This observational study reports the dermoscopic examination of 134 cases of melanoma that clinically mimics seborrheic keratosis.
Key Points
Question
What is the dermoscopic appearance of melanomas that clinically simulate seborrheic keratosis?
Findings
In this observational study of 134 cases of melanoma, 82% of clinically seborrheic keratosis–like melanomas were correctly detected by dermoscopy, despite the presence of features typically observed in seborrheic keratosis. The presence of the blue-black sign was the most helpful criterion and was independently associated with a correct diagnosis.
Meaning
Seborrheic keratosis–like melanomas can be clinically and dermoscopically challenging to detect, but careful dermoscopic examination leads to the recognition and correct diagnosis of melanoma.
Abstract
Importance
Melanomas that clinically mimic seborrheic keratosis (SK) can delay diagnosis and adequate treatment. However, little is known about the value of dermoscopy in recognizing these difficult-to-diagnose melanomas.
Objective
To describe the dermoscopic features of SK-like melanomas to understand their clinical morphology.
Design, Setting, and Participants
This observational retrospective study used 134 clinical and dermoscopic images of histopathologically proven melanomas in 134 patients treated in 9 skin cancer centers in Spain, France, Italy, and Austria. Without knowledge that the definite diagnosis for all the lesions was melanoma, 2 dermoscopy-trained observers evaluated the clinical descriptions and 48 dermoscopic features (including all melanocytic and nonmelanocytic criteria) of all 134 images and classified each dermoscopically as SK or not SK. The total dermoscopy score and the 7-point checklist score were assessed. Images of the lesions and patient data were collected from July 15, 2013, through July 31, 2014.
Main Outcomes and Measures
Frequencies of specific morphologic patterns of (clinically and dermoscopically) SK-like melanomas, patient demographics, and interobserver agreement of criteria were evaluated.
Results
Of the 134 cases collected from 72 men and 61 women, all of whom were white and who had a mean (SD) age of 55.6 (17.5) years, 110 (82.1%) revealed dermoscopic features suggestive of melanoma, including pigment network (74 [55.2%]), blue-white veil (72 [53.7%]), globules and dots (68 [50.7%]), pseudopods or streaks (47 [35.1%]), and blue-black sign (43 [32.3%]). The remaining 24 cases (17.9%) were considered likely SKs, even by dermoscopy. Overall, lesions showed a scaly and hyperkeratotic surface (45 [33.6%]), yellowish keratin (42 [31.3%]), comedo-like openings (41 [30.5%]), and milia-like cysts (30 [22.4%]). The entire sample achieved a mean (SD) total dermoscopy score of 4.7 (1.6) and a 7-point checklist score of 4.4 (2.3), while dermoscopically SK-like melanomas achieved a total dermoscopy score of only 4.2 (1.3) and a 7-point checklist score of 2.0 (1.9), both in the range of benignity. The most helpful criteria in correctly diagnosing SK-like melanomas were the presence of blue-white veil, pseudopods or streaks, and pigment network. Multivariate analysis found only the blue-black sign to be significantly associated with a correct diagnosis, while hyperkeratosis and fissures and ridges were independent risk markers of dermoscopically SK-like melanomas.
Conclusions and Relevance
Seborrheic keratosis–like melanomas can be dermoscopically challenging, but the presence of the blue-black sign, pigment network, pseudopods or streaks, and/or blue-white veil, despite the presence of other SK features, allows the correct diagnosis of most of the difficult melanoma cases.
Introduction
Seborrheic keratosis (SK) is among the most common benign skin neoplasms that are universally distributed and frequently treated without biopsy by dermatologists and cosmeticians. A 2002 retrospective study of lesions sent to a pathologist as SKs found that 0.7% of these SKs were melanomas. Another study found that of the 9% of SKs excised during the study observation year, 8.2% were associated with melanomas.
After the introduction of dermoscopy in 1998, the diagnostic accuracy for skin tumors and SKs has significantly improved, reaching a sensitivity of 95.7% and a specificity of 78.3%. Dermoscopy helps confirm the clinical diagnosis of SK by assessing specific dermoscopic features such as milia-like cysts (white clods), comedo-like openings (black to brown clods), and fissures and ridges (thick lines), to name a few. Moreover, Braun and colleagues showed that only 10% of 203 SKs they prospectively evaluated were judged equivocally enough to merit histopathologic confirmation.
Cases of melanoma that arise within or adjacent to SK or that clinically mimic SK suggest that, sometimes, melanoma may be difficult to differentiate from SK and vice versa. Melanoma lesions can have a verrucous appearance and/or any of the features of nonmelanocytic tumors, including scaly surface, hyperkeratosis, epidermal hyperplasia, milia-like cysts, and comedo-like openings, especially when folliculotropism is presented. The biological behavior and outcome of these verrucous melanomas seem to be similar to other melanoma subtypes.
Apart from insights from a few single case reports, little else is known about the role of dermoscopy in the diagnosis of SK-like melanomas. Our primary goal for this study was to describe the dermoscopic features of a large series of retrospectively collected and histopathologically diagnosed melanomas for which the clinical differential diagnosis included SK. Our secondary goal was to identify the main dermoscopic features that enable the correct recognition of SK-like melanomas and to highlight features that have caused clinicians to misinterpret melanomas as SKs.
Methods
Lesion Selection
Between July 15, 2013, and July 31, 2014, we gathered a multicentric collection of cases of melanomas that could mimic SKs. Nine skin cancer centers from Spain (Hospital Clínic de Barcelona and Hospital del Mar de Barcelona, Barcelona; Hospital Sant Pau i Santa Tecla, Tarragona; and Hospital Son Llatzer, Palma Mallorca), France (Centre Hospitalier Lyon Sud, Lyon), Italy (Arcispedale Santa Maria Nuova, Reggio Emilia; Città della Salute e della Scienza, Turin; and Ca’ Granda Ospedale Maggiore Policlinico, Milano), and Austria (Medical University of Graz, Graz) contributed clinical and dermoscopic images of pathologically proven melanomas, which included SK in the routine presurgical clinical differential diagnosis. Patient demographics included age at diagnosis of melanoma, sex, personal and family history of melanoma, and clinical presence of widespread true SK and/or sun-damaged skin. Histopathologic features reported in medical records were lesion site, histopathologic subtype, Breslow thickness, associated nevus, ulceration, folliculotropism, hyperkeratotic epidermal hyperplasia, and elastosis. No further histopathological revisions were performed. The institutional review board of the Hospital Clinic of Barcelona exempted this study from review because all of the images were anonymous or not identifiable, and patients gave written informed consent to be photographed in their respective clinical centers.
Tumor Evaluation
The evaluation was performed by 2 independent, dermoscopy-trained observers with more than 10 years of experience each (S.S. and P.A.). The observers were asked to evaluate “seborrheic keratosis–like tumors” in a blind design, without knowing that the definite diagnosis for all of them was melanoma. In addition, the observers evaluated clinical descriptions and 48 dermoscopic features (including all the melanocytic and nonmelanocytic criteria described in the literature to date) and classified all tumors dermoscopically as SK or not SK. Lesions that were assigned to the former category are referred to as dermoscopically SK-like melanomas. According to the presence or absence of the dermoscopic criteria evaluated, the ABCD (asymmetry, border, colors, and dermoscopic structure) total dermoscopy score (TDS) and the 7-point checklist score were assessed. To simplify our reporting, we randomly chose observer 1 as the main evaluator, shown in the Results section, and observer 2 as the assessor of the interobserver agreement.
Statistical Analysis
Statistical analysis was performed with PASW Statistics 22.0 software (IBM). Fisher exact test or Pearson χ2 analysis, when appropriate, was used to describe the tumors and to evaluate the association between the dermoscopic features of SK-like melanomas and those of clearly identified melanomas. Mean comparison by the 2-tailed t test was used to assess the quantitative study of size, TDS, 7-point checklist, Breslow thickness, and mitotic index. Multivariate analysis was performed, including all significant associated features in the univariate analyses, and a logistic regression multistep model was assessed to study the features associated with dermoscopically SK-like melanomas (Hosmer-Lemeshow test). The κ statistic and percentage of positive concordance were calculated for interobserver agreement analysis. Two-sided P < .05 was considered statistically significant.
Results
Characteristics of Patients and Tumors
Our study consisted of a total of 134 tumors from 134 patients, including 72 men (54.1%) and 61 women (45.9%) (sex and age data were missing for 2 patients). The race/ethnicity of all patients was white, their mean (SD) age was 55.6 (17.5) years, and the most common location of the tumor was the posterior trunk (52 [39.1%]) and limbs (47 [35.6%]). Of note, while 34 men (47.2%) developed melanoma on their posterior trunk, 29 women (48.3%) had melanoma on their limbs. The mean (SD) diameter of lesions available by scale was 10.4 (4.9) mm. In the whole set, only 22 cases (17.5%) were in situ melanomas. The mean (SD) Breslow thickness among the invasive cases was 1.5 (1.4) mm. The distribution between histological subtypes was 98 superficial spreading melanomas (75.4%), 20 lentigo maligna melanomas (15.4%), and 9 nodular melanomas (6.9%). Ulceration was recorded in 20 cases (17.1%), an associated nevus was reported in 19 (24.1%), and folliculotropism was noted in 21 (18.8%).
Dermoscopic Characterization
Most lesions presented with sharply demarcated borders (86 [64.7%]) and as raised tumors (89 [66.4%]). Dermoscopic features observed in the whole sample are summarized in Table 1. The mean (SD) number of colors present was 3.9 (1.2). Dermoscopic criteria were classified into 3 categories: (1) usually associated with SKs, (2) usually associated with melanocytic tumors, and (3) vascular structures.
Table 1. Dermoscopic Characterization of SK-like Melanomas and Interobserver Agreementa.
| Dermoscopic Features | Total, No. (%) (N = 134) |
Interobserver Agreement, κ Valueb |
Concordance Between Both Readers: Positive Agreement, %/Presence According to Both Readers, % |
|---|---|---|---|
| General characteristics | |||
| Melanoma diagnosis on overall dermoscopic evaluation | 110 (82.1) | 0.41 | 88.1/77.6 |
| SK diagnosis on overall dermoscopic evaluation | 24 (17.9) | 0.41 | 62.5/7.5 |
| Symmetry | 47 (36.7) | 0.30 | 25.5/9.4 |
| Asymmetry in 2 axes | 44 (34.4) | 0.31 | 90.9/31.3 |
| Palpable or raised | 89 (66.4) | 0.45 | 92.1/61.2 |
| Ulceration | 25 (18.7) | ND | |
| Melanocytic features | |||
| Pigment network | 74 (55.2) | 0.69 | 87.3/46.3 |
| Atypical pigment network | 66 (49.3) | 0.50 | 79.6/32.1 |
| Facial pseudonetwork | 16 (11.9) | ND | |
| Negative network | 11 (8.2) | ND | |
| Pigmented globules and dots | 68 (50.7) | 0.50 | 69.7/46.3 |
| Irregular globules and dots | 64 (47.8) | 0.47 | 67.1/41.0 |
| Pseudopods or streaks | 47 (35.1) | 0.70 | 83.7/26.9 |
| Blotches | 44 (32.8) | 0.40 | 54.1/24.6 |
| Blue-white veil (raised area) | 72 (53.7) | 0.66 | 87.7/42.5 |
| Blue-black sign | 43 (32.3) | 0.50 | 59.0/27.1 |
| Blue gray regression | 39 (29.1) | ND | |
| Shiny white structures | 49 (36.6) | 0.47 | 49.0/17.9 |
| SK features | |||
| Sharply demarcated borders | 86 (64.7) | 0.12 | 38.4/24.8 |
| Comedo-like openings | 41 (30.6) | 0.50 | 70.6/18.0 |
| Fissures and ridges | 15 (11.2) | ND | |
| Cerebriform-like pattern | 6 (4.5) | ND | |
| Network-like structures | 12 (9) | ND | |
| Yellowish keratin | 42 (31.3) | 0.44 | 60.0/20.1 |
| Scale and hyperkeratosis | 45 (33.6) | 0.51 | 56.3/33.6 |
| Milia-like cysts | 30 (22.4) | ND | |
| ≥3 Milia-like cysts | 17 (12.7) | ND | |
| Mouth-eaten border | 6 (4.5) | ND | |
| Fingerprint-like areas | 4 (3.0) | ND |
Abbreviations: ND, not done; SK, seborrheic keratosis.
Dermoscopic description is shown according to observer 1, an independent blinded expert who evaluated the 134 cases. Interobserver agreement between the 2 observers (both experts in the field [S.S. and P.A.]) was assessed for criteria presented in at least 30% of the 134 cases, according to observer 1 and is expressed as κ values, the percentage of positive concordance for each feature, and the percentage of the total series where both observers found the criteria.
The κ values were assessed for features observed in at least 30% of cases. Meaning of the level of agreement depending on κ value ranges: 0.01 to 0.20, slight agreement; 0.21 to 0.40, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.80, substantial agreement; 0.81 to 0.99, almost perfect agreement.
In the first category (SK criteria), comedo-like openings were observed in 41 cases (30.6%), scale and hyperkeratosis surface in 45 (33.6%), yellowish surface areas in 42 (31.3%), milia-like cysts in 30 (22.4%), and fissure and ridges in 15 (11.2%). In the second category (melanocytic criteria), a pigment network was seen in 74 cases (55.2%), pigmented globules and dots in 68 (50.7%), and pseudopods or streaks in 47 (35.1%). Blue-white veil was observed in 72 cases (53.7%), and both black blotches (44 cases) and the blue-black sign (43 cases) were observed in more than 30% of melanomas. In the third category (vascular structures), atypical vessels were observed in 56 cases (41.8%), among which 29 (21.6%) had milky red areas, 28 (20.9%) had polymorphous vessels, and 19 (14.2%) had dotted vessels. Only 7 of the lesions (5.2%) presented with hairpin vessels. The interobserver agreement (κ statistic) and percentage of positive concordance among the criteria found in at least 30% of cases are shown in Table 1.
Melanoma Discrimination by Dermoscopy and Interobserver Agreement
Overall, 110 of 134 cases (82.1%) were correctly diagnosed as melanomas by dermoscopic evaluation (referred to as dermoscopically non–SK-like melanomas), while the remaining 24 (17.9%) were judged as dermoscopically SK-like melanomas. Observer 2 categorized 118 of 134 (88.1%) correctly as melanomas and 16 (11.9%) as dermoscopically SK-like melanomas. The percentage of concordance of cases correctly diagnosed as melanoma by both observers 1 and 2 was 77.6% (κ value, 0.42).
When we applied the ABCD rule, 65 cases (48.5%) gave a TDS greater than 4.75 and would have been considered suspicious. Of note, observer 2 found a TDS greater than 4.75 in 100 cases (74.6%) (P = .002), with a positive concordance of 41.8% (κ value, 0.22). According to the 7-point checklist (cutoff, 3 points), 92 lesions (68.7%) would have been considered as melanoma by observer 1 and 93 lesions (69.4%) by observer 2, with a 55.2% of positive concordance (κ value, 0.35). The mean (SD) TDS for all lesions was 4.71 (1.6); for those dermoscopically considered SK, the mean (SD) was 4.22 (1.3) (P = .07). The mean (SD) score on the 7-point checklist was 4.37 (2.3) for the whole series and was 1.96 (1.9) for dermoscopically SK-like melanomas (P < .001) (eTable 2 in the Supplement). No differences were found in diameter, number of colors, or abrupt borders between the dermoscopically SK-like melanomas and the other cases.
Dermoscopic Pitfalls and Clues Related to the Diagnosis of Melanomas as SK
Dermoscopic features associated with a risk of diagnosing melanomas as SKs by dermoscopy are summarized in Table 2. The highest risk was found for hairpin vessels (odds ratio [OR], 36.3; 95% CI, 4.1-319.8; P < .001), fingerprint-like structures (OR, 15.6; 95% CI, 1.5-157.0; P = .02), network-like structures (brown circles) (OR, 8.6; 95% 2.5-30.4; P < .001), and fissures and ridges (OR, 7.3; 95% 2.3-23.1; P < .001). Other common misleading dermoscopic features (with interobserver agreement concordance >60%) were scaly or hyperkeratotic surface (OR, 7.1; 95% 2.6-18.9; P < .001), yellowish keratin (OR, 5.1; 95% 2.0-13.0; P < .001), comedo-like openings (OR, 5.4; 95% CI, 2.1-13.7; P < .001), and presence of milia-like cysts (OR, 2.6; 95% CI, 0.98-6.6; P = .05).
Table 2. Dermoscopic Features Associated With Risk of Incorrect Diagnosis of Melanoma as Seborrheic Keratosis and With Correct Diagnosis of SK–like Melanoma .
| Feature | Dermoscopically Non–SK-like MM, No. (%) (n = 110 [82.1%]) |
Dermoscopically SK-like MM, No. (%) (n = 24 [17.9%]) |
Risk of Diagnosing MM as SK by Dermoscopy, OR (95% CI) |
P Value |
|---|---|---|---|---|
| Dermoscopic Features Associated With Risk of Incorrect Diagnosis | ||||
| Hairpin vessels | 1 (0.9) | 6 (25) | 36.3 (4.1-319.8) | <.001 |
| Fingerprint-like areas | 1 (0.9) | 3 (12.5) | 15.6 (1.5-157) | .02 |
| White halo surrounding vessels | 3 (2.7) | 6 (25) | 11.9 (2.7-51.9) | <.001 |
| Cerebriform-like pattern | 2 (1.8) | 4 (16.7) | 10.8 (1.8-62.9) | .01 |
| Network-like structures | 5 (4.5) | 7 (29.2) | 8.6 (2.5-30.4) | <.001 |
| Fissures and ridges | 7 (6.4) | 8 (33.3) | 7.3 (2.3-23.1) | .001 |
| Scale and hyperkeratosis surface | 28 (25.5) | 17 (70.8) | 7.1 (2.6-18.9) | <.001 |
| Comedo-like openings | 26 (23.6) | 15 (62.5) | 5.4 (2.1-13.7) | <.001 |
| Yellowish keratin | 27 (24.5) | 15 (62.5) | 5.1 (2.0-13.0) | <.001 |
| Milia-like cysts | 21 (19.1) | 9 (37.5) | 2.6 (0.98-6.6) | .05 |
| Dermoscopic Features Associated With Correct Diagnosis | ||||
| Pigment network | 73 (66.4) | 1 (4.2) | 0.02 (0.003-0.17) | <.001 |
| Pseudopods or streaks | 45 (40.9) | 2 (8.3) | 0.13 (0.3-0.59) | .002 |
| Blue-black sign | 41 (37.6) | 2 (8.3) | 0.15 (0.03-0.6) | .005 |
| Irregular globules and dots | 58 (52.7) | 6 (25) | 0.30 (0.1-0.8) | .01 |
| Blue-white veil (raised area) | 64 (58.2) | 8 (33.3) | 0.36 (0.14-0.91) | .03 |
| Atypical vessels | 50 (45.5) | 6 (25) | 0.3 (0.1-0.9) | .06 |
Abbreviations: MM, melanoma; OR, odds ratio; SK, seborrheic keratosis.
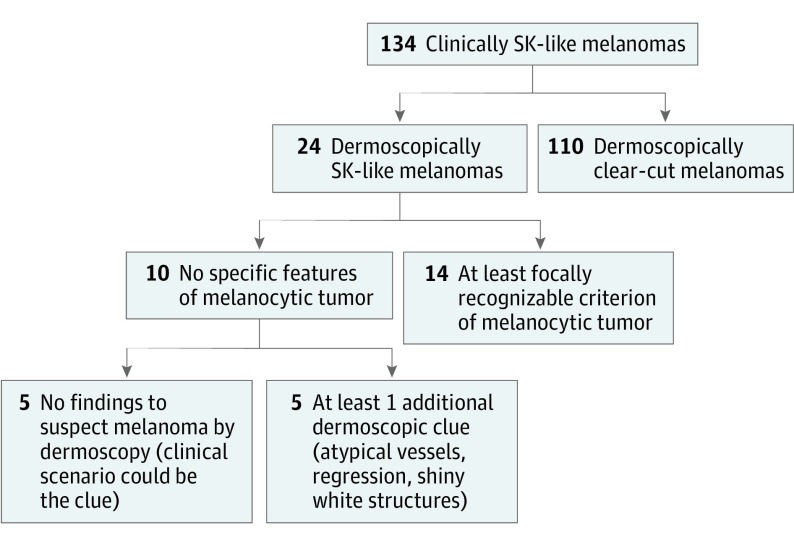
The most frequent dermoscopic features that helped to correctly classify clinically SK-like melanomas were pigment network (OR, 0.02; 95% CI, 0.003-0.17; P < .001), blue-white veil (OR, 0.36; 95% CI, 0.14-0.91; P = .03), irregular globules and dots (OR, 0.30; 95% CI, 0.1-0.8; P = .01), pseudopods or streaks (OR, 0.13; 95% CI, 0.3-0.59; P = .002), and the blue-black sign (OR, 0.15; 95% CI, 0.03-0.6; P = .005). The most protective and robust factors against diagnosing SK-like melanomas were blue-white veil, pseudopods or streaks, and pigment network. These criteria were also identified with high concordance between both observers (superior to 80%). Only 10 of the 134 cases (7.4%) presented with none of these 5 melanoma specific features. Of those 10 cases, 5 (3.7%) showed at least 1 additional dermoscopic clue for malignancy—namely, shiny white streaks, regression, or atypical vessels. However, the remaining 5 tumors (3.7%) were excised despite showing no dermoscopically identifiable suspicious features (Figures 1, 2, and 3).
Figure 1. Diagram of Seborrheic Keratosis (SK)–like Melanoma Detection by Different Dermoscopic Clues.
A total of 134 clinically SK-like melanomas were evaluated; among them, 24 cases (17.9%) were considered possible SK (dermoscopically SK-like melanomas). Of these, 14 showed at least 1 recognizable criterion of a melanocytic lesion: pigment network, globules or dots, pseudopods, blue-white veil, and/or the blue-black sign.
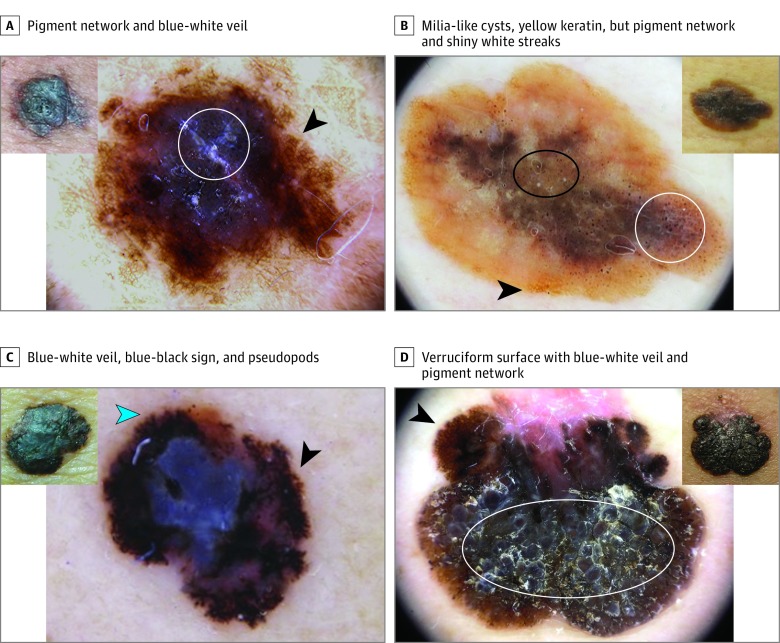
Figure 2. Four Seborrheic Keratosis (SK)–like Melanomas Belonging to the Group of 110 Lesions Easily Detected by Dermoscopy.
Insets, Pigmented lesions with some degree of hyperkeratotic surface and sharp demarcation that clinically can simulate SKs. Larger images, Dermoscopy shows features suggestive of melanocytic lesions and therefore melanoma. A, Presence of pigment network at the periphery (arrowhead) with hyperkeratosis and blue-white veil in the center (circle). B, Brownish lesion with notable milia-like cysts (black oval) and yellowish keratin, pigment network (arrowhead), irregular globules and dots, and shiny white streaks (white circle). C, Blue-white veil and the blue-black sign in the center, in addition to atypical network (black arrowhead) and pseudopods at the periphery (blue arrowhead). D, Markedly hyperkeratotic tumor with verruciform surface and blue-white veil (white oval); the clue is at the periphery, with atypical network (arrowhead) and regression.
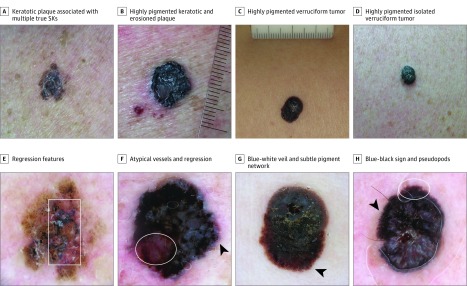
Figure 3. Four Seborrheic Keratosis–like Melanomas Needing Careful Dermoscopic Evaluation to Be Correctly Diagnosed.
A-D, Clinical photographs. In B and C, the rules are in millimeters. E-H, Dermoscopic images of the same lesions. Pigmented lesions with marked hyperkeratotic surface partially impeding the easy observation of the dermoscopic clues. The proper use of immersion liquid and enough light may help the evaluation (compare G, which did not have enough liquid, and H, which is a proper image that allows the detection of the pigment network [circle]). The presence of subtle pigment network (arrowheads in G and H) and globules (arrowhead in F) at the periphery of the lesions and the blue-black sign are the main key features. Regression features (box in E; circle in F) can be another clue to recommend excision.
Multivariate analysis for predictive and negative dermoscopic features associated with SK-like melanomas showed that the presence of hyperkeratosis was an independent risk marker to misclassify SK-like melanomas (OR, 12.05; 95% CI, 2.08-69.86; P = .005), while the blue-black sign was an independent feature to avoid missing SK-like melanomas (OR, 0.20; 95% CI, 0.001-0.316; P = .006) by dermoscopy. The presence of fissures and ridges was a risk marker with an OR of 9.2 but was not statistically significant (95% CI, 0.77-110.45; P = .08).
Associations of Presentation of SK-like Melanomas
The presence of comedo-like openings was associated with folliculotropism on histology (38.2% of those showing a comedo-like opening vs 10.3% of those without comedo-like openings; OR, 5.4; 95% CI, 1.9-14.8; P < .001). Blue-black sign was associated with invasive melanoma (97.6% of cases) in contrast to those without the blue-black sign (74.7%) (P = .001). Blue-white veil was related to invasive melanoma (100% vs 65.6% of those without blue-white veil; P = .001), and folliculotropism was associated with melanomas showing blue-white veil (29.3% vs 7.4% of those without veil; OR, 5.2; 95% CI, 1.6-16.6; P = .003). Patients with few or no true SK on their body had a higher risk of presenting pseudopods or streaks (43.7% vs 14.6% of those with many SKs; P = .002) and blue-white veil (55.2% vs 36.6% of those in patients with many SKs; P = .05) in their SK-like melanomas. Limb location was significantly associated with the absence of network (63.8% vs 32.9% of nonlimb locations) and hyperkeratotic (59.6% of limb location tumors) presentation on dermoscopy.
Discussion
We investigated the role of dermoscopy in the diagnosis of clinically SK-like melanomas. Besides describing the morphologic patterns associated with these tumors, we also elaborate practical clues and rules to help clinicians correctly manage such challenging melanomas.
One main result of our study is that the addition of dermoscopy allows the correct diagnosis of melanoma in more than 80% of clinically SK-like melanomas with a high concordance between both observers 1 and 2 (ie, agreement in more than 77% of all cases). On the other hand, our study also suggests that dermoscopic algorithms may be inappropriate for the diagnosis of SK-like melanomas. Although the mean (SD) TDS was 4.71 (1.6) and 7-point checklist score for the whole sample was 4.37 (2.3), these numbers would have missed 69 (51.5%) and 42 (31.3%) of the melanomas in our series, respectively. The reason for this oversight may be that thick melanomas (in our series, mean [SD] tumor thickness was 1.5 mm [1.4]) or melanomas with a verrucous surface often lack classic melanocytic criteria (ie, pigment network, globules, or pseudopods or streaks) or, as shown in our study, exhibit benign patterns associated with SK. In line with our findings, a recent study testing the validity and reliability of dermoscopic features and algorithms concluded that dermoscopists do better in distinguishing benign from malignant lesions when a global image is shown than when specific features and scores are evaluated.
The recognition of SK-like melanomas requires a careful analysis of all criteria, even if they are not immediately obvious. At first glance, the keratotic aspect of such tumors could induce a confident but false clinical diagnosis of SK. Our study, however, highlights that dermoscopic melanoma clues could be found in most of the cases we evaluated, allowing a correct diagnosis.
In addition to the well-known melanocytic criteria, the blue-black sign seems to be the most important independent feature associated with SK-like melanomas. With the exception of angiokeratoma, the blue-black sign (referring to the presence of blue and black in a hyperpigmented tumor) has been shown to be an important clue in diagnosing nodular melanoma. In the context of nonspecific raised tumors, both blue-white veil and the blue-black sign could be considered a warning to rule out melanoma. However, their specific histopathologic counterpart is different: Black, other than what is seen in comedo-like openings or lacunae, is thought to be caused by the highly pigmented neoplastic cells being eliminated through a thin epidermis (pagetoid elimination of cells and pigment), and blue is the deep dermal component of a pigmented tumor. Blue-white veil is thought to be caused by the hyperkeratotic surface over a pigmented tumor invading the deep dermis. Other important features that can help to detect melanomas that mimic SKs are atypical vessels (other than hairpin; ie, polymorphic, dotted, and/or milky-red areas), regression features, blue-white veil, and shiny white structures. All of them, observed in an otherwise equivocal or nonspecific tumor, make the histopathologic diagnosis necessary.
Of the 134 cases, only 5 (3.7%) presented with none of these dermoscopic clues. In those cases, the clinical information, with nodular or inflamed appearance, or the given history of changes should lead physicians to rule out squamous cell carcinoma or nodular melanoma even in the absence of suspicious dermoscopic features. In the case of tumors with verrucous presentation, it has been shown that 50% of verrucous melanomas can simulate benign tumors. Verrucous melanomas are considered a specific histopathologic subtype of melanoma, for which it is mandatory to find hyperkeratosis and pseudoepitheliomatous hyperplasia of the epidermis that is intermingled with atypical melanocytes. On the other hand, the term SK-like melanoma refers to a clinical and/or dermoscopic appearance that makes the melanoma look like SK, being verrucous or not on the histopathology report. The most important and independent findings in dermoscopically SK-like melanomas were hyperkeratosis and fissures and ridges.
After the introduction of dermoscopy in general dermatology, the expectation was that the possibility of mistreating melanoma as SK would be reduced. However, it is well known that about 10% of pigmented SK can display 1 or more melanocytic features, the most frequent of which is a “false” pigment network. Recently, a series of atypical appearance of SKs by dermoscopy has been reported, highlighting the importance of pathological confirmation in the case of any atypical finding. Lin and colleagues proposed a modified dermoscopic algorithm for SK diagnosis. It is necessary to (1) carefully evaluate the entire lesion to ensure that the dermoscopic findings are all consistent with the diagnosis and (2) remember that none of the SK dermoscopic features is specific to SK—ie, any of these features can be found in other skin tumors.
As has been reported in previous studies, the existence of melanocytic criteria (pigment network, globules and dots, streaks), blue-black color, blue-white veil, and regressive phenomena in an otherwise solitary SK-like lesion should be of concern. In addition to these dermoscopic clues, good-quality dermoscopic examination by means of immersion liquid and enough light is important in cases of a heavily keratinized or pigmented surface, respectively.
The association between comedo-like openings and folliculotropism is interesting because this histopathologic feature could imply a pitfall for Breslow thickness and for the response to nonsurgical treatments. According to our data, patients without true SK (57 [44.5%]) are more likely to present SK-like melanomas with clear-cut features of melanoma. On the other hand, those patients with hundreds of SKs could present melanomas that completely resemble their other SKs.
Limitations
Limitations of this study derive from its retrospective design, which is based on excised melanomas and thus does not allow further insights into their clinical history or overall clinical context. The interobserver agreement for both the single dermoscopic findings and the scores of algorithms was weak, which could be caused by the inherent difficulty to diagnose such lesions; this was the reason we randomly chose only 1 observer’s evaluations. Further prospective analysis of SKs could provide an accurate description of the clinical and dermoscopic features in SK-like melanomas described here. Moreover, we did not test the diagnostic accuracy of criteria in a mixed sample containing other lesions, such as SKs, keratinocyte skin cancers, and basal cell carcinomas.
Conclusions
Many practitioners, even nondermatologists, remove typical-looking SKs without the use of dermoscopy and without confirming the diagnosis with pathologic analysis. This study supports the view that a proportion of melanomas may resemble SK. Dermoscopy enables clinicians to correctly identify the melanocytic nature of these tumors by exhibiting either the criteria associated with them or the blue-black sign. Furthermore, many patients older than 50 years frequently are examined for multiple widespread SKs combined with solar lentigines and other sun-induced skin conditions. In this context, it can be challenging to perform a thorough whole-skin examination and to detect suspicious lesions easily hidden among so many SKs. All lesions of patients with numerous SKs need to be assessed dermoscopically to increase the possibility of detecting the odd SK-like melanoma.
eTable 1. Demographics and Characteristics of Melanomas Resembling Seborrheic Keratosis
eTable 2. Total Dermoscopy Score (TDS) and 7-Point Checklist Score for the Whole Series and Specifically for Those 24 Cases Considered as SKs also by Dermoscopy (Referred as Dermoscopically SK-like MMs)
References
- 1.Izikson L, Sober AJ, Mihm MC Jr, Zembowicz A. Prevalence of melanoma clinically resembling seborrheic keratosis: analysis of 9204 cases. Arch Dermatol. 2002;138(12):1562-1566. [DOI] [PubMed] [Google Scholar]
- 2.Lim C. Seborrhoeic keratoses with associated lesions: a retrospective analysis of 85 lesions. Australas J Dermatol. 2006;47(2):109-113. [DOI] [PubMed] [Google Scholar]
- 3.Braun RP, Rabinovitz HS, Krischer J, et al. Dermoscopy of pigmented seborrheic keratosis: a morphological study. Arch Dermatol. 2002;138(12):1556-1560. [DOI] [PubMed] [Google Scholar]
- 4.Lin J, Han S, Cui L, et al. Evaluation of dermoscopic algorithm for seborrhoeic keratosis: a prospective study in 412 patients. J Eur Acad Dermatol Venereol. 2014;28(7):957-962. [DOI] [PubMed] [Google Scholar]
- 5.Kuehnl-Petzoldt C, Berger H, Wiebelt H. Verrucous-keratotic variations of malignant melanoma: a clinicopathological study. Am J Dermatopathol. 1982;4(5):403-410. [PubMed] [Google Scholar]
- 6.Kamino H, Tam ST, Alvarez L. Malignant melanoma with pseudocarcinomatous hyperplasia—an entity that can simulate squamous cell carcinoma: a light-microscopic and immunohistochemical study of four cases. Am J Dermatopathol. 1990;12(5):446-451. [DOI] [PubMed] [Google Scholar]
- 7.Zabel RJ, Vinson RP, McCollough ML. Malignant melanoma arising in a seborrheic keratosis. J Am Acad Dermatol. 2000;42(5, pt 1):831-833. [DOI] [PubMed] [Google Scholar]
- 8.Argenziano G, Rossiello L, Scalvenzi M, et al. Melanoma simulating seborrheic keratosis: a major dermoscopy pitfall. Arch Dermatol. 2003;139(3):389-391. [DOI] [PubMed] [Google Scholar]
- 9.Thomas I, Kihiczak NI, Rothenberg J, Ahmed S, Schwartz RA. Melanoma within the seborrheic keratosis. Dermatol Surg. 2004;30(4, pt 1):559-561. [DOI] [PubMed] [Google Scholar]
- 10.Carrera C, Segura S, Palou J, et al. Seborrheic keratosislike melanoma with folliculotropism. Arch Dermatol. 2007;143(3):373-376. [DOI] [PubMed] [Google Scholar]
- 11.Braga JCT, Scope A, Klaz I, Mecca P, Spencer P, Marghoob AA. Melanoma mimicking seborrheic keratosis: an error of perception precluding correct dermoscopic diagnosis. J Am Acad Dermatol. 2008;58(5):875-880. [DOI] [PubMed] [Google Scholar]
- 12.Haruna K, Suga Y, Mizuno Y, Ikeda S. Malignant melanoma with a seborrheic keratosis-like clinical presentation. Indian J Dermatol. 2009;54(4):387-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defazio J, Zalaudek I, Busam KJ, Cota C, Marghoob A. Association between melanocytic neoplasms and seborrheic keratosis: more than a coincidental collision? Dermatol Pract Concept. 2012;2(2):202a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salerni G, Alonso C, Gorosito M, Fernández-Bussy R. Seborrheic keratosis-like melanoma. J Am Acad Dermatol. 2015;72(1)(suppl):S53-S55. [DOI] [PubMed] [Google Scholar]
- 15.Longo C, Moscarella E, Piana S, et al. Not all lesions with a verrucous surface are seborrheic keratoses. J Am Acad Dermatol. 2014;70(6):e121-e123. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira A, Arzberger E, Massone C, Carrera C, Zalaudek I. Verrucous melanoma simulating melanoacanthoma: dermoscopic, reflectance confocal microscopic and high-definition optical coherence tomography presentation of a rare melanoma variant. Australas J Dermatol. 2016;57(1):72-73. [DOI] [PubMed] [Google Scholar]
- 17.Nachbar F, Stolz W, Merkle T, et al. The ABCD rule of dermatoscopy: high prospective value in the diagnosis of doubtful melanocytic skin lesions. J Am Acad Dermatol. 1994;30(4):551-559. [DOI] [PubMed] [Google Scholar]
- 18.Argenziano G, Fabbrocini G, Carli P, De Giorgi V, Sammarco E, Delfino M. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions: comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134(12):1563-1570. [DOI] [PubMed] [Google Scholar]
- 19.Carrera C, Marchetti MA, Dusza SW, et al. Validity and reliability of dermoscopic criteria used to differentiate nevi from melanoma: a web-based International Dermoscopy Society study. JAMA Dermatol. 2016;152(7):798-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argenziano G, Longo C, Cameron A, et al. Blue-black rule: a simple dermoscopic clue to recognize pigmented nodular melanoma. Br J Dermatol. 2011;165(6):1251-1255. [DOI] [PubMed] [Google Scholar]
- 21.Balagula Y, Braun RP, Rabinovitz HS, et al. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and nonmelanocytic lesions. J Am Acad Dermatol. 2012;67(2):194.e1-194.e8. [DOI] [PubMed] [Google Scholar]
- 22.Shitara D, Ishioka P, Alonso-Pinedo Y, et al. Shiny white streaks: a sign of malignancy at dermoscopy of pigmented skin lesions. Acta Derm Venereol. 2014;94(2):132-137. [DOI] [PubMed] [Google Scholar]
- 23.Lallas A, Apalla Z, Moscarella E, et al. Extensive regression in pigmented skin lesions: a dangerous confounding feature. Dermatol Pract Concept. 2012;2(2):202a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy, part II: nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):377-386. [DOI] [PubMed] [Google Scholar]
- 25.de Giorgi V, Grazzini M, Savarese I, et al. What can hide under exophytic verrucous appearance? Acta Derm Venereol. 2011;91(1):100-101. [DOI] [PubMed] [Google Scholar]
- 26.Blessing K, Evans AT, al-Nafussi A. Verrucous naevoid and keratotic malignant melanoma: a clinico-pathological study of 20 cases. Histopathology. 1993;23(5):453-458. [DOI] [PubMed] [Google Scholar]
- 27.De Giorgi V, Massi D, Stante M, Carli P. False “melanocytic” parameters shown by pigmented seborrheic keratoses: a finding which is not uncommon in dermoscopy. Dermatol Surg. 2002;28(8):776-779. [DOI] [PubMed] [Google Scholar]
- 28.Squillace L, Cappello M, Longo C, Moscarella E, Alfano R, Argenziano G. Unusual dermoscopic patterns of seborrheic keratosis. Dermatology. 2016;232(2):198-202. [DOI] [PubMed] [Google Scholar]
- 29.Puig S, Argenziano G, Zalaudek I, et al. Melanomas that failed dermoscopic detection: a combined clinicodermoscopic approach for not missing melanoma. Dermatol Surg. 2007;33(10):1262-1273. [DOI] [PubMed] [Google Scholar]
- 30.Seidenari S, Longo C, Giusti F, Pellacani G. Clinical selection of melanocytic lesions for dermoscopy decreases the identification of suspicious lesions in comparison with dermoscopy without clinical preselection. Br J Dermatol. 2006;154(5):873-879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographics and Characteristics of Melanomas Resembling Seborrheic Keratosis
eTable 2. Total Dermoscopy Score (TDS) and 7-Point Checklist Score for the Whole Series and Specifically for Those 24 Cases Considered as SKs also by Dermoscopy (Referred as Dermoscopically SK-like MMs)