Abstract
Introduction
3-Methyl crotonyl CoA carboxylase (3MCC) deficiency is an inborn error of leucine metabolism whose detection was increased with the advent of expanded newborn screening. While most NBS-identified infants appear clinically normal, prior studies suggest a possible increased risk for developmental or metabolic abnormalities. As yet, no predictive markers are known that can identify children at risk for biochemical or developmental abnormalities.
Method
All available 3-MCC cases diagnosed by newborn screening in the Inborn Errors of Metabolism Information System (IBEM-IS) were reviewed for markers that might be predictive of outcome.
Results
A limited number of cases were identified with traditional biochemical symptoms including acidosis, hyperammonemia or lactic acidosis, and 15% of those with available developmental information had recorded developmental disabilities not clearly attributable to other causes. There was no correlation between newborn screening (NBS) C5OH level and presence of metabolic, newborn, later-life or developmental abnormalities in these cases.
Discussion
This sample, obtained from the IBEM-IS database, attempts to avoid some of the ascertainment bias present in retrospective studies. An increase in developmental abnormalities and in traditionally described metabolic symptoms remains apparent, although no specific biochemical markers appear predictive of outcome. The role that prevention of fasting plays in outcome cannot be ascertained. These data suggest that C5OH level found on newborn screening by itself is not sufficient for diagnostic or predictive purposes.
Keywords: 3-MCC Deficiency, Newborn Screening, Outcomes, Database, Symptoms
1. Introduction
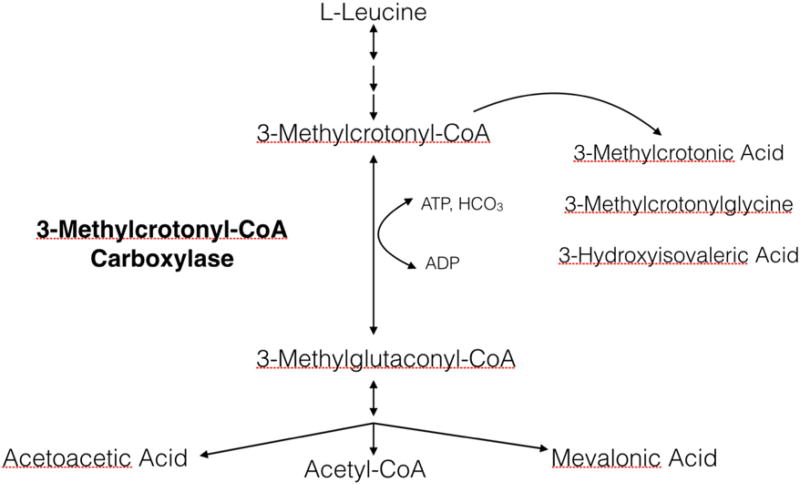
With the introduction of expanded newborn screening (NBS) using tandem mass spectrometry in the 1990s, the number of inborn errors of metabolism (IEMs) that could be detected soon after birth increased five-fold [6]. Now, most states screen for more than thirty disorders so that a diagnosis can be made before a child develops symptoms [27]. As a result, the clinical phenotype of IEMs has broadened to include apparently asymptomatic and more mildly affected individuals as well as those with classic severe presentations. This is particularly true for 3-methylcrotonyl-CoA carboxylase (3-MCC) deficiency (OMIM 210200), a disorder in the metabolism of leucine that causes elevations of 3-hydroxyisovalerylcarnitine (C5OH) among other substrates (Fig. 1).
Fig. 1.
Leucine metabolism. A genetic defect in 3-methylcrotonyl-CoA carboxylase leads to elevation of 3-methylcrotonyl CoA, 3-methylcrotonyl glycine and 3-OH isovaleric acid
3-MCC deficiency is one of the most common IEMs diagnosed by NBS with a prevalence ranging from 1:2400 to 1:68,000 depending on the population [28]. This frequency is much higher than what was predicted based on the number of cases who presented clinically before 3-MCC deficiency was added to the NBS panels [4]. Children with 3-MCC deficiency were diagnosed clinically after being evaluated for developmental delay, failure to thrive, hypotonia, seizures, or metabolic disturbances such as hypoglycemia, hyperammonemia, ketoacidosis, or Reye syndrome [3,8–10,13,19,20,24]. More than 90% of cases with 3-MCC deficiency diagnosed by NBS, however, appear to remain asymptomatic. Among the <10% who do exhibit health or developmental abnormalities these are not always obviously attributable to their 3-MCC deficiency [12,21–23], Healthy mothers have also been identified as having 3-MCC deficiency from their infant's NBS, having passed the abnormal metabolites to their infants through the placenta [18,21], again suggesting that this disorder is not always clinically significant. However, in a few cases childhood hypotonia, or maternal muscular symptoms in affected mothers have been reported [14,21]. Variation in phenotype even occurs among family members with the disorder even though they share genetics and a common environment [11].
At this time, there is no predictive factor that clinicians can use to determine which cases will be symptomatic from 3-MCC deficiency. Several retrospective studies failed to identify a correlation between genotype or biochemical phenotype and outcomes [2,15]. These studies reported cases for publication retrospectively and voluntarily. This raises concern that physicians are more likely to report symptomatic cases rather than asymptomatic cases. Also, the role fasting prevention after pre-symptomatic identification plays in suppressing symptoms is not known. Prospective studies would help to alleviate some of the data bias in outcome studies [1]. The goal of this study was to use the Inborn Errors of Metabolism Information System (IBEM-IS) (https://www.ibem-is.org) to gather outcome data on a prospective sample of cases with 3-MCC deficiency and determine if there were factors that predict the likelihood of developing symptoms secondary to this disorder.
2. Method
The Inborn Errors of Metabolism Information System (IBEM-IS) was established in 2007 to allow the capture and management of longitudinal data from individuals identified with an inborn error of metabolism (IEM) [5]. Data contained in the IBEM-IS are collected and managed using REDCap electronic data capture tools hosted at Michigan Public Health Institute (MPHI) [16]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies. The goal of the Inborn Errors of Metabolism Collaborative (IBEMC) is to use the IBEM-IS to accumulate data that will inform about survival, medical status, and long-term outcomes of cases with IEMs to develop evidence-based practice in patient care (ibem-is.org). As of June 23, 2015 1896 subjects representing 41 conditions have data entered into the IBEM-IS and 1312 of these subjects were identified by NBS.
After this study was deemed to be exempt by the University of Pittsburgh's IRB (PRO13060460), anonymized data were extracted from the IBEM-IS database. These data included information about subject demographics, identification method and diagnosis confirmation, mutation status, neonatal health history, development, and interim health history including other health issues, biochemical labs, diet, and medications. Data were captured using electronic forms developed using Research Electronic Data Capture (REDCap) REFXX [16] by the IBEMC in collaboration with the Newborn Screening Translational Research Network, creating the Longitudinal Pediatric Data Resource REFXX (https://www.nbstrn.org/research-tools/longitudinal-pediatric-data-resource).
Data are reported as they were submitted to the database. In some cases the submitting centers were queried by anonymized case number in an attempt to clarify or seek additional data.
3. Results
3.1. Demographics
All available cases were ascertained from the IBEM-IS. Thirty-seven cases of 3-MCC deficiency were present in the database, including ten males and twenty females, (gender was not reported in seven cases). Diagnoses were established and confirmatory studies were done at the discretion of the submitting center. At the time of enrollment, subject's ages ranged from 1.5 months to 43 years with a median age of 6 years. The thirty-seven subjects had an average of one follow-up visit after enrollment (range: 1–4).
3.2. Identification method and case selection
Of the thirty-seven cases, three were maternal cases identified through their infant's NBS, one was a sibling of a NBS identified case, and one was diagnosed clinically, but the specific symptoms prompting metabolic evaluation were not reported. The remaining thirty-two were identified by NBS. Two of the NBS-identified cases had normal enzyme analysis (DNA analysis not done) and one NBS-identified case had only one mutation identified (no enzyme activity performed). In three cases the confirmatory C5OH level was in the normal range (though urine organic acid analysis did detect some 3OH-IVA and 3-MCG) and in one other case no NBS or confirmatory data were available. Thus, a total of twelve cases were not included in the data analysis; all remaining 25 cases were identified through newborn screening. Of the eight cases without proof of diagnosis, the urine organic acid analysis was reported as abnormal (one case not reported) (Table 1).
Table 1.
Cases without proof of diagnosis.
| Case number |
Newborn C5OH (µmol) |
Confirmatory C5OH (µmol) |
Confirmatory plasma acylcarnitine |
Confirmatory urine organic acid | Outcome |
|---|---|---|---|---|---|
| 5 | (3X normal) | NA | Abnormal | Abnormal | No abnormalities reported |
| 19 | NA | NA | NA | Abnormal | Developmental Delays |
| Special education | |||||
| 21 | 2.22 | 7.25 | Abnormal | Abnormal | No abnormalities reported |
| 27 | 4.24 | NA | NA | Elevated 3-HIVA and 3-MCG | Lactic acidosis and hyperammonemia at birth |
| 30 | NA | NA | NA | Elevated lactic, pyruvic, alpha-ketoglutaric acids | No abnormalities reported |
| 32 | 6.2 | 7.07 | NA | NA | NICU (details unspecified) |
| 33 | 4.2 | NA | NA | Elevated 3-HIVA and 3-MCG | No abnormalities reported |
| 34 | 1.74 | 2 | Abnormal | Elevated 3-HIVA and 2-oxoglutarate | No reported abnormalities |
3.3. Confirmation of diagnosis
Verification of confirmation of diagnosis was a significant concern in these database-ascertained cases. Seventeen cases had the diagnosis confirmed either by enzymology or molecular analysis. Enzyme assay was performed in fourteen of the cases, eight on leukocytes, five on fibroblasts, and one on both. Residual enzyme activity was reported in only a few cases, thus no data analysis using enzyme activity was possible. Seven cases had DNA analysis demonstrating two mutations (listed in Table 2); four of these had both DNA and enzyme analysis, and three were diagnosed only by DNA analysis. The remaining eight cases had no proof of diagnosis beyond biochemical assessment (Table 2). Abnormal metabolites are listed as they were reported in the database, with many variables reported simply as “normal” or “abnormal”.
Table 2.
DNA mutations identified and presence of various symptoms.
| Case number | Mutation | Previous description | Neonatal period | Development |
|---|---|---|---|---|
| 2 | c.517dupT (p.S173Ffs*25) on MCCC2 | [4]; asymptomatic | Normal | Delayed |
| c.433T>C on MCC2 | None | |||
| 3 | c.1155A>C (p.R385S) on MCCC1 | [4]; variable phenotype | Jaundiced | Normal |
| c.1263dupG (p.Q421Afs*10) on MCCC1 | [4]; mild phenotype | |||
| 16 | c.1015G>A (p.V339M) on MCCC2 | [4] | Jaundiced | Delayed - Trisomy 21 |
| Deletion in MCC2 | ||||
| 23 | c.214C>T (p.R72X) on MCCC2 | [7]; asymptomatic | Premature and jaundiced | Delayed |
| c.1676G>A (p.G559D) on MCC2 | None | |||
| 26 | c.95G>T on MCC2 | None | Normal | Normal |
| c.295G>C (p.?) on MCCC2 | [4]; variable phenotype | |||
| 29 | c.929C>G (p.P310R) on MCCC2 | [4]; mild muscle weakness | Jaundiced | Normal |
| c.1559A>G (p.Y520C) on MCCC2 | [30] - no details | |||
| 31 | p.R188Xon MCC2 | None | Normal | N/A |
| c.1065A>T (p.L355F) on MCCC2 | [30] - no details |
Limiting the analyses to only the 17 cases with proof of diagnosis did not change the statistical outcomes (data not shown).
3.4. C5OH level
Each state NBS program uses a unique cut-off range for C5OH. All cases had an elevated C5OH for their state lab, however the state of birth and the normal range for that state were not included in the database. For comparison sake, Huang et al. [17] reports an upper limit of 0.73 µM is commonly used. At least one laboratory in these data used a lower cut-off level (NBS C5OH of 0.6 in a case with enzyme deficiency).
Of the 25 cases included in the final data analysis, a numerical value for NBS C5OH level was reported in 22 of the cases (Table 3). The analysis of these 22 available NBS C5OH levels revealed a mean of 4.67 µM (Table 2). The NBS C5OH level was not significantly different between those with enzymatic or DNA proof of diagnosis vs. those without proof (4.41 vs. 5.30 µM respectively) (p < 0.58). A numerical value for both NBS and confirmatory C5OH level were available in five cases. A paired comparison of NBS vs. confirmatory C5OH level on these 5 cases demonstrated no significant difference between the NBS and confirmatory level (2.90 vs 4.46 µM) (p < 0.16).
Table 3.
Comparison of NBS C5OH between outcome groups.
| N | NBS C5OH Mean (µM) |
SD | Range | p | |
|---|---|---|---|---|---|
| All | 22 | 4.68 | 3.10 | 0.6–13.2 | |
| Diagnosis confirmed | 16 | 4.41 | 2.64 | 0.6–9.42 | p < 0.58 |
| Diagnosis unconfirmed | 6 | 5.30 | 4.15 | 1.74–13.2 | |
| Neonatal complications | 3 | 4.51 | 1.57 | 3.09–6.2 | p < 0.87 |
| No neonatal complications | 15 | 4.87 | 3.46 | 1.74–13.2 | |
| Hospital admissions | 7 | 4.42 | 3.22 | 0.6–9.42 | p < 0.92 |
| No hospital admissions | 9 | 4.38 | 3.60 | 1.74–13.2 |
3.5. Other biochemical labs
Follow-up confirmatory testing included plasma acylcarnitine and urine organic acid analyses. Only five cases reported the actual numerical value for the confirmatory C5OH as the numerical value is not a required entry element. Other cases were reported as “abnormal”, one as “normal” (in a case with two previously reported DNA mutations), and two as “elevated C5OH”. Confirmatory urine organic acid analyses were available on twenty-one cases. All were described as “abnormal” with additional details available on a few including six reporting 3-hydroxyisovaleric acid and 3-methylcrotonylglycine, one with 3-hydroxyisovaleric acid and 2-oxoglutarate, and one with lactate, pyruvate and alpha-ketoglutarate.
3.6. Neonatal period
There were no neonatal complications in most cases. Seven infants had jaundice reported – these included one with Down Syndrome and one who was premature and also had congenital bilateral sensorineural hearing loss, these seven cases were not included as having neonatal symptoms. One infant had lactic acidosis and hyperammonemia requiring high-dose intravenous glucose. Two other infants required intravenous fluids for unknown reasons. One infant was reported as having some type of complication during the neonatal period, but there were no specifics regarding the complication available. For the cases having recorded information on neonatal outcome, eighteen reported the numerical NBS C5OH level, and these were not significantly different between those with a neonatal complication vs. those without any complications (4.51 vs 4.87 µM), respectively (p < 0.87). (Table 3).
3.7. Developmental outcome
At least one report of developmental outcome was available on all cases but one. Six cases were reported to have developmental delay, including one with Down Syndrome and one who was premature and had hearing loss. Excluding these two cases, overall two were reported with gross motor delays, three with speech delay, and three required special education (though one reportedly “caught up” and re-entered mainstream education) (Table 4).
Table 4.
Cases reported to have abnormal developmental outcome (excluding the infant with Down Syndrome and the infant with prematurity/hearing loss.)
| Case number |
NBS C5OH (µM) |
Neonatal period |
Gross motor |
Fine motor |
Speech | Social & emotional |
Special education |
Other |
|---|---|---|---|---|---|---|---|---|
| 2 | 3.21 | Normal | x | x | x | x | Sees neurology and is on antiepileptic medication | |
| 14 | 9.42 | Normal | x | x | Later normalized | |||
| 19 | 13.2 | Normal | x | x | ||||
| 28 | 2.2 | Jaundiced | x |
3.8. Interval health history
Follow-up health information was available on 21 cases. Three admissions for nephrotic syndrome in one case were not included in the analyses. Otherwise there were eight hospital admissions reported to be associated with 3-MCC deficiency. One was for metabolic acidosis and hyperammonemia (in the same case who had lactic acidosis and hyperammonemia as a newborn), two were for metabolic acidosis, one for fever and vomiting, one for jaundice, one for a gastrointestinal parasitic infection, and two for unknown reasons. Of these 21 cases reporting a hospital admission, a numerical NBS C5OH level was available in 16 and there was no significant difference in the NBS C5OH level between the cases having an admission vs. those not reporting a hospital admission (442 vs 4.38 µM respectively) (p < 0.92) (Table 3).
3.9. Growth
Growth parameters were available on eleven cases, but there were insufficient data to calculate accurate percentiles.
3.9.1. Carnitine supplementation
At the intake plasma carnitine was reported in sixteen cases, with nine reported as low or abnormal and seven as normal. Eighteen cases were reported to receive carnitine supplementation. Three of the eighteen had normal carnitine levels on follow-up, while one still had “abnormal levels.” The remaining fourteen had no follow-up carnitine levels reported.
3.9.2. Protein restriction
Ten cases were placed on a restricted diet (low leucine). These cases were from multiple centers. In six cases the child had developmental delay in at least one domain, or a history of acidosis or hyperammonemia. No abnormalities were reported in three cases, and no outcome information was available in one.
4. Discussion
3-MCC deficiency is a common inborn error of metabolism diagnosed by NBS, but the spectrum of disease is quite variable. Historically, 3-MCC deficiency was reported to be associated with developmental delays, hypoglycemia, acidosis, failure to thrive and other poor outcomes [3,8–10,13,19,20,24]. Rat models have noted the metabolites of 3-MCC deficiency are associated with oxidative damage in the brain [29]. With NBS, the incidence of this disorder is much higher than previously expected with the majority of cases having largely normal medical and developmental outcome. This suggests that this disorder might represent a biochemical phenotype, but not a disease, may have a low penetrance or require other genetic or environmental influences in order to manifest a phenotype, or constitute a “predisposition” disorder. Many prior studies have suffered from a bias of ascertainment in that metabolic physicians may be more motivated to report cases with abnormal outcome. This study was designed to use the IBEM-IS in order to eliminate some of the retrospective bias given that cases would be enrolled before long-term outcomes were known. The objective of this database is to enroll cases at first identification, and then follow them prospectively. However, as a database study it is still, in essence, retrospective.
Database studies have inherent limitations in that data are frequently missing or erroneous. We did in fact not agree that a number of the patients in the database actually have 3-MCC deficiency, and the status of some are still unclear. Missing numerical values (that is, reporting as “normal” or “abnormal”) are also a significant limitation. It is clear that diagnostic and management standards are needed, as well as consideration of more stringent database requirements for data entry. The latter is difficult without a substantial increase in funding for such projects.
To date studies have failed to elicit a prognostic factor that could predict outcome or preemptively identify cases that may be symptomatic. Reported outcome concerns have included fasting abnormalities, failure to thrive and other problems [2,15]. Shepard et al. [22] proposed a difference between symptoms suggestive of leucine toxicity such as hypoglycemia, acidosis and others, and “non-specific” symptoms such as intellectual disability or seizures. That study found that cases with “non-specific” symptoms were more likely to have increased regions of homozygosity, and 5/10 “non-specific” symptom cases studied by exome sequencing had another gene identified that likely explained the patient's symptoms. In our data we found “specific” symptoms including recurrent acidosis with hyperammonemia and lactic acidosis in one case, and hospitalizations for metabolic acidosis in two others. Even when the cases with Down Syndrome and prematurity are removed from our analysis, there remain four cases of 27 (14.8%) having a “non-specific” delay in at least one developmental domain, a number consistent with prior reports [2,15]. Wilcken [26], cited this concerning persistence of developmental disabilities between studies even though the disabilities can frequently be attributed to other causes such as prematurity [21]. The possibility remains that 3-MCC deficiency might constitute a predisposition to developmental disabilities in the presence of other stressors. Medical concerns in mothers diagnosed after their infant's abnormal NBS have also been reported, and one of the three maternal cases in this study reported symptoms of muscle cramping and decreased exercise endurance.
Twenty-five NBS ascertained cases were included in this study for data analysis. Of the seven cases with molecular studies, four of the fourteen mutations were not previously reported. Three of the four mutations that had not been previously described were missense mutations and one was a nonsense mutation. Six of the seven cases with reported mutations had some type of neonatal complication, developmental abnormality, or medical event in their health history, potentially expanding the phenotype of each of the previously reported mutations. However, there is insufficient information to adequately define genotype/phenotype relationships for the reported mutations. Comprehensive definition of the pathogenic state of these mutations would require further in vitro study. While NBS C5OH was useful to confirm the diagnosis, these levels did not appear to correlate with the presence of neonatal complications, developmental abnormalities, or subsequent disease-related hospitalization. Further, it does not appear that a mild elevation of C5OH is sufficient grounds to establish a diagnosis, in that two reported “cases” dropped from the final analysis had normal enzyme activity, while another with the lowest C5OH level (that might have been considered within normal limits in some states) had abnormal enzyme analysis. Three other cases were dropped from analysis because no confirmatory testing was done and the NBS C5OH level dropped into the normal range on repeat. However it is not known if, like in VLCAD deficiency, confirmatory levels can drop into the normal range in the presence of enzyme deficiency.
As has been previously reported, carnitine deficiency appears relatively common in this disorder at approximately 50% of cases. Few reports have commented on the biochemical and clinical benefit of carnitine supplementation [25]. Eighteen cases received supplementation, but carnitine levels were not recorded, preventing a determination of how many subjects had levels that were medically concerning.
At present, the role of dietary leucine restriction remains unproven, and only a minority of cases were on a restricted diet. Most of these cases had some developmental or metabolic abnormalities, suggesting clinicians may be more conservative in management regarding these cases. The extent to which early surveillance for catabolism during intercurrent illnesses afforded by newborn screening might be affecting outcome cannot be determined.
5. Conclusion
Overall, this study again illustrates that a small percentage of cases with 3-MCC deficiency will be symptomatic either in the newborn period or later in life with either “specific” or “non-specific” symptoms, or in terms of developmental progress. Unfortunately, no prognostic factors that could predict who would become symptomatic could be identified in this sample. The nature of the database at this time still yields more retrospective analysis. Further, the extent to which pre-symptomatic management (largely by prevention of fasting) affects outcome is not known. The IBEM-IS database, however, has great potential to contribute to future studies on the disorders that are diagnosed via newborn screen as subjects can have outcome measures documented from time of diagnosis. As we continue to learn more about the spectrum of these disorders, continued ascertainment into databases like IBEM-IS and ongoing improvements in data gathering are crucial, so that these limitations may be overcome.
Acknowledgments
NIH: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), National Institutes of Health under award number 5R01HD069039.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
NBSTRN: This research was facilitated by the Newborn Screening Translational Research Network (“NBSTRN”), which is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (HHSN275201300011C).
HRSA: This research is facilitated by the Health Resource and Services Administration (HRSA) Maternal and Child Health Bureau (MCHB) Regional Genetic and Newborn Screening Services Collaboratives, Heritable Disorders Program through grants to: Region 2 - New York Mid-Atlantic Consortium for Genetic and Newborn Screening Services (NYMAC) (H46MC24094), Region 4 Midwest Genetics and Newborn Screening Collaborative (H46MC24092), Region 5 Heartland Genetic Services Collaborative (H46MC24089), and Region 6 - Mountain States Genetics Regional Collaborative (H46MC24095).
RedCap: Study data were collected and managed using REDCap electronic data capture tools hosted at the Michigan Public Health Institute (MPHI) [16]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. http://www.project-redcap.org/cite.php.
References
- 1.Arnold GL, Koeberl DD, Matern D, Barshop B, Braverman N, Burton B, Cederbaum S, Fiegenbaum A, Garganta C, Gibson J, Goodman SI, Harding C, Kahler S, Kronn D, Longo N. A Delphi-based consensus clinical practice protocol for the diagnosis and management of 3-methylcrotonyl CoA carboxylase deficiency. Mol. Genet. Metab. 2008;93(4):363–370. doi: 10.1016/j.ymgme.2007.11.002. http://dx.doi.org/10.1016/j.ymgme.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Arnold GL, Salazar D, Neidich JA, Suwannarat P, Graham BH, Lichter-Konecki U, Bosch AM, Cusmano-Ozog K, Enns G, Wright EL, Lanpher BC, Owen NN, Lipson MH, Cerone R, Levy P, Wong LJ, Dezsofi A. Outcome of infants diagnosed with 3-methyl-crotonyl-CoA-carboxylase deficiency by newborn screening. Mol. Genet. Metab. 2012;106(4):439–441. doi: 10.1016/j.ymgme.2012.04.006. http://dx.doi.org/10.1016/j.ymgme.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett K, Bennett MJ, Hill RP, Lashford LS, Pollitt RJ, Worth HG. Isolated biotin-resistant 3-methylcrotonyl CoA carboxylase deficiency presenting with life-threatening hypoglycemia. J. Inherit. Metab. Dis. 1984;7(4):182. doi: 10.1007/BF01805608. http://dx.doi.org/10.1007/bf01805608. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner MR, Almashanu S, Suormala T, Obie C, Cole RN, Packman S, Baumgartner ER, Valle D. Themolecular basis of human 3-methylcrotonyl-CoA carboxylase deficiency. J. Clin. Investig. 2001;107(4):495–504. doi: 10.1172/JCI11948. http://dx.doi.org/10.1172/JCI11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry SA, Jurek AM, Anderson C, Bentler K. Region 4 Genetics Collaborative Priority W. The Inborn Errors of Metabolism Information System: a project of the region 4 genetics collaborative priority 2 workgroup. Genetics in Medicine. 2010;12:S215–S219. doi: 10.1097/GIM.0b013e3181fe5d23. http://dx.doi.org/10.1097/GIM0b013e3181fe5d23. [DOI] [PubMed] [Google Scholar]
- 6.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin. Chem. 2003;49(11):1797–1817. doi: 10.1373/clinchem.2003.022178. http://dx.doi.org/10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 7.Dantas MF, Suormala T, Randolph A, Coelho D, Fowler B, Valle D. Baumgartner MR. 3-Methylcrotonyl-CoA carboxylase deficiency: mutation analysis in 28 probands, 9 symptomatic and 19 detected by newborn screening. Hum. Mutat. 2005 Aug;26(2):164. doi: 10.1002/humu.9352. [DOI] [PubMed] [Google Scholar]
- 8.de Kremer RD, Latini A, Suormala T, Baumgartner ER, Larovere L, Civallero G, Guelbert N, Paschini-Capra A, Depetris-Boldini C, Mayor CQ. Leukodystrophy and CSF purine abnormalities associated with isolated 3-methylcrotonyl-CoA carboxylase deficiency. Metab. Brain Dis. 2002;17(1):13–18. doi: 10.1023/a:1014096112916. [DOI] [PubMed] [Google Scholar]
- 9.Eldjarn L, Jellum E, Stokke O, Pande H, Waaler PE. Beta-hydroxyisovaleric aciduria and beta-methylcrotonylglycinuria: a new inborn error of metabolism. Lancet. 1970;2(7671):521–522. doi: 10.1016/s0140-6736(70)90140-6. http://dx.doi.org/10.1016/S0140-6736(70)90140-6. [DOI] [PubMed] [Google Scholar]
- 10.Elpeleg ON, Havkin S, Barash V, Jakobs C, Glick B, Shalev RS. Familial hypotonia of childhood caused by isolated 3-methylcrotonyl-coenzyme A carboxylase deficiency. J. Pediatr. 1992;121(3):407–410. doi: 10.1016/s0022-3476(05)81796-2. http://dx.doi.org/10.1016/s0022-3476(05)81796-2. [DOI] [PubMed] [Google Scholar]
- 11.Eminoglu FT, Ozcelik AA, Okur I, Tumer L, Biberoglu G, Demir E, Hasanoglu A, Baumgartner MR. 3-Methylcrotonyl-CoA carboxylase deficiency: phenotypic variability in a family. J. Child Neurol. 2009;24(4):478–481. doi: 10.1177/0883073808324536. http://dx.doi.org/10.1177/0883073808324536. [DOI] [PubMed] [Google Scholar]
- 12.Ficicioglu C, Payan I. 3-Methylcrotonyl-CoA carboxylase deficiency: metabolic de-compensation in a noncompliant child detected through newborn screening. Pediatrics. 2006;118(6):2555–2556. doi: 10.1542/peds.2006-1659. http://dx.doi.org/10.1542/peds.2006-1659. [DOI] [PubMed] [Google Scholar]
- 13.Finnie MDA, Cottrall K, Seakins JW, Snedden W. Massive excretion of 2-oxoglutaric acid and 3-hydroxyisovaleric acid in a patient with a deficiency of 3-methylcrotonyl-CoA carboxylase. Clin. Chim. Acta. 1976;73(3):513–519. doi: 10.1016/0009-8981(76)90155-8. http://dx.doi.org/10.1016/0009-8981(76)90155-8. [DOI] [PubMed] [Google Scholar]
- 14.Gibson KM, Bennett MJ, Naylor EW, et al. 3-MethylCrotonyl-CoA carboxylase deficiency in Amish/Mennonite adults identified by detection of increased acylcarnitines in blood spots of their children. J. Pediatr. 1998;132:519. doi: 10.1016/s0022-3476(98)70032-0. [DOI] [PubMed] [Google Scholar]
- 15.Grünert SC, Stucki M, Morscher RJ, Suormala T, Bürer C, Burda P, Christensen E, Ficicioglu C, Herwig J, Kölker S, Möslinger D, Pasquini E, Santer R, Schwab KO, Wilcken B, Fowler B, Yue WW, Baumgartner MR. 3-Methylcrotonyl-CoA carboxylase deficiency: clinical, biochemical, enzymatic and molecular studies in 88 individuals. Orphanet Journal of Rare Diseases. 2012;7:31. doi: 10.1186/1750-1172-7-31. http://dx.doi.org/10.1186/1750-1172-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. http://dx.doi.org/10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Yang L, Tong F, Yang R, Zheng Z. Screening for inborn errors of metabolism in high-risk children: a 3-year pilot study in Zhejiang Province, China. BMC Pediatr. 2012;12:18. doi: 10.1186/1471-2431-12-18. http://dx.doi.org/10.1186/1471-2431-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kör D, Mungan NÖ, Yılmaz BŞ, Öktem M. An asymptomatic mother diagnoses with 3-methylcrotonyl-CoA carboxylase deficiency after newborn screening. J. Pediatr. Endocrinol. Metab. 2015;28(5–6):669–671. doi: 10.1515/jpem-2014-0302. http://dx.doi.org/10.1515/jpem-2014-0302. [DOI] [PubMed] [Google Scholar]
- 19.Layward EM, Tanner MS, Pollitt RJ, Bartlett L. Isolated biotin-resistant 3-methylcrotonyl CoA carboxylase deficiency presenting as a Reye syndrome-like illness. J. Inherit. Metab. Dis. 1989;12(3):339–340. doi: 10.1007/BF01799234. http://dx.doi.org/10.1007/bf01799234. [DOI] [PubMed] [Google Scholar]
- 20.Murayama K, Kimura M, Yamaguchi S, Shinka T, Kodama K. Isolated 3-methylcrotonyl-CoA carboxylase deficiency in a 15-year-old girl. Brain Dev. 1997;19(4):303–305. doi: 10.1016/s0387-7604(97)86920-3. http://dx.doi.org/10.1016/s0387-7604(97)86920-3. [DOI] [PubMed] [Google Scholar]
- 21.Rips J, Almashanu S, Mandel H, Josephsberg S, Lerman-Sagie T, Zerem A, Podeh B, Anikster Y, Shaag A, Luder A, Staretz Chacham O, Spiegel R. Primary and maternal 3-methylcrotonyl-CoA carboxylase deficiency: insights from the Israel newborn screening program. J. Inherit. Metab. Dis. 2015 Nov;13 doi: 10.1007/s10545-015-9899-4. [DOI] [PubMed] [Google Scholar]
- 22.Shepard PJ, Barshop BA, Baumgartner MR, Hansen J-B, Jepen K, Smoth EN, Frazer KA. Consanguinity and rare mutations outside of MCCC genes underlie nonspecific phenotypes of MCCD. Genetics in Medicine. 2015;17:660–667. doi: 10.1038/gim.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadler SC, Polanetz R, Maier EM, Heidenreich SC, Niederer B, Mayerhofer PU, Lagler F, Koch HG, Santer R, Fletcher JM, Ranieri E, Das AM, Spiekerkötter U, Schwab KO, Pötzsch S, Marquardt I, Hennermann JB, Knerr I, Mercimek-Mahmutoglu S, Kohlschmidt N, Liebl B, Fingerhut R, Olgemöller B, Muntau AC, Roscher AA, Röschinger W. Newborn screening for 3-methylcrotonyl-CoA carboxylase deficiency: population heterogeneity of MCCA and MCCB mutation and impact on risk assessment. Hum. Mutat. 2006;27(8):748–759. doi: 10.1002/humu.20349. http://dx.doi.org/10.1002/humu.20349. [DOI] [PubMed] [Google Scholar]
- 24.Steen C, Baumgartner ER, Duran M, Lehnert W, Suormala T, Fingerhut R, Stehen M, Kohlschütter A. Metabolic stroke in isolated 3-methylcrotonyl-CoA carboxylase deficiency. Eur. J. Pediatr. 1999;158(9):730–733. doi: 10.1007/s004310051189. http://dx.doi.org/10.1007/978-3-662-02613-7_25. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen JA, Lund AM, Olesen JH, Mohr M, Rasmussen J. Is L-carnitine supplementation beneficial in 3-methylcrotonyl-CoA carboxylase deficiency? J. Inherit. Metab. Dis. 2015;21:79–88. doi: 10.1007/8904_2014_393. http://dx.doi.org/10.1056/NEJMoa025225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcken B. 3-Methylcrotonyl-CoA Carboxylase Deficiency: To Screen or not to Screen? J Inherit Metab Dis. 2015 Dec 11; doi: 10.1007/s10545-015-9906-9. (Epub 2015 Dec 11) [DOI] [PubMed] [Google Scholar]
- 27.Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N. Engl. J. Med. 2003 Jun 5;348(23):2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Yang J, Zhang T, Weng C, Hong F, Tong F, Yang R, Yin X, Yu P, Huang X, Qi M. Identification of eight novel mutations and transcript analysis of two splicing mutations in Chinese newborns with MCC deficiency. Clin. Genet. 2015;88(5):484–488. doi: 10.1111/cge.12535. http://dx.doi.org/10.1111/cge.12535. [DOI] [PubMed] [Google Scholar]
- 29.Zanatta A, Moura AP, Tonin AM, Knebel LA, Grings M, Lobato VA, Ribeiro CAJ, Dutra-Filho CS, Leipnitz G, Wajner M. Neurochemical evidence that the metabolites accumulating in 3 methylcrotonyl CoA carboxylase deficiency induce oxidative damage in cerebral cortex of young rats. Cell. Mol. Neurobiol. 2013 Jan 28;33(1):137–146. doi: 10.1007/s10571-012-9879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen KV, Naviaux RK, Patra S, Barshop BA, Nyhan WL. Novelmutations in the human MCA and MCCB gene causing methylcrotonylglycinuria. Mol. Genet. Metab. 2011;102(2):218–221. doi: 10.1016/j.ymgme.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Accessed 1 July 2015.
- 32. [Accessed February 12, 2016]; Longitudinal Pediatric Data Resource – https://www.nbstrn.org/research-tools/longitudinal-pediatric-data-resource.