Abstract
Objective
Several studies have reported repetitive transcranial magnetic stimulation (rTMS) therapy as an effective treatment for the control of motor symptoms in Parkinson disease. The objective of the study is to quantify the overall efficacy of this treatment.
Types
Systematic review and meta-analysis.
Literature survey
We reviewed the literature on clinical rTMS trials in Parkinson disease since the technique was introduced in 1980. We used the following databases: MEDLINE, Web of Science, Cochrane, and CINAHL.
Methodology
Patients and setting: Patients with Parkinson disease who were participating in prospective clinical trials that included an active arm and a control arm and change in motor scores on Unified Parkinson’s Disease Rating Scale as the primary outcome. We pooled data from 21 studies that met these criteria. We then analyzed separately the effects of low- and high-frequency rTMS on clinical motor improvements.
Synthesis
The overall pooled mean difference between treatment and control groups in the Unified Parkinson’s Disease Rating Scale motor score was significant (4.0 points, 95% confidence interval, 1.5, 6.7; P = .005). rTMS therapy was effective when low-frequency stimulation (≤ 1 Hz) was used with a pooled mean difference of 3.3 points (95% confidence interval 1.6, 5.0; P = .005). There was a trend for significance when high-frequency stimulation (≥5 Hz) studies were evaluated with a pooled mean difference of 3.9 points (95% confidence interval, −0.7, 8.5; P = .08). rTMS therapy demonstrated benefits at short-term follow-up (immediately after a treatment protocol) with a pooled mean difference of 3.4 points (95% confidence interval, 0.3, 6.6; P = .03) as well as at long-term follow-up (average follow-up 6 weeks) with mean difference of 4.1 points (95% confidence interval, −0.15, 8.4; P = .05). There were insufficient data to statistically analyze the effects of rTMS when we specifically examined bradykinesia, gait, and levodopa-induced dyskinesia using quantitative methods.
Conclusion
rTMS therapy in patients with Parkinson disease results in mild-to-moderate motor improvements and has the potential to be used as an adjunct therapy for the treatment of Parkinson disease. Future large, sample studies should be designed to isolate the specific clinical features of Parkinson disease that respond well to rTMS therapy.
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disease, manifesting with tremors, rigidity, bradykinesia, and postural instability [1]. Pharmacologic therapies such as dopaminergic medications form the mainstay treatment for the control of motor symptoms [2]. In addition, deep-brain stimulation surgery, which is approved by Food and Drug Administration for select indications such as medication-refractory tremors and motor complications arising from chronic dopaminergic treatments [3], does not necessarily improve gait and balance disturbances in many patients with PD [4]. Alternate treatments like repetitive transcranial magnetic stimulation (rTMS) are used increasingly in research settings, but their exact therapeutic potential is not clearly established.
Transcranial magnetic stimulation (TMS) is a painless, noninvasive, well-tolerated technique of brain stimulation based on the theory of electromagnetic induction [5]. rTMS is the repetitious application of TMS pulses over a predefined target with ability to modulate the excitability of the brain and therefore serve a therapeutic role in control of PD symptoms. rTMS therapy is offered at low and at high frequency of stimulation with distinct mechanisms of action. rTMS at frequencies of 5 Hz and greater enhance motor cortex excitability [6], whereas rTMS at frequencies of 1 Hz and lower depresses the cortical excitability [7]. Several controlled and uncontrolled studies have tested the therapeutic application of rTMS in PD and have found beneficial effects [8]; however, most studies have involved small sample sizes and varied greatly in terms of rTMS dosing regimens, outcome measures, inclusion/exclusion criteria, use of sham-TMS, brain sites for stimulation, and the rigor in monitoring safety and tolerability. A meta-analysis of pooled results from 10 controlled trials found that there was an effect size of −0.58 on the Unified Parkinson’s Disease Rating Scale (UPDRS) for the use of high-frequency rTMS, whereas there were no significant effects seen for low-frequency rTMS studies [9]. Recently, many more studies, mostly using high-frequency stimulation parameters, have been published [10,11]. Here we present a systematic review and analysis of rTMS studies that investigated the motor benefits in PD.
Methods
We searched the literature for articles on the use of rTMS in PD published between the period 1980 and 2013. We used the following databases: MEDLINE, Web of Science, Cochrane, and CINAHL using the following key search terms: “Parkinson’s disease,” “transcranial magnetic stimulation,” “brain stimulation,” “repetitive transcranial magnetic stimulation,” and “noninvasive brain stimulation.” We retrieved 130 articles. We then searched the reference lists in systematic reviews, searched conference abstracts, and searched ClinicalTrials.gov for any ongoing trials in this field. As a first step, we reviewed the abstracts to screen articles deemed relevant and subsequently read the full articles for extraction of outcome measures. We removed all the duplicate articles based on the abstract. Articles were excluded if the score information was missing [12–15], the method of TMS stimulation was not clear [16], or the article reflected duplication of results [17].
Selection Criteria for Meta-Analysis
We used the following inclusion criteria: (1) prospective studies that evaluated the effects of rTMS on motor function in PD; (2) studies that used the UPDRS motor section to measure the motor symptoms; (3) manuscripts or findings reported in English language; (4) findings that were published in a peer-reviewed journal, book, or proceedings; (5) findings for the motor section were reported as a continuous variable with mean and standard deviation (SD) before and after treatment, or provided other parameters that could be used to derive these values; and (6) we also included studies that reported objective motor measurements such as finger tapping speed, Pegboard test, gait speed, and studies that recorded control of levodopa-induced dyskinesia.
We used a semistructured form to extract data and plot the final findings on a master worksheet. We then created separate worksheets for studies that included UPDRS score as a motor outcome measure, reported rTMS effects on dyskinesia, and those that reported objective measurements for bradykinesia and gait assessments. For each study, the data were extracted and checked independently by 2 authors. If there were disagreements, these were resolved with the help of oral discussions and consensus. Data were analyzed with the help of a biostatistician. The following variables were extracted: (1) demographic and clinical characteristics (eg, number of patients, age, disease duration, medication status); (2) study design; (3) baseline Hoehn and Yahr stage; (4) TMS parameters (frequency, intensity, number of pulses, number of sessions, coil type used, evaluation time after TMS); (5) mean and SD of the motor section (part III) of the UPDRS for baseline and after treatment for the active and placebo group (some studies used sham stimulation as control); (6) mean and SD for the follow-up period evaluations (if these data were available); and (7) mean and SD of the outcome measures used for evaluation of dyskinesia and bradykinesia. Our primary analyses examined the effects of low- (≤1 Hz) and high- (≥5 Hz) frequency rTMS studies separately. We then conducted additional analysis for studies that had a control group, studies that included a specific sham coil in the control group, and finally we analyzed the short- and long-duration benefits of rTMS.
Statistical Methods
The primary analysis was based on all controlled studies with baseline and final results for both the control group and treatment group. The end point (metric) was the difference in changes: baseline minus final for the treated group less that for the control group. Other analyses were done to confirm qualitatively that the point and interval estimates were consistent with the main analysis. These included analyzing subsets of studies and analyzing the posttest results only (second metric), whether there was a baseline value reported or not.
Because we lacked patient-level data, and because some studies either lacked Cohen D data [18] for either metric or lacked standard error information for either metric, we were restricted from using either Cohen D or inverse variance weighted random effects methods. In addition, without these patient-level data, it was not possible to construct tests for heterogeneity, forest plats, or funnel plots.
We therefore adopted a minor modification (explained below) of the patient-weighted random effects method of Shuster [19], Section 3. Conceptually, we have presumed that we have a large urn of studies, from which we extracted a sample of studies. Our inference is aimed to be applied to the entire urn, not to the actual sample. The target parameter was the following: Pick a study at random from the urn with probability proportional to its “effective sample size” defined below. What is the average difference in the metric if the subject was assigned to treatment versus assigned to control? If there were no repeated evaluations and no 3-arm studies, this would be straightforward, but the reality is that both issues exist. For studies with 3 treatment arms (including 1 control arm), we defined the effective sample size as the number of controls plus the average number of treated subjects for the 2 active treatment groups. For example, if there were 10 controls, 12 on A, and 13 on B, the effective sample size would be 10 + 12.5 = 22.5. The metric would be defined as the difference between the arithmetic mean for the 2 active treatments less the mean for the controls [19]. For repeated time points, the effective sample size would be the average sample size for controls (the same controls were used, but some may lack the later endpoint) plus the average sample size for treatment. If both issues exist, we first calculated the metric at each time point, and then combined them as discussed previously. The metric within studies in all of these cases would represent the arithmetic mean of the treatment values less the arithmetic mean for the control values (equally weighted to timing and treatment groups). Although somewhat complex, we do have a clean population interpretation of the effect size along with a random effects interpretation. Each study contributes one and only one result to the meta-analysis. This approach was followed by a similar meta-analysis [20].
Criteria for Classifying Study Quality and Strength of Evidence
We applied 3 standardized methods to grade the quality of studies and strength of evidence. Although all 3 scales are designed for grading the quality of clinical trial, there are differences when one considers users and their specialty for each of these scales, and the approach each scale takes while assigning points to the quality of study. Because there are no guidelines currently available to unify these scales, we will list them individually in Table 1. First we used the Oxford Centre for Evidence-Based Medicine levels [21]. According to this grading scale, evidence for quality is rated from level 1 (best quality) to level 5 (lowest quality) based on criteria listed in table. These criteria examine the quality of evidence based on design of the study. We then used the Physiotherapy Evidence Database (PEDro) scale. This scale is used commonly in physical therapy-based systematic reviews [22]. The scale includes 11 questions and is based on a scale of 0–10 to assess the overall quality of the randomized controlled trial. The first question is used to determine external validity and is not graded in the scale. The PEDro scale is described in the Table 2. Finally, we followed the guidelines recommended by American Academy of Neurology for the evaluation of quality of evidence (see Table 2). These guidelines use the following quality-of-evidence indicators: use of a comparison (control) group, method of treatment allocation (randomized versus other), method of allocation concealment, proportion of patients with complete follow-up, use of intent-to-treat methodologies, and use of masking throughout the study (single-blind, double-blind, independent assessment). Details of these guidelines are available at the Web site www.aan.com/Guidelines/.
Table 1.
Criteria used for scoring in PEDro scale, Oxford scale, and AAN classification
| The Oxford Centre for Evidence Based Medicine Levels | PEDro Scale | AAN Guidelines for Therapeutic Intervention |
|---|---|---|
|
|
Class I - Randomized, RCT in a representative population
|
PEDro = Physiotherapy Evidence Database; AAN = American Academy of Neurology; RCT = randomized controlled trial.
Table 2.
Study characteristics of high-frequency stimulation studies
| Study | Age (Mean ± SD) |
Disease Duration (Mean ± SD) |
Medication | HY Stage (Mean ± SD) |
Design | Site | Coil | Intensity | Frequency | No. of Stimuli | Duration | Evaluation Time | Total Sample |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siebner 2000 [37] | 57 ± 11 | 5.5 ± 3.4 | OFF | Controlled | M1 | F8 | 90% MT | 5 | 2250 | 1 h | 12 | ||
| Khedr 2003 [24] | 57.8 ± 9.2 | 3.5 ± 2.3 | OFF | RCT blinded | M1 | F8 | 120% RMT | 5 | 2000 | 10 d | Immediate and 1 mo | 36 | |
| Lefauheur 2004 [25] | 64 ± 2 | 11 ± 1 | OFF | 3.4 ± 0.2 | RCT, blinded | Left M1 | F8 | 80% RMT | 10 | 2000 | 1 | Immediate | 12 |
| Fregni 2004 [38] | 65.7 ± 7.8 | 7.5 ± 8.3 | OFF | 2.1 ± 1.2 | RCT blinded | Left DLPFC | F8 | 110% RMT | 15 | 3000 | 2 wk (5/wk) | Immediate | 42 |
| Mir 2005 [39] | 63.2 ± 6.8 | 5.8 ± 3.2 | ON(rTMS) | 2.2 ± 0.3 | Controlled blinded | PMD | F8 | 90% AMT | 5 | 1500 | 1 | Immediate | 20 |
| Khedr 2006 [41] | 58 ± 10.6 | 3.6 ± 2.1 | OFF | 2.6 ± 0.6 | Controlled | Bilateral M1 | F8 | 100% MT | 25 | 3000 | 6 d | 1 mo | 55 |
| Lomarev 2006 [26] | 63 ± 10 | 13.8 ± 6.8 | ON | RCT double blind | Bilateral DLPFC | F8 | 100% RMT | 25 | 1200 | 4 wk (2/wk) | Immediate and 1 mo | 18 | |
| del Olmo 2007 [30] | 61.7 ± 5.2 | 8.1 ± 5.2 | ON | 2.2 ± 0.6 | Randomized controlled | DLPFC | F8 | 90% RMT | 10 | 450 | 10 d | Immediate | 13 |
| Sedlackova 2009 [40] | 63.7 ± 6.7 | 7.8 ± 2.3 | OFF | Controlled unblinded | PMD | F8 | 100% RMT | 10 | 1350 | 1 | Immediate (30 min) and 1 mo | 10 | |
| Benninger 2009 [34] | 62.6 ± 9.6 | ON | 2.3 ± 0.4 | Yncontrolled | M1 | C | 90% AMT | 50 | 1000 | 1 | Immediate | 10 | |
| Pal 2010 [28] | 68.5 ± 7.9 | 6 ± 2.9 | ON | 2 ± 0.5 | RCT double blind | DLPFC | F8 | 90% RMT | 5 | 600 | 10 | 1 and 30 d | 22 |
| Benninger 2011 [10] | 62.1 ± 6.9 | 10.8 ± 7.1 | ON | 2.6 ± 0.2 | RCT double blind | M1 + DLPFC bilater ally | C | 80% AMT | 50Hz theta burst | 2 (4/wk) | 1 d and 1 mo | 26 | |
| Shirota 2013 [11] | 67.9 ± 8.4 | 7.8 ± 6.6 | ON | 2.8 ± 1.3 | RCT double blind | SMA | F8 | 110% AMT | 10 | 1000 | 8 (1/wk) | 1 and 12 wk | 106 |
HY = Hoehn and Yahr scale; M1 = primary motor cortex; AMT = active motor threshold; RCT = randomized controlled trial; RMT = rest motor threshold; DLPFC = dorsolateral prefrontal cortex; MD = premotor dorsal cortex; SMA = supplementary motor area.
Results
We found 21 studies that satisfied the aforementioned inclusion criteria. There were 10 randomized controlled studies [10,11,23–30] and 4 studies with uncontrolled design [31–34]. There were 10 studies (Table 3) that tested the effects of low-frequency (≤1 Hz) rTMS [11,23,25,27,29,31–33,35,36] and 13 studies (Table 2) used high frequency (≥5 Hz) for stimulation [10,11,24–26,28–30,34,38–40].
Table 3.
Study characteristics of low-frequency stimulation studies
| Study | Age (Mean ± SD) |
Disease Duration (Mean ± SD) |
Medication | HY Stage (Mean ± SD) |
Design | Site | Coil | Intensity | Frequency | No. of Stimuli | Duration | Evaluation Time | Total Sample |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shimamato 2001 [35] | 65.1 ± 28 | 7 ± 4.2 | ON | 3.1 ± 0.9 | Controlled blinded | Frontal | C | 0.4T | 0.2 | 60 | 8 wk (1/wk) | Immediate | 18 |
| Dragasevic 2002 [31] | 59.9 ± 8.5 | ON | 2 ± 0.7 | Uncontrolled | Bilateral frontal | C | 110% AMT | 0.5 | 200 | 10 | 2 h and 20 d | 10 | |
| Ikeguchi 2003 [36] | 68.8 ± 6.8 | 7.8 ± 4.5 | ON | 2.9 ± 1.1 | Controlled | Bilateral frontal | C | 70% output | 0.2 | 60 | 2 wk (3/wk) | Immediate | 16 |
| Okabe 2003 [23] | 67.2 ± 8.2 | 8.8 ± 5.1 | OFF | 3.1 ± 0.9 | RCT blinded | Vertex | C | 110% RMT | 0.2 | 100 | 8 wk (1/wk) | 4 and 8 wk | 85 |
| Buhmann 2004 [32] | 58.4 ± 10.5 | ON | 2.1 ± 0.6 | Uncontrolled | PMD | F8 | 80% AMT | 1 | 1200 | 1 | Immediate | 19 | |
| Lefauheur 2004 [25] | 64 ± 6.9 | 11 ± 3.4 | OFF | 3.4 ± 0.7 | RCT blinded | Left M1 | F8 | 80% RMT | 0.5 | 600 | 1 | Immediate (20 min) | 12 |
| Baumer 2009 [33] | 62.2 ± 6.5 | 10.7 ± 2.9 | OFF | 3.1 ± 0.6 | Uncontrolled | PMD | F8 | 80% AMT | 1 | 1200 | 1 | Immediate | 15 |
| Arias 2010 [29] | ON | RCT | Vertex | C | 90% RMT | 1 | 100 | 1 | Immediate and 1 wk | 18 | |||
| Filipovic 2010 [27] | 64.5 ± 9.4 | 15.6 ± 5.6 | OFF | 3.3 ± 2.2 | Controlled blinded | M1 | F8 | 90% RMT | 1 | 1800 | 1 | 1 d | 10 |
| Shirota 2013 [11] | 68.8 ± 7.6 | 8.5 ± 7.3 | ON | 2.9 ± 1.1 | RCT, double blind | SMA | F8 | 110% AMT | 1 | 1000 | 8 wk (1/wk) | 1 and 12 wk | 106 |
HY = Hoehn and Yahr scale; AMT = active motor threshold; RCT = randomized controlled trial; RMT = rest motor threshold; PMD = premotor dorsal cortex; M1 = primary motor cortex; SMA = supplementary motor area; MT = rest motor threshold; 5PMD = premotor dorsal cortex; 6M1 = primary motor cortex; 7SMA = supplementary motor area.
We conducted a separate analysis for studies that evaluated the rTMS effects at 2 different time points after the intervention (short term and long term). These studies included: Dragasevic et al 2002 [31] (2 hours and 20 days), Okabe et al 2003 (1 month and 2 months) [23], Khedr et al 2003 (immediate and at 1 month) [24], Pal et al 2010 (1 day and at 30 days) [28], Fregni et al 2004 (immediate and at 6 weeks) [38], Lomarev et al 2006 (1 day and at 1 month) [26], Aria et al 2010 (immediate and at 1 week) [29], Benninger et al 2011 (1 day and at one month) [10], and Shirota et al 2013 (1 week and at 12 weeks) [11]. For those studies that had more than 1 active group, for example, when 2 different doses of TMS were administered, we considered each arm as 1 study in the quantitative analysis. This approach was used for 2 studies: Khedr et al 2006 [41] and Shirota et al 2013 [11]. Then, some studies used objective instruments for assessment of bradykinesia along with UPDRS [24–26,34], whereas others [10,30,32,36] included objective gait measures in their analysis. Finally, rTMS studies that investigated therapeutic effects on levodopa-induced dyskinesia included Koch et al 2005 [42], Brusa et al 2006 [43], Wagle-Shukla et al 2007 [44], and Filipovic et al 2009 [45].
Pooled Weighted Effects of rTMS Therapy
The overall pooled mean difference between treatment and control groups for controlled studies was significant (4.0 points, 95% confidence interval [95% CI], 1.5, 6.7, P = .005). Figure 1 includes analysis was studies in which the baseline pretreatment UPDRS scores were available. As seen in Figure 1, the point estimates were similar regardless of whether one considered subgroups with low- or high-frequency stimulation, short- or long-term follow-up, and use of sham coil as the control arm. rTMS therapy was effective in the low-frequency stimulation group, the pooled mean difference was significant (3.3 points, 95% CI, 1.6, 5.0, P = .005), and it showed a trend for significance in the high-frequency stimulation group (3.9 points, 95% CI, −0.7, 8.5, P = .08). rTMS therapy showed definite short-term benefits with a pooled mean difference of 3.4 points (95% CI, 0.3, 6.6, P = .04) and the mean difference of 4.1 points, approached significance at long-term follow-up (95% CI, −0.15, 8.4, P = .05). In studies that specifically used a sham coil in their control arm, we found the pooled mean difference showed a significant trend (4.1 points, 95% CI −0.08, 8.4, P = .05).
Figure 1.
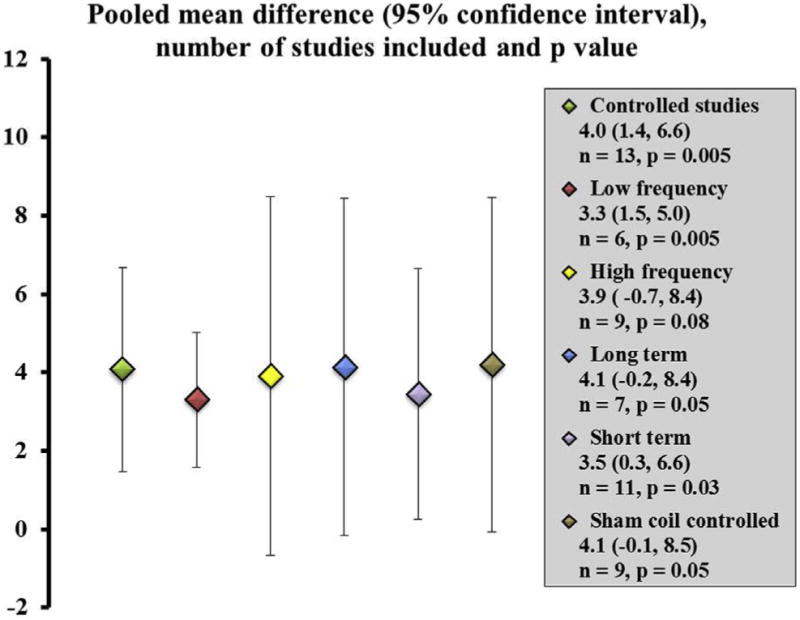
Pooled mean difference between treatment and control groups when comparing baseline and posttreatment motor scores. The figure shows all controlled studies together and then presents data when individual factors such as low-frequency, high-frequency, long- and short-term follow-up, and studies that specifically included a sham coil were separately examined. The scatter plot shows the point estimates with 95% confidence interval error bars. The number of studies included, and the P value for each comparison is presented.
Figure 2 reflects our posttest analysis in which the baseline values of the treatment and control arms were ignored. The results of this analysis were qualitatively consistent with previous analysis; the overall pooled mean difference of 4 points achieved significance (95% CI, 0.5, 7.3, P = .02); however, in individual subgroup analysis of stimulation frequency, time to follow-up, and presence of sham coil, we found the mean difference was significant only for high-frequency stimulation group (4.6 points, 95% CI, 1.2, 8.0, P = .01) and the sham coil group (4.8 points, 95% CI, 1.3, 8.2, P = .01).
Figure 2.
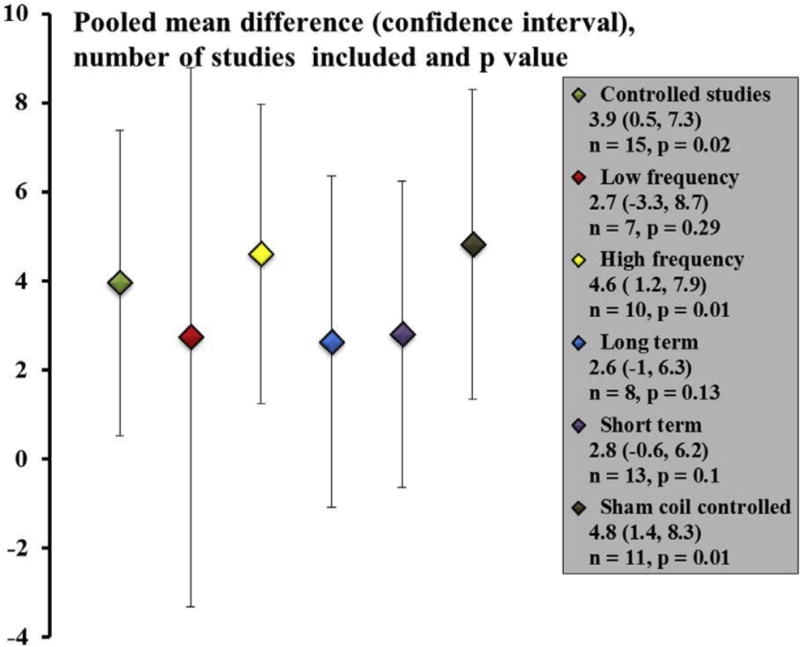
Pooled mean difference between treatment and control groups with baseline values ignored. The figure shows all controlled studies together and then presents data when individual factors such as low-frequency, high-frequency, long- and short-term follow-up, and studies that specifically included a sham coil were separately examined. The scatter plot shows the point estimates with 95% confidence interval error bars. The number of studies included, and the P value for each comparison is presented.
Objective Assessment of Bradykinesia and Gait
Few studies were found to include objective assessment of bradykinesia as an outcome measure; however, they all used variable stimulation protocols. Dragasevic et al 2002 [31] delivered low-frequency (0.5 HZ) rTMS for a period of 10 days to the bilateral dorsolateral prefrontal cortex (DLPFC). Although the study was open labeled, rTMS was seen to improve the finger tapping performance in 7 patients on day 10 (2.2 ± 0.6 and 3.0 ± 1.5 on day 1 versus 3.0 ± 1.1 and 3.7 ± 1.3 for the right and left hand, respectively). Similar improvements in finger tapping performance were seen by Sommer and Paulus [46], where 900 pulses at low frequency were delivered in a single session of 15 minutes to the left primary motor cortex (M1) in 11 subjects. Although the use of low frequency was promising for finger tapping performance, the effect on gait was highly variable. Ikeguchi et al [36] stimulated the frontal region at a frequency of 0.2 Hz for a period of 2 weeks where 30 stimuli were delivered every day for 10 minutes with the help of coil placed over the vertex. At the end of therapy, the gait speed recorded over a 10-minute walking distance revealed no significant improvements. In contrast, Lefaucheur et al [25] delivered rTMS to the M1 at frequency of 0.5 Hz and found improvements in both gait speed and arm rigidity.
Many studies used high frequency for stimulation, for example, Pascual-Leone et al [47] found positive improvements on the pegboard test when 5-Hz rTMS was delivered to the M1 and Khedr et al [24] noted improvement in walking when suprathreshold 5-Hz rTMS was applied to the leg areas of the M1. Subsequently, Lomarev et al [26] published their experience with a greater frequency (25 Hz) of rTMS when delivered to the bilateral M1 and DLPFC once a week for a period of 8 weeks. They found a cumulative improvement in gait and upper-extremity bradykinesia, which they postulated were a result of repeated episodes of long-term potentiation and remodeling of circuits. These results were replicated by Khedr et al [41], who tested both 10-Hz and 25-Hz rTMS, with the latter demonstrating greater benefits, thus suggesting benefits of rTMS to be more potent with a greater frequency of stimulation. However, a recent study that used intermittent theta burst stimulation (50 Hz), a pattern of stimulation known to induce long-term potentiation effects, to the M1 and DLPFC surprisingly did not show any improvements in gait and timed motor tests.
Clinical Outcomes in Patients With Levodopa-Induced Dyskinesias
Similar to bradykinesia and gait, rTMS benefits for levodopa-induced dyskinesias have been evaluated in only few small sample studies with variable stimulation protocols. Koch et al [42] found alleviation of dyskinesias that lasted for only about 30 minutes when they delivered a low-frequency rTMS (1-Hz frequency, 900 stimuli over 15 minutes) over the supplementary motor cortex. In their subsequent study, they delivered stimulation for 5 consecutive days (daily sessions for 15 minutes); however, to their surprise, no cumulative benefits developed at the end of therapy [43]. In another similar study, Wagle-Shukla et al [44] used the same parameters (900 stimuli at 1 Hz over 15 minutes) for a period of 2 weeks but targeted the M1 instead of the supplementary motor cortex. Although the study was open labeled, patients were evaluated with blinded video assessments at 3 time points of 1 day, 2 weeks, and at 4 weeks after therapy. They found significant improvements at 1 day and 2 weeks assessment in the dyskinesia rating scale and the scores based off a diary maintained by patients; however, the benefits were seen to be lost at the 4 weeks’ follow-up. Subsequently, Filipovic et al [45] conducted a randomized controlled study on 10 patients with severe levodopa-induced dyskinesias using real and sham rTMS (1800 pulses; 1-Hz rate over 4 days). Although the real and sham groups responded in same proportions, only the real group demonstrated significant improvements at the end of therapy. The investigators felt the stimulation parameters and the overall dose used in the study were probably too low to establish significant differences between the real and sham group.
Quality of Evidence
We graded the quality of studies by using 3 different scales as reported in Table 4. When considering the Oxford Centre for Evidence-Based Medicine scale, we found the average score ranged from 2 to 4, with 9 of 21 studies scoring 2. None of the studies had a score of 1, which corresponds with the greatest level of evidence. According to the PEDro scale, a randomized controlled trial is assigned high quality if its total score is 6 of 10 or better. In our study we did not include the first question on the PEDro scale that describes the source of the participants and eligibility criteria used. With this scale, we found the grading of articles revealed a wide inter-article variability, with total scores ranging from 3 to 9 on a scale of 0 to 10. We had 9 controlled studies which scored 6 or greater, only one of the articles could be scored 10. We then used the American Academy of Neurology criteria according to which, there were 2 randomized controlled studies meeting criteria for a Class I evidence (Benninger et al [10], Shirota et al [11]), and 5 other studies meeting criteria for Class II evidence (Khedr et al [24], Okabe et al [23], Fregni et al [38], Lomarev et al [26], Pal et al [28]). In summary, there were 9 studies that could be assigned a high-quality status using one or the other grading scheme and in general studies that received the highest score on the PEDro scale appeared to also receive the greatest scores on other classification scales.
Table 4.
Scoring of articles according to Oxford scale, PEDro scale, and AAN classification
| Study | Oxford Scale | PEDro Scale | AAN Classification | |
|---|---|---|---|---|
| 1 | Siebner et al 2000 [37] | Level 4 | 3/10 | Class III |
| 2 | Shimamato et al 2001 [25] | Level 4 | 5/10 | Class III |
| 3 | Dragasevic et al 2002 [31] | Level 4 | 3/10 | Class III |
| 4 | Khedr et al 2003 [24] | Level 2 | 8/10 | Class II |
| 5 | Okabe et al 2003 [23] | Level 2 | 7/10 | Class II |
| 6 | Ikeguchi et al 2003 [36] | Level 4 | 4/10 | Class III |
| 7 | Buhmann et al 2004 [32] | Level 4 | 3/10 | Class III |
| 8 | Lefauheur et al 2004 [25] | Level 3 | 6/10 | Class III |
| 9 | Fregni et al 2004 [38] | Level 2 | 7/10 | Class II |
| 10 | Mir et al 2005 [39] | Level 3 | 5/10 | Class III |
| 11 | Lomarev et al 2006 [26] | Level 2 | 7/10 | Class II |
| 12 | Khedr et al 2006 [41] | Level 3 | 4/10 | Class III |
| 13 | del olmo et al 2009 | Level 2 | 5/10 | Class III |
| 14 | Sedlackova et al 2009 [40] | Level 3 | 4/10 | Class III |
| 15 | Benninger et al 2009 [34] | Level 4 | 4/10 | Class III |
| 16 | Baumer et al 2009 [33] | Level 4 | 3/10 | Class III |
| 17 | Pal et al 2010 [28] | Level 2 | 9/10 | Class II |
| 18 | Filipovic et al 2010 [27] | Level 4 | 3/10 | Class III |
| 19 | Arias et al 2010 [29] | Level 2 | 7/10 | Class III |
| 20 | Benninger et al 2011 [10] | Level 2 | 9/10 | Class I |
| 21 | Shirota et al 2013 [11] | Level 2 | 9/10 | Class I |
PEDro = Physiotherapy Evidence Database; AAN = American Academy of Neurology.
Discussion
Several studies have shown the therapeutic benefits of rTMS therapy for control of motor symptoms in PD [9,48]. Because rTMS therapy is offered at low- and high-frequency stimulation we analyzed the results separately. In contrast to a previous meta-analysis [9], we found rTMS therapy as a beneficial treatment with the use of low-frequency stimulation, whereas there was only a trend for significance in the high-frequency group. In the low-frequency group, there were 2 large sample studies by Okabe et al [23] (n = 85, results were negative) and Shirota et al [11] (n =106, results were positive). A contrast between their findings was possibly related to the dose of stimulation used. Shirota et al [11] used a greater dose of stimulation of 1000 stimuli per session in their protocol.
Recently, several publications have reported the effects of high-frequency stimulation, including the use of theta burst stimulation in which multiple stimuli are delivered either as a continuous or an intermittent train. The enthusiasm for high-frequency stimulation primarily developed from the rationale that under-activation of the M1, supplementary motor area, and the DLPFC can potentially be corrected by increases in excitability induced by high-frequency stimulation [49]. The high-frequency group of studies also consisted of 2 large sample Class I studies, and interestingly their findings were conflicting as well. Benninger et al [10] (n = 26, results negative) used high-frequency theta burst stimulation, whereas Shirota et al [11] (n = 106, results positive) had positive findings with 10-Hz stimulation. A variation in stimulation pattern might have accounted for the difference in outcome.
Despite these conflicting results, the net analysis supported rTMS therapy as beneficial. It should be noted based on previous work showing a difference of 2.7 points in the UPDRS scale as minimal and 6.7 points as moderate [50], our pooled mean estimate difference between the treatment and the control group of 4 points was consistent with only mild beneficial changes. An important consideration is the heterogeneity of stimulation parameters that were used in these studies including the sites of stimulation, coil type, number of pulses delivered, pattern of stimulation used (theta burst), and the number of sessions used. Most of the high-frequency studies, unlike the low-frequency stimulation group, seemed to use a focal figure-of-eight coil to achieve a greater precision in targeted stimulation. Nearly 50% of high-frequency stimulation studies chose M1 as the target for rTMS therapy with DLPFC noted to be the second preferred choice. Studies in the low-frequency stimulation group chose the supplementary motor cortex, dorsal pre-motor cortex, DLPFC, and M1 in nearly equal proportions. Interestingly, stimulation of a nonmotor target such as the DLPFC, a target that is approved by the Food and Drug Administration for treatment of depression, was noted to demonstrate motor improvements that were likely related to the spread of stimulation effects along specific neural connections to distant cortical and subcortical regions [38].
An important consideration in the trial design was the control group included for comparison. The majority of studies chose sham stimulation for the control arm to offset the potential placebo effect of the rTMS intervention. Some studies kept the same patient group but had a control site (occipital) for stimulation, and some included healthy controls as their control group. The method used for ideal sham stimulation has been debated. Sham stimulation comprises 3 main methods. One of the earlier methods has been the use of a real TMS coil tilted at an angle, presumably not discharging substantial amounts of magnetic energy into the brain. The second method has been the use of a sham coil that is similar in appearance and making the same sound as a real TMS coil. One problem with this method was the potential of unmasking participants at high TMS intensities (>90% intensity) as the regular coil induced a twitching sensation on the scalp. The third method comprises electrical stimulation of the scalp muscles over the targeted site and a clicking sound is created by a real coil placed close to the site and not over the site [51]. We found all studies except for 2 used a proper sham coil for stimulation. In our subgroup analysis of studies with sham coil, the overall rTMS benefits continued to show significance.
Medication status is another important consideration when one interprets the effects of rTMS, although the role of dopaminergic medications currently is not clear. According to a previous notion, dopaminergic medications were proposed to have a potential to mask the effects of rTMS therapy, which was referred to as a “ceiling effect” [8]. A recent large study by Shirota et al [11] noted benefits from rTMS while the patients took their dopaminergic medications Similarly, Aria et al [29] found positive results with rTMS regardless of the medication status.
Indeed, a treatment intervention with significant impact on clinical practice must demonstrate benefits that are clinically meaningful, long lasting, and outweigh the side effects. After the success of single rTMS session, many studies began to use multiple sessions based on the widely held belief that repeated sessions resulted in cumulative benefits [48]. We conducted a separate analysis for such studies to determine whether rTMS therapy had cumulative and long-term benefits. We found motor improvements were sustained for an average follow-up of 6 weeks after the therapeutic sessions were completed. On specific examination of adverse effects, we found no report of serious effects. Some studies reported benign side effects, such as mild headache, neck pain, a mild burning sensation over the scalp, and increased salivation [31]. For example, Dragasevic et al [31] reported 4 of 10 patients developed a light burning sensation over the scalp during stimulation and 3 patients developed a mild tension headache. Most studies excluded patients with a seizure disorder to comply with the safety guidelines for rTMS [52]. In the theta burst stimulation study, special attention was provided to the possibility of increased seizure risk; electroencephalography electrodes were applied over the scalp and the forearm to monitor any increase in cortical excitability or epileptiform activity during the course of treatment [34].
The literature on the use of rTMS for levodopa-induced dyskinesia, objective bradykinesia, and gait measures is sparse and overall disappointing [25,31]. On the basis of the current available information, the results are conflicting, and no clear treatment protocol has yet been defined. Although some of the previous high-frequency studies in the range of 25 Hz demonstrated positive improvements [26], a Class I study that used theta burst stimulation (50 Hz) failed to demonstrate any significant improvements in gait and bradykinesia [10]. The authors felt these discrepancies were largely related to methodologic differences in that the circular coil used in the theta burst study has a wider spread of stimulation, which may have offset the benefits of stimulating focal leg and hand areas.
In summary, with recent publication of several large sample studies, rTMS therapy has been demonstrated to be an effective treatment for motor symptoms in PD. The benefits are sustained at a follow-up period of about 6 weeks. Although the rTMS therapy requires a specialized setup and skilled personnel, it is easy to administer and is well tolerated by most patients. Although studies included in our analysis reported improvements in the UPDRS motor scale regardless of stimulation frequency, it was not clear whether any particular item of the scale was more likely to demonstrate a treatment response. The mechanisms underlying the actions of rTMS remain largely unknown; the individual differences in pathophysiology likely play an important role in impacting the treatment outcomes. Future studies should be directed towards determination of optimal stimulation parameters. It may also be reasonable to conclude that rTMS therapy may have greater benefits if the dose and stimulation parameters are personalized in individuals to address specific symptoms.
Footnotes
Disclosure
A.W.S. Disclosures related to this publication: grant, National Institutes of Health (NIH) KL2 (#TR000065) (money to institution)
J.J.S. Disclosures related to this publication: grant, NIH, National Center for Advancing Translational Sciences (#1UL1TR000064) (money to institution)
J.W.C. Disclosure: nothing to disclose
D.E.V. Disclosures related to this publication: grants, NIH (#R01 NS052318, R01 NS075012), Bachmann-Strauss, Tyler’s Hope Foundations
Disclosures outside this publication: personal fees, NIH Study Section Member, National Parkinson’s Foundation, UT Southwestern Medical Center and University of Illinois at Chicago, University of Colorado, University of Pittsburgh
C.P. Disclosure: nothing to disclose
J.O. Disclosures outside this publication: consultancy, Medtronic, Allergan, Merz, Boston Scientific (money to institution); expert testimony (money to institution); grants/grants pending, St. Jude, MRI interventions; payment for lectures including service on speakers bureaus, Allergan (money to institution)
M.S.O. Disclosures related to this publication: grant, NIH, Bachmann-Strauss Foundation (money to author and institution); consulting fee or honorarium, National Parkinson Foundation (NPF); Tyler’s Hope Dystonia Center, National Parkinson Foundation
Disclosures outside this publication: board membership, associate editor for New England Journal of Medicine Journal Watch Neurology (money to author); consultancy, NPF (money to author); grants/grants pending, NPF, Michael J Fox, Smallwood, Tourette Syndrome Association, UF foundation (money to author); royalties, Demos, Manson, Amazon, Smashwords, Cambridge (money to author and institution); payment for development of educational presentations, Peer-View, Prime, Vanderbilt University (money to author)
Contributor Information
Aparna Wagle Shukla, Department of Neurology and Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Road, Gainesville, FL 32607.
Jonathan J. Shuster, Department of Health Outcomes and Policy, Clinical and Translational Science Institute, University of Florida, Gainesville, FL
Jae Woo Chung, Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL.
David E. Vaillancourt, Department of Neurology and Center for Movement Disorders and Neurorestoration, University of Florida, Gainesville, FL Department of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL.
Carolynn Patten, Brain Rehabilitation Research Center of Excellence and Department of Physical Therapy, University of Florida, Gainesville, FL.
Jill Ostrem, Department of Neurology and Surgical Movement Disorders, University of California, San Francisco, CA.
Michael S. Okun, Department of Neurology and Center for Movement Disorders and Neurorestoration, University of Florida, Gainesville, FL
References
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72(21 Suppl 4):S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: Initiation of treatment for Parkinson’s disease: An evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58:11–17. doi: 10.1212/wnl.58.1.11. [DOI] [PubMed] [Google Scholar]
- 3.Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: An open-label randomised controlled trial. Lancet Neurol. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 4.Wagle Shukla A, Okun MS. Surgicaltreatment of Parkinson’s disease: Patients, targets, devices, and approaches. Neurotherapeutics. 2014;11:47–59. doi: 10.1007/s13311-013-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagle Shukla A, Vaillancourt DE. Treatment and physiology in Parkinson’s disease and dystonia: Using transcranial magnetic stimulation to uncover the mechanisms of action. Curr Neurol Neurosci Rep. 2014;14:449. doi: 10.1007/s11910-014-0449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson’s disease: A systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry. 2005;76:1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function—systematic review of controlled clinical trials. Mov Disord. 2009;24:357–363. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- 10.Benninger DH, Berman BD, Houdayer E, Pal N, Luckenbaugh DA, Schneider L, et al. Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology. 2011;76:601–609. doi: 10.1212/WNL.0b013e31820ce6bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirota Y, Ohtsu H, Hamada M, Enomoto H, Ugawa Y. Research Committee on rTMS Treatment of Parkinson’s Disease. Supplementary motor area stimulation for Parkinson disease: A randomized controlled study. Neurology. 2013;80:1400–1405. doi: 10.1212/WNL.0b013e31828c2f66. [DOI] [PubMed] [Google Scholar]
- 12.Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol. 2001;112:259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 13.Dias AE, Barbosa ER, Coracini K, Maia F, Marcolin MA, Fregni F. Effects of repetitive transcranial magnetic stimulation on voice and speech in Parkinson’s disease. Acta Neurol Scand. 2006;113:92–99. doi: 10.1111/j.1600-0404.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 14.Epstein CM, Davey KR. Iron-core coils for transcranial magnetic stimulation. J Clin Neurophysiol. 2002;19:376–381. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hamada M, Ugawa Y, Tsuji S. Effectiveness of rTMS on Parkinson’s Disease Study Group, Japan. High-frequency rTMS over the supplementary motor area improves bradykinesia in Parkinson’s disease: Subanalysis of double-blind sham-controlled study. J Neurol Sci. 2009;287:143–146. doi: 10.1016/j.jns.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Kimura H, Kurimura M, Kurokawa K, Nagaoka U, Arawaka S, Wada M, et al. A comprehensive study of repetitive transcranial magnetic stimulation in Parkinson’s disease. ISRN Neurol. 2011;2011:845453. doi: 10.5402/2011/845453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boggio PS, Fregni F, Bermpohl F, Mansur CG, Rosa M, Rumi DO, et al. Effect of repetitive TMS and fluoxetine on cognitive function in patients with Parkinson’s disease and concurrent depression. Mov Disord. 2005;20:1178–1184. doi: 10.1002/mds.20508. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein M, Hedges L, Higgins J, Rothstein H, editors. Introduction to Meta-Analysis. New York: Wiley Publications; 2008. [Google Scholar]
- 19.Shuster JJ. Empirical vs natural weighting in random effects meta-analysis. Stat Med. 2010;29:1259–1265. doi: 10.1002/sim.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuster JJ, Guo JD, Skyler JS. Meta-analysis of safety for low event-rate binomial trials. Res Synth Methods. 2012;3:30–50. doi: 10.1002/jrsm.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips B, Ball C, Sackett D. Levels of evidence and grades of recommendations. Available at www.cebm.net/. Accessed July 11, 2011.
- 22.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 23.Okabe S, Ugawa Y, Kanazawa I. Effectiveness of rTMS on Parkinson’s Disease Study Group. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson’s disease. Mov Disord. 2003;18:382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- 24.Khedr EM, Farweez HM, Islam H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur J Neurol. 2003;10:567–572. doi: 10.1046/j.1468-1331.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115:2530–2541. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Lomarev MP, Kanchana S, Bara-Jimenez W, Iyer M, Wassermann EM, Hallett M. Placebo-controlled study of rTMS for the treatment of Parkinson’s disease. Mov Disord. 2006;21:325–331. doi: 10.1002/mds.20713. [DOI] [PubMed] [Google Scholar]
- 27.Filipovic SR, Rothwell JC, Bhatia K. Slow (1 Hz) repetitive transcranial magnetic stimulation (rTMS) induces a sustained change in cortical excitability in patients with Parkinson’s disease. Clin Neurophysiol. 2010;121:1129–1137. doi: 10.1016/j.clinph.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal E, Nagy F, Aschermann Z, Balazs E, Kovacs N. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: A randomized, double-blind, placebo-controlled study. Mov Disord. 2010;25:2311–2317. doi: 10.1002/mds.23270. [DOI] [PubMed] [Google Scholar]
- 29.Arias P, Vivas J, Grieve KL, Cudeiro J. Controlled trial on the effect of 10 days low-frequency repetitive transcranial magnetic stimulation (rTMS) on motor signs in Parkinson’s disease. Mov Disord. 2010;25:1830–1838. doi: 10.1002/mds.23055. [DOI] [PubMed] [Google Scholar]
- 30.del Olmo MF, Bello O, Cudeiro J. Transcranial magnetic stimulation over dorsolateral prefrontal cortex in Parkinson’s disease. Clin Neurophysiol. 2007;118:131–139. doi: 10.1016/j.clinph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Dragasevic N, Potrebic A, Damjanovic A, Stefanova E, Kostic VS. Therapeutic efficacy of bilateral prefrontal slow repetitive transcranial magnetic stimulation in depressed patients with Parkinson’s disease: An open study. Mov Disord. 2002;17:528–532. doi: 10.1002/mds.10109. [DOI] [PubMed] [Google Scholar]
- 32.Buhmann C, Gorsler A, Baumer T, Hidding U, Demiralay C, Hinkelmann K, et al. Abnormal excitability of premotor-motor connections in de novo Parkinson’s disease. Brain. 2004;127(Pt 12):2732–2746. doi: 10.1093/brain/awh321. [DOI] [PubMed] [Google Scholar]
- 33.Baumer T, Hidding U, Hamel W, Buhmann C, Moll CK, Gerloff C, et al. Effects of DBS, premotor rTMS, and levodopa on motor function and silent period in advanced Parkinson’s disease. Mov Disord. 2009;24:672–676. doi: 10.1002/mds.22417. [DOI] [PubMed] [Google Scholar]
- 34.Benninger DH, Lomarev M, Wassermann EM, Lopez G, Houdayer E, Fasano RE, et al. Safety study of 50 Hz repetitive transcranial magnetic stimulation in patients with Parkinson’s disease. Clin Neurophysiol. 2009;120:809–815. doi: 10.1016/j.clinph.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimamoto H, Takasaki K, Shigemori M, Imaizumi T, Ayabe M, Shoji H. Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson’s disease. J Neurol. 2001;248(Suppl 3):III48–52. doi: 10.1007/pl00007826. [DOI] [PubMed] [Google Scholar]
- 36.Ikeguchi M, Touge T, Nishiyama Y, Takeuchi H, Kuriyama S, Ohkawa M. Effects of successive repetitive transcranial magnetic stimulation on motor performances and brain perfusion in idiopathic Parkinson’s disease. J Neurol Sci. 2003;209:41–46. doi: 10.1016/s0022-510x(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 37.Siebner HR, Rossmeier C, Mentschel C, Peinemann A, Conrad B. Short-term motor improvement after sub-threshold 5-Hz repetitive transcranial magnetic stimulation of the primary motor hand area in Parkinson’s disease. J Neurol Sci. 2000;178:91–94. doi: 10.1016/s0022-510x(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 38.Fregni F, Santos CM, Myczkowski ML, Rigolino R, Gallucci-Neto J, Barbosa ER, et al. Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1171–1174. doi: 10.1136/jnnp.2003.027060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mir P, Matsunaga K, Gilio F, Quinn NP, Siebner HR, Rothwell JC. Dopaminergic drugs restore facilitatory premotor-motor interactions in Parkinson disease. Neurology. 2005;64:1906–1912. doi: 10.1212/01.WNL.0000163772.56128.A8. [DOI] [PubMed] [Google Scholar]
- 40.Sedlackova S, Rektorova I, Srovnalova H, Rektor I. Effect of high frequency repetitive transcranial magnetic stimulation on reaction time, clinical features and cognitive functions in patients with Parkinson’s disease. J Neural Transm. 2009;116:1093–1101. doi: 10.1007/s00702-009-0259-0. [DOI] [PubMed] [Google Scholar]
- 41.Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Hamdy A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov Disord. 2006;21:2201–2205. doi: 10.1002/mds.21089. [DOI] [PubMed] [Google Scholar]
- 42.Koch G, Brusa L, Caltagirone C, Peppe A, Oliveri M, Stanzione P, et al. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology. 2005;65:623–625. doi: 10.1212/01.wnl.0000172861.36430.95. [DOI] [PubMed] [Google Scholar]
- 43.Brusa L, Versace V, Koch G, Iani C, Stanzione P, Bernardi G, et al. Low frequency rTMS of the SMA transiently ameliorates peak-dose LID in Parkinson’s disease. Clin Neurophysiol. 2006;117:1917–1921. doi: 10.1016/j.clinph.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 44.Wagle-Shukla A, Angel MJ, Zadikoff C, Enjati M, Gunraj C, Lang AE, et al. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology. 2007;68:704–705. doi: 10.1212/01.wnl.0000256036.20927.a5. [DOI] [PubMed] [Google Scholar]
- 45.Filipovic SR, Rothwell JC, van de Warrenburg BP, Bhatia K. Repetitive transcranial magnetic stimulation for levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2009;24:246–253. doi: 10.1002/mds.22348. [DOI] [PubMed] [Google Scholar]
- 46.Sommer M, Paulus W. Pulse configuration and rTMS efficacy: A review of clinical studies. Suppl Clin Neurophysiol. 2003;56:33–41. doi: 10.1016/s1567-424x(09)70207-7. [DOI] [PubMed] [Google Scholar]
- 47.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Wu AD, Fregni F, Simon DK, Deblieck C, Pascual-Leone A. Noninvasive brain stimulation for Parkinson’s disease and dystonia. Neurotherapeutics. 2008;5:345–361. doi: 10.1016/j.nurt.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 50.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67:64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 51.Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, et al. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2007;118:709–716. doi: 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


