Abstract
Objectives
To determine the incidence of bacterial meningitis (BM) among all febrile infants 29–56 days old undergoing a lumbar puncture (LP) in the emergency department (ED) of a tertiary care children’s hospital and the number of low-risk FYI with BM, in order to reassess the need for routine LP in these infants.
Study design
Retrospective cohort study using a quality improvement registry from July 2007-April 2014. Infants included were 29–56 days old with fever and who had an LP in the ED. Low-risk criteria were adapted from the Philadelphia criteria. 4BM was defined as having a bacterial pathogen isolated from the cerebrospinal fluid (CSF). A medical record review of one-third of randomly selected patients in the cohort determined the proportion who met low-risk criteria.
Results
One of 1188 (0.08%) FYI had BM; this patient did not meet low-risk criteria. An additional 40 (3.4%) had positive CSF cultures; all were contaminants. Sub-analysis of one-third of the study population revealed that 45.6% met low-risk criteria; the most common reasons for failing low-risk classification included abnormal white blood cell count or urinalysis.
Conclusions
In a cohort of FYI, BM is uncommon and no cases of BM would have been missed had LPs not been performed in those meeting low-risk criteria.
Keywords: febrile infant, lumbar puncture, quality of care, pediatric emergency department
Fever is a common reason for young infants to seek care in the emergency department (ED). Febrile infants less than 56 days old are at increased risk for serious bacterial infection (SBI),1–3 and clinicians are unable to consistently discriminate between a viral illness and SBI by physical examination alone.5,6 This led to the adoption of a conservative practice of routinely acquiring blood, urine and cerebrospinal fluid (CSF) cultures, empirically treating with antibiotics and hospitalization.7 Subsequently, protocols were developed that defined subsets of febrile young infants at low risk for SBI; these patients, generally greater than 28 days old, were candidates for outpatient management, typically without antibiotics.8–10 The principle difference among protocols was whether CSF analysis was used as a low-risk criterion. Guidelines originating in Philadelphia and Boston recommended routinely performing the lumbar puncture (LP) while the Rochester protocol did not mandate CSF analysis.8–10
The lack of a universal approach has led to wide variation in clinical practice with respect to laboratory testing and patient disposition and inconsistent adherence to specific guidelines.11 Since publication of these protocols in the early 1990s, universal use of conjugate pneumococcal and Haemophilus influenza (Hib) vaccines have changed the epidemiology of these infections and the incidence of Listeria monocytogenes has fallen.12–14 Further, the routine acquisition of an LP may be contrary to the goals of value-based medicine by leading to false-positive CSF cultures, unnecessary hospitalizations, increased costs and lengths of ED stays, pain for the patients, and parental anxiety.
On the other hand, infants managed in a tertiary care referral center may warrant a more conservative approach because they likely have a higher incidence of SBI and bacterial meningitis (BM). The goal of this study was to reassess our standard practice of routinely performing LPs for all febrile infants 29–56 days old (FYI). The objectives of this study were (1) to determine the incidence of BM among all FYI undergoing an LP in the ED of a tertiary care referral center; (2) to determine the ratio of contaminants to true pathogens among those with positive CSF cultures; (3) to determine the proportion of study subjects who met low-risk criteria in the absence of CSF analysis; and (4) to determine the number of infants meeting low-risk criteria who had BM.
Methods
This was a retrospective cohort study in the pediatric ED of an urban, tertiary care children’s hospital with an annual patient volume of over 90,000 visits.
Infants were included if they were 29–56 days of age, cared for in the ED between July 1, 2007 and April 20, 2014, had a rectal temperature of 38° F or higher measured either at home, in a physician’s office, or in the ED, and had an LP and CSF analysis performed in the ED. Infants who had CSF collected from a ventriculoperitoneal shunt were excluded.
Data for this study were extracted from an existing clinical pathway quality improvement (QI) registry. The QI registry was created using data extracted from the electronic medical record and collated in a Database Warehouse using SAS (v9.2) software. Patients were identified based on a qualifying date of birth and orders placed for an LP or CSF lab specimens. The following data were extracted: medical record number, encounter identification, date of birth, date and time of arrival to the ED, disposition from the ED (admission versus discharge), date and time of hospital discharge, and CSF results.
Two study investigators were randomly assigned to any patient with a positive CSF culture result and independently performed a manual review of those medical records to determine if the patient met low-risk criteria and if the organism was a true pathogen or a contaminant. Medical records were either electronic or scanned copies of paper records, depending on the year that the patient sought ED care. These data were abstracted and entered into a web-based data management tool (Research Electronic Data Capture) for analysis. Data collected included: date and time of LP, highest documented fever, identification of CSF bacteria and time (in hours) for growth of CSF bacteria. Additional data were collected for risk stratification, and an infant at low risk for SBI was defined as one meeting all criteria listed in Table I. The two investigators determined if the bacteria in the CSF represented a pathogen or a contaminant based on the specific organism, time to positivity, and detailed analysis of the patient’s management by hospital physicians. In the event of a disagreement for any data point, a consensus was reached by the two investigators. In addition, crosscheck was performed between this database and microbiology laboratory records identifying all children of any age diagnosed with BM over the study period.
Table 1.
Factors that define an infant at low risk for serious bacterial infection
| Past Medical History | Physical Examination | Laboratory Results |
|---|---|---|
|
| ||
|
|
|
A secondary outcome was to determine the proportion of study subjects who met low-risk criteria (Table I). The low-risk criteria used in the study were a modification of the original Philadelphia criteria.4 During the 7-year study period, these data (along with CSF analysis) were used to define low risk. The existing protocol was for patients judged to be low-risk to be discharged home without antibiotics. For the study, we sought to determine if we could safely omit the CSF analysis in identifying low-risk infants.
The rate of low-risk subjects was estimated by performing a medical record review on a sample of approximately one-third (401) of study infants randomly selected from the original QI registry. Based on previous work, we estimated that 35–40% of subjects would meet low-risk criteria. An analysis prior to this study determined that a sample size of 400 subjects produced a two-sided 95% confidence interval (CI) with a width equal to 0.1, if 40% of the sample population was low risk. The data collected for this analysis were the same as those described above for infants with a positive CSF culture; data were abstracted and entered into a web-based data management tool (Research Electronic Data Capture) for analysis. Each patient was randomly assigned to two study investigators who independently performed a manual review of the infants’ medical records; any disagreements were reviewed and consensus determined by the study investigators.
Statistical Analyses
Demographic characteristics were summarized by standard descriptive summaries (e.g. means and standard deviations were used for continuous variables and percentage for categorical variables). Chi2 test was used to compare categorical variables of interest. The magnitude of association between these variables was approximated by calculating odds ratios (OR) and 95% confidence intervals. P value of ≤ 0.05 was considered statistically significant. All analyses were conducted using Stata version 13 (College Station, TX).
This study was approved by the hospital’s institutional review board. The requirement of informed consent/assent was waived.
Results
There were 1188 infants evaluated during the study period. The mean age was 43 days (SD ±8) and 160 (13.5%) (95% CI: 12.6,16.6) had known chronic medical conditions at the time of enrollment. The overall admission rate was 73.0% (95% CI: 71.4, 76.3). Additionally, during the 7-year study period there was little variation in the rates of LP performance or hospital admission (Figure).
Figure 1.
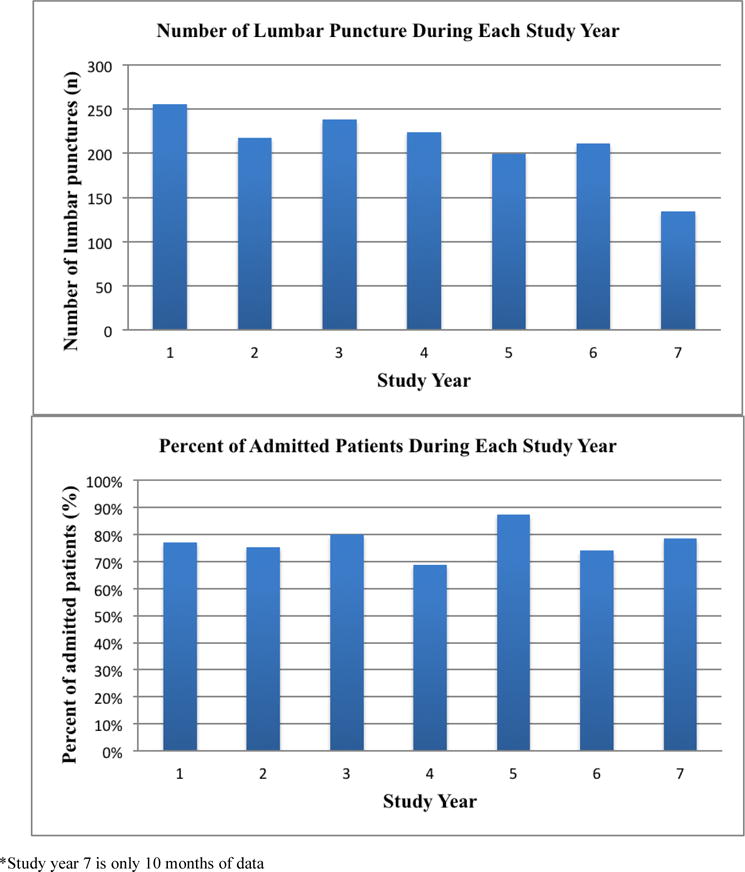
Number of lumbar punctures and admission rates during each study year
One (0.08%) infant (#41, Table II; available at www.jpeds.com) was diagnosed with BM but did not meet low-risk criteria. This patient was a 36-day-old infant born after 36 weeks of gestation who was febrile and brought to the ED because of crying. The infant was described by clinicians as “crying inconsolably”, “very fussy”, and had a distended abdomen; group B Streptococcus was isolated from the blood and CSF.
Table 2.
Characteristics of infants with positive CSF culture results
| Age (days) |
CSF culture (organism) |
Time to growth (hours) |
Low risk criteria failed | Actions of Clinicians | Contaminant (Yes or No) |
|
|---|---|---|---|---|---|---|
| 1 | 46 | Acinetobacter | 54 | Abnormal blood WBC | No antibiotics | Yes |
| 2 | 46 | Staphylococcus epidermidis | 76 | None | Discharged from the ED No follow up | Yes |
| 3 | 38 | Staphylococcus epidermidis | 18 | Chronic medical condition Abnormal B/N* and urine WBC | No antibiotics | Yes |
| 4 | 55 | Staphylococcus epidermidis | 23 | Abnormal blood WBC And CSF WBC | Antibiotics stopped after CSF culture speciation | Yes |
| 5 | 49 | Staphylococcus epidermidis | 33 | Prematurity Abnormal CSF protein | Antibiotics stopped after CSF culture speciation | Yes |
| 6 | 47 | α-hemolytic Streptococcus | 21 | LP only successful for culture | Antibiotics stopped after CSF culture speciation | Yes |
| 7 | 30 | Staphylococcus epidermidis | Unknown | Abnormal CSF protein No blood culture sent | No antibiotics, diagnosed with Influenza | Yes |
| 8 | 48 | Corynebacterium sp. | 84 | Abnormal blood WBC and urine WBC | Discharged on hospital day 2, culture grew after discharge, not readmitted | Yes |
| 9 | 40 | Staphylococcus epidermidis | 34 | Prematurity Abnormal CSF protein | Antibiotics stopped after CSF speciation. | Yes |
| 10 | 30 | Staphylococcus haemolvticus | 40 | Abnormal CSF WBC and protein | Antibiotics stopped after CSF culture speciation | Yes |
| 11 | 40 | Streptococcus mitis group | 14 | Abnormal CSF protein | Discharged from the ED, readmitted. ID consult, treatment for meningitis discontinued. | Yes |
| 12 | 51 | Staphylococcus capitis | 27 | Chronic medical condition Abnormal blood WBC and CSF WBC | Antibiotics stopped after CSF culture speciation | Yes |
| 13 | 51 | Staphylococcus epidermidis | 49 | None | No antibiotics, discharged on hospital day 2 | Yes |
| 14 | 36 | Staphylococcus epidermidis | 34 | Abnormal CSF WBC, protein, and glucose | Antibiotics stopped after CSF culture speciation | Yes |
| 15 | 54 | Streptococcus mitis group | 26 | None | Antibiotics stopped after CSF culture speciation | Yes |
| 16 | 45 | Bacillus species | 60 | None | Unknown | Yes |
| 17 | 46 | Bacillus species | 33 | Abnormal CSF glucose | Antibiotics stopped after CSF culture speciation | Yes |
| 18 | 36 | Micrococcus species | 41 | Hypothermia Abnormal blood WBC and B/N* | ID consult, treatment for meningitis discontinued | Yes |
| 19 | 54 | Staphylococcus epidermidis | 49 | None | No antibiotics, culture grew after discharge | Yes |
| 20 | 32 | Staphylococcus epidermidis | 62.5 | Abnormal CXR | No treatment | Yes |
| 21 | 47 | Staphylococcus auricularis | 60.5 | None | No antibiotics, diagnosed with Influenza | Yes |
| 22 | 53 | Staphylococcus hominis | 82 | Unable to obtain CBC or blood culture | ID consult, treatment for meningitis discontinued | Yes |
| 23 | 54 | Staphylococcus epidermidis | 46 | Abnormal B/N* and CSF WBC | Antibiotics stopped after CSF culture speciation | Yes |
| 24 | 49 | Staphylococcus hominis | 53 | Abnormal CSF WBC, protein, and glucose | Discharged on hospital day 2, culture grew after discharge | Yes |
| 25 | 38 | Staphylococcus epidermidis | 24 | Abnormal urine WBC, CSF WBC and protein | No antibiotics | Yes |
| 26 | 29 | Non-hemolytic Streptococcus | 31 | Abnormal CSF WBC, protein, and glucose | Antibiotics stopped after CSF culture speciation | Yes |
| 27 | 49 | Staphylococcus epidermidis | 45 | None | Discharged from the ED, grew after discharge | Yes |
| 28 | 37 | Bacillus species | 37 | None | Discharged from the ED, grew after discharge | Yes |
| 29 | 35 | Staphylococcus hominis | 43 | Abnormal blood WBC | Antibiotics stopped after CSF culture speciation | Yes |
| 31 | 33 | Staphylococcus epidermidis and Staphylococcus capitis | 19 | Abnormal CSF glucose | Discharged from the ED, grew after discharge. Readmitted and antibiotics stopped after CSF culture speciation | Yes |
| 31 | 29 | Staphylococcus warneri | 56 | Abnormal blood WBC, urine WBC, CSF WBC and glucose | Discharged from hospital on UTI treatment, no treatment for meningitis | Yes |
| 32 | 36 | Streptococcus parasanguinis | 21 | Abnormal B/N* | ID consult, treatment for meningitis discontinued | Yes |
| 33 | 37 | Gram-positive cocci | 48 | Abnormal CSF protein and glucose | Unknown | Yes |
| 34 | 47 | Escherichia coli | 29 | Abnormal blood WBC | ID consult, treatment for meningitis discontinued Grew in enrichment broth | Yes |
| 35 | 40 | Polymicrobial species | 22 | Abnormal B/N* and CSF protein | Treatment discontinued | Yes |
| 36 | 53 | Bacillus species | 15 | Chronic medical condition Prematurity | Antibiotics stopped after CSF culture speciation | Yes |
| 37 | 42 | Escherichia coli | 24 | None | Discharged from ED, readmitted. ID consult, treatment for meningitis discontinued. Grew in enrichment broth | Yes |
| 38 | 36 | Staphylococcus epidermidis and Enterococcus sp. | 25 | Abnormal urinalysis | Treated for UTI | Yes |
| 39 | 44 | Single gram-negative rod | 53 | LP only successful for culture | Discharged on hospital day 2, culture grew after discharge. Not readmitted. | Yes |
| 40 | 35 | Staphylococcus warneri | 27.5 | Prematurity Abnormal blood WBC and urine gram stain | ID consult, treatment for meningitis discontinued | Yes |
| 41 | 36 | Group B Streptococcus | 23 | Ill-appearing Prematurity | +blood culture Treated for meningitis | No |
Crosscheck with data from the microbiology laboratory identified one additional infant with BM during the study period. This 33-day-old infant initially evaluated in the ED was not a study subject because the LP was performed after hospitalization. The infant was critically ill upon ED arrival, brought directly to the ED resuscitation room, and ultimately died in the NICU. Salmonella group G was isolated from the CSF. This infant would have failed low-risk criteria based on being ill appearing, hypoglycemic and having an abnormal band-to-neutrophil ratio.
An additional 40 infants (3.4%) had positive CSF results, all of which were contaminants (Table II). The most common contaminants were Staphylococcus epidermidis (n=16) and Bacillus species (n=4). Of note, 2 patients (#34 and #37) had isolation of Escherichia coli from the CSF, an organism traditionally considered to be a pathogen. Patient #34 was described as well-appearing and had normal CSF tests including gram stain, WBC count, protein and glucose levels. This subject failed low-risk criteria based on an abnormal blood WBC and was hospitalized for empiric antibiotic therapy. Escherichia coli grew in the enrichment broth only, 29 hours after CSF was obtained. Infectious Disease (ID) physicians were consulted and concluded that this organism was a contaminant. The patient was discharged after 48 hours, without antibiotics and without sequelae. Patient #37 had a completely normal evaluation during the initial ED encounter, including normal results of screening laboratory and CSF tests, and was discharged home without antibiotics. However, at 24 hours the patient was called back for the positive CSF culture result pending speciation. ID physicians were consulted and concluded that this organism was likely a contaminant given the patient’s initial presentation and normal laboratory test results. Furthermore, the patient had been without antibiotics for over 24 hours and remained afebrile and well-appearing.
A medical record review of 401 randomly selected febrile infants 29–56 days old revealed that 45.6% (95% CI: 40.7, 50.7) met low-risk criteria, excluding CSF analysis. Of those not meeting low-risk criteria, a majority failed only one criterion (Table III). The most common reasons to fail low-risk criteria were abnormal WBC and abnormal urinalysis (Table III).
Table 3.
Number and most common low-risk criteria failed (N = 401 patients)
| Number of failed criteria | N (%) | 95% CI |
|---|---|---|
| One criterion | 118 (29.6) | 25.2 to 34.4 |
| Two criteria | 73 (18.2) | 14.5 to 22.3 |
| Three criteria | 33 (8.2) | 5.7 to 11.4 |
| Failed low-risk criteria* | N (%) | |
| Abnormal blood WBC | 85 (21.1) | |
| Abnormal urinalysis | 54 (13.5) | |
| Abnormal blood band-to-neutrophil ratio | 38 (9.5) | |
| Ill appearance | 34 (8.5) | |
| Prematurity | 32 (8.0) | |
| Chronic medical condition | 21 (5.2) | |
| Hypothermia | 4 (1.0) | |
Patients may have failed more the one low-risk criteria
There were no significant differences between the 401 randomly selected subjects and the QI registry cohort, respectively, for mean age [43 versus 43 days (p=0.1)] or proportion with chronic medical conditions [12.2% versus 13.5% (p=0.7]). Fifty-nine percent (95% CI: 51.4, 66.4) of low-risk FYI were hospitalized versus 89.6% (95% CI: 84.4, 93.4) of FYI who did not meet all low-risk criteria [RR 0.67 (95% CI: 0.60, 0.75)].
Discussion
We found a low incidence of BM among a large cohort of febrile infants 29–56 days old managed by protocol to undergo an LP in the ED of a tertiary care children’s hospital. The vast majority of positive CSF cultures were due to contaminants. Further, over the course of almost seven years, not a single case of BM occurred among infants who otherwise met low-risk criteria for SBI. Approximately 45% fewer infants would have undergone an LP during the course of the study had the procedure been restricted to those failing to meet other low-risk criteria in this practice setting. These data, combined with that reported by others, have led to a re-assessment of the strategy of routinely performing an LP in this cohort.
Routinely performing an LP can be associated with false-positive CSF analysis of pleocytosis and culture, unnecessary hospitalization with risk of nosocomial infection, increased cost and resource utilization, increased length of ED stay, and parental anxiety.15 In one study, among almost 1,000 low-risk febrile infants 29–60 days old about one-fifth had either a traumatic or unsuccessful LP in the ED.15 Hospitalizations rates were substantially higher for this group compared with those with an interpretable CSF profile (72% vs 18%) and median hospital charges were 2.5 times greater. To avoid these problems, criteria have traditionally been employed in EDs to identify a subset of infants at such low risk for SBI that they can safely be managed as outpatients, typically without antibiotics.8–10 Protocols developed in the early 1990s used a combination of historical/demographic, physical examination, and laboratory criteria to classify infants at high and low risk for SBI; protocols differed in age criteria and management strategies, but the principle difference was that those developed in Philadelphia and Boston used the CSF analysis as a criterion for assignment of an infant as low-risk while the Rochester protocol did not.8–10 Of note, in the original Rochester, Boston, and Philadelphia reports, none of 1227 infants meeting their respective low-risk criteria had BM.
Subsequently, optimal management of well-appearing febrile infants has been debated.16 The lack of a universal standard has led to substantial practice variability, especially with respect to performing LPs.11 A recent study assessed the management of greater than 35,000 febrile infants evaluated in 37 pediatric EDs.11 There was significant inter-hospital variation in laboratory testing including LP rates, treatment, and hospitalization rates. In a separate report, among 16 pediatric EDs with clinical practice guidelines for the management of febrile infants 29–56 days old, exactly half recommended an LP for all patients while the other half recommended an LP only for high-risk patients.17 Further, there is poor adherence to guidelines even among those who report following a specific guideline. One study surveyed members of the American Academy of Pediatrics Section on Emergency Medicine.18 Two-thirds of respondents reported following a specific protocol yet they deviated markedly from the guidelines when reporting management strategies for three fictitious cases. For example, among the respondents who reported following the Philadelphia protocol, 44% neglected to perform an LP in a well-appearing febrile 44-day-old patient. In another survey, just two-thirds reported using published guidelines to manage FYI.19
Our results are consistent with those reported by other investigators assessing the performance of low-risk criteria. Byington et al used the modified Rochester criteria to assess well-appearing febrile infants 1–90 days old; CSF analysis was not used to define infants as low-risk.20 Over the course of 2 years after implementation of a care process model, about one-half of infants underwent an LP. Just 7 of 2,987 (0.2%) infants had BM; importantly, none met low-risk criteria. Of note, the total number of low-risk infants cared for during the implementation phase was not reported although 55% of all infants cared for before and after model implementation were low-risk. A literature review by Huppler et al reported results from 22 prospective and retrospective studies published since the mid-1980s.21 These studies assessed the accuracy of the low-risk criteria in excluding SBI in febrile infants 0–90 days old; the total number of low-risk infants in the 29–56 day old subset was not reported, however. Of 3,984 infants who met low-risk criteria, just 2 (0.05%) had BM and they were both neonates less than 29 days old. Specifically, among 11 studies in which CSF analysis was not used to define low-risk, none of the 2,114 infants who met low-risk criteria had BM. Martinez et al reported on the prevalence of BM among febrile infants less than 90 days old.22 Of the almost 2,000 well-appearing febrile infants greater than 21 days old, none had BM. However, only about one-fifth of these study subjects had an LP performed and the total number of infants 29–56 days old was not reported. In a study performed in Spain, there was no missed case of BM among 676 febrile infants 22–90 days old who met low-risk criteria.23
In the present study, we sought to re-assess our practice of routinely performing an LP among FYI cared for in a tertiary care referral center. We theorized that young infants would be protected from pneumococcal and Hib infections by herd immunity, because older children are now immunized with vaccines that were not available at the time that previous protocols were developed. In fact, there have been dramatic decline in invasive Hib infections13, although Hib was not a major cause of meningitis in infants less than 8 weeks of age. Likewise, pneumococcal infections have fallen drastically among those 0–90 days old one study found, while a separate study did not; neither study was powered to determine vaccine impact on rates of BM in this age group.12,24 There has also been a concurrent decrease in Listeria monocytogenes infection14, possibly related to avoidance of certain foods. On the other hand, incidence of late onset group B streptococcal infections has not been impacted by intrapartum antibiotic chemoprophylaxis.25
It is highly unlikely that any case of BM was missed by this retrospective review. We included infants who had LPs performed at an outside hospital just prior to transfer, if the CSF analysis was performed in our hospital’s laboratories. We did not attempt to capture those FYI who were managed outside of the existing clinical practice, because any such patients, managed without an LP, likely met low-risk criteria, including them likely would have further supported the practice of not routinely performing an LP.
Our study has several limitations. Given the retrospective nature, it was possible that some patients may have been misclassified with respect to their SBI risk. However, efforts were made to minimize this including having two investigators independently perform chart reviews. Also, much of the risk classification criteria are quantitative rather than qualitative measures. Other investigators have included biomarkers such as procalcitonin as one component of a screening tool for SBI.26,27 In the current study, procalcitonin levels were not performed as part of the diagnostic evaluation of infants during the study period. Given the low overall incidence of BM, the sample size was relatively small. Importantly, these data do not prove that febrile infants 29–56 days of age meeting low-risk criteria are at substantially lower risk for BM compared with others. However, this nearly 7 year experience demonstrates that febrile infants 29–56 days old cared for in a tertiary care referral center who otherwise met low-risk criteria are at very low risk for BM. A multicenter study including hundreds of thousands of infants would need to be performed to determine the true prevalence of BM in low-risk patients compared with others and to assess the impact of excluding CSF analysis as a variable used to define low risk. Absent such a study, clinicians must continue to weigh the risks (LP failure rates, traumatic LPs leading to unnecessary hospitalizations, increased length of stays in the ED, and costs) and benefits (earlier detection of BM) of routinely performing the LP in otherwise low-risk febrile infants greater than 28 days of age.
Acknowledgments
We thank Catherine Botos, BS for her assistance with data collection, management, and analysis. We also thank Damaris Amaya, MSN, CRNP, Ashley Woodford, BS, and Mary Kate Funari, MSN, RN, CPEN for their assistance with data collection and analysis.
F. B. supported by NIH NHLBI (K12-HL109009) and NICHD (K23-HD082368).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Edited by SSL and WFB
The authors declare no conflicts of interest.
References
- 1.Baskin MN. The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22:462–6. doi: 10.3928/0090-4481-19930801-06. [DOI] [PubMed] [Google Scholar]
- 2.Baraff LJ, Oslund SA, Schriger DL, Stephen ML. Probability of bacterial infections in febrile infants less than three months of age: a meta-analysis. Pediatr Infect Dis J. 1992;11:257–64. doi: 10.1097/00006454-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Byington CL, Enriquez FR, Hoff C, Tuohy R, Taggart EW, Hillyard DR, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatr. 2004;113:1662–6. doi: 10.1542/peds.113.6.1662. [DOI] [PubMed] [Google Scholar]
- 4.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. New Engl J Med. 1993;329:1437–41. doi: 10.1056/NEJM199311113292001. [DOI] [PubMed] [Google Scholar]
- 5.Baker MD, Avner JR, Bell LM. Failure of infant observation scales in detecting serious illness in febrile 4–8 week old infants. Pediatr. 1990;85:1040–3. [PubMed] [Google Scholar]
- 6.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatr. 2001;108:311–6. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
- 7.Long SS. Approach to the febrile patient with no obvious focus of infection. Pediatr Rev. 1984;5:305–315. [Google Scholar]
- 8.Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120:22–7. doi: 10.1016/s0022-3476(05)80591-8. [DOI] [PubMed] [Google Scholar]
- 9.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–41. doi: 10.1056/NEJM199311113292001. [DOI] [PubMed] [Google Scholar]
- 10.Jaskiewicz JA, McCarthy CA, Richardson AC, White KC, Fisher DJ, Dagan R, et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the rochester criteria and implications for management. Pediatrics. 1994;94:390–6. [PubMed] [Google Scholar]
- 11.Aronson PL, Thurm C, Alpern ER, Alessandrini EA, Williams DJ, Shah SS, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatr. 2014;134:667–77. doi: 10.1542/peds.2014-1382. [DOI] [PubMed] [Google Scholar]
- 12.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 13.Peltola H, Salo E, Saxen H. Incidence of haemophilus influenzae type b meningitis during 18 years of vaccine use: observational study using routine hospital data. BMJ. 2005;330:18–9. doi: 10.1136/bmj.38301.657014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cioffredi L, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170:794–800. doi: 10.1001/jamapediatrics.2016.0596. [DOI] [PubMed] [Google Scholar]
- 15.Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28–60 days of age. Acad Emerg Med. 2015;22:240–3. doi: 10.1111/acem.12582. [DOI] [PubMed] [Google Scholar]
- 16.ACEP Clinical Policies Committee and Clinical Policies Subcommittee on Pediatric Fever. Clinical Policy for Children Younger Than Three Years Presenting to the Emergency Department With Fever. Ann Emerg Med. 2003;42:530–45. doi: 10.1067/s0196-0644(03)00628-0. [DOI] [PubMed] [Google Scholar]
- 17.Aronson PL, Thurm C, Williams DJ, Nigrovic LE, Alpern ER, Tieder JS, et al. Association of clinical practice guidelines with emergency department management of febrile infants < 56 days of age. J Hosp Med. 2015 doi: 10.1002/jhm.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meehan WP, Fleegler E, Bachur RG. Adherence to guidelines for managing the well-appearing febrile infant. Pediatr Emerg Care. 2010;26:875–80. doi: 10.1097/PEC.0b013e3181fe90d1. [DOI] [PubMed] [Google Scholar]
- 19.Jain S, Cheng J, Alpem ER, Thurm C, Schroeder L, Black K, et al. Management of febrile neonates in US pediatric emergency departments. Pediatr. 2014;133:187–195. doi: 10.1542/peds.2013-1820. [DOI] [PubMed] [Google Scholar]
- 20.Byington CL, Reynolds CC, Korgenski K, Sheng X, Valentine KJ, Nelson RE, et al. Costs and infant outcomes after implementation of a care process model for febrile infants. Pediatr. 2012;130:e16–24. doi: 10.1542/peds.2012-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huppler AR, Eickhoff JC, Wald ER. Performance of low risk criteria in the evaluation of young infants with fever: review of the literature. Pediatr. 2010;125:228–33. doi: 10.1542/peds.2009-1070. [DOI] [PubMed] [Google Scholar]
- 22.Martinez E, Mintegi S, Vilar B, Martinez MJ, Lopez A, Catediano E, et al. Prevalence and predictors of bacterial meningitis in young infants with fever without a source. Pediatr Infect Dis J. 2015;34:494–8. doi: 10.1097/INF.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 23.Mintegi S, Gomez B, Martinez-Virumbrales L, Morientes O, Benito J. Outpatient management of selected young febrile infants without antibiotics. Arch Dis Child. 2016;0:1–6. doi: 10.1136/archdischild-2016-310600. [DOI] [PubMed] [Google Scholar]
- 24.Olarte L, Ampofo K, Stockman C, Mason EO, Daly JA, Pavia AT, et al. Invasive pneumococcal disease in infants younger than 90 days before and after introduction of PCV7. Pediatr. 2013;132:e17–e24. doi: 10.1542/peds.2012-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 26.Gomez B, Mintegi S, Bressan S, Da Dalt L, Gervaix A, Lacroix L. Validation of the “Step by Step” approach in the management of young febrile infants. Pediatr. 2016;138:e21054381. doi: 10.1542/peds.2015-4381. [DOI] [PubMed] [Google Scholar]
- 27.Milcent K, Faesch S, Gras-Le Guen C, Dubus F, Poulalhon C, Badier I, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62–69. doi: 10.1001/jamapediatrics.2015.3210. [DOI] [PubMed] [Google Scholar]


