Key Points
Question
What are the prevalence of both genital human papillomavirus (HPV) infection and the HPV vaccination rate among adult men in the United States?
Findings
In this cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2013-2014, the overall genital HPV infection prevalence among 1868 men was 45.2%, which appears to be widespread among all age groups, with a low HPV vaccination rate of 10.7%.
Meaning
Male HPV vaccination may have a greater effect on HPV transmission and cancer prevention in men and women than previously estimated.
Abstract
Importance
Human papillomavirus (HPV) is a common sexually transmitted infection that is a major cause of noncervical anogenital and oropharyngeal cancers. Prophylactic HPV vaccine is available for primary prevention. However, the population prevalence data for male genital HPV infection is not well known, while the HPV vaccination coverage is low in the United States.
Objectives
To estimate the prevalence of genital HPV infection and the HPV vaccination rate in the United States among adult men and to examine potential risk factors for HPV infection.
Design, Setting, and Participants
The National Health and Nutrition Examination Survey (NHANES) samples a representative cross-section of the US population. Men aged 18 to 59 years were examined in mobile examination centers during the NHANES 2013-2014. DNA was extracted from self-collected penile swab specimens, and HPV genotyping was performed by polymerase chain reaction amplification. Demographic and vaccination information was gathered via self-report during home-based standardized interviews. Binary multivariable logistic regression was used to estimate the odds of HPV infection.
Main Outcomes and Measures
The prevalence of genital HPV infection and the HPV vaccination coverage rate among adult men.
Results
During the NHANES 2013-2014, a total of 1868 men aged 18 to 59 years were examined. The overall genital HPV infection prevalence was 45.2% (95% CI, 41.3%-49.3%). The infection prevalence with at least 1 high-risk HPV subtype defined by DNA testing was 25.1% (95% CI, 23.0%-27.3%). In vaccine-eligible men, the prevalence of infection with at least 1 HPV strain targeted by the HPV 4-valent vaccine and HPV 9-valent vaccine was 7.1% (95% CI, 5.1%-9.5%) and 15.4% (95% CI, 11.7%-19.6%), respectively. Among vaccine-eligible men, the HPV vaccination coverage was 10.7% (95% CI, 7.8%-14.6%).
Conclusions and Relevance
Among men aged 18 to 59 years in the United States, the overall prevalence of genital HPV infection was 45.2% (95% CI, 41.3%-49.3%). The overall genital HPV infection prevalence appears to be widespread among all age groups of men, and the HPV vaccination coverage is low.
This cross-sectional study of the NHANES 2013-2014 estimates the prevalence of genital human papillomavirus (HPV) infection and the HPV vaccination rate in the United States among adult men and examines potential risk factors for HPV infection.
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States. Epidemiological studies have established HPV infection as the central cause of various cancers. In the United States alone, 79 million persons are estimated to be infected with some type of HPV, with approximately half of new infections occurring before age 24 years. It is estimated that more than 9000 cases of HPV-related cancers occur in men annually, responsible for 63% of penile, 91% of anal, and 72% of oropharyngeal cancers, in addition to being an indirect causal factor for cervical cancer via men serving as reservoirs for HPV transmission. Low-risk HPV infection is not without consequence, with HPV-6 and HPV-11 responsible for 90% of genital warts, and 160 000 men are affected annually with low-risk HPV. In addition, HPV infection may lead to recurrent respiratory papillomatosis.
Since 2006, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) has recommended HPV vaccination for adolescent girls at age 11 years, with catch-up vaccination to age 26 years. However, male vaccination was not approved by the US Food and Drug Administration until 2009, and the ACIP provided guidance on male HPV vaccination for genital warts prevention. This guidance was further expanded in 2011 to include routine HPV vaccination to decrease HPV transmission and cancer prevention in male individuals aged 11 to 26 years. Recently, a 9-valent HPV vaccine has been approved for HPV-related cancers that includes high-risk HPV subtypes 31, 33, 45, 52, and 58, in addition to strains 6, 11, 16, and 18 of HPV. Furthermore, only 2 doses of HPV vaccine are recommended for young adolescents instead of the traditional 3-shot series. Although male HPV vaccination programs have been available to the public since 2009, the male genital HPV infection prevalence is still not clear epidemiologically, and the vaccination rate remains low in the United States.
The prevalence of oral HPV infection is low compared with genital infections, occurring in 10.1% of men and 3.6% of women, although HPV-associated oropharyngeal cancers have significantly increased over time. With the current trend, the annual incidence of HPV-related oropharyngeal cancers is expected to surpass the annual number of cervical cancers by 2020 despite the availability of an efficacious prophylactic vaccine against HPV.
While the prevalence of female genital HPV infection and the female HPV vaccination coverage in the United States are well known, there are limited population data regarding genital HPV infection in male individuals. In this study, we report the first population-based study to date of the genital HPV infection prevalence among US male adults.
Methods
Study Population and Design
The National Health and Nutrition Examination Survey (NHANES) is a series of ongoing cross-sectional surveys conducted by the National Center for Health Statistics (NCHS) of the CDC. The NHANES sampling procedure oversampled targeted populations, such as Hispanics, non-Hispanic blacks, non-Hispanic Asians, older adults, and low-income persons, to obtain adequate samples for meaningful subgroup analyses and more reliable variable estimates. Demographic information, including race/ethnicity, educational level, household income, and sexual history, was obtained during the home-based interview via self-report. A total of 1868 men aged 18 to 59 years participated in both the NHANES 2013-2014 interview and the mobile examination center component. Of these individuals, 111 men had inadequate laboratory samples and were excluded from the HPV infection analysis, but they were retained for analyses of the self-reported vaccination rate. To account for unequal selection probabilities among participants and adjustments for nonresponse, all estimates were weighted as provided by the NCHS. The protocol was approved by the NCHS institutional review board, and written informed consent was obtained from all participants. The design and weighting methods have been previously described.
Specimen Collection and Processing
During examinations, participants self-collected a penile swab sample from the glans. These swabs were stored under refrigerated conditions (2°C-8°C) before shipment to the CDC for HPV genotyping.
HPV Risk
We used the established classification of high-risk and low-risk HPV to categorize those that cause or are associated with various cancers. The following HPV types were labeled as low-risk HPV: types 6, 11, 40, 42, 54, 55, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, 89, and IS39. High-risk subtypes included 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82. In addition, participants positive for at least 1 of the 14 high-risk HPV subtypes assessed in DNA testing (subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) were grouped for separate analysis. Low risk is classified as the presence of at least 1 low-risk type without a high-risk subtype. Participants with no detected HPV infections but with valid positive β-globin control were considered negative for genital HPV infection. Samples negative for HPV with negative β-globin control were considered inadequate for interpretation.
Statistical Analysis
All analyses were conducted using mobile examination center sample weights to account for the complex survey design and provide accurate estimates of the HPV prevalence among the noninstitutionalized US population. The prevalence estimates are reported as percentages with 95% CIs. Rao-Scott χ2 tests of independence were performed in accord with CDC analytic guidelines. Odds ratios (ORs) with 95% Wald CIs were estimated using binary multivariable logistic regression models to assess potential associations between the genital HPV infection prevalence and respondent characteristics. Covariates in the reduced model included age, race/ethnicity, educational level, marital status, circumcision, and age at first sexual intercourse. Men aged 18 to 32 years were considered eligible for vaccination, while the remainder (aged 33-59 years) were not eligible.
Univariate statistical analyses were weighted and conducted using statistical software (SPSS Complex Samples, version 21; IBM). Multivariable analyses were conducted with another software program (SAS, version 9.4; SAS Institute Inc). Variances were adjusted by Taylor series linearization. Two-tailed P < .05 was considered statistically significant. An institutional review board waiver was obtained in accord with the use of publicly available deidentified data.
Results
Demographic characteristics of 1757 male participants (age range, 18-59 years) in the NHANES 2013-2014 with valid HPV genotyping are listed in eTable 1 in the Supplement. Characteristics of these participants were similar to those with noninterpretable HPV genotyping results.
Prevalence
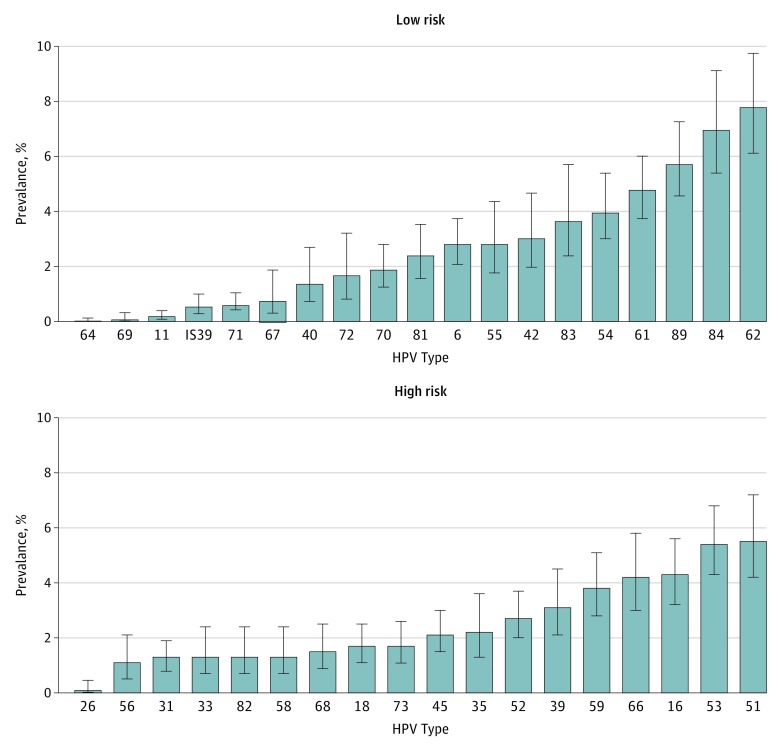
The overall genital HPV infection prevalence for men aged 18 to 59 years was 45.2% (95% CI, 41.3%-49.3%), and the high-risk HPV prevalence defined by DNA testing was 25.1% (95% CI, 23.0%-27.3%). The genital HPV infection prevalence appeared to follow a bimodal pattern, with a peak in infection among men aged 28 to 32 years and a second higher peak among men aged 58 to 59 years (eTable 1 in the Supplement). Prevalence rates of specific high-risk and low-risk HPV are shown in the Figure. The most prevalent high-risk HPV subtype detected was 51 (5.5%; 95% CI, 4.2%-7.2%). The overall prevalence of HPV 6, 11, 16, and 18 found in the 4-valent vaccine was 2.7% (95% CI, 2.0%-3.6%), 0.2% (95% CI, 0.1%-0.4%), 4.3% (95% CI, 3.2%-5.6%), and 1.7% (95% CI, 1.1%-2.5%), respectively. The overall prevalence of the additional 5 HPV subtypes 31, 33, 45, 52, and 58 found in the 9-valent vaccine was 1.3% (95% CI, 0.8%-1.9%), 1.3% (95% CI, 0.7%-2.4%), 2.1% (95% CI, 1.5%-3.0%), 2.7% (95% CI, 2.0%-3.7%), and 1.3% (95% CI, 0.7%-2.4%), respectively.
Figure. Prevalence of HPV Strains Among Men Aged 18 to 59 Years in the NHANES 2013-2014.
Error bars show 95% CIs. HPV indicates human papillomavirus; NHANES, National Health and Nutrition Examination Survey.
The HPV prevalence with at least 1 of the 4-valent HPV types among adults aged 18 to 59 years was 8.5% (95% CI, 7.1%-10.0%), representing more than 6.5 million men in the United States. The overall prevalence of infection with at least 1 of the 9-valent HPV subtypes was 15.1% (95% CI, 13.7%-16.6%) (eTable 2 in the Supplement). Among those aged 18 to 32 years who are or would have been eligible for the vaccine, the prevalence of infection with at least one 4-valent HPV type or 9-valent HPV subtype was 7.1% (95% CI, 5.1%-9.5%) and 15.4% (95% CI, 11.7%-19.6%), respectively. The prevalence of 9-valent HPV was similarly elevated among the vaccine-ineligible group at 14.6% (95% CI, 12.5%-16.9%). Bivariate analysis indicated no difference in prevalence based on vaccine age eligibility among 4-valent HPV types (P = .17 for comparison) and 9-valent HPV subtypes (P = .73 for comparison).
We found that the overall HPV-16 prevalence was 4.3% (95% CI, 3.2%-5.6%), which represents 3.3 million men in the United States. The HPV-18 prevalence was 1.7% (95% CI, 1.1%-2.5%), which reflects 1.3 million men. The infection prevalence solely among those aged 18 to 32 years was 3.3% (95% CI, 2.2%-5.0%) for HPV-16 and 1.3% (95% CI, 0.6%-2.6%) for HPV-18. There was no difference between vaccine-eligible and vaccine-ineligible groups (P = .27 for comparison for HPV-16 and P = .25 for comparison for HPV-18).
The distribution and prevalence of genital high-risk HPV infection were similar between vaccine-eligible and vaccine-ineligible groups at 29.5% (95% CI, 25.0%-34.3%) and 29.1% (95% CI, 26.4%-33.0%), respectively (eTable 2 in the Supplement). The overall prevalence of HPV infection was lowest among men aged 18 to 22 years at 28.9% (95% CI, 22.2%-36.8%). Most men infected with only low-risk HPV (79.1%; 95% CI, 73.0%-84.2%) showed single-strain infection compared with the high-risk group, who showed single-strain infection at 36.4% (95% CI, 31.1%-42.1%) (Table 1).
Table 1. HPV Infections by Classified Risk.
| No. of HPV Strains Detected | HPV Infection, % (95% CI) | |
|---|---|---|
| Low Risk | High Risk | |
| 1 | 79.1 (73.0-84.2) | 36.4 (31.1-42.1) |
| 2 | 16.3 (11.1-23.2) | 26.3 (22.6-30.3) |
| 3 | 3.0 (1.2-7.4) | 16.9 (13.0-21.7) |
| 4 | 1.6 (0.5-5.1) | 9.7 (6.7-13.9) |
| ≥5 | 0.0 (0.0-0.0) | 10.7 (7.7-14.5) |
Abbreviation: HPV, human papillomavirus.
Factors Associated With HPV Infection
Demographic characteristics associated with genital HPV infection in univariate analysis included age (P < .001), race/ethnicity (P < .001), marital status (P < .001), educational level (P < .002), and age at first sexual intercourse (P < .001) (P value for trend). Annual household income (P = .05) and smoking status (P = .05) were marginally associated with genital HPV infection. Circumcision was also marginally associated with such infection (P = .03). The results were similar in the high-risk HPV DNA testing group except for circumcision (P = .01) and smoking status (P = .35). The genital HPV infection prevalence was highest among non-Hispanic black men (65.0%; 95% CI, 59.7%-70.0%) and lowest among non-Hispanic Asian men (24.4%; 95% CI, 18.4%-31.5%).
In multivariable analyses, men in older age groups were approximately twice as likely to have genital HPV infection compared with those aged 18 to 22 years. This increased risk was apparent in those aged 23 to 32 years only when the analysis was limited to the 14 high-risk HPV subtypes (Table 2). Compared with those with less than a high school education, those with a high school diploma or general equivalency diploma were approximately 40% more likely to have these infections (OR, 1.4; 95% CI, 1.0-2.1) in the high-risk group. Compared with married men, men who reported never having been married, living with a partner, or being widowed, divorced, or separated from a spouse were twice as likely to have genital HPV infections. In the high-risk group, this prevalence increased to 2.8 times (OR, 2.8; 95% CI, 1.7-4.7) if widowed, divorced, or separated from a spouse.
Table 2. Multivariable Analysis of Characteristics Associated With All HPV and High-Risk Only HPV Infections Among Men Aged 18-59 Yearsa.
| Characteristic | Any HPV | High Risk by DNA Testingb | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age, y | ||||
| 8-22 | 1 [Reference] | .01 | 1 [Reference] | .04 |
| 23-32 | 2.3 (1.3-3.9) | 2.0 (0.9-4.5) | ||
| 33-42 | 1.8 (1.0-3.1) | 1.5 (0.8-2.9) | ||
| 43-59 | 2.6 (1.4-4.6) | 0.9 (1.0-4.4) | ||
| Race/ethnicity | ||||
| Non-Hispanic white | 1 [Reference] | .93 | 1 [Reference] | .76 |
| Hispanic | 0.8 (0.5-1.3) | 1.1 (0.8-1.6) | ||
| Non-Hispanic black | 1.6 (1.1-2.4) | 1.7 (1.2-2.5) | ||
| Non-Hispanic Asian | 0.7 (0.5-1.2) | 0.9 (0.4-1.7) | ||
| Other, including multiracial | 0.7 (0.3-1.7) | 0.6 (0.3-1.5) | ||
| Educational level | ||||
| <High school | 1 [Reference] | .09 | 1 [Reference] | .01 |
| High school or general equivalency diploma | 1.5 (0.9-2.3) | 1.4 (1.0-2.1) | ||
| >High school | 0.9 (0.6-1.3) | 0.8 (0.6-1.0) | ||
| Marital status | ||||
| Married | 1 [Reference] | <.001 | 1 [Reference] | <.001 |
| Never married | 1.9 (1.2-3.0) | 2.7 (1.9-4.0) | ||
| Living with partner | 1.8 (1.2-2.7) | 2.3 (1.4-3.7) | ||
| Widowed, divorced, or separated | 2.3 (1.4-4.0) | 2.8 (1.7-4.7) | ||
| Circumcision | ||||
| Circumcised | 1 [Reference] | .14 | 1 [Reference] | .05 |
| Uncircumcised | 0.8 (0.6-1.1) | 0.7 (0.5-1.1) | ||
| Age at first sexual intercourse, y | ||||
| <16 | 1 [Reference] | <.001 | 1 [Reference] | .02 |
| 16-18 | 0.7 (0.6-1.1) | 0.8 (0.6-1.2) | ||
| >18 | 0.5 (0.4-0.7) | 0.5 (0.3-1.0) | ||
Abbreviations: HPV, human papillomavirus; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio.
Includes 1430 participants with complete information.
High-risk HPV defined by DNA testing. Participants were positive for at least 1 of the following HPV subtypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
Vaccination
The overall rate of HPV vaccination among men who are or were vaccine eligible was 10.7% (95% CI, 7.8%-14.6%) among an unweighted sample of 729 participants. This sample represents 3 million men aged 18 to 32 years in the noninstitutionalized US population. Among men aged 18 to 22 years, 22.0% (95% CI, 15.5%-30.3%) reported having received an HPV vaccination, and 48.1% (95% CI, 26.0%-71.0%) completed the series, with a mean age at HPV vaccination of 17 years (95% CI, 16.4-17.8 years).
Discussion
In this nationally representative sample of men aged 18 to 59 years, the prevalence of overall genital HPV infection in the United States was 45.2% (95% CI, 41.3%-49.3%), representing 34.8 million men, with the high-risk HPV prevalence at 25.1% (95% CI, 23.0%-27.3%). The lowest prevalence was 28.9% (95% CI, 22.2%-36.8%) among men aged 18 to 22 years, which increased to 46.5% (95% CI, 38.4%-54.7%) in the next age group (23-27 years) and then remained high and constant in older age groups (P < .001 for trend). This finding may reflect the current practice of providing HPV vaccination to younger male age groups. This result is in contrast to the female HPV prevalence, which was higher among the age group younger than 20 years and then decreased in later years.
The prevalence of genital HPV infection followed a bimodal pattern, with a peak in prevalence among men aged 28 to 32 years and a second higher peak among men aged 58 to 59 years. This pattern of infection is similar to the prevalence of oral HPV infection from the NHANES 2009-2010 previously reported. The overall HPV prevalence is similar to recent research from Denmark, which reported a 41.8% overall HPV prevalence, with the same high-risk HPV subtype 51 as the most prevalent. In female individuals, the most prevalent HPV subtype was 53, and subtype 51 was the fourth most common. Furthermore, the most prevalent HPV subtype may not reflect the putative potency of carcinogenesis. This inconsistency of the most prevalent high-risk HPV subtype in infection and in cancer may reflect different aggressiveness of HPV subtypes because common HPV subtypes in cancer are 16 and 18, which are responsible for 70% of all anogenital cancers. This overall HPV prevalence was found to be lower than that among the multinational population study that combined Mexico, Brazil, and the United States, which reported a prevalence of 65.2%. The prevalence difference may also be owing to the types of tests that were used, the location of the male genital area swabbed, or the study population.
In addition, the Denmark study showed the highest HPV infection prevalence among men aged 20 to 29 years in contrast to our study, which demonstrated a lower prevalence among younger groups, with a higher and persistent prevalence among older age groups. It may be that the cumulative prevalence of persistent infections would be expected to increase with age, as was shown in oral HPV infection. It is thought that the lower immune response to natural infection in male individuals may explain the higher prevalence of HPV infections, with a wide age range in boys and men.
Clearance of genital HPV infection in men has been reported to occur between 6 and 18 months, which is comparable to that in female individuals. However, male individuals have lower circulating HPV antibodies than female individuals despite a higher genital HPV infection prevalence. This finding may explain the difference in HPV immune responses between the sexes. The HPV Infection in Men (HIM) cohort study reported that the number of partners and new partners in the last 3 months was similar for all age groups, hence potentially providing continued exposure to HPV throughout life in male individuals. Therefore, if men generate a lower and weaker HPV immune response in the setting of remaining at high risk of acquiring new HPV infections throughout their lives, then vaccination programs for older men might be warranted.
Our study revealed independent risk factors for high-risk genital HPV infection, including demographic characteristics of age, educational level, marital status, and sexual behavior at an early age. An analysis of the number of lifetime sex partners was not possible because of a low response rate, suggesting that the self-reported sexual history may not be accurate. In addition, current tobacco use was not associated with male genital HPV infection, although it is a known risk for female genital and oropharyngeal infection. Educational level was independently associated with the high-risk HPV group, but the increased risk decreased to baseline in the group with higher education, with a 95% CI close to 1.0. With the high prevalence of HPV among the general population, sexually active male individuals are at risk of transmission. Therefore, educational level may not lower the risk of HPV infection as seen in other sexually transmitted infections.
Historically, circumcision is known to lower HPV infection risk. Our analysis revealed a higher risk of HPV infection with circumcision, although statistical significance was not met in multivariable analysis. This finding may be due to increased exposure of the mucosal surface of the glans in circumcised male individuals, which is the area obtained for genital HPV swab specimens in this study. In general, circumcision is known to lower the risk of sexually transmitted infections, which may not be as clear in HPV secondary to subclinical relevance with a high prevalence rate. When high-risk and low-risk HPV strains are classified separately, most low-risk HPV represented infection of a single type (79.1%; 95% CI, 73.0%-84.2%) compared with high-risk HPV infection, which frequently involved multiple strains (63.6%; 95% CI, 57.9%-68.9%). High-risk HPV subtypes may be more aggressive secondary to concurrent infections of multiple strains.
The 9-valent HPV vaccine was approved by the US Food and Drug Administration in late 2014 for male and female individuals up to age 26 years to cover 90% of HPV types responsible for cervical cancer, with recent requirement of only 2 vaccines for young adolescents. The present investigation is the first population-based study to date in the United States to examine the prevalence of the 9-valent HPV subtypes (15.1%; 95% CI, 13.7%-16.6%) among men, which remained elevated throughout all ages. To our knowledge, we are also the first to report the national HPV vaccination coverage among US adult men (10.7%; 95% CI, 7.8%-14.6%), which is significantly lower than the current female vaccination rate.
Only 5.6% (95% CI, 3.2%-8.2%) of the adult male population reported completing the HPV vaccine series. This vaccination rate may reflect low public awareness and the stigma of a sexually transmitted infection vaccine, with concerns about the vaccine’s effect on sexual behavior. The initial HPV vaccine approval for male individuals was obtained for genital warts only, which was expanded later.
The principal strength of this study is that the data come from the NHANES database, which represents 76.9 million male Americans. Limitations include that vaccination history and sexual history were obtained from self-reports, which can lead to underreporting or overreporting. In addition, HPV samples were self-collected. However, there is evidence to suggest that self-collected specimens provide sensitivity and specificity comparable to clinician-collected samples.
Using the NHANES 2013-2014 data, we estimated that more than 25 million eligible male Americans did not receive HPV vaccination. The high HPV prevalence among male individuals suggests that there is opportunity for increased vaccine effectiveness as the HPV vaccination coverage in the population increases. Human papillomavirus vaccination may have a profound effect on the prevention of HPV-related cancers in male and female individuals because one serves as host for the other, in addition to being a direct cause of anogenital and oropharyngeal cancers. Only when vaccination rates are significantly increased will progress be made in eradicating most HPV-related cancers in the United States.
Conclusions
Our study provides the first national estimate to date of the genital HPV infection prevalence among men aged 18 to 59 years in the United States. The overall genital HPV infection prevalence was 45.2% (95% CI, 41.3%-49.3%), and the oncogenic high-risk HPV prevalence was 29.5% (95% CI, 25.0%-34.3%) among the vaccine-eligible group. The overall vaccination rate was only 10.7% (95% CI, 7.8%-14.6%). Our study indicates that male HPV vaccination may have a greater effect on HPV infection transmission and cancer prevention in men and women than previously estimated. Further studies may be warranted to evaluate the rationale regarding the current male vaccination age cutoff.
eTable 1. Prevalence of HPV Infection by Demographic Characteristics
eTable 2. HPV Infection in US Men: Prevalence in the Population and in Defined Risk Groups
References
- 1.Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10. [DOI] [PubMed] [Google Scholar]
- 2.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072-1079. [DOI] [PubMed] [Google Scholar]
- 6.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-193. [DOI] [PubMed] [Google Scholar]
- 7.Juckett G, Hartman-Adams H. Human papillomavirus: clinical manifestations and prevention. Am Fam Physician. 2010;82(10):1209-1213. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Human papillomavirus (HPV). http://www.cdc.gov/hpv/parents/questions-answers.html. Updated November 28, 2016. Accessed August 8, 2016.
- 9.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(suppl 3):35-41. [DOI] [PubMed] [Google Scholar]
- 10.Wiatrak BJ, Wiatrak DW, Broker TR, Lewis L. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope. 2004;114(11, pt 2)(suppl 104):1-23. [DOI] [PubMed] [Google Scholar]
- 11.De Vincenzo R, Ricci C, Conte C, Scambia G. HPV vaccine cross-protection: highlights on additional clinical benefit. Gynecol Oncol. 2013;130(3):642-651. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59(20):630-632. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males: Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention CDC recommends only two HPV shots for younger adolescents. https://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html. Updated October 20, 2016. Accessed November 5, 2016.
- 15.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. National Health and Nutrition Examination Survey: analytic guidelines, 2011-2012. http://www.cdc.gov/nchs/data/nhanes/analytic_guidelines_11_12.pdf. Published September 30, 2013. Accessed August 2, 2016.
- 17.Parsons VL, Moriarity C, Jonas K, Moore TF, Davis KE, Tompkins L. Design and estimation for the National Health Interview Survey, 2006-2015. Vital Health Stat 2. 2014;(165):1-53. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention MEC Laboratory Procedures Manual http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_MEC_Laboratory_Procedures_Manual.pdf. Published January 2013. Accessed August 2, 2016.
- 19.Muñoz N, Bosch FX, de Sanjosé S, et al. ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Key concepts about chi-square test. http://www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/HypothesisTesting/Info3.htm. Accessed August 2, 2016.
- 21.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813-819. [DOI] [PubMed] [Google Scholar]
- 22.Hebnes JB, Munk C, Nøhr B, et al. Human papillomavirus infection among 2460 men in Denmark: prevalence in relation to age using 2 human papillomavirus DNA testing methods. Sex Transm Dis. 2015;42(8):463-467. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The Human Papillomavirus Infection in Men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2036-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males [published correction appears in N Engl J Med. 2011;364(15):1481]. N Engl J Med. 2011;364(5):401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuliano AR, Lu B, Nielson CM, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198(6):827-835. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital Human Papillomavirus Infection in Men (HIM): a cohort study [published correction appears in Lancet. 2011;377(9782):2006]. Lancet. 2011;377(9769):932-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009;200(7):1059-1067. [DOI] [PubMed] [Google Scholar]
- 28.Brouwer AF, Eisenberg MC, Carey TE, Meza R. Trends in HPV cervical and seroprevalence and associations between oral and genital infection and serum antibodies in NHANES 2003-2012. BMC Infect Dis. 2015;15:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194(8):1044-1057. [DOI] [PubMed] [Google Scholar]
- 30.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porras C, Hildesheim A, González P, et al. ; CVT Vaccine Group . Performance of self-collected cervical samples in screening for future precancer using human papillomavirus DNA testing. J Natl Cancer Inst. 2014;107(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Prevalence of HPV Infection by Demographic Characteristics
eTable 2. HPV Infection in US Men: Prevalence in the Population and in Defined Risk Groups