Key Points
Question
What are the clinical features of patients presenting with intravenous drug abuse–related endogenous fungal endophthalmitis during the height of New England’s opioid crisis?
Findings
Medical record review revealed 10 patients treated for intravenous drug abuse–related endogenous fungal endophthalmitis at New England Eye Center from May 2014 to May 2016. All patients were ambulatory at presentation, and 90% had isolated ocular symptoms without systemic signs of infection.
Meaning
Patients with intravenous drug abuse–related endogenous fungal endophthalmitis are typically systemically well on presentation, thus requiring health care professionals to maintain a high index of suspicion to facilitate timely diagnosis and initiation of treatment.
Abstract
Importance
Intravenous drug abuse (IVDA) is a known risk factor for endogenous fungal endophthalmitis (EFE), a severe intraocular infection caused by hematogenous seeding of mycotic organisms to the eye. Reporting significant increases in heroin-related deaths since 2014, the New England region is in the midst of an opioid crisis that has led to a substantial increase in patients at risk for this vision-threatening disease.
Objective
To present an update on characteristics, management, and visual outcomes in patients with EFE.
Design, Setting, and Participants
Medical records review was initiated on July 1, 2016, evaluating all patients with EFE referred to New England Eye Center at Tufts Medical Center, a tertiary care ophthalmology practice distributed throughout Massachusetts, from May 1, 2014, to May 1, 2016. Patients with a history of IVDA and culture-proven or clinical evidence of fungal endophthalmitis were included.
Exposures
Intravenous drug use.
Main Outcomes and Measures
Patient demographics, comorbidities, presenting symptoms and vision, vitreoretinal findings, treatment regimens, culture data, and final visual acuities.
Results
Ten patients (5 women) with IVDA-related EFE were identified between May 1, 2014, and May 1, 2016—an increase from 3 patients treated from May 2012 to April 2014. The mean (SD) patient age was 34 (11) years (range, 24-60 years). Presenting visual acuities ranged from 20/25 to hand motion. The most common presenting symptoms were floaters (n = 8), reduced vision (n = 6), and pain (n = 5). Initial treatment included systemic antifungals in all patients and intravitreal antifungals in 9 eyes. Five patients required pars plana vitrectomy for worsening vitritis. The most commonly isolated pathogen was Candida albicans in 20% of the patients. Final visual acuity ranged from 20/40 to 20/300.
Conclusions and Relevance
The data provided in this report suggest that EFE represents severe end organ damage associated with IVDA and portends poor visual outcomes. Health care professionals must maintain a high suspicion for EFE, as patients are typically ambulatory on presentation without systemic signs of infection.
This case series describes 10 patients with intravenous drug abuse who developed and underwent treatment for endogenous fungal endophthalmitis.
Introduction
During the past decade, increasing rates of opioid abuse have developed in regions throughout the United States, including the New England area (Massachusetts, Maine, Connecticut, New Hampshire, Rhode Island, and Vermont). A sharp rise in opioid-related deaths since 2014 has signaled a crisis throughout Massachusetts, prompting an increased focus on the morbidity, mortality, and health care utilization associated with intravenous drug abuse (IVDA). Opioid abusers are at increased risk for serious infections related to the intravenous route of administration. Viral hepatitis, human immunodeficiency virus, infectious endocarditis, and septic arthritis are all well-described sequelae of IVDA. Similarly, IVDA can lead to a vision-threatening endogenous fungal endophthalmitis (EFE) caused by the hematogenous dissemination of pathogens to the eye. This infection represents end-organ damage of IVDA and portends a poor visual outcome and substantial morbidity.
In EFE, mycotic organisms reach the eye by seeding of the choroidal circulation, leading to an infectious chorioretinitis. Funduscopic examination may demonstrate a variety of findings, including creamy chorioretinal lesions in the posterior pole, diffuse vitreous inflammation, and/or focal vitreous opacities forming a “string of pearls.” Unfortunately, clinical findings can also be nonspecific, emulating noninfectious forms of uveitis. In addition, patients often experience chronic, vague symptoms of reduced vision, redness, and pain, leading to a delay in diagnosis and treatment. At the New England Eye Center at Tufts Medical Center in Boston, a substantial increase in cases of EFE has been noted since May 2014, prompting investigation into predisposing risk factors, specifically IVDA. Given the morbidity associated with EFE, an update on patient characteristics, treatment, and outcomes is crucial to the management of care for future patients with this vision-threatening disease.
Methods
A medical records review beginning July 1, 2016, was performed identifying all patients referred with endogenous endophthalmitis from May 1, 2014, to May 1, 2016, to the New England Eye Center, which is the ophthalmology department for Tufts Medical Center in Boston and includes 7 satellite offices throughout Massachusetts. This study was approved by the institutional review board at Tufts Medical Center with waiver of informed consent. The research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996.
Billing and electronic health records were screened by International Classification of Diseases, Ninth Revision code 360 and International Statistical Classification of Diseases, Tenth Revision code H44 for endophthalmitis. Patients presenting with an exogenous cause of endophthalmitis, such as postsurgical or posttraumatic etiology, were excluded. Only patients with a history of intravenous drug use who had fundus findings characteristic of endogenous endophthalmitis and/or positive ocular or systemic fungal cultures were included. Medical records were reviewed for patient demographics, comorbidities, presenting symptoms, presenting visual acuity (VA), treatment regimens, and culture data. Vitreoretinal findings on initial presentation and throughout treatment were reviewed. The main outcome measured was final best-corrected VA.
Results
Ten eyes of 10 patients met all inclusion criteria over the study interval for this analysis. Two patients with a history of IVDA and endophthalmitis were excluded because cultures were nondiagnostic for a fungal cause, and clinical evidence suggested a polymicrobial infection. In 1 patient, blood and ocular cultures grew Serratia marcescens and sputum cultures grew Candida albicans; in the other patient, blood cultures grew methicillin-sensitive Staphylococcus aureus and ocular cultures were negative. One patient with a history of recent IVDA, who presented with a retinal detachment and clinical evidence of endophthalmitis following prolonged therapy for uveitis at another institution, was excluded given an inability to qualitatively describe clinical findings, perform imaging, or accurately monitor response to therapy. Two patients presenting with endophthalmitis after recent ureteral stent placement were excluded since there was no history of IVDA.
Patient characteristics and clinical findings are detailed in the Table. The mean (SD) patient age was 34 (11) years (range, 24-60 years); 5 patients were female. All patients resided in the New England region: Massachusetts (n = 7), Maine (n = 2), and Vermont (n = 1). All patients were referred to New England Eye Center for retinal evaluation. Three patients initially denied intravenous drug use; however, all patients eventually reported prior IVDA. Six patients had a history of intravenous heroin use, 1 heroin user also reported intravenous cocaine use, and 1 patient used intravenous buprenorphine with naloxone. Three patients did not specify which intravenous drug they used. All patients were ambulatory at presentation. Nine patients were clinically well, lacking systemic signs or symptoms of infection, and the tenth patient reported fevers and night sweats. One woman was pregnant on presentation. All patients were negative for human immunodeficiency virus. Four patients were known to be hepatitis C positive, and another patient received a new diagnosis of hepatitis C during her inpatient hospitalization for endophthalmitis. Other comorbidities included type 1 diabetes (n = 2), immunomodulatory therapy (n = 1), end-stage renal disease (n = 1), immunodeficiency (n = 1), laparotomy (n = 1), and indwelling line removal (n = 2).
Table. Clinical Characteristics of Intravenous Drug Abusers Presenting With Endogenous Fungal Endophthalmitis.
| Patient No./Sex/Age, y | Drug Use History | Comorbid Conditions | Presenting VA | Vitreous Findings | Location of Retinal Lesion | Surgical Intervention (Days After Presentation) | Culturesa | Final BCVA | Follow-up, d |
|---|---|---|---|---|---|---|---|---|---|
| 1/Female/30sb | IV heroin | Hepatitis C | 20/100 | Fluffy opacities | Maculac | None | Vitreous tap: Candida dubliniensis Blood culture: coagulase-negative Staphylococcus in 1 of 2 bottles, repeat culture negative Urine culture: negative |
20/300 | 6 |
| 2/Male/40s | IVDA | Type 1 diabetes, psoriatic arthritis (receiving TNF inhibitor) | 20/25 | String of pearls | Peripapillary | None | Vitreous tap: negative Blood culture: negative |
20/200 | 12 |
| 3/Male/20s | IV heroin | None | HM | White opacities | No view | PPV (23 d) Phaco/PPV (58 d) |
AC tap: negative Vitreous biopsy: negative Blood culture: MSSA in 2 of 4 bottles; Candida tropicalis in 1 of 2 bottles |
20/100 | 294 |
| 4/Female/20s | IV heroin | Hepatitis C; recent exploratory laparotomy | 20/200 | Fluffy opacities | Macula | PPV (7 d) | Vitreous tap: negative Vitreous biopsy: negative Blood culture: negative Urine culture: negative |
20/100 | 82 |
| 5/Male/40s | IV heroin | Hepatitis C | 20/400 | Linear opacities | Macula | PPV (7 d) | AC tap: negative Vitreous biopsy: negative Blood culture: negative Urine culture: negative |
20/70 | 208 |
| 6/Female/60s | IVDA | Type 1 diabetes, ESRD, recent indwelling catheter | 20/200 | String of pearls | None | None | Vitreous tap: dry Blood culture: negative |
Lost to follow-up | None |
| 7/Female/20s | IV heroin | None | CF 1.2 m | Debris | Peripapillary, maculac | PPV (10 d) | Vitreous tap: negative Vitreous biopsy: Candida albicans Blood culture: negative |
20/40 | 49 |
| 8/Female/30s | IVDA | Hepatitis C, aplastic anemia (port removal 3 mo prior) |
CF 0.9 m | Fluffy opacities | Macula | None | Vitreous tap: Candida albicans Blood culture: negative |
20/300 | 36 |
| 9 /Male/20s | IV buprenorphine and naloxone | None | CF 0.6 m | String of pearls | None | None | Vitreous tap: negative Refused systemic workup |
Lost to follow-up | None |
| 10/Male/20s | IV cocaine and heroin | Hepatitis C | CF 3.0 m | String of pearls | Macula | PPV (45 d) | Vitreous biopsy: negative Blood culture: negative |
20/50 | 68 |
Abbreviations: AC, anterior chamber; BCVA, best-corrected visual acuity; CF, counting fingers; ESRD, end-stage renal disease; HM, hand motion; IV, intravenous; IVDA, intravenous drug abuse; MSSA, methicillin-sensitive Staphylococcus aureus; Phaco, phacoemulsification (cataract surgery); PPV, pars plana vitrectomy; TNF, tumor necrosis factor; VA, visual acuity.
Vitreous biopsy indicates vitrectomy-based sample; vitreous tap, office-based sample.
Pregnant on presentation.
Multifocal lesions.
All patients were unilaterally affected. The most common presenting symptoms were floaters (n = 8), reduced vision (n = 6), pain (n = 5), and photophobia (n = 3). Presenting VA ranged from 20/25 to the detection of hand motions only. Three patients had a hypopyon on initial slitlamp examination. On dilated fundus examination, 5 eyes presented with a single chorioretinal lesion, 2 eyes with multifocal lesions, and 2 eyes with an isolated vitritis (Figure 1). In 1 eye, a dense vitritis precluded a view of the underlying retina. All eyes had evidence of vitreous inflammation, 4 eyes had the classically described string of pearls formation, and 3 eyes had fluffy “snowball” opacities. Initial chorioretinal lesions were located in the macula of 5 eyes and in the peripapillary region of 1 eye. One eye had both peripapillary and macular lesions.
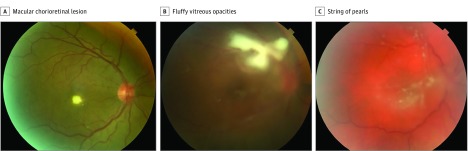
Figure 1. Initial Fundus Examination in 3 Intravenous Drug Abusers.
A, Single macular chorioretinal lesion with localized overlying vitritis (patient 5). B, Chorioretinal lesion in the superior macula with overlying fluffy vitreous opacities (patient 4). C, String of pearls vitritis (patient 10).
Nine patients were admitted to the hospital for inpatient workup and administration of systemic antifungal therapy. One patient refused admission. Nine patients underwent intravitreal injection with an antifungal agent as the primary treatment within 3 days of presentation. The choice of intravitreal agent was at the discretion of the treating retina physician, with 7 eyes receiving voriconazole, 100 μg/0.1 mL, and 2 eyes receiving amphotericin B, 5 μg/0.1 mL. All 9 patients underwent vitreous tap prior to intravitreal injection; 2 patients required anterior chamber tap after dry vitreous tap. One patient initially refused intravitreal treatment but underwent subsequent injection at the time of pars plana vitrectomy (PPV) 45 days after initial presentation. All patients were initially treated with a systemic antifungal agent at the discretion of the consulting infectious disease clinician: intravenous voriconazole in 8 patients, oral voriconazole in 1 patient, and oral fluconazole in 1 patient. Patients were discharged home with oral antifungal therapy for a total mean (SD) treatment duration of 55 (17) days (range, 35-76 days) in patients followed up for more than 2 weeks; 1 patient was followed up by an outside infectious disease clinician.
Five eyes eventually required PPV; the indication in all 5 of these patients was worsening vitritis and/or VA despite intravitreal and systemic antifungal therapy. Visual acuity at the time of PPV ranged from 20/100 to counting fingers at 0.3 m. The mean time from presentation to surgery was 18 (16) days (range, 7-45 days). Four patients had negative vitreous or anterior chamber tap cultures at the time of decision to proceed with PPV. One patient had a blood culture positive for Candida tropicalis at the time of surgery. Another patient, who refused initial tap and intravitreal injection, had negative blood cultures at the time of surgery. All patients underwent vitreous biopsy and received an intravitreal antifungal injection at the time of surgery. One patient required repeat vitrectomy at the time of subsequent cataract extraction. Five patients required a total of 2 intravitreal injections throughout the course of their treatment (all on presentation, 4 with subsequent injection at the time of PPV, and 1 with subsequent injection at a follow-up office visit 6 days after the initial presentation for worsening vitritis). Five patients required only 1 intravitreal injection throughout the course of their treatment (4 on presentation and 1 at the time of PPV). Surgical complications included vitreous hemorrhage (n = 1), cystoid macular edema (n = 1), and cataract (n = 1).
Vitreous or anterior chamber tap sample, taken at the time of initial intravitreal injection, was positive in 2 eyes. A vitreous sample for biopsy taken at the time of PPV was positive in 1 patient who had a previously negative vitreous tap. Blood cultures were positive for fungus in 1 patient. Causative organisms were identified as C albicans (n = 2), C tropicalis (n = 1), and Candida dubliniensis (n = 1). The most commonly isolated pathogen was C albicans in 20% of the patients. Final VA ranged from 20/40 to 20/300. The 5 patients who underwent PPV had a median final VA of 20/70. The 3 patients monitored in our series who did not undergo PPV had a median final VA of 20/300. No patients required enucleation. Mean time from presentation to last follow-up was 94 (103) days (range, 6-294 days). Two patients failed to follow up after initial evaluation. Only 1 patient is still actively receiving follow-up with the New England Eye Center; all other patients were eventually nonadherent to follow-up.
Discussion
Increased prescription of opioids by health care professionals, followed by the growing availability and lower cost of heroin, has contributed to the rise of opioid abuse throughout the New England region over the past 2 decades. A sharp increase in unintentional opioid-associated deaths in 2014 signaled an opioid crisis within Massachusetts communities, prompting increased focus on the morbidity and mortality of opioid addiction. Serious infections related to IVDA, such as endocarditis, human immunodeficiency virus, hepatitis C, and osteomyelitis, have been extensively studied. Endogenous fungal endophthalmitis similarly represents severe end-organ damage associated with IVDA. The New England Eye Center noted a substantial increase in cases of EFE since May 2014, prompting investigation into predisposing risk factors. Intravenous drug abuse was the most common risk factor for the development of EFE among the studied patient population. From May 2014 to May 2016, 10 cases of EFE were attributable to IVDA, which is an increase from only 3 cases seen from May 2012 to April 2014. This occurrence represents a 233% increase in IVDA-associated EFE over the past 2 years, mirroring regional trends of heroin-associated mortality and hospital admissions. This noted increase may also be influenced by the referral patterns to a tertiary care medical center. To our knowledge, there have been no series investigating the clinical characteristics of IVDA-associated EFE in light of the growing opioid epidemic. An update on clinical features and outcomes is mandatory to limit the severe morbidity related to this increasingly prevalent disease.
This present review included only cases of EFE in which a history of intravenous drug use was elicited. The most frequently used substance was heroin. One patient reported recent injection of buprenorphine with naloxone, a medication used to treat opioid addiction. Three patients initially denied any history of use when specifically asked about drug use on presentation. Once patients were informed that knowledge of intravenous drug use was vital to making appropriate treatment decisions, all admitted IVDA. Eliciting a history of IVDA can prove to be vital since the often nonspecific intraocular inflammation seen in EFE can pose a diagnostic challenge. Physicians can utilize systemic clues, such as needle tracks, skin ulcerations, or related comorbidities (eg, history of hepatitis C infection as seen in 5 of our patients) to aid in making the diagnosis. The nonadherence seen in this patient population is important when making treatment decisions. Two patients in our series did not follow up after their initial examination, and an additional 2 patients were lost to follow-up within 2 weeks of diagnosis. All patients received intravitreal antifungal agents at the time of presentation, which may have reduced morbidity from EFE in these transitory individuals. Additional challenges included medication and drop nonadherence, intervention refusal (hospital admission and intravitreal injection), and intermittent follow-up.
First extensively studied during a European heroin outbreak in the 1980s, the association between Candida endophthalmitis and IVDA has now been confirmed in multiple series. Candida species were the only causative organisms identified in our patient population, with 3 patients having positive ocular cultures. Two patients had positive vitreous tap cultures, and 1 patient had a positive vitreous biopsy culture. Seven patients had negative ocular cultures. This seemingly low diagnostic yield is characteristic of Candida endophthalmitis, with histopathologic studies showing that Candida preferentially sequesters within inflammatory nodules, insulating the organism and limiting the yield of culturing techniques. Initial vitreous tap often has low diagnostic yield given the small sample volume, limited aspiration from the middle of the vitreous cavity, and inability to direct sampling to areas of focal inflammation that are typically close to the retina surface. Even with vitrectomy affording access to larger volumes of vitreous and areas of active inflammation, the yield of vitreous cultures remained low in our series. This low yield is likely related to the intravitreal and systemic antifungal therapy given before vitrectomy. Both patients with multifocal lesions had positive ocular cultures, which may suggest a higher pathogen burden leading to an increased yield of culturing techniques. It is important that health care professionals not interpret negative cultures as sterile but rather as a consequence of the sequestered, localized inflammation characteristic of Candida endophthalmitis.
All patients in our series were ambulatory on presentation, and 90% did not report systemic symptoms. Only 1 patient had positive fungal blood cultures on presentation. These findings support the notion that IVDA results in a transient fungemia, which seeds the vascular choroid soon after inoculation. An otherwise healthy, immunocompetent patient may quickly clear the fungemia; however, once seeded to the choroid, Candida causes infectious destruction, invading Bruch’s membrane and the retinal pigment epithelium. Disruption of the tight junctions between retinal pigment epithelium cells permits infection to cross the outer blood-retinal barrier, isolating it from the systemic immune system and allowing for indolent fungal growth. This transient fungemia with brief exposure to the vascular choroid likely accounts for the unilateral involvement of all patients in our series. In contrast, systemically ill patients with sustained fungemia demonstrate a much higher rate of bilateral infection.
There are limited clinical studies on the role of antifungals and vitrectomy in the treatment of EFE secondary to IVDA. In our series, the duration of systemic therapy did not correlate with a need for surgical intervention or final VA. Systemic antifungal dosing is limited by penetration across the blood-retinal barrier. For example, amphotericin B, which is effective in treatment of systemic Candida infections, is unable to achieve therapeutic intraocular levels when administered systemically. This lack of therapeutic levels can be overcome by intravitreal administration of amphotericin B, although retinal toxicity is a potential adverse effect. Voriconazole, a newer-generation triazole, demonstrates broad-spectrum coverage and better barrier penetration, with a reported intraocular concentration of 70% of plasma levels with systemic dosing. In addition, intravitreal dosing of voriconazole is a safe and effective means of achieving localized, high concentrations of antifungal therapy, which may prove to be prudent in patients with sight-threatening macular lesions.
In patients with worsening inflammation despite local and systemic treatment, there has been increasing interest in the diagnostic and therapeutic role of early vitrectomy. In other cases of infectious and noninfectious uveitis, vitrectomy has been associated with improved visual outcomes. Proponents of early vitrectomy cite the therapeutic ability of PPV to clear infectious organisms, remove inflammatory mediators, and increase penetration of intravitreal and systemic therapies. It is important to consider the implications of operating on an acutely inflamed eye, with studies showing high rates of retinal detachment in patients with EFE despite PPV. In our small series, patients who underwent vitrectomy had improved visual outcomes compared with those treated with intravitreal and systemic antifungals alone. The 5 patients who underwent vitrectomy had a median final VA of 20/70; the 3 patients who did not undergo PPV had a median final VA of 20/300. In addition, patients who underwent PPV tended to have earlier resolution of inflammation and infectious lesions (Figure 2).
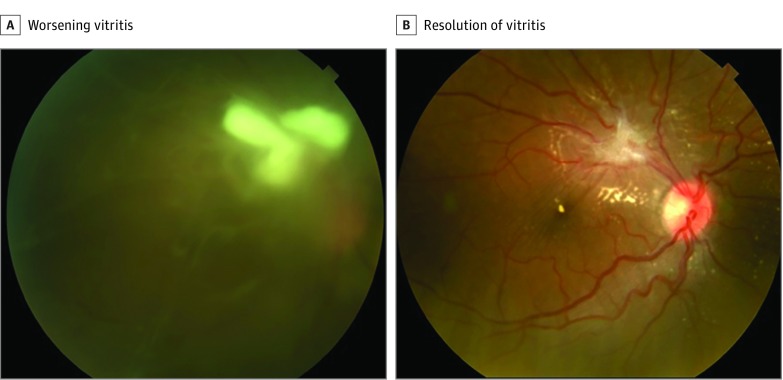
Figure 2. Fungal Endophthalmitis in an Intravenous Drug Abuser in Her 20s.
A, Worsening vitritis 5 days after intravitreal voriconazole. B, Eleven weeks after vitrectomy, there was resolution of vitritis with residual fibrosis and exudates.
Limitations
To our knowledge, this is the first series to investigate the clinical trends of EFE and IVDA in light of the growing opioid crisis. Our study is limited in that the series is retrospective and may be influenced by referral patterns to a tertiary care medical center.
Conclusions
Increasing rates of IVDA in the New England region have placed more patients at risk for vision-threatening EFE. However, the marked increase in cases of EFE seen at our institution parallels the increasing IVDA-associated mortality and hospital admissions throughout Massachusetts. Patients presenting with EFE in the setting of IVDA were typically systemically well and ambulatory at time of presentation, with only ocular symptoms. A high clinical suspicion, detailed history taking, and open discussion with the patient are necessary for early and accurate diagnosis. These data suggest that prompt treatment, especially in a nonadherent patient population, can lead to improved visual outcomes and decreased morbidity.
References
- 1.Massachusetts Department of Public Health. Data brief: opioid-related overdose deaths among Massachusetts residents. http://www.mass.gov/eohhs/docs/dph/stop-addiction/current-statistics/data-brief-overdose-deaths-nov-2016-ma-residents.pdf. Published November 2016. Accessed February 1, 2017.
- 2.Hedegaard H, Chen LH, Warner M Drug poisoning deaths involving heroin: United States, 2000–2013. National Center for Health Statistics. https://www.cdc.gov/nchs/products/databriefs/db190.htm. NCHS data brief, no. 190. Published March 2015. Accessed February 1, 2017. [PubMed]
- 3.Commonwealth of Massachusetts Health Policy Commission. Opioid use disorder in Massachusetts: an analysis of its impact on the health care system, availability of pharmacologic treatment, and recommendations for payment and care delivery reform. http://www.mass.gov/anf/budget-taxes-and-procurement/oversight-agencies/health-policy-commission/publications/opioid-use-disorder-report.pdf. Published 2016. Accessed February 1, 2017.
- 4.Wurcel AG, Anderson JE, Chui KKH, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3(3):ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh N, Nonnemacher MR, Pirrone V, Block T, Mehta A, Wigdahl B. Substance abuse, HIV-1 and hepatitis. Curr HIV Res. 2012;10(7):557-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Vázquez C, Fernández-Ulloa J, Bordón J, et al. Candida albicans endophthalmitis in brown heroin addicts: response to early vitrectomy preceded and followed by antifungal therapy. Clin Infect Dis. 1998;27(5):1130-1133. [DOI] [PubMed] [Google Scholar]
- 8.Connell PP, O’Neill EC, Amirul Islam FM, et al. Endogenous endophthalmitis associated with intravenous drug abuse: seven-year experience at a tertiary referral center. Retina. 2010;30(10):1721-1725. [DOI] [PubMed] [Google Scholar]
- 9.Edwards JE Jr, Montgomerie JZ, Foos RY, Shaw VK, Guze LB. Experimental hematogenous endophthalmitis caused by Candida albicans. J Infect Dis. 1975;131(6):649-657. [DOI] [PubMed] [Google Scholar]
- 10.Donahue SP, Greven CM, Zuravleff JJ, et al. Intraocular candidiasis in patients with candidemia: clinical implications derived from a prospective multicenter study. Ophthalmology. 1994;101(7):1302-1309. [DOI] [PubMed] [Google Scholar]
- 11.Smiddy WE. Treatment outcomes of endogenous fungal endophthalmitis. Curr Opin Ophthalmol. 1998;9(3):66-70. [DOI] [PubMed] [Google Scholar]
- 12.Samiy N, D’Amico DJ. Endogenous fungal endophthalmitis. Int Ophthalmol Clin. 1996;36(3):147-162. [DOI] [PubMed] [Google Scholar]
- 13.Smith SR, Kroll AJ, Lou PL, Ryan EA. Endogenous bacterial and fungal endophthalmitis. Int Ophthalmol Clin. 2007;47(2):173-183. [DOI] [PubMed] [Google Scholar]
- 14.Lingappan A, Wykoff CC, Albini TA, et al. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol. 2012;153(1):162-166.e1. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 16.Bharel M. An assessment of opioid related death in Massachusetts (2013-2014). Massachusetts Department of Public Health. http://www.mass.gov/eohhs/docs/dph/stop-addiction/chapter-55-opioid-overdose-study-data-brief-9-15-2016.pdf. Published September 2016. Accessed February 1, 2017.
- 17.US Department of Justice Drug Enforcement Administration. 2013 National drug threat assessment summary. https://www.dea.gov/resource-center/DIR-017-13%20NDTA%20Summary%20final.pdf. Published November 2013:5-7. Accessed February 1, 2017.
- 18.Shankland GS, Richardson MD. Possible role of preserved lemon juice in the epidemiology of Candida endophthalmitis in heroin addicts. Eur J Clin Microbiol Infect Dis. 1989;8(1):87-89. [DOI] [PubMed] [Google Scholar]
- 19.Shankland GS, Richardson MD. Epidemiology of an outbreak of Candida endophthalmitis in heroin addicts: identification of possible source of infection by biotyping. J Med Vet Mycol. 1988;26(3):199-202. [DOI] [PubMed] [Google Scholar]
- 20.Patel SN, Rescigno RJ, Zarbin MA, Langer P, Bhagat N. Endogenous endophthalmitis associated with intravenous drug abuse. Retina. 2014;34(7):1460-1465. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar GL, Blumenkrantz MS, Egbert PR, McCulley JP. Candida endophthalmitis after intravenous drug abuse. Arch Ophthalmol. 1979;97(1):96-100. [DOI] [PubMed] [Google Scholar]
- 22.Lavine JA, Mititelu M. Multimodal imaging of refractory Candida chorioretinitis progressing to endogenous endophthalmitis. J Ophthalmic Inflamm Infect. 2015;5(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essman TF, Flynn HW Jr, Smiddy WE, et al. Treatment outcomes in a 10-year study of endogenous fungal endophthalmitis. Ophthalmic Surg Lasers. 1997;28(3):185-194. [PubMed] [Google Scholar]
- 24.Tanaka H, Ishida K, Yamada W, Nishida T, Mochizuki K, Kawakami H. Study of ocular candidiasis during nine-year period. J Infect Chemother. 2016;22(3):149-156. [DOI] [PubMed] [Google Scholar]
- 25.Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol. 2008;92(7):871-878. [DOI] [PubMed] [Google Scholar]
- 26.Tod M, Lortholary O, Padoin C, Chaine G. Intravenous penetration of fluconazole during endophthalmitis. Clin Microbiol Infect. 1997;3(1):143-144. [DOI] [PubMed] [Google Scholar]
- 27.Kim DY, Moon HI, Joe SG, Kim JG, Yoon YH, Lee JY. Recent clinical manifestation and prognosis of fungal endophthalmitis: a 7-year experience at a tertiary referral center in Korea. J Korean Med Sci. 2015;30(7):960-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birnbaum FA, Gupta G. The role of early vitrectomy in the treatment of fungal endogenous endophthalmitis. Retin Cases Brief Rep. 2016;10(3):232-235. [DOI] [PubMed] [Google Scholar]
- 29.Becker M, Davis J. Vitrectomy in the treatment of uveitis. Am J Ophthalmol. 2005;140(6):1096-1105. [DOI] [PubMed] [Google Scholar]
- 30.Peyman GA, Raichand M, Bennett TO. Management of endophthalmitis with pars plana vitrectomy. Br J Ophthalmol. 1980;64(7):472-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ness T, Pelz K, Hansen LL. Endogenous endophthalmitis: microorganisms, disposition and prognosis. Acta Ophthalmol Scand. 2007;85(8):852-856. [DOI] [PubMed] [Google Scholar]