ABSTRACT
Increased Prostate Specific Membrane Antigen expression promotes tumor progression in prostate epithelium by dysregulating the β1-integrin/type I insulin-like growth factor receptor axis, resulting in a shift in signaling from the less aggressive mitogen-activated protein kinase-extracellular signal-regulated kinases 1 and 2 pathway to the pro-survival protein kinase B(AKT)/phosphatidylinositol 3-kinase pathway.
KEYWORDS: MAPK, PI3K-AKT, Prostate Cancer, PSMA, Tumor
Prostate cancer (PC) is the second leading cause of cancer death in males worldwide (https://seer.cancer.gov). Currently, patients with metastatic spread receive chemotherapy combined with targeted pharmacological manipulation of the androgen receptor and its signaling pathways. Although this regimen improves the survival and quality of life, most tumors ultimately develop drug resistance1 with no effective treatment, consistent with an adaptation to a more pro-survival phenotype to support tumor growth, tissue remodeling and cancer metastasis, thus highlighting the need for improved methods of diagnosis, detection and management of PC.
The persistence of cancerous cells commonly involves changes in the activation of receptor tyrosine kinases (RTK), such as the type I insulin-like growth factor receptor (IGF-1R). Increased abundance of IGF-1R on the cell surface of primary and metastatic tumors is frequently observed in many cancers, including PC, suggesting that it could be a potential target for drug therapies. Unfortunately, results from recent clinical trials targeting the IGF-1R pathway have been disappointing,2 signifying a more complex system is involved. Interestingly, unsolicited crosstalk between critical oncogenic signaling pathways and the abnormal activation of distinct pathways have both been implicated in cancer promotion, demonstrated by the aberrant interaction between the IGF-1R and the β-integrin signaling pathways discovered in PC,3 among others.4 Therefore, identification of the global and unique contributors to these pathways and their role in disease progression are critical for the improvement of PC patient outcome.
Prostate Specific Membrane Antigen (PSMA) is a type II transmembrane peptidase enzyme that is encoded by the folate hydrolase 1 (FOLH1) gene. Although there are many names for this enzyme, those studying PC use the term PSMA, which we use here. Historically, PSMA has been used as a reliable clinical biomarker for the detection and localization of PC due to its progressive upregulation on tumor cells during PC progression which correlates negatively with prognosis.5 A wide variety of imaging agents that target PSMA have been described with many currently in clinical trials (www.clinicaltrials.gov), highlighting the continued interest in PSMA in biomedical, translational medicine and pharmaceutical fields.6 While we have previously shown that endothelial-expressed PSMA regulates angiogenesis7 and retinal neovascularization8 primarily via β1 integrin-mediated cell adhesion, an important functional role for PSMA in PC had not yet been established.
In our recent work published in Science Signaling,9 we demonstrate that expression of PSMA in prostatic epithelial cells directly underlies prostate tumor progression in vivo by crossing FOLH1 global knockout mice10 (hereafter called PSMA knockout) with the well-characterized TRansgenic Adenocarcinoma of the Mouse Prostate (TRAMP) murine model to study PC tumor progression in the absence of PSMA expression. We observed that the primary tumors in wild-type animals were larger than tumors in the PSMA knockout animals and were highly vascularized, with higher rates of progression from hyperplasia to adenocarcinoma.
To quantitatively confirm our observation that the lack of PSMA delayed tumor development, we analyzed markers of hypoxia (carbonic anhydrase IX), apoptosis (survivin, caspase-3, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling(TUNEL), cleaved poly(adenosine-5′-diphosphate-ribose) polymerase(PARP)-Asp241) and proliferation (Ki67). We found that PSMA-positive tumor cells were able to survive at greater distances from the vasculature with less cell death than PSMA knockout cells, suggesting that PSMA provides important cell-intrinsic survival components that also contribute to tumor growth.
Tumor cells often escape apoptosis by modifying the expression or activation status of RTKs to induce hyper-activation of pro-survival signal transduction pathways and preserve cell viability. We found that wild-type tumors expressed greater amounts of IGF-1R and exhibited greater activation of the oncogenic phosphatidylinositol 3-kinase (PI3K)–protein kinase B (AKT) pathway, whereas tumors lacking PSMA not only had decreased IGF-1R expression but also had diverted signaling downstream of PI3K-AKT to the mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathway, consistent with a PSMA-dependent signaling switch. Manipulation of PSMA expression by clustered regularly interspaced short palindromic repeats (CRISPR), short interfering RNA (siRNA) or overexpression in mouse and human PC cell lines mimicked this switch.
As mentioned previously, disrupted interactions between components of signaling pathways are frequently responsible for alterations in downstream signaling pathways during tumorigenesis. We found that when PSMA was present in a protein complex with the cell scaffolding protein receptor for activated C kinases (RACK1), signaling between β1-integin and IGF-1R was disrupted, resulting in activation of the PI3K-AKT pathway instead of the canonical MAPK-ERK1/2 pathway (Fig. 1).
Figure 1.
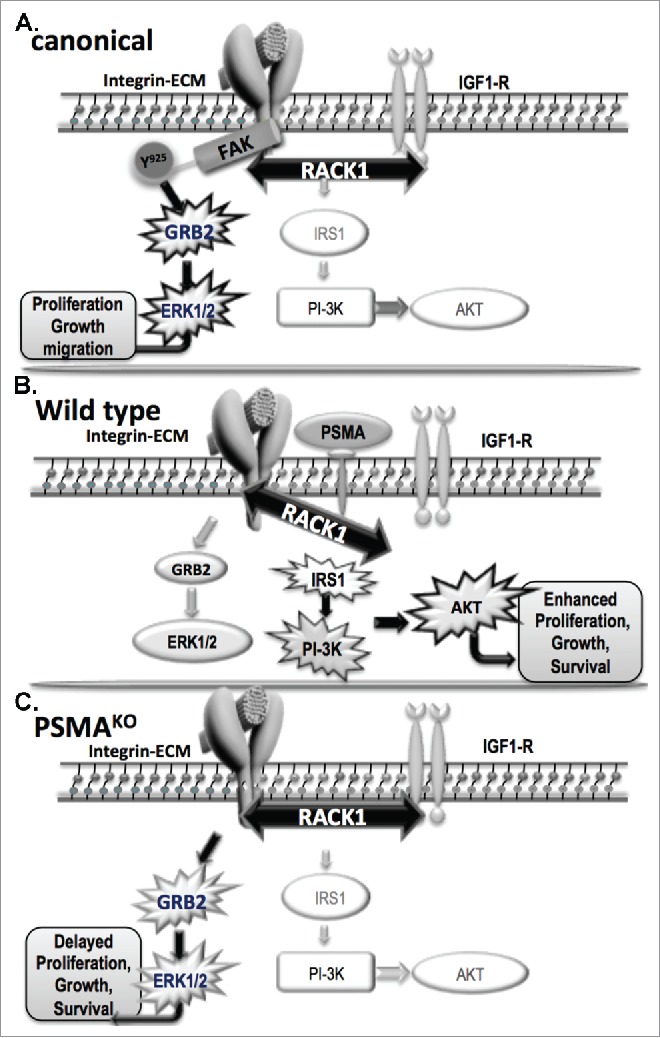
PSMA-dependent regulation of signal transduction in prostate cancer: In the canonical (A) pathway, a stable complex comprised of IGF-1R, RACK1 and β1-integrin activate FAK by phosphorylation at tyrosine925 leading to the activation of the GRB2-ERK1/2 pathway resulting in proliferation, growth and migration of tumor cells. When PSMA is present, such as in our wild-type (B) model, it physically associates with RACK1 and signaling between β1-integin and IGF-1R is disrupted, resulting in activation of the PI3K-AKT pathway instead of the canonical GRB2-ERK 1/2 pathway. In the absence of PSMA, such as in our PSMAKO (C.), the complex of IGF-R1, RACK1, β1-integrin is allowed to form and activates the GRB2-ERK1/2 pathway resulting in delayed proliferation, growth and survival and therefore smaller tumors. Key: Prostate Specific Membrane Antigen (PSMA); type I insulin growth factor receptor (IGF-1R); receptor for activated C kinases (RACK1); focal adhesion kinase (FAK); growth factor receptor–bound protein 2 (GRB2); extracellular signal-regulated kinases 1 and 2 (ERK1/2); phosphatidylinositol 3-kinase (PI3K); protein kinase B (AKT); insulin receptor substrate 1 (IRS-1). PSMAKO refers to PSMA knockout in mouse prostate and prostate cancer cell lines.
To investigate the link between PSMA expression levels and tumor progression in human PC, we examined publically available PC gene expression data sets (www.ncbi.nlm.nih.gov/geo/ and http://seek.princeton.edu). Analysis confirmed that high PSMA expression was predictive of a high Gleason score. Additionally, 59 patient samples with high PSMA expression and high Gleason scores displayed a pro-survival gene expression signature with increased expression of the anti-apoptotic marker survivin as well as IGF-1R when compared to matched, benign controls, which is consistent with a role for PSMA in the regulation of signal transduction in human PC disease as well.
In addition to its role as a PC marker and target, our results indicate that increasing amounts of PSMA in prostate tumor epithelium serve to drive pro-survival mechanisms and thus identify it as a functional regulator of prostate tumor progression. These findings also suggest that PSMA-positive tumors may be more sensitive to PI3K pathway inhibitors and less sensitive to MAPK pathway inhibitors, thus providing an alternative approach to treatment of PC patients as well as patients with other PSMA-positive cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH NCI Mentored Research Scientist Development Award to Promote Diversity (K01CA188412) to L.A.C. and the Prostate Cancer Foundation and the Department of Defense Prostate Cancer Program (PC073976) to L.H.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015; 15:701-11; PMID:26563462; https://doi.org/ 10.1038/nrc4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen HX, Sharon E. IGF-1R as an anti-cancer target–trials and tribulations. Chin J Cancer 2013; 32:242-52; PMID:23601239; https://doi.org/ 10.5732/cjc.012.10263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trerotola M, Li J, Alberti S, Languino LR. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the beta1 integrin-RACK1 axis. J Cell Physiol 2012; 227:3670-7; PMID:22378065; https://doi.org/ 10.1002/jcp.24074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edderkaoui M, Hong P, Lee JK, Pandol SJ, Gukovskaya AS. Insulin-like growth factor-I receptor mediates the prosurvival effect of fibronectin. J Biol Chem 2007; 282:26646-55; PMID:17627944; https://doi.org/ 10.1074/jbc.M702836200 [DOI] [PubMed] [Google Scholar]
- 5.Heston WD. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: A novel folate hydrolase. Urology 1997; 49:104-12; PMID:9123729; https://doi.org/ 10.1016/S0090-4295(97)00177-5 [DOI] [PubMed] [Google Scholar]
- 6.Dassie JP, Hernandez LI, Thomas GS, Long ME, Rockey WM, Howell CA, Chen Y, Hernandez FJ, Liu XY, Wilson ME, et al.. Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen. Mol Ther 2014; 22:1910-22; PMID:24954476; https://doi.org/ 10.1038/mt.2014.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway RE, Joiner K, Patterson A, Bourgeois D, Rampp R, Hannah BC, McReynolds S, Elder JM, Gilfilen H, Shapiro LH. Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis 2013; 16:847-60; PMID:23775497; https://doi.org/ 10.1007/s10456-013-9360-y [DOI] [PubMed] [Google Scholar]
- 8.Grant CL, Caromile LA, Ho V, Durrani K, Rahman MM, Claffey KP, Fong GH, Shapiro LH. Prostate specific membrane antigen (PSMA) regulates angiogenesis independently of VEGF during ocular neovascularization. PLoS One 2012; 7:e41285; PMID:22815987; https://doi.org/ 10.1371/journal.pone.0041285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caromile LA, Dortche K, Rahman MM, Grant CL, Stoddard C, Ferrer FA, Shapiro LH. PSMA redirects cell survival signaling from the MAPK to the PI3K-AKT pathways to promote the progression of prostate cancer. Sci Signal 2017; 10:eaag3326; PMID:28292957; https://doi.org/ 10.1126/scisignal.aag3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacich DJ, Ramadan E, O'Keefe DS, Bukhari N, Wegorzewska I, Ojeifo O, Olszewski R, Wrenn CC, Bzdega T, Wroblewska B, et al.. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J Neurochem 2002; 83:20-9; PMID:12358725; https://doi.org/ 10.1046/j.1471-4159.2002.01117.x [DOI] [PubMed] [Google Scholar]


