This study examines the feasibility of using laparoscopic total pancreatectomy with islet autotransplantation in the treatment of chronic pancreatitis.
Key Points
Question
Is laparoscopic total pancreatectomy with islet autotransplantation and intraoperative islet separation a feasible treatment for patients with chronic pancreatitis?
Findings
In this case series of 20 patients, patients undergoing laparoscopic total pancreatectomy with islet autotransplantation experienced shorter total operative and islet isolation times, reduced length of stay, and quicker opioid independence compared with patients undergoing open or robotic-assisted total pancreatectomy with islet autotransplantation.
Meaning
Laparoscopic total pancreatectomy with islet autotransplantation is a feasible alternative treatment for patients with chronic pancreatitis.
Abstract
Importance
Pain management of patients with chronic pancreatitis (CP) can be challenging. Laparoscopy has been associated with markedly reduced postoperative pain but has not been widely applied to total pancreatectomy with islet autotransplantation (TPIAT).
Objective
To examine the feasibility of using laparoscopic TPIAT (L-TPIAT) in the treatment of CP.
Design, Setting, and Participants
Thirty-two patients with CP presented for TPIAT at a tertiary hospital from January 1, 2013, through December 31, 2015. Of the 22 patients who underwent L-TPIAT, 2 patients converted to an open procedure because of difficult anatomy and prior surgery. Pain and glycemic outcomes were recorded at follow-up visits every 3 to 6 months postoperatively.
Main Outcomes and Measures
Operative outcomes included operative time, islet isolation time, warm ischemia time, islet equivalent (IE) counts, estimated blood loss, fluid resuscitation, and blood transfusions. Postoperative outcomes included length of stay, all-cause 30-day readmission rate, postoperative complications, mortality rate, subjective pain measurements, opioid use, random C-peptide levels, insulin requirements, and glycated hemoglobin level.
Results
Of the 32 patients who presented for TPIAT, 20 underwent L-TPIAT (8 men and 12 women; mean [SD] age, 39 [13] years; age range, 21-58 years). Indication for surgery was CP attributable to genetic mutation (n = 9), idiopathic pancreatitis (n = 6), idiopathic pancreatitis with pancreas divisum (n = 3), and alcohol abuse (n = 2). Mean (SD) operative time was 493 (78) minutes, islet isolation time was 185 (37) minutes, and warm ischemia time was 51 (62) minutes. The mean (SD) IE count was 1325 (1093) IE/kg. The mean (SD) length of stay was 11 (5) days, and the all-cause 30-day readmission rate was 35% (7 of 20 patients). None of the patients experienced postoperative surgical site infection, hernia, or small-bowel obstruction, and none died. Eighteen patients (90%) had a decrease or complete resolution of pain, and 12 patients (60%) no longer required opioid therapy at a median follow-up period of 6 months. Postoperative random insulin C-peptide levels were detectable in 19 patients (95%) at a median follow-up of 10.4 months. At a median follow-up of 12.5 months, 5 patients (25%) were insulin independent, whereas 9 patients (45%) required 1 to 10 U/d, 5 patients (25%) required 11 to 20 U/d, and 1 patient (5%) required greater than 20 U/d of basal insulin. The mean (SD) glycated hemoglobin level was 7.4% (0.5%).
Conclusions and Relevance
This study represents the first series of L-TPIAT, demonstrating its safety and feasibility. Our approach enables patients to experience shorter operative times and the benefits of laparoscopy, including reduced length of stay and quicker opioid independence.
Introduction
Patients with chronic pancreatitis (CP) can experience long-standing and intractable abdominal pain. Total pancreatectomy is a treatment option after medical and endoscopic approaches have failed, but it results in lifelong insulin dependence. Total pancreatectomy with islet autotransplantation (TPIAT) can be an effective treatment for select patients to maintain endocrine function and prevent postoperative brittle diabetes and diabetes-associated complications.
Laparoscopy has been found in other operations to be associated with markedly reduced pain and fewer postoperative complications. For example, patients undergoing laparoscopic radical hysterectomy or cholecystectomy report significantly lower postoperative pain intensity compared with patients undergoing the open versions of these procedures. In addition, some studies have observed that patients undergoing laparoscopic distal pancreatectomy or colon surgery experience significantly lower morbidity and shorter hospital stays than patients undergoing the open versions of these procedures. We hypothesized that a totally laparoscopic TPIAT (L-TPIAT) with simultaneous intraoperative islet isolation can be a safe and effective approach to select patients with severe CP, enabling them to experience the benefits of minimally invasive surgery, such as reduced length of stay and quicker opioid independence.
Methods
From January 1, 2013, through December 31, 2015, a total of 32 consecutive patients with CP presented for TPIAT and were considered for the laparoscopic approach with intraoperative islet isolation. The indications for TPIAT are acute recurrent idiopathic pancreatitis, painful chronic pancreatitis, and eliminating the risk of pancreatic cancer in a patient at high risk. Although some of these patients are at increased risk for pancreatic cancer development, there has only been a single case report of autotransplantation of a pancreatic tumor after TPIAT. Patient demographic data collected included sex, age, body mass index, prior abdominal operative history, and origin of CP. This study was approved by the Johns Hopkins Institutional Review Board. All patients provided written informed consent. Patient data were not deidentified.
Operative outcomes documented were operative time, islet isolation time, warm ischemia time, islet equivalent (IE) counts, estimated blood loss, fluid resuscitation, and blood transfusions. Operative time was defined from incision time to closure. Islet isolation time was defined from time of cessation of blood flow to the pancreas to time of injection of islets back into the patient. Warm ischemia time was defined from time of cessation of blood flow to the pancreas to time of flushing with cold University of Wisconsin solution. The islet counting method changed during the study period from automated to manual counting because of falsely elevated islet counts using the automated method. As a result, the reported mean IE count only represents the IEs measured from 4 of the most recent patients undergoing L-TPIAT.
Postoperative outcomes included length of stay, all-cause 30-day readmission rate, postoperative complications, mortality rate, subjective pain measurements, opioid use, random C-peptide levels (laboratory assay with lower limit of 0.1 ng/mL [to convert to nanomoles per liter, multiply by 0.331]), insulin requirements, and glycated hemoglobin (HbA1c) levels. Pain measurements were collected at follow-up visits using a questionnaire that asked patients to rate their pain on a 100-point visual analog scale (with 0 indicating no pain and 100 indicating the most pain).
Surgical Technique
We began by placing a 12-mm port inferior to the umbilicus, followed by a pair of ports on each side of the abdomen using a 12-mm port in the surgeon’s right hand and a 5-mm port in the surgeon’s left hand from each respective side. A 10-mm, 45° laparoscope was used for visualization. The lesser sac was accessed through the gastrocolic ligament. The gastroduodenal artery was skeletonized; two 5-mm white, plastic locking clips were placed on the proximal artery; and a 10-mm metal clip applier was applied distal to these clips. The gastroduodenal artery was divided with a vessel sealant device distally. The portal vein was then dissected from above the pancreas, and the superior mesenteric vein was dissected from below. A tunnel behind the pancreatic neck was created. The stomach was divided just proximal to the pylorus with a laparoscopic stapling device with green or black loads. A cholecystectomy was performed, and the common bile duct was divided proximal to the entry site of the cystic duct, which enabled the gallbladder to remain with the specimen for subsequent extraction.
The neck of the pancreas was divided after ensuring the safety of the portal vein and the superior mesenteric vein. Division of the pancreas neck facilitates division of the superior mesenteric artery margin, allows a staged removal for the back table team, and enables easy cannulation of the pancreatic duct for collagenase infusion into the duct. We then performed a Kocher maneuver to free the ligament of Treitz from the right side, marching distally on the duodenum. After approximately 20 cm of jejunum was delivered to the right side of the ligament of Treitz, the jejunum was divided with a laparoscopic stapling device with a white load (2.5 mm). The small-bowel mesentery was divided in the proximal direction to the uncinate and along the superior mesenteric artery margin until the Whipple specimen was free. It was extracted through the umbilicus by extending the 12-mm port slightly. The specimen was passed to the laboratory team to infuse the gastroduodenal artery with collagenase and to clean off the pancreas head and cut it into pieces.
Distal Pancreatectomy
The body and tail of the pancreas were mobilized along with the spleen. The splenic vessels were divided, beginning, when possible, with the splenic artery followed by the splenic vein. Once extracted, the distal pancreas was also perfused, cleaned, and dissected into small pieces on the back table before being processed by the islet laboratory personnel.
We previously reported our hepaticojejunostomy reconstruction technique with a single layer of 4-0 barbed sutures in the anterior with interrupted barbed sutures and suture clips. The gastrojejunostomy was then performed in an antecolic retrogastric manner along the posterior wall of the stomach in a stapled side-to-side technique with a laparoscopic stapler. Beginning in 2014, we routinely added a Braun jejunojejunostomy to the procedure to mitigate bile acid reflux gastritis unopposed by pancreatic bicarbonate secretion, which is eliminated with the total pancreatectomy.
Intraoperative Laboratory Isolation
Pancreas digestion and autologous islet isolation followed a modified version of the method described by Ricordi et al. On the back table in the operating room, the head was flushed through the gastroduodenal artery and the tail through the splenic arteries with cold University of Wisconsin solution. The pancreatic duct of each half was cannulated and distended via the duct with a prewarmed mixture of collagenase and thermolysin (GMP Grade Liberase MTF C/T, F. Hoffmann-La Roche Ltd). The pancreas was then divided into 1- to 2-cm2 pieces, placed in a 600-mL digestion chamber (Biorep Technologies), and transferred to a Ricordi Islet Isolator (Biorep Technologies). Collagenase was maintained at 37°C and circulated through the digestion chamber while samples were periodically removed, stained with dithizone (Sigma-Aldrich Co), and viewed under a microscope. Digestion was stopped by the introduction of cold RPMI 1640 (Mediatech Inc). The mixture of islets and acinar tissue was collected, washed, and resuspended in 5% human serum albumin supplemented with 70 U/kg of heparin. Final islet counts were initially determined using an automated islet counter (Biorep Technologies). Later in the study, the standard manual counting method was used instead. The islets were transferred to an infusion bag and delivered into the portal circulation by gravity flow.
Islet Infusion
Simultaneous with the hepaticojejunostomy and gastrojejunostomy, the islets were prepared in the laboratory in the operating room. When the islet solution was ready for autotransplantation into the liver, a metal, hollow-bore, 16-gauge needle with intravenous tubing attached was placed through a 12-mm port site. From within the peritoneal cavity, laparoscopic instruments were used to place the needle into the splenic or portal vein. The islet infusion was then delivered, the needle was subsequently removed, and direct pressure was applied to the puncture site of the vein to achieve hemostasis. Pressures in the portal vein were constantly monitored. Of note, for all advanced laparoscopic pancreas procedures, a No. 10 blade scalpel and Mayo scissors were always kept on the Mayo stand in case a rapid conversion to an open procedure was necessary.
Glycemic Control
All participants were postoperatively admitted to a surgical intensive care unit and administered intravenous insulin infusion and dextrose-containing intravenous fluids for tight glucose control. On postoperative day 3, patients were transitioned to subcutaneous insulin injections. When stable for discharge, all patients were maintained with at least basal insulin unless contraindicated because of hypoglycemia. This common practice is thought to rest the islets after transplant. Insulin therapy was continued for at least 3 months after TPIAT, at which time patients underwent a 75-g oral glucose tolerance test. If testing indicated no diabetes, an attempt was made to discontinue use of insulin or to transition to oral hypoglycemic therapy. All participants returned for regular clinic visits and additional oral glucose tolerance testing every 3 to 6 months after TPIAT.
Statistical Analysis
We used a univariate linear regression model and the Pearson correlation coefficient to analyze the trends in L-TPIAT operative times and islet isolation times during the 2013 to 2015 study period. The level of significance used was P < .05. R statistical software (version 3.0.2; The R Foundation) was used for all statistical analyses.
Results
Patient Demographic Data and Indications for TPIAT
Of the 32 patients who presented for TPIAT, 20 underwent L-TPIAT (8 men and 12 women; mean [SD] age, 39 [13] years; age range, 21-58 years). Two patients converted to the open procedure because of difficult anatomy and prior surgery. Causes of CP included gene mutation(s) (n = 9), idiopathic pancreatitis (n = 6), idiopathic pancreatitis with pancreas divisum (n = 3), and alcohol abuse (n = 2) (Table 1). The 9 patients with gene mutations included 2 with protease, serine 1 (PRSS1) (OMIM 276000), 2 with serine peptidase inhibitor, Kazal type 1 (SPINK1) (OMIM 167790), 2 with cystic fibrosis transmembrane conductance regulator (CFTR) (OMIM 602421), 2 transheterozygotes with both SPINK1 and CFTR, and 1 transheterozygote with both SPINK1 and PRSS1.
Table 1. Patient Demographic Characteristics and Indicationsa .
| Demographic Characteristic | Finding (N = 20) |
|---|---|
| Male/female | 8/12 |
| Age, mean (SD) [range], y | 39 (13) [21-58] |
| Body mass index, mean (SD) [range]b | 25.1 (3.8) [18.4-32.8] |
| Prior abdominal operations | 17 |
| Cause of chronic pancreatitis | |
| Gene mutation(s) | 9 |
| PRSS1 | 2 |
| SPINK1 | 2 |
| CFTR | 2 |
| SPINK1 and CFTR | 2 |
| SPINK1 and PRSS1 | 1 |
| Idiopathic pancreatitis | 6 |
| Idiopathic pancreatitis with pancreas divisum | 3 |
| Alcohol abuse | 2 |
Data are presented as number of patients unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Operative Factors and Postoperative Outcomes
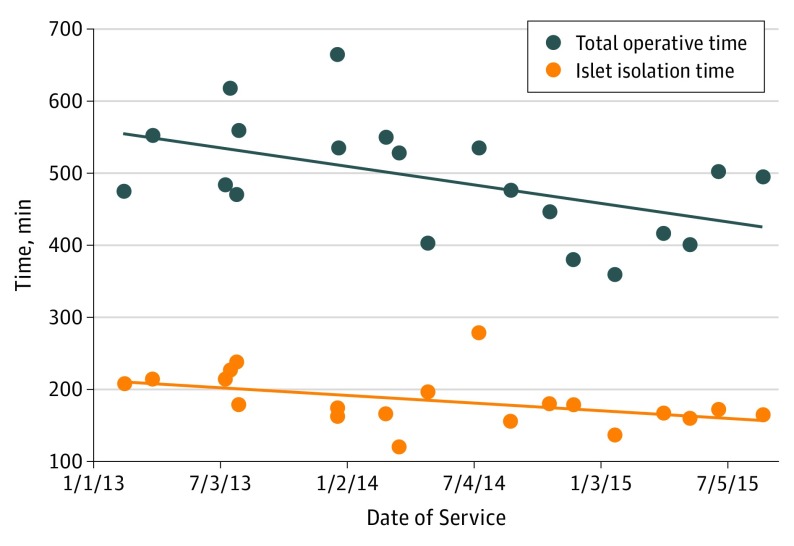
The mean (SD) operative time was 493 (78) minutes (Table 2). Islet isolation time was limited to a mean (SD) of 185 (37) minutes with a mean (SD) warm ischemia time of 51 (62) minutes. The mean (SD) IE count was 1325 (1093) IE/kg. The mean (SD) length of stay was 11 (5) days, and all-cause 30-day readmission rate was 35% (7 of 20 patients). None of the patients experienced a postoperative surgical site infection, hernia, small-bowel obstruction, or mortality. Eighteen patients (90%) had a decrease or complete resolution of pain, and 12 patients (60%) no longer required opioid therapy at a median follow-up period of 6 months. Preoperative pain scores ranged from 0 to 100, with a mean score of 40.75. Postoperative pain scores ranged from 0 to 50, with a mean score of 7.5. As a result, the mean decrease in pain score postoperatively was 33.25. Furthermore, the Figure illustrates the downward trend in L-TPIAT operative times (R = −0.51, P = .02) and islet isolation times (R = −0.45, P = .04) from 2013 to 2015.
Table 2. Operative and Postoperative Outcomesa .
| Outcome | Finding (N = 20) |
|---|---|
| Operative time, mean (SD) [range], min | 493 (78) [360-664] |
| Islet isolation time, mean (SD) [range], min | 185 (37) [121-279] |
| Warm ischemia time, mean (SD) [range], min | 51 (62) [3-181] |
| IE count, mean (SD), IE/kgb | 1325 (1093) |
| Estimated blood loss, mean, mL | 627.5 |
| Fluids, mean, L | 6.2 |
| Patients requiring blood transfusions | 3 (15) |
| Length of postoperative stay, mean (SD) [range], d | 11 (5) [5-27] |
| All-cause readmissions within 30 d | 7 (35) |
| Postoperative surgical site infection, hernia, or small-bowel obstruction | 0 |
| Mortality | 0 |
| Patients with a decrease or complete resolution of pain at a median follow-up of 6 mo | 18 (90) |
| Preoperative pain score, mean (range) | 40.75 (0-100) |
| Postoperative pain score, mean (range) | 7.5 (0-50) |
| Patients no longer requiring opioids at a median follow-up of 6 mo | 12 (60) |
| Patients with a detectable random C-peptide level at a median follow-up of 10.4 mo | 19 (95) |
| Insulin requirement at a median follow-up of 12.5 mo, U/d | |
| 0 | 5 (25) |
| 1-10 | 9 (45) |
| 11-20 | 5 (25) |
| >20 | 1 (5) |
| Glycated hemoglobin at a median follow-up of 12.5 mo, mean (SD) | 7.4 (0.5) |
Abbreviation: IE, islet equivalent.
Data are presented as number (percentage) of patients unless otherwise indicated.
Represents mean IE count of 4 of the patients most recently undergoing laparoscopic total pancreatectomy with islet autotransplantation.
Figure. Total Operative and Islet Isolation Times.
Decrease in total operative times (R = −0.51, P = .02) and islet isolation times (R = −0.45, P = .04) for laparoscopic total pancreatectomy with islet autotransplantation from 2013 to 2015.
Glycemic Outcomes
The time point for glycemic outcomes was 1 year after L-TPIAT or at last follow-up visit for those with more recent operations (all patients had undergone surgery in at least the previous 6 months, and 3 were not yet at 1 year after surgery). The median follow-up time was 12.5 months (range, 199-482 days; interquartile range, 333-406 days). At follow-up, 5 patients (25%) were insulin independent. Of the remaining 15 patients who required insulin, all were receiving basal insulin therapy with a median daily dose of 10 U (range, 4-34 U; interquartile range, 7-13 U): 9 patients (60%) required 1 to 10 U/d, 5 patients (33%) required 11 to 20 U/d, and 1 patient (7%) required greater than 20 U/d of basal insulin. Furthermore, of the 15 patients requiring insulin therapy at follow-up, 6 (40%) required no bolus insulin, 2 (13%) required sliding scale insulin as needed, and 7 (47%) required nutritional insulin. Of note, at follow-up, 5 cohort participants (25%) were receiving oral hypoglycemic therapy: 2 of 5 patients (40%) in the insulin-independent group who had prediabetes and 3 of 15 patients (20%) in the insulin-dependent group who had diabetes.
At follow-up, the overall mean (SD) HbA1c level was 7.4% (0.5%) (to convert to proportion of hemoglobin, multiply by 0.01), with measurement of HbA1c occurring within 1 month of the assessment of insulin regimen for all patients except for 1. When categorized by insulin status, the mean (SD) HbA1c level was 5.6% (0.2%) for the insulin-independent group and 8.0% (0.6%) for the insulin-dependent group. Finally, 19 (95%) of the 20 patients had a detectable random C-peptide level at a median follow-up of 10.4 months (range, 26-626 days; interquartile range, 224-414 days).
Discussion
Safe and Feasible
This study represents the first series of L-TPIAT. Table 3 summarizes our study compared with other surgical approaches. In our series, we observed a shorter mean operative time, islet isolation time, and length of stay and quicker opioid independence. Recently, robotic-assisted TPIAT has been proposed as an approach that enables patients to receive the benefits of minimally invasive surgery. Although this is one strategy, we describe the feasibility of a totally laparoscopic approach that avoids the high costs and consumption of operating room resources associated with robotic-assisted surgery.
Table 3. Outcomes of Open, Robotic, and Laparoscopic TPIATs.
| Source | No. of Patients | Operation | Mean Operative Time, min | Mean Islet Isolation Time, min | Mean Islet Count, IE/kg | Mean EBL, mL | No. of Patients Requiring Blood Transfusions/Total No. of Patients (%) | Mean LOS, d | No. of Deaths Before Initial Discharge | No. of Patients With Pain Control at Follow-up/Total No. of Patients (%) | No of Patients With Controlled Glucose at Follow-up/Total No. of Patients (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Desai et al, 2011 | 12 | Open TPIAT | 637.2 | ≥250 | 2538.2a | 641.7 | 6/12 (50) | 16 | 0 | NR | NR |
| Ahmad et al, 2005 | 45 | Open TPIAT | 533 | Approximately 240 | 3799 in ID patients 6635 in II patients |
563 | 24/45 (53) | 12.6 | 0 | 23/32 (72) Opioid independent at 23-mo follow-upb | 18-mo Follow-up: 18/45 (40) at insulin dose of 0 U/d, 11/45 (24) at 1-20 U/d, 9/45 (20) at 21-40 U/d, and 7/45 (16) at >40 U/d |
| Sutherland et al, 2012 | 409 | Open TPIAT | NR | 270a | 3260a in last 253 cases | NR | NR | NR | 5 | 186/207 (90) With preoperative opioid use, 122/207 (59) opioid independent at 24-mo follow-up |
36-mo Follow-up at <2500 IE/kg transplanted: 4/33 (12) II, 11/33 (33) PF, and 18/33 (55) ID; at 2500-5000 IE/kg transplanted: 8/37 (22) II, 23/37 (62) PF, and 6/37 (16) ID; and at >5000 IE/kg transplanted: 18/25 (72) II, 6/25 (24) PF, and 1/25 (4) ID patients |
| Galvani et al, 2014 | 6 | Robotic TPIAT | 712 | 272.6 | 2301 | 630 | 1/6 (17) | 12.6 | 0 | All 5/5 (100) patients with intractable chronic pain syndrome were in the process of successfully weaning off narcotics at 1 mo follow-up | NR |
| Current study | 20 | Laparoscopic TPIAT | 493 | 185 | 1325c | 627.5 | 3/20 (15) | 11 | 0 | 17/20 (85) With preoperative opioid use, 12/20 (60) opioid independent at 6-mo follow-up |
12.5-mo Follow-up: 5/20 (25) at insulin dose of 0 U/g, 9/20 (45) at 1-10 U/d, 5/20 (25) at 11-20 U/d, and 1/20 (5) at >20 U/d |
Abbreviations: EBL, estimated blood loss; ID, insulin dependent; IE, islet equivalent; II, insulin independent; LOS, length of stay; NR, not reported; PF, partial function; TPIAT, total pancreatectomy with islet autotransplantation.
Reported as median.
Subset of patients with longer than 5 mo of follow-up (n = 32).
Represents mean IE count in the 4 patients most recently undergoing laparoscopic TPIAT.
CP Pain and Benefits of Laparoscopy
Pain associated with CP continues to be a major clinical challenge. It is recognized that ongoing pain in patients with CP may induce central sensitization or pronociceptive pain modulation. This pain modulation may manifest as spreading hyperalgesia. Ultimately, this process may become entirely independent of nociceptive input and inhibitory pain modulation, leading to an autonomous pain state. The treatment of pain in patients with CP is usually based on the World Health Organization pain treatment ladder, which typically ends with opioid treatment. Opioids may provide effective analgesia in some patients with CP but may have considerable adverse effects and may even induce more hyperalgesia. Pain treatments recommended in these cases should directly target the central nervous system (eg, tricyclic antidepressants or gabapentin and intravenous ketamine). Patients undergoing TPIAT usually present with severe chronic pain, which can make surgical pain difficult to manage. In addition, the spike in postoperative pain can complicate short-term and long-term pain management. In addition, L-TPIAT can be particularly beneficial to patients with chronic pain because it may reduce the surgical pain spike at the time. In other operations, laparoscopy is associated with markedly reduced pain, morbidity, and length of hospital stay. In our series, opioid independence after L-TPIAT was achieved more quickly than in the other TPIAT series (Table 3).
Role of Intraoperative Isolation
We hypothesized that the islet isolation laboratory could be set up in the operating room to effectively eliminate transit time, rapidly isolate islets, and enable real-time coordination of intraoperative infusion. Although it is unknown whether added transit time to a neighboring laboratory has an effect on outcomes, performing the isolation in the operating room streamlines the process and may reduce the risk of hand-off error. Some centers may already have negligible transit times given the proximity of their laboratory to the operating room; however, other centers still use remote facilities. Intraoperative isolation may have contributed to our efficient total operative times. In addition, islet isolation time includes warm and cold ischemia time. Corlett and Scharp found that after 45 minutes of warm ischemia time, islet counts decreased to 45.7% in rats and 52.5% in dogs. After 90 minutes, islet counts decreased to 15.6% in rats and 23.9% in dogs. Pileggi et al found that islet yield and viability were significantly reduced in rat models in the long, cold ischemia time group. Thus, minimizing islet isolation time increases islet count and viability, which is positively correlated with insulin independence after TPIAT.
Learning Curve
A difficult learning curve is associated with laparoscopic pancreaticoduodenectomy. We noted that our experience in performing more than 400 laparoscopic distal pancreatectomies and more than 100 laparoscopic pancreaticoduodenectomies aided our performance in L-TPIAT. As we continue to perform laparoscopic pancreas surgery, we are constantly revising our technique to be more efficient and safe. During the 3 years we have been performing L-TPIATs, our operative times have decreased significantly (Figure). This improvement is most likely attributable to multiple factors, including surgeon experience and more efficient islet isolation, which also led to a significant decrease in islet isolation times (Figure).
Limitations
This series has some important limitations. First, our method for IE measurement changed during the study period from automated to manual counting. Despite the low mean IE count of 1325 IE/kg for the 4 valid manual islet counts, our glycemic outcomes were similar to 1-year insulin independence rates reported in the literature. Second, not all patients were candidates for L-TPIAT. During the study period, 6 patients were only offered open surgery because of extensive disease or previous complex surgery. In particular, we found that prior necrotizing and severe pancreatitis can be a relative contraindication to the minimally invasive approach. Third, we do not report 5- or 10-year outcomes given the recent description of L-TPIAT.
Conclusions
We describe the feasibility and safety in this first series of L-TPIAT with intraoperative islet isolation. Although L-TPIAT tends to be considered after medical, endoscopic, and traditional surgical approaches have failed, it should be considered earlier given that early surgical intervention of 2 to 3 years or less is associated with improved pain control in patients with CP. Prior pancreatic surgery has also been found to reduce islet yields and increase insulin requirements in patients who subsequently undergo TPIAT. Overall, the L-TPIAT approach enables patients to experience shorter operative times and the benefits of laparoscopy, including reduced length of stay and quicker opioid independence.
References
- 1.Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377(9772):1184-1197. [DOI] [PubMed] [Google Scholar]
- 2.Trikudanathan G, Navaneethan U, Vege SS. Modern treatment of patients with chronic pancreatitis. Gastroenterol Clin North Am. 2012;41(1):63-76. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58(2):365-382. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez Rilo HL, Ahmad SA, D’Alessio D, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7(8):978-989. [DOI] [PubMed] [Google Scholar]
- 5.Campos LS, Limberger LF, Stein AT, Kalil AN. Postoperative pain and perioperative outcomes after laparoscopic radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer: a randomised controlled trial. Trials. 2013;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enes H, Semir I, Sefik H, Husnija M, Goran I. Postoperative pain in open vs. laparoscopic cholecystectomy with and without local application of anaesthetic. Med Glas (Zenica). 2011;8(2):243-248. [PubMed] [Google Scholar]
- 7.Sui CJ, Li B, Yang JM, Wang SJ, Zhou YM. Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg. 2012;35(1):1-8. [DOI] [PubMed] [Google Scholar]
- 8.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255(6):1048-1059. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? a meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci. 2013;20(4):421-428. [DOI] [PubMed] [Google Scholar]
- 10.Braga M, Vignali A, Gianotti L, et al. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236(6):759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. ; International Hereditary Pancreatitis Study Group . Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst. 1997;89(6):442-446. [DOI] [PubMed] [Google Scholar]
- 12.Muratore S, Zeng X, Korc M, et al. Metastatic pancreatic adenocarcinoma after total pancreatectomy islet autotransplantation for chronic pancreatitis. Am J Transplant. 2016;16(9):2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edil BH, Cooper MA, Makary MA. Laparoscopic pancreaticojejunostomy using a barbed suture: a novel technique. J Laparoendosc Adv Surg Tech A. 2014;24(12):887-891. [DOI] [PubMed] [Google Scholar]
- 14.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413-420. [DOI] [PubMed] [Google Scholar]
- 15.Kissler HJ, Niland JC, Olack B, et al. Validation of methodologies for quantifying isolated human islets: an Islet Cell Resources study. Clin Transplant. 2010;24(2):236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai CS, Stephenson DA, Khan KM, et al. Novel technique of total pancreatectomy before autologous islet transplants in chronic pancreatitis patients. J Am Coll Surg. 2011;213(6):e29-e34. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201(5):680-687. [DOI] [PubMed] [Google Scholar]
- 19.Galvani CA, Rodriguez Rilo H, Samamé J, Porubsky M, Rana A, Gruessner RW. Fully robotic-assisted technique for total pancreatectomy with an autologous islet transplant in chronic pancreatitis patients: results of a first series. J Am Coll Surg. 2014;218(3):e73-e78. [DOI] [PubMed] [Google Scholar]
- 20.Radomski M, Zureikat AH. Total pancreatectomy and islet cell autotransplantation: outcomes, controversies and new techniques. JOP. 2015;16(1):1-10. [DOI] [PubMed] [Google Scholar]
- 21.Amodeo A, Linares Quevedo A, Joseph JV, Belgrano E, Patel HR. Robotic laparoscopic surgery: cost and training. Minerva Urol Nefrol. 2009;61(2):121-128. [PubMed] [Google Scholar]
- 22.Andrén-Sandberg A, Hoem D, Gislason H. Pain management in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14(9):957-970. [DOI] [PubMed] [Google Scholar]
- 23.Drewes AM, Krarup AL, Detlefsen S, Malmstrøm ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut. 2008;57(11):1616-1627. [DOI] [PubMed] [Google Scholar]
- 24.Drewes AM, Gratkowski M, Sami SA, Dimcevski G, Funch-Jensen P, Arendt-Nielsen L. Is the pain in chronic pancreatitis of neuropathic origin? support from EEG studies during experimental pain. World J Gastroenterol. 2008;14(25):4020-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouwense SA, Olesen SS, Drewes AM, Frøkjær JB, van Goor H, Wilder-Smith OH. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? an exploratory study. PLoS One. 2013;8(2):e55460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouwense SA, Buscher HC, van Goor H, Wilder-Smith OH. S-ketamine modulates hyperalgesia in patients with chronic pancreatitis pain. Reg Anesth Pain Med. 2011;36(3):303-307. [DOI] [PubMed] [Google Scholar]
- 27.Bouwense SA, Olesen SS, Drewes AM, Poley JW, van Goor H, Wilder-Smith OH. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS One. 2012;7(8):e42096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buscher HC, Wilder-Smith OH, van Goor H. Chronic pancreatitis patients show hyperalgesia of central origin: a pilot study. Eur J Pain. 2006;10(4):363-370. [DOI] [PubMed] [Google Scholar]
- 29.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corlett MP, Scharp DW. The effect of pancreatic warm ischemia on islet isolation in rats and dogs. J Surg Res. 1988;45(6):531-536. [DOI] [PubMed] [Google Scholar]
- 31.Pileggi A, Ribeiro MM, Hogan AR, et al. Effects of pancreas cold ischemia on islet function and quality. Transplant Proc. 2009;41(5):1808-1809. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Zhang M, Qin Y, et al. Systematic review and meta-analysis of islet autotransplantation after total pancreatectomy in chronic pancreatitis patients. Endocr J. 2015;62(3):227-234. [DOI] [PubMed] [Google Scholar]
- 33.Yang CJ, Bliss LA, Freedman SD, et al. Surgery for chronic pancreatitis: the role of early surgery in pain management. Pancreas. 2015;44(5):819-823. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed Ali U, Nieuwenhuijs VB, van Eijck CH, et al. ; Dutch Pancreatitis Study Group . Clinical outcome in relation to timing of surgery in chronic pancreatitis: a nomogram to predict pain relief. Arch Surg. 2012;147(10):925-932. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Desai KD, Dong H, et al. Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation. 2013;95(8):1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]