circulation strokes present with alteration in level of consciousness and require emergent intubation for airway protection. However, the optimal IAT anesthesia treatment for the remaining population remains unknown. We sought to determine whether the use of MAC for ET of posterior circulation strokes was safe and to compare the differences in clinical and angiographic outcomes between the GA and MAC.
Methods
Patients and Variables
We reviewed prospectively collected databases for consecutive vertebrobasilar occlusion strokes (VBOS) treated with IAT at 2 tertiary care academic institutions (from September 2005 to September 2015 at University of Pittsburgh Medical Center Stroke Institute, Pittsburgh, Pennsylvania, and from September 2010 to September 2015 at the Marcus Stroke and Neuroscience Center at Grady Memorial Hospital, Atlanta, Georgia). Patients who required emergent intubation prior to arrival at the neuroendovascular suite were excluded from the analysis. The remaining patients were categorized into 2 groups: (1) those who had MAC and (2) those who had elective intubation for the procedure (elective GA). Patients who were converted from MAC to GA during the procedure were included in the MAC group (intention to treat). Sedation was provided by an anesthesia care team at both institutions.
The anesthesia protocol was comparable across both centers. Essentially, MAC was performed with a dexmedetomidine infusion with supplemental fentanyl and midazolam as needed. For patients requiring GA, induction was achieved with propofol and a paralytic agent (succinylcholine or rocuronium). Anesthesia was then maintained with volatile anesthetics (sevoflurane, isoflurane, or desflorane) along with fentanyl or remifentanil. Further paralytics were typically avoided but were used for a subset of patients and monitored by qualitative train-of-4 measurements. Additional agents used included ondansetron (as needed) and phenylephrine (to maintain systolic blood pressure greater than or within 20% of the patient’s presenting pressure).
The 2 groups were subsequently matched using a weighted Euclidian distance method to obtain a pair of patients considered to be the nearest neighbors in a 3-dimensional space of age, baseline National Institutes of Health Stroke Scale score, and pretreatment glucose levels as previously described. These parameters are known to be important predictors of outcomes in stroke. The distance between each MAC-GA pair was computed using the %FIND_NEIGHBORS Macro in SAS, University Edition (SAS Institute). The distribution of Euclidian distances was then studied and a threshold was determined as follows:
| Threshold = Q75 + 1.5 × (Q75 − Q25) |
where Q25 and Q75 are the 25th and 75th percentile, respectively. Pairs with distances greater than the threshold were considered outliers and eliminated from further consideration.
In-between group comparisons were made for baseline characteristics, procedural, and outcome parameters. The primary outcome measure was the shift in the degree of disability among the 2 matched anesthesia modality groups as measured by the modified Rankin scale score (mRS) at 90 days. Secondary end points included the rates of successful reperfusion (modified Thrombolysis in Cerebral Infarction score, 2b-3) and functional independence (mRS, 0-2) at 90 days. Safety end points were 90-day mortality and rates of any parenchymal hematoma as defined in the European Cooperative Acute Stroke Study. The study was approved by both the University of Pittsburgh and Emory University institutional review boards. Consent was waived because this was a retrospective medical record review and no additional intervention was performed.
Statistical Analysis
Continuous variables were reported as mean (SD) or median (IQR) as appropriate. Categorical variables were reported as proportions. Between groups, comparisons for continuous/ordinal variables were made with paired t test or Wilcoxon rank sum test as appropriate. Categorical variables were compared by McNemar test for discordant pairs. The overall distribution of 90-day mRS was compared between groups (shift in disability levels) using the Wilcoxon signed rank test to account for the matching. Ordinal and conditional logistic regressions were computed for odds ratio to assess the association between the anesthesia modality and mRS.
Significance was set at P less than .05, and all P values were 2-sided. Statistical analyses were performed using IBM SPSS Statistics 23 (IBMCorp) and SAS (SAS Institute).
Results
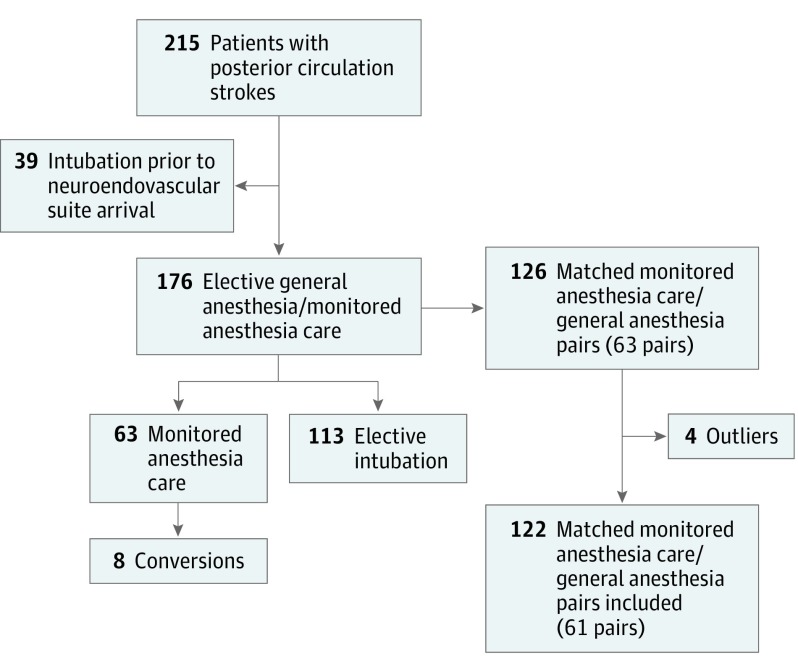
A review of the prospectively maintained databases identified 215 patients with posterior circulation strokes. Thirty-nine patients were excluded owing to emergent intubation prior to arrival at the neuroendovascular suite. Sixty-three patients underwent MAC (35.8%) and 113 underwent elective GA (64.2%). The conversion rate from MAC to GA during the procedure was 13% (n = 8) (Figure 1). Reasons for conversion were as follows: extreme motion (n = 2), mental status deterioration (n = 2), increased intracranial pressure (n = 1), and undetermined (n = 3).
Figure 1. Flowchart of the Study Sample.
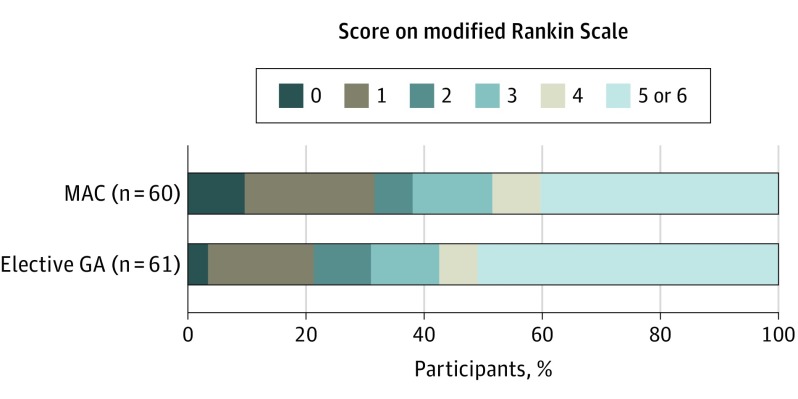
Of the 63 pairs generated by the matching algorithm, 2 had a Euclidean distance higher than the defined threshold, leaving 61 matched pairs for the analysis (Figure 1). Baseline characteristics, procedural, efficacy, and safety parameters were well balanced between the 2 groups. Median age was 69 years (interquartile range, 60-75 years) in the MAC group vs 67 years (interquartile range, 55.5-78.5 years) in the GA group (P = .83). Fifty-four percent of the MAC group were men vs 41% in the GA group (P = .44). The modality of anesthesia was not associated with any significant changes in the overall distribution of 90-day mRS (MAC: OR, 1.52; 95% CI, 0.80-2.90; P = .19) (Figure 2). Adjusting for the matching variables yielded similar results (MAC: OR, 1.37; 95% CI, 0.71-2.63; P = .35). There were no statistically significant differences in rates of successful reperfusion, any PH, wire perforations, good outcomes, or mortality at 90 days (Table). Multivariate conditional logistic regression confirmed that neither GA nor MAC was associated with good outcome (MAC: OR, 1.60; 95% CI, 0.73-3.53; P = .24).
Figure 2. Functional Outcome at 90 Days According to the Score on the Modified Rankin Scale.
Shift analysis as assessed by the Wilcoxon signed rank test, P = .19. MAC indicates monitored anesthesia care and GA indicates general anesthesia.
Table. Conscious Sedation vs Elective GA: Baseline Characteristics, Procedural Variables, and Outcomes.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Conscious Sedation (n = 61) |
Elective GA (n = 61) |
||
| Age, median (IQR), y | 69 (60-75) | 67 (55.5-78.5) | .83a |
| Glucose, median (IQR), mg/dL | 121 (108-153) | 129 (113.157.5) | .46a |
| bNIHSS, mean (SD) | 15.20 (7.32) | 17.47 (9.31) | .40a |
| Sex, male | 33 (54.1) | 25 (41) | .44 |
| Hypertension | 43 (70.5) | 39 (63.9) | .21 |
| Dyslipidemia | 26 (42.6) | 26 (42.6) | .17 |
| Atrial fibrillation | 14 (23) | 13 (21.3) | .80 |
| Diabetes | 12 (19.7) | 14 (23) | .65 |
| Smoking | 18 (29.5) | 18 (29.5) | .99 |
| Hemoglobin A1c, median (IQR), % of total hemoglobin | 5.8 (5.5-6.2) | 5.8 (5.5-6.2) | .90 |
| LDL cholesterol, median (IQR), mg/dL | 91 (66-113) | 89 (68.5-117.5) | .70 |
| Creatinine, median (IQR), mg/dL | 0.9 (0.8-1.1) | 0.99 (0.7-1.1) | .89 |
| Platelets, median (IQR) | 230 (181-257) | 208 (180-280) | .69 |
| INR, median (IQR) | 1.1 (1.0-1.2) | 1.05 (1.0-1.2) | .96 |
| Baseline SBP, mean (SD), mm Hg | 157.38 (28.26) | 147.64 (27.25) | .19 |
| IV thrombolyis | 8 (13.8) | 14 (23.7) | .10 |
| Etiology | |||
| Cardioembolic | 22 (36.1) | 20 (32.8) | .73 |
| Large vessel atherosclerosis | 19 (31.9) | 24 (39.3) | |
| Other determined etiology | 4 (6.6) | 4 (6.6) | |
| Undetermined | 16 (26.2) | 13 (21.3) | |
| Procedure | |||
| LKN to puncture, median (IQR) | 620 (266-1306) | 567 (316-846.5) | .56 |
| LKN to reperfusion, median (IQR) | 703 (357-1510) | 705 (408-1157) | .48 |
| Procedure length, median (IQR)b | 99 (61-135) | 86.5 (67-126) | .77 |
| Conversions | 8 (13.1) | NA | NA |
| Stentriever | 23 (37.7) | 19 (31.1) | .49 |
| Penumbra aspiration | 7 (11.5) | 10 (16.7) | .46 |
| Merci retriever | 11 (18) | 13 (21.3) | .63 |
| Intracranial stenting | 16 (26.7) | 18 (29.5) | .67 |
| Reperfusion | |||
| mTICI 2b-3 | 53 (86.9) | 54 (88.5) | .78 |
| mTICI 3 | 30 (49.2) | 32 (52.5) | .72 |
| Wire perforation | 4 (6.6) | 3 (4.9) | .99 |
| Any PH | 6 (9.8) | 6 (9.8) | .99 |
| mRS 0-2 at 90 d | 23 (38.3)c | 19 (31.1) | .23 |
| Mortality at 90 d | 21 (35)c | 26 (42.6) | .33 |
Abbreviations: bNIHSS, baseline National Institutes of Health Stroke Scale score; GA, generalized anesthesia; INR, international normalized ratio; IV, intravenous; LDL, low-density lipoprotein; LKN, last known normal; mRS, modified Rankin scale score; mTICI, modified thrombolysis in cerebral infarction score; NA, not applicable; PH, parenchymal hematoma; SBP, systolic blood pressure.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; to convert glucose to millimoles per liter, multiply by 0.0555; to convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01; to convert LDL cholesterol to millimoles per liter, multiply by 0.0259.
Control individuals were matched for these criteria.
Procedure length: time from groin puncture to reperfusion or last run if reperfusion not achieved. Intubation time included for the converted monitored anesthesia care cases.
n = 60.
Discussion
We report what is, to our knowledge, the largest series of patients undergoing ET for posterior circulation with MAC and demonstrate that MAC is feasible and appears to be as safe and effective as GA. Additionally, we found comparable rates of recanalization and procedure times in the GA and MAC groups.
Historically, some clinicians feared that patient movement during conscious sedation would increase periprocedural complications, such as hemorrhage, and delay or prevent recanalization by compromising digital subtraction imaging. Moreover, VBOS may lead to bulbar weakness and/or depressed level of consciousness with concerns for clearing oral secretions and airway protection. Conversely, the routine application of GA may be harmful in a subset of patients because it may lead to decreased blood pressure and delays in achieving reperfusion. In the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) study, for example, the mean time from door to groin puncture was 32 minutes longer in patients who underwent GA vs non-GA. Accordingly, there was larger infarct growth in the GA population, with a 51% estimated decreased treatment effect compared with the non-GA group. In MR CLEAN, it was noted that procedural times were comparable in the GA and non-GA groups, and there was an absolute difference in recanalization of 11% in favor of the non-GA group.
The generalizability of MR CLEAN and other studies of anesthesia modality in stroke outcomes after thrombectomy to the posterior circulation is unclear because most of the published literature has focused on the anterior circulation. The 2016 randomized clinical trial Sedation vs Intubation for Endovascular Stroke Treatment (SIESTA) (n = 150) showed no differences in outcomes between the GA and conscious sedation groups for anterior circulation strokes. Two other ongoing randomized clinical trials (Sedation Versus General Anesthesia for Endovascular Therapy in Acute Stroke: Impact on Neurological Outcome and General or Local Anaestesia in Intra Arterial Therapy) are studying outcomes in patients undergoing ET with GA vs non-GA. However, all of these studies have limited enrollment to patients with anterior circulation occlusions. While these trials should provide insight into choice of anesthesia modality in the anterior stroke population, they will not address the safety and feasibility of non-GA for basilar occlusions.
Many published series have excluded VBOS because a significant proportion of those patients who presented to the angiography suite were already intubated. Consequently, the data on the safety and feasibility of MAC in patients with posterior circulation strokes are scarce and limited to small, nonrandomized observational studies. Basilar strokes were part of larger cohorts, including both posterior and anterior circulation strokes, and although these studies showed that patients under conscious sedation behaved similarly to those under GA with similar recanalization rates, acceptable incidence of hemorrhagic complications, and similar or higher rates of good functional outcomes, they failed to evaluate the effect of stroke location on outcomes. Several studies studied the feasibility of MAC for elective endovascular procedures either in anterior or posterior circulations and demonstrated high technical success with low rates of periprocedural complications and mortality. Although they included a relatively large proportion of posterior circulation interventions and showed promising results, these studies were not able to compare outcomes with GA in the emergent setting and in particular in the presence of severe brainstem ischemia.
Our study shows that patients who underwent MAC for acute VBOS achieved similar rates of good angiographic and clinical outcomes as those who had GA without an increased risk of parenchymal hematoma or mortality. These results suggest that MAC is a viable option for endovascular stroke therapy of VBOS whenever feasible. In the patient experiencing acute stroke, GA has a number of potential adverse effects. For example, periprocedural hypotension is associated with worse outcomes and appears to be more common and severe with GA. Intubation may also increase the risk of pneumonia and create challenges regarding extubation.
Limitations
There are important limitations to our study, most of which are inherent to the retrospective design and relatively limited sample size. We also did not investigate potentially significant procedural variables (ie, door-to-puncture/reperfusion times and periprocedural blood pressure fluctuations), imaging parameters, and intermediate outcomes (rates of aspiration pneumonia and subsequent intubation and hospital length of stay) that may have influenced our results. Moreover, it is possible that our results could have been different had we included a larger cohort of early-treated patients. The different types of non-GA were not addressed in this study (ie, MAC with anesthesia personnel, nursing conscious sedation, and local anesthesia) because all our cases were performed in the presence of the anesthesia team. Additionally, the retrospective nonrandomized design of our study may have introduced undetected biases that may have influenced our results; thus, confirmation with a randomized clinical trial is needed. The SIESTA trial remains, to our knowledge, the only randomized clinical study to evaluate the effect of anesthesia modality on clinical outcomes of patients undergoing endovascular therapy. While SIESTA provided strong evidence that GA and MAC are comparable, it only included patients with anterior circulation strokes. As such, the influence of anesthesia modality on the outcomes of vertebrobasilar occlusion strokes remains elusive. This study represents, to our knowledge, the first systematic matched case-control analysis and the largest report addressing this critical issue. This becomes particularly important in face of the decline in the use of GA for stroke thrombectomy. In fact, GA was used in less than 40% of the patients treated in the 5 randomized clinical trials published in 2015. Remarkably, GA was used in less than 10% of the patients with ET in 2 of the aforementioned trials. Given this trend and in face of our data, the routine use of GA for posterior circulation thrombectomy should be reconsidered and warrants further investigation.
Conclusions
Our study suggests that, in properly selected patients, MAC appears to be as safe and effective as GA for posterior circulation acute ischemic stroke interventions. Future prospective studies will hopefully further improve our understanding of the effect of the anesthesia modality in patients with VBOS undergoing ET.
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Murad MH, Rabinstein AA, Cloft HJ, Lanzino G, Kallmes DF. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(3):525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonagh DL, Olson DM, Kalia JS, Gupta R, Abou-Chebl A, Zaidat OO. Anesthesia and sedation practices among neurointerventionalists during acute ischemic stroke endovascular therapy. Front Neurol. 2010;1:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jumaa MA, Zhang F, Ruiz-Ares G, et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41(6):1180-1184. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Chebl A, Lin R, Hussain MS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41(6):1175-1179. [DOI] [PubMed] [Google Scholar]
- 6.Mandava P, Kalkonde YV, Rochat RH, Kent TA. A matching algorithm to address imbalances in study populations: application to the National Institute of Neurological Diseases and Stroke Recombinant Tissue Plasminogen Activator acute stroke trial. Stroke. 2010;41(4):765-770. [DOI] [PubMed] [Google Scholar]
- 7.Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC; German Stroke Study Collaboration . Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35(1):158-162. [DOI] [PubMed] [Google Scholar]
- 8.Bruno A, Williams LS, Kent TA. How important is hyperglycemia during acute brain infarction? Neurologist. 2004;10(4):195-200. [DOI] [PubMed] [Google Scholar]
- 9.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hacke W, Kaste M, Fieschi C, et al. ; The European Cooperative Acute Stroke Study (ECASS) . Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. JAMA. 1995;274(13):1017-1025. [PubMed] [Google Scholar]
- 11.Berkhemer OA, van den Berg LA, Fransen PS, et al. ; MR CLEAN investigators . The effect of anesthetic management during intra-arterial therapy for acute stroke in MR CLEAN. Neurology. 2016;87(7):656-664. [DOI] [PubMed] [Google Scholar]
- 12.Schönenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. [DOI] [PubMed] [Google Scholar]
- 13.Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J Neurointerv Surg. 2010;2(1):67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langner S, Khaw AV, Fretwurst T, Angermaier A, Hosten N, Kirsch M. Endovascular treatment of acute ischemic stroke under conscious sedation compared to general anesthesia: safety, feasibility and clinical and radiological outcome [in German]. Rofo. 2013;185(4):320-327. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Deshaies EM, Singla A, et al. Impact of anesthesia on mortality during endovascular clot removal for acute ischemic stroke. J Neurosurg Anesthesiol. 2014;26(4):286-290. [DOI] [PubMed] [Google Scholar]
- 16.Davis MJ, Menon BK, Baghirzada LB, et al. ; Calgary Stroke Program . Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116(2):396-405. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Chebl A, Krieger DW, Bajzer CT, Yadav JS. Intracranial angioplasty and stenting in the awake patient. J Neuroimaging. 2006;16(3):216-223. [DOI] [PubMed] [Google Scholar]
- 18.Chamczuk AJ, Ogilvy CS, Snyder KV, et al. Elective stenting for intracranial stenosis under conscious sedation. Neurosurgery. 2010;67(5):1189-1193. [DOI] [PubMed] [Google Scholar]
- 19.Whalin MK, Lopian S, Wyatt K, et al. Dexmedetomidine: a safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014;6(4):270-275. [DOI] [PubMed] [Google Scholar]
- 20.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015;46(9):2678-2680. [DOI] [PubMed] [Google Scholar]
- 21.Wendell LC, Raser J, Kasner S, Park S. Predictors of extubation success in patients with middle cerebral artery acute ischemic stroke. Stroke Res Treat. 2011;2011:248789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 23.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. [DOI] [PubMed] [Google Scholar]